Review on Activated Carbons by Chemical Activation with FeCl3
Abstract
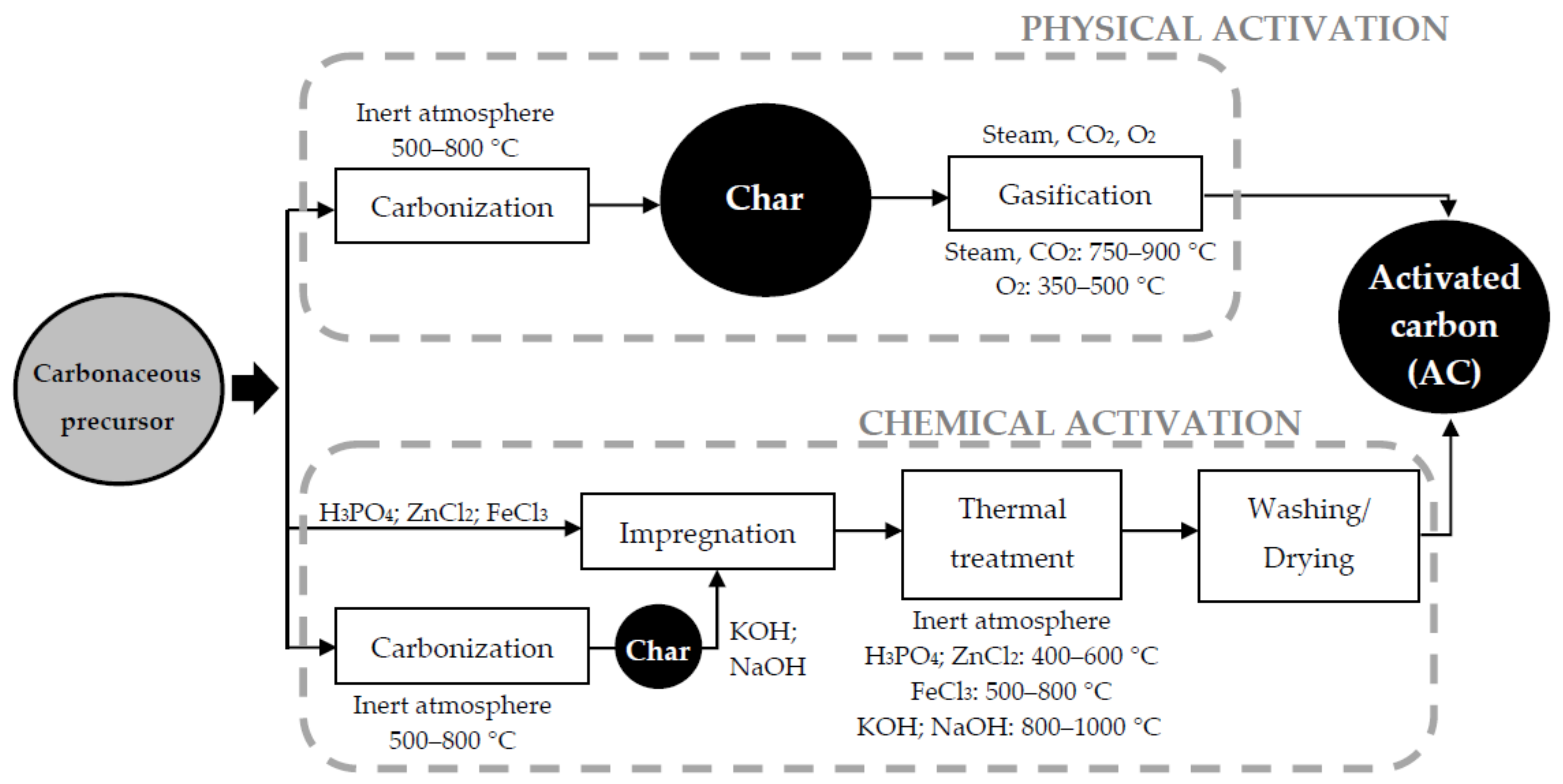
1. Activated Carbons
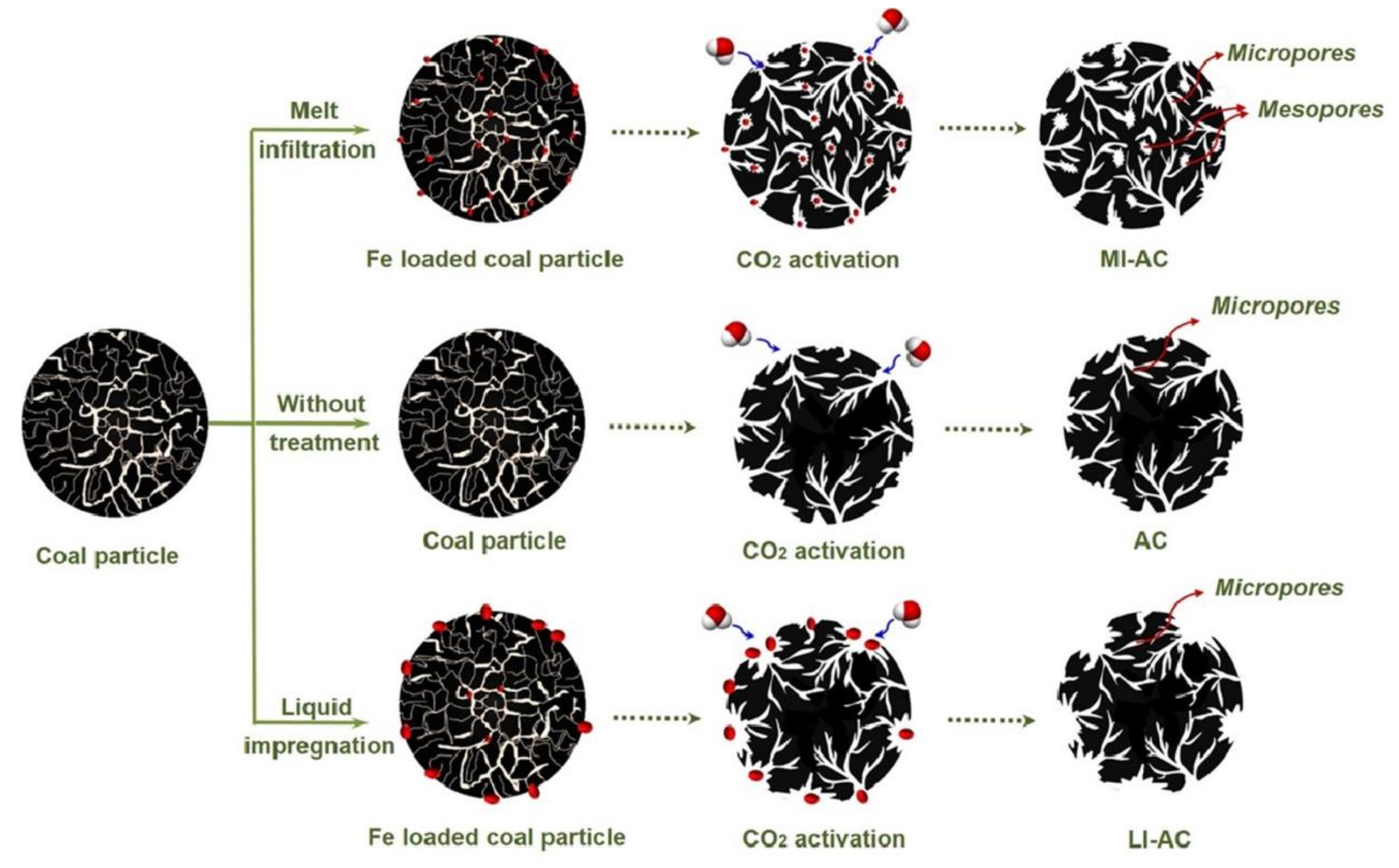
2. Activation Mechanism
3. Conditions of Chemical Activation with FeCl3
Mixtures of Activating Agents
4. Characterization of the Activated Carbons
4.1. Porous Texture
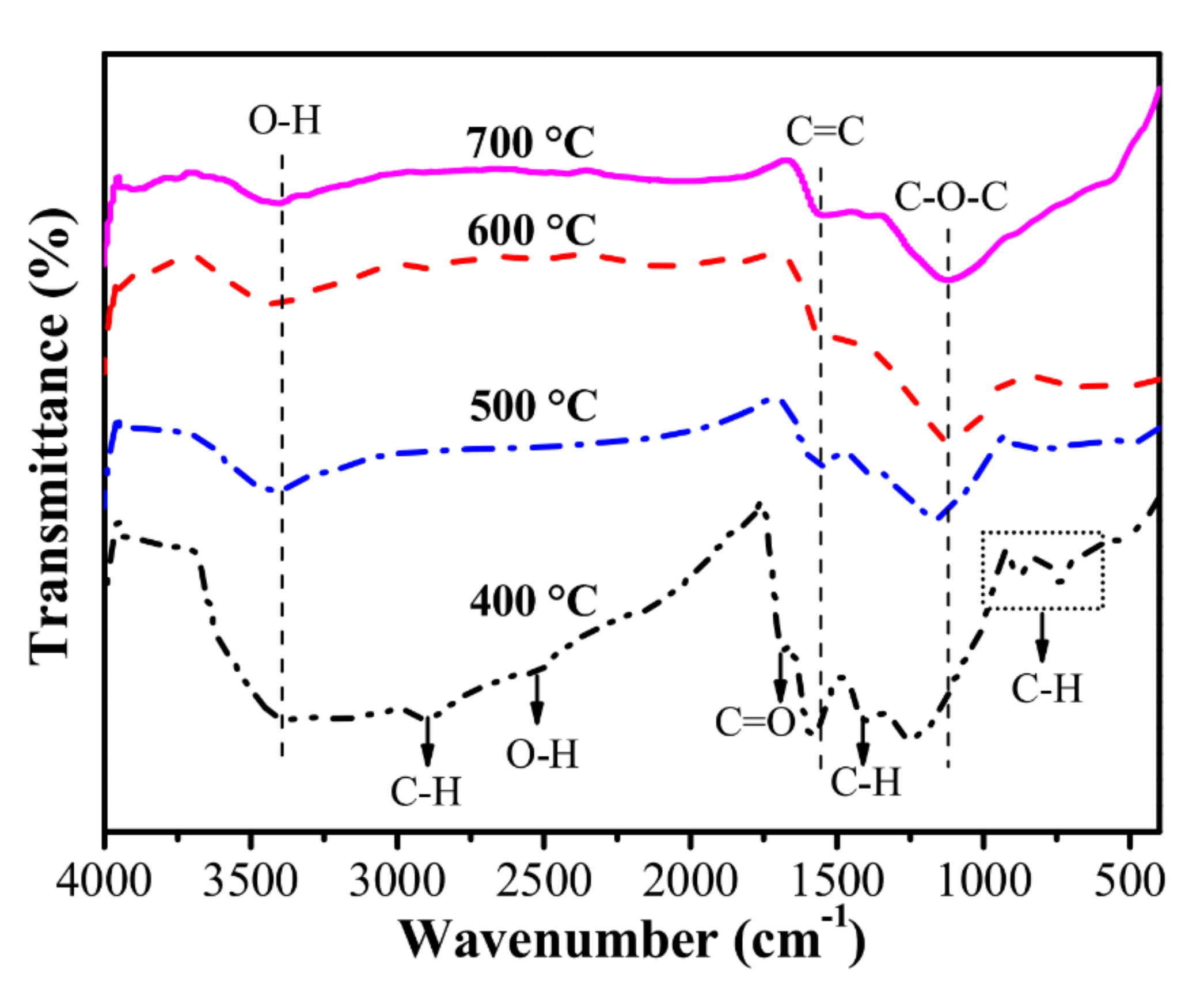
4.2. Surface Chemistry
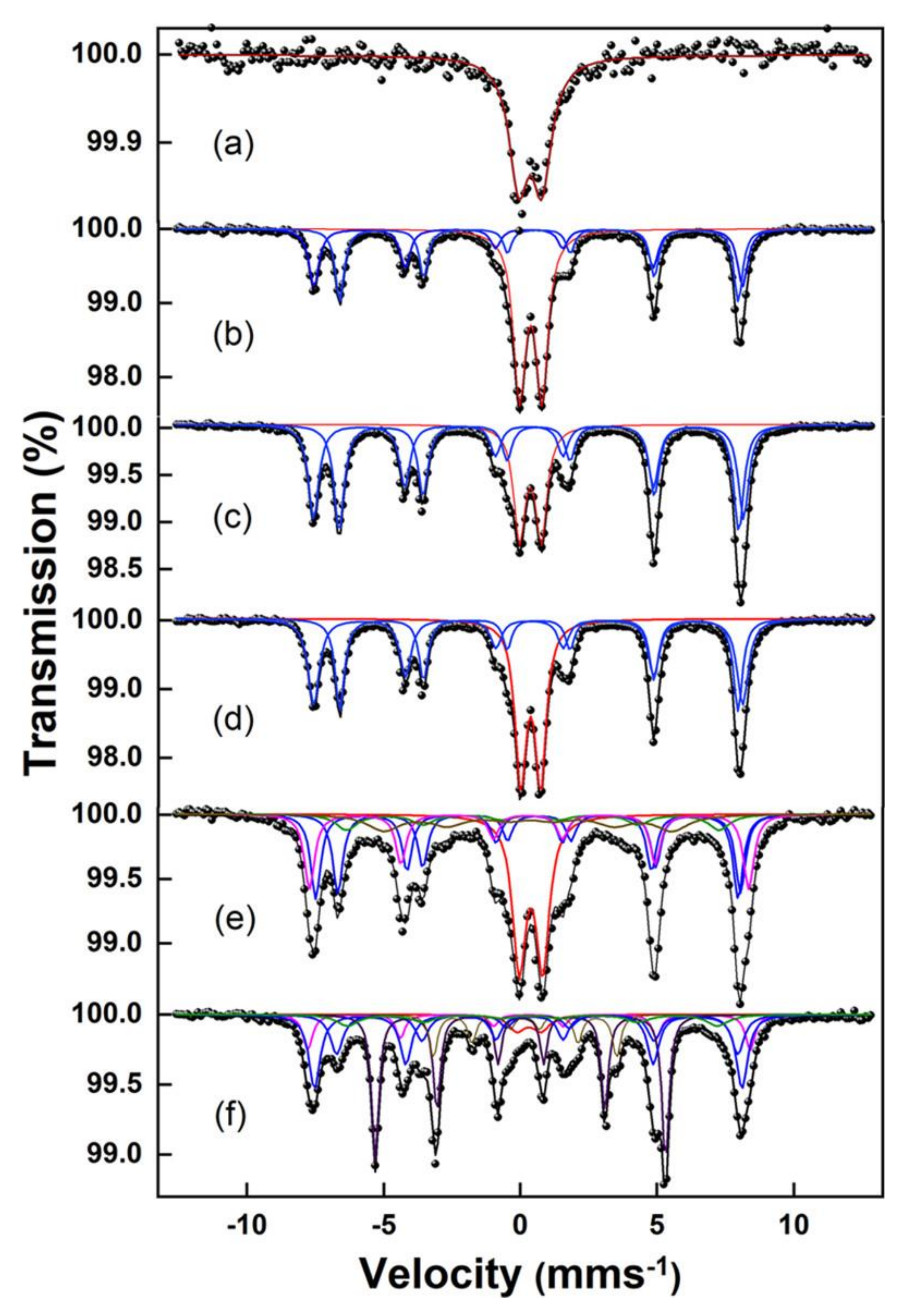
4.3. Magnetic Properties
5. Applications
5.1. Adsorption
5.2. Catalysis
5.3. Energy Storage
6. Conclusions and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Elsevier Ltd.: Amsterdam, The Netherlands, 2006; ISBN 9780080444635. [Google Scholar]
- Çeçen, F.; Aktaş, Ö. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9783527324712. [Google Scholar]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9781420028812. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780080970356. [Google Scholar]
- Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd supported on mesoporous activated carbons with high oxidation resistance as catalysts for toluene oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Martin-Martinez, M.; Gómez-Sainero, L.M.; Alvarez-Montero, M.A.; Bedia, J.; Rodriguez, J.J. Comparison of different precious metals in activated carbon-supported catalysts for the gas-phase hydrodechlorination of chloromethanes. Appl. Catal. B Environ. 2013, 132–133, 256–265. [Google Scholar] [CrossRef]
- Palomo, J.; Rodríguez-Cano, M.A.; Rodríguez-Mirasol, J.; Cordero, T. On the kinetics of methanol dehydration to dimethyl ether on Zr-loaded P-containing mesoporous activated carbon catalyst. Chem. Eng. J. 2019, 378, 122198. [Google Scholar] [CrossRef]
- Fang, R.; Huang, H.; Ji, J.; He, M.; Feng, Q.; Zhan, Y.; Leung, D.Y.C. Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature. Chem. Eng. J. 2018, 334, 2050–2057. [Google Scholar] [CrossRef]
- Rey, A.; Hungria, A.B.; Duran-Valle, C.J.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Rodriguez, J.J. On the optimization of activated carbon-supported iron catalysts in catalytic wet peroxide oxidation process. Appl. Catal. B Environ. 2016, 181, 249–259. [Google Scholar] [CrossRef]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of the decomposition of 2-butanol on carbon-based acid catalyst. AIChE J. 2010, 56, 1557–1568. [Google Scholar] [CrossRef]
- Cordero, T.; Rodríguez-Mirasol, J.; Bedia, J.; Gomis, S.; Yustos, P.; García-Ochoa, F.; Santos, A. Activated carbon as catalyst in wet oxidation of phenol: Effect of the oxidation reaction on the catalyst properties and stability. Appl. Catal. B Environ. 2008, 81, 122–131. [Google Scholar] [CrossRef]
- Valero-Romero, M.J.; Calvo-Muñoz, E.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus-Containing Mesoporous Carbon Acid Catalyst for Methanol Dehydration to Dimethyl Ether. Ind. Eng. Chem. Res. 2019, 58, 4042–4053. [Google Scholar] [CrossRef]
- Rey, A.; Faraldos, M.; Bahamonde, A.; Casas, J.A.; Zazo, J.A.; Rodríguez, J.J. Role of the Activated Carbon Surface on Catalytic Wet Peroxide Oxidation. Ind. Eng. Chem. Res. 2008, 47, 8166–8174. [Google Scholar] [CrossRef]
- Shi, H. Activated carbons and double layer capacitance. Electrochim. Acta 1996, 41, 1633–1639. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef]
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Ibeh, P.O.; García-Mateos, F.J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Activated carbon monoliths from lignocellulosic biomass waste for electrochemical applications. J. Taiwan Inst. Chem. Eng. 2019, 97, 480–488. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Cordero, T.; Rodríguez-Mirasol, J. Carbon Materials from Lignin and Their Applications. In Production of Biofuels and Chemicals from Lignin; Springer: Berlin/Heidelberg, Germany, 2016; pp. 217–262. ISBN 978-981-10-1964-7. [Google Scholar]
- Pui, W.K.; Yusoff, R.; Aroua, M.K. A review on activated carbon adsorption for volatile organic compounds (VOCs). Rev. Chem. Eng. 2019, 35, 649–668. [Google Scholar] [CrossRef]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.; Belver, C. A Review on the Synthesis and Characterization of Biomass-Derived Carbons for Adsorption of Emerging Contaminants from Water. C. J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef]
- Lakshmi, S.D.; Avti, P.K.; Hegde, G. Activated carbon nanoparticles from biowaste as new generation antimicrobial agents: A review. Nano-Struct. Nano-Objects 2018, 16, 306–321. [Google Scholar] [CrossRef]
- Labus, K.; Gryglewicz, S.; Machnikowski, J. Granular KOH-activated carbons from coal-based cokes and their CO2 adsorption capacity. Fuel 2014, 118, 9–15. [Google Scholar] [CrossRef]
- Chingombe, P.; Saha, B.; Wakeman, R.J. Surface modification and characterisation of a coal-based activated carbon. Carbon N. Y. 2005, 43, 3132–3143. [Google Scholar] [CrossRef]
- Bagreev, A.; Menendez, J.A.; Dukhno, I.; Tarasenko, Y.; Bandosz, T.J. Bituminous coal-based activated carbons modified with nitrogen as adsorbents of hydrogen sulfide. Carbon N. Y. 2004, 42, 469–476. [Google Scholar] [CrossRef]
- Acevedo, B.; Barriocanal, C.; Lupul, I.; Gryglewicz, G. Properties and performance of mesoporous activated carbons from scrap tyres, bituminous wastes and coal. Fuel 2015, 151, 83–90. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S.; Tyagi, I. Potential of activated carbon from waste rubber tire for the adsorption of phenolics: Effect of pre-treatment conditions. J. Colloid Interface Sci. 2014, 417, 420–430. [Google Scholar] [CrossRef]
- Ramírez-Arias, A.M.; Moreno-Piraján, J.C.; Giraldo, L. Adsorption of Triton X-100 in aqueous solution on activated carbon obtained from waste tires for wastewater decontamination. Adsorption 2020, 26, 303–316. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of SO2 removal over lignin-based activated carbon. Chem. Eng. J. 2017, 307, 707–721. [Google Scholar] [CrossRef]
- Cotoruelo, L.M.; Marqués, M.D.; Díaz, F.J.; Rodríguez-Mirasol, J.; Rodríguez, J.J.; Cordero, T. Adsorbent ability of lignin-based activated carbons for the removal of p-nitrophenol from aqueous solutions. Chem. Eng. J. 2012, 184, 176–183. [Google Scholar] [CrossRef]
- Rodríguez-Mirasol, J.; Bedia, J.; Cordero, T.; Rodríguez, J. Influence of water vapor on the adsorption of VOCs on lignin-based activated carbons. Sep. Sci. Technol. 2005, 40, 3113–3135. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, Y.; Fu, Y.; Zhang, N. Activated carbons synthesized from unaltered and pelletized biomass wastes for bio-tar adsorption in different phases. Renew. Energy 2020, 146, 1700–1709. [Google Scholar] [CrossRef]
- Liou, T.H. Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Rosas, J.M.; Bedia, J.; Rodríguez-Mirasol, J.; Cordero, T. Preparation of Hemp-Derived Activated Carbon Monoliths. Adsorption of Water Vapor. Ind. Eng. Chem. Res. 2008, 47, 1288–1296. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Ye, N.; Wang, Z.; Wang, S.; Peijnenburg, W.J.G.M. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef]
- Holmes, A.M.; Mackenzie, L.; Roberts, M.S. Disposition and measured toxicity of zinc oxide nanoparticles and zinc ions against keratinocytes in cell culture and viable human epidermis. Nanotoxicology 2020, 14, 263–274. [Google Scholar] [CrossRef]
- Demarchi, C.A.; Michel, B.S.; Nedelko, N.; Ślawska-Waniewska, A.; Dłużewski, P.; Kaleta, A.; Minikayev, R.; Strachowski, T.; Lipińska, L.; Dal Magro, J.; et al. Preparation, characterization, and application of magnetic activated carbon from termite feces for the adsorption of Cr(VI) from aqueous solutions. Powder Technol. 2019, 354, 432–441. [Google Scholar] [CrossRef]
- Cesano, F.; Cravanzola, S.; Brunella, V.; Damin, A.; Scarano, D. From Polymer to Magnetic Porous Carbon Spheres: Combined Microscopy, Spectroscopy, and Porosity Studies. Front. Mater. 2019, 6, 84. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, Z.; Zhou, Y.; Chen, W.; Zhang, T.; Huang, Y.; Zhang, D. Insights into the pyrolysis behavior and adsorption properties of activated carbon from waste cotton textiles by FeCl3-activation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123934. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Sun, Z.; Zhang, D.; Huang, Y.; Gu, S.; Chen, W. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon. Chemosphere 2020, 241, 125120. [Google Scholar] [CrossRef]
- Zhu, X.; Qian, F.; Liu, Y.; Matera, D.; Wu, G.; Zhang, S.; Chen, J. Controllable synthesis of magnetic carbon composites with high porosity and strong acid resistance from hydrochar for efficient removal of organic pollutants: An overlooked influence. Carbon N. Y. 2016, 99, 338–347. [Google Scholar] [CrossRef]
- Vinu, R.; Broadbelt, L.J. A mechanistic model of fast pyrolysis of glucose-based carbohydrates to predict bio-oil composition. Energy Environ. Sci. 2012, 5, 9808–9826. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Pezoti, O.; Bedin, K.C.; Silva, T.L.; Paesano Junior, A.; Asefa, T.; Almeida, V.C. Magnetic Activated Carbon Derived from Biomass Waste by Concurrent Synthesis: Efficient Adsorbent for Toxic Dyes. ACS Sustain. Chem. Eng. 2016, 4, 1058–1068. [Google Scholar] [CrossRef]
- Gong, X.; Guo, Z.; Wang, Z. Variation of char structure during anthracite pyrolysis catalyzed by Fe2O3 and its influence on char combustion reactivity. Energy Fuels 2009, 23, 4547–4552. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Falco, C.; Perez Caballero, F.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.M.; Baccile, N. Hydrothermal carbon from biomass: Structural differences between hydrothermal and pyrolyzed carbons via 13C solid state NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, Y.; Luo, G.; Qian, F.; Zhang, S.; Chen, J. Facile fabrication of magnetic carbon composites from hydrochar via simultaneous activation and magnetization for triclosan adsorption. Environ. Sci. Technol. 2014, 48, 5840–5848. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Li, R.; Wu, Y.; Yang, M. A modified kinetic analysis method of cellulose pyrolysis based on TG–FTIR technique. Thermochim. Acta 2018, 665, 20–27. [Google Scholar] [CrossRef]
- Stoeva, S.; Karaivanova, M.; Benev, D. Poly(vinyl chloride) composition. II. Study of the flammability and smoke-evolution of unplasticized poly(vinyl chloride) and fire-retardant additives. J. Appl. Polym. Sci. 1992, 46, 119–127. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Han, X.; Zhang, X.; Yi, M.; Yang, S.; Yu, D.; Liu, W. Catalytic Dechlorination and Charring Reaction of Polyvinyl Chloride by CuAl Layered Double Hydroxide. Energy Fuels 2018, 32, 2407–2413. [Google Scholar] [CrossRef]
- Zazo, J.A.; Bedia, J.; Fierro, C.M.; Pliego, G.; Casas, J.A.; Rodriguez, J.J. Highly stable Fe on activated carbon catalysts for CWPO upon FeCl3 activation of lignin from black liquors. Catal. Today 2012, 187, 115–121. [Google Scholar] [CrossRef]
- Mohedano, A.F.; Monsalvo, V.M.; Bedia, J.; Lopez, J.; Rodriguez, J.J. Highly stable iron catalysts from sewage sludge for CWPO. J. Environ. Chem. Eng. 2014, 2, 2359–2364. [Google Scholar] [CrossRef]
- Andrijanto, E.; Purwaningsih, I.; Silvia, L.; Subiyanto, G.; Hulupi, M. The conversion of biomass into carbon electrode material using FeCl3 as an activating agent for battery application. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019; Volume 299, p. 012001. [Google Scholar]
- Tian, D.; Xu, Z.; Zhang, D.; Chen, W.; Cai, J.; Deng, H.; Sun, Z.; Zhou, Y. Micro–mesoporous carbon from cotton waste activated by FeCl3/ZnCl2: Preparation, optimization, characterization and adsorption of methylene blue and eriochrome black T. J. Solid State Chem. 2019, 269, 580–587. [Google Scholar] [CrossRef]
- Chen, C.; Mi, S.; Lao, D.; Shi, P.; Tong, Z.; Li, Z.; Hu, H. Single-step synthesis of eucalyptus sawdust magnetic activated carbon and its adsorption behavior for methylene blue. RSC Adv. 2019, 9, 22248–22262. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, N.; Feng, C.; Gao, Y. Mechanisms of Cr(VI) removal by FeCl3-modified lotus stem-based biochar (FeCl3@LS-BC) using mass-balance and functional group expressions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 17–24. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Ma, S.; Zhu, B.; Zhang, J.; Zheng, C. Mercury Removal by Magnetic Biochar Derived from Simultaneous Activation and Magnetization of Sawdust. Environ. Sci. Technol. 2016, 50, 12040–12047. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Adsorptive removal of p-nitrophenol on microporous activated carbon by FeCl3 activation: Equilibrium and kinetics studies. Desalin. Water Treat. 2015, 55, 522–531. [Google Scholar] [CrossRef]
- Theydan, S.K.; Ahmed, M.J. Adsorption of methylene blue onto biomass-based activated carbon by FeCl3 activation: Equilibrium, kinetics, and thermodynamic studies. J. Anal. Appl. Pyrolysis 2012, 97, 116–122. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Sun, Y.; Wang, Y.; Li, Q.; Zhao, P.; Chen, S. Physicochemical and adsorptive properties of activated carbons from Arundo donax Linn utilizing different iron salts as activating agents. J. Taiwan Inst. Chem. Eng. 2014, 45, 3007–3015. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. A comparative study of chemical treatment by FeCl3, MgCl2, and ZnCl2 on microstructure, surface chemistry, and double-layer capacitance of carbons from waste biomass. J. Mater. Res. 2010, 25, 1451–1459. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Pereira, E.; Guimaraes, I.R.; Vallone, A.; Pereira, M.; Mesquita, J.P.; Sapag, K. Preparation of activated carbons from coffee husks utilizing FeCl3 and ZnCl2 as activating agents. J. Hazard. Mater. 2009, 165, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Diaz, E.; Manzano, F.J.; Villamil, J.; Rodriguez, J.J.; Mohedano, A.F. Low-Cost Activated Grape Seed-Derived Hydrochar through Hydrothermal Carbonization and Chemical Activation for Sulfamethoxazole Adsorption. Appl. Sci. 2019, 9, 5127. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Suárez-Ruiz, I.; Marco, J.F.; Blanco, J.; Fuente, E. Sustainable Thermochemical Single-Step Process to Obtain Magnetic Activated Carbons from Chestnut Industrial Wastes. ACS Sustain. Chem. Eng. 2019, 7, 17293–17305. [Google Scholar] [CrossRef]
- Bedia, J.; Monsalvo, V.M.; Rodriguez, J.J.; Mohedano, A.F. Iron catalysts by chemical activation of sewage sludge with FeCl3 for CWPO. Chem. Eng. J. 2017, 318, 224–230. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Yuan, H.; Chen, Y. Alfalfa Leaf-Derived Porous Heteroatom-Doped Carbon Materials as Efficient Cathodic Catalysts in Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2017, 5, 9766–9773. [Google Scholar] [CrossRef]
- Bedia, J.; Belver, C.; Ponce, S.; Rodriguez, J.; Rodriguez, J.J. Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem. Eng. J. 2018, 333, 58–65. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, C.; Bedia, J.; Bonal, P.; Rodriguez, J.J.; Gómez-Sainero, L.M. Chloroform conversion into ethane and propane by catalytic hydrodechlorination with Pd supported on activated carbons from lignin. Catal. Sci. Technol. 2018, 8, 3926–3935. [Google Scholar] [CrossRef]
- Mojoudi, N.; Soleimani, M.; Mirghaffari, N.; Belver, C.; Bedia, J. Removal of phenol and phosphate from aqueous solutions using activated carbons prepared from oily sludge through physical and chemical activation. Water Sci. Technol. 2019, 80, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Jiang, X.; Jia, X.; Liang, S.; Qian, L.; Rao, Z. Synthesis of biomass carbon electrode materials by bimetallic activation for the application in supercapacitors. J. Electroanal. Chem. 2019, 844, 105–115. [Google Scholar] [CrossRef]
- Thue, P.S.; Adebayo, M.A.; Lima, E.C.; Sieliechi, J.M.; Machado, F.M.; Dotto, G.L.; Vaghetti, J.C.P.; Dias, S.L.P. Preparation, characterization and application of microwave-assisted activated carbons from wood chips for removal of phenol from aqueous solution. J. Mol. Liq. 2016, 223, 1067–1080. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Dai, Y.; Fu, F. High-performance magnetic carbon materials in dye removal from aqueous solutions. J. Solid State Chem. 2016, 239, 265–273. [Google Scholar] [CrossRef]
- Qian, F.; Zhu, X.; Liu, Y.; Hao, S.; Ren, Z.J.; Gao, B.; Zong, R.; Zhang, S.; Chen, J. Synthesis, characterization and adsorption capacity of magnetic carbon composites activated by CO2: Implication for the catalytic mechanisms of iron salts. J. Mater. Chem. A 2016, 4, 18942–18951. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Mao, Y.; Gao, B.; Gao, Y.; Huang, L. Enhanced adsorption of chromium onto activated carbon by microwave-assisted H3PO4 mixed with Fe/Al/Mn activation. J. Hazard. Mater. 2014, 265, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Gómez, J.J.; Villarroel-Rocha, D.; de Freitas-Araújo, K.C.; Martínez-Huitle, C.A.; Sapag, K. Applicability of activated carbon obtained from peach stone as an electrochemical sensor for detecting caffeine. J. Electroanal. Chem. 2018, 822, 171–176. [Google Scholar] [CrossRef]
- Wang, L.; Sun, F.; Gao, J.; Pi, X.; Pei, T.; Qie, Z.; Zhao, G.; Qin, Y. A novel melt infiltration method promoting porosity development of low-rank coal derived activated carbon as supercapacitor electrode materials. J. Taiwan Inst. Chem. Eng. 2018, 91, 588–596. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Wang, L.; Hunt, A.J.; Song, H.; Shang, J.; Ok, Y.S.; Poon, C.S. Aluminium-biochar composites as sustainable heterogeneous catalysts for glucose isomerisation in a biorefinery. Green Chem. 2019, 21, 1267–1281. [Google Scholar] [CrossRef]
- Yang, X.; Yu, I.K.M.; Cho, D.W.; Chen, S.S.; Tsang, D.C.W.; Shang, J.; Yip, A.C.K.; Wang, L.; Ok, Y.S. Tin-Functionalized Wood Biochar as a Sustainable Solid Catalyst for Glucose Isomerization in Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 4851–4860. [Google Scholar] [CrossRef]
- Wan, Z.; Sun, Y.; Tsang, D.C.W.; Yu, I.K.M.; Fan, J.; Clark, J.H.; Zhou, Y.; Cao, X.; Gao, B.; Ok, Y.S. A sustainable biochar catalyst synergized with copper heteroatoms and CO2 for singlet oxygenation and electron transfer routes. Green Chem. 2019, 21, 4800–4814. [Google Scholar] [CrossRef]
- Boudou, J.P.; Bégin, D.; Alain, E.; Furdin, G.; Marêché, J.F.; Albiniak, A. Effects of FeCl3 (intercalated or not in graphite) on the pyrolysis of coal or coal tar pitch. Fuel 1998, 77, 601–606. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Preparation and characterization of activated carbons from Sterculia alata nutshell by chemical activation with zinc chloride to remove phenol from wastewater. Adsorption 2006, 12, 119–132. [Google Scholar] [CrossRef]
- Fu, K.; Yue, Q.; Gao, B.; Wang, Y.; Li, Q. Activated carbon from tomato stem by chemical activation with FeCl2. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 842–849. [Google Scholar] [CrossRef]
- Chiu, K.L.; Ng, D.H.L. Synthesis and characterization of cotton-made activated carbon fiber and its adsorption of methylene blue in water treatment. Biomass Bioenergy 2012, 46, 102–110. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, T.; Yuan, Z.; Zhang, D.; Sun, Z.; Huang, Y.X.; Chen, W.; Tian, D.; Deng, H.; Zhou, Y. Fabrication of cotton textile waste-based magnetic activated carbon using FeCl3 activation by the Box-Behnken design: Optimization and characteristics. RSC Adv. 2018, 8, 38081–38090. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, D.; Sun, Z.; Zhang, D.; Zhou, Y.; Chen, W.; Deng, H. Highly porous activated carbon synthesized by pyrolysis of polyester fabric wastes with different iron salts: Pore development and adsorption behavior. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 180–187. [Google Scholar] [CrossRef]
- Liu, W.J.; Tian, K.; He, Y.R.; Jiang, H.; Yu, H.Q. High-yield harvest of nanofibers/mesoporous carbon composite by pyrolysis of waste biomass and its application for high durability electrochemical energy storage. Environ. Sci. Technol. 2014, 48, 13951–13959. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Ren, J.; Rostam-Abadi, M.; Chang, R. Preparation of functionalized and metal-impregnated activated carbon by a single-step activation method. Appl. Surf. Sci. 2014, 290, 92–101. [Google Scholar] [CrossRef]
- Luo, J.J.; Lu, J.; Niu, Q.; Chen, X.; Wang, Z.; Zhang, J. Preparation and characterization of benzoic acid-modified activated carbon for removal of gaseous mercury chloride. Fuel 2015, 160, 440–445. [Google Scholar] [CrossRef]
- Prakash, R.; Mishra, A.K.; Roth, A.; Kübel, C.; Scherer, T.; Ghafari, M.; Hahn, H.; Fichtner, M. A ferrocene-based carbon-iron lithium fluoride nanocomposite as a stable electrode material in lithium batteries. J. Mater. Chem. 2010, 20, 1871–1876. [Google Scholar] [CrossRef][Green Version]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- Volgmann, K.; Voigts, F.; Maus-Friedrichs, W. The interaction of oxygen molecules with iron films studied with MIES, UPS and XPS. Surf. Sci. 2010, 604, 906–913. [Google Scholar] [CrossRef]
- Murad, E.; Johnston, J.H. Mössbauer Spectroscopy Applied to Inorganic Chemistry; Long, G.J., Grandjean, F., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 507–582. ISBN 9780306430732. [Google Scholar]
- Schaaf, P.; Wiesen, S.; Gonser, U. Mössbauer study of iron carbides: Cementite (Fe, M)3C (M = Cr, Mn) with various manganese and chromium contents. Acta Metall. Mater. 1992, 40, 373–379. [Google Scholar] [CrossRef]
- Rocha, L.S.; Pereira, D.; Sousa, É.; Otero, M.; Esteves, V.I.; Calisto, V. Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: A review. Sci. Total Environ. 2020, 718, 137272. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Chen, H.; Li, H.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B. Preparation, characterization, and application of magnetic activated carbon for treatment of biologically treated papermaking wastewater. Sci. Total Environ. 2020, 713, 136423. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Q.; Li, W.; Xie, X.; Zhang, W.; Zhang, X.; Chai, H.; Huang, Y. Engineering magnetic N-doped porous carbon with super-high ciprofloxacin adsorption capacity and wide pH adaptability. J. Hazard. Mater. 2020, 388, 122059. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Tang, L.; Zhang, F.; Zeng, G.; Peng, X.; Luo, L.; Deng, Y.; Pang, Y.; Zhang, J. Insight into highly efficient co-removal of p-nitrophenol and lead by nitrogen-functionalized magnetic ordered mesoporous carbon: Performance and modelling. J. Hazard. Mater. 2017, 333, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cheng, Y.; Lv, H.; Ji, G.; Du, Y. A novel hierarchically porous magnetic carbon derived from biomass for strong lightweight microwave absorption. Carbon N. Y. 2019, 142, 245–253. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Preparation and characterization of corn cob activated carbon coated with nano-sized magnetite particles for the removal of Cr(VI). Bioresour. Technol. 2013, 134, 94–100. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Cui, H.J.; Cai, J.K.; Zhao, H.; Yuan, B.; Ai, C.; Fu, M.L. One step solvothermal synthesis of functional hybrid γ-Fe2O3/carbon hollow spheres with superior capacities for heavy metal removal. J. Colloid Interface Sci. 2014, 425, 131–135. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Dhedan, S.K. Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib. 2012, 317, 9–14. [Google Scholar] [CrossRef]
- Rashid, J.; Tehreem, F.; Rehman, A.; Kumar, R. Synthesis using natural functionalization of activated carbon from pumpkin peels for decolourization of aqueous methylene blue. Sci. Total Environ. 2019, 671, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-X.; Zhou, Y.-R.; Wang, M.; Zhang, Q.; Ji, T.; Chen, T.-Y.; Yu, D.-C. Adsorption of methylene blue from aqueous solution onto viscose-based activated carbon fiber felts: Kinetics and equilibrium studies. Adsorpt. Sci. Technol. 2019, 37, 312–332. [Google Scholar] [CrossRef]
- Bedin, K.C.; Souza, I.P.A.F.; Cazetta, A.L.; Spessato, L.; Ronix, A.; Almeida, V.C. CO2-spherical activated carbon as a new adsorbent for Methylene Blue removal: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2018, 269, 132–139. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Li, S.; Tong, Y.; Ye, B. Synthesis of Magnetic Microspheres with Sodium Alginate and Activated Carbon for Removal of Methylene Blue. Materials 2017, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zheng, Z.; Lin, K.; Luan, J.; Zhang, J. Adsorption of p-nitrophenol from aqueous solutions onto activated carbon fiber. J. Hazard. Mater. 2007, 143, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Daud, W.M.A.W.; Ahmad, M.A.; Radzi, R. Using cocoa (Theobroma cacao) shell-based activated carbon to remove 4-nitrophenol from aqueous solution: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 178, 461–467. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Laxmi Gayatri, S. Batch adsorption of 4-nitrophenol by acid activated jute stick char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Zheng, H.; Guo, W.; Li, S.; Chen, Y.; Wu, Q.; Feng, X.; Yin, R.; Ho, S.H.; Ren, N.; Chang, J.S. Adsorption of p-nitrophenols (PNP) on microalgal biochar: Analysis of high adsorption capacity and mechanism. Bioresour. Technol. 2017, 244, 1456–1464. [Google Scholar] [CrossRef]
- Shi, Z.L.; Liu, F.M.; Yao, S.H. Adsorptive removal of phosphate from aqueous solutions using activated carbon loaded with Fe(III) oxide. Xinxing Tan Cailiao N. Carbon Mater. 2011, 26, 299–306. [Google Scholar] [CrossRef]
- Dabrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Marqués, M.D.; Cotoruelo, L.M.; Rodríguez-Mirasol, J.; Cordero, T. Removal of paracetamol on biomass-derived activated carbon: Modeling the fixed bed breakthrough curves using batch adsorption experiments. Chem. Eng. J. 2015, 279, 18–30. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon N. Y. 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Wang, X.S.; Chen, L.F.; Li, F.Y.; Chen, K.L.; Wan, W.Y.; Tang, Y.J. Removal of Cr(VI) with wheat-residue derived black carbon: Reaction mechanism and adsorption performance. J. Hazard. Mater. 2010, 175, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H. Synthesis of graphene oxide decorated with magnetic cyclodextrin for fast chromium removal. J. Mater. Chem. 2012, 22, 24577–24583. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Liu, T.; Jiang, J.; Yang, L.; Fan, Y.; Wee, A.T.S.; Chen, J.P. Characterization of hexavalent chromium interaction with Sargassum by X-ray absorption fine structure spectroscopy, X-ray photoelectron spectroscopy, and quantum chemistry calculation. J. Colloid Interface Sci. 2011, 356, 741–748. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for chromium removal based on graphene oxide functionalized with magnetic cyclodextrin-chitosan. Colloids Surf. B Biointerfaces 2013, 107, 76–83. [Google Scholar] [CrossRef]
- Siddique, A.; Nayak, A.K.; Singh, J. Synthesis of FeCl3-activated carbon derived from waste Citrus limetta peels for removal of fluoride: An eco-friendly approach for the treatment of groundwater and bio-waste collectively. Groundw. Sustain. Dev. 2020, 10, 100339. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Bedia, J.; Rodriguez, J.J.; Belver, C. Effect of activating agent on the properties of TiO2/activated carbon heterostructures for solar photocatalytic degradation of acetaminophen. Materials 2019, 12, 378. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Belver, C.; Rodriguez, J.J.; Bedia, J. Degradation pathways of emerging contaminants using TiO2-activated carbon heterostructures in aqueous solution under simulated solar light. Chem. Eng. J. 2020, 124867. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for supercapacitors: When Li-ion battery power is not enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 424–473. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage. In Energy and Environmental Science; Royal Society of Chemistry: London, UK, 2014; Volume 7, pp. 1250–1280. [Google Scholar]
- Yudasaka, M.; Kikuchi, R. Graphitization of Carbonaceous Materials by Ni, Co and Fe. In Supercarbon—Synthesis, Properties and Applications; Springer: Berlin, Germany, 1998; ISBN 978-3-642-08405-8. [Google Scholar]
- Sajitha, E.P.; Prasad, V.; Subramanyam, S.V.; Eto, S.; Takai, K.; Enoki, T. Synthesis and characteristics of iron nanoparticles in a carbon matrix along with the catalytic graphitization of amorphous carbon. Carbon N. Y. 2004, 42, 2815–2820. [Google Scholar] [CrossRef]
- Zhai, D.; Du, H.; Li, B.; Zhu, Y.; Kang, F. Porous graphitic carbons prepared by combining chemical activation with catalytic graphitization. Carbon N. Y. 2011, 49, 725–729. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.J.; Ulrich, S.; Araujo, P.T.; Bakker, M.G. Catalytic graphitization in nanocast carbon monoliths by iron, cobalt and nickel nanoparticles. Carbon N. Y. 2018, 134, 452–463. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, X.; Su, R.; Hao, Z.; Jia, B.; Li, S.; Dong, L. Highly porous graphitic activated carbons from lignite via microwave pretreatment and iron-catalyzed graphitization at low-temperature for supercapacitor electrode materials. Processes 2019, 7, 300. [Google Scholar] [CrossRef]







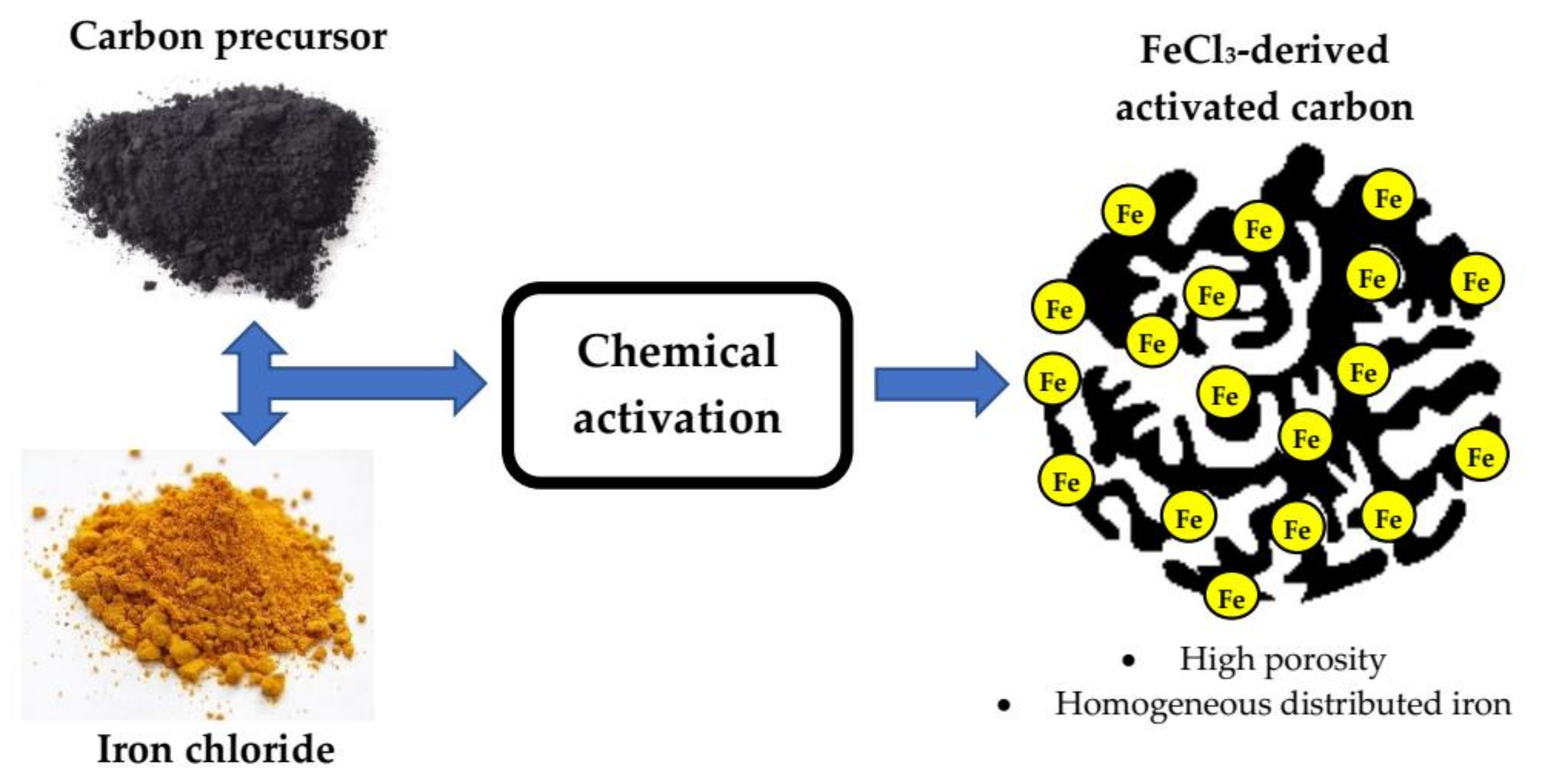
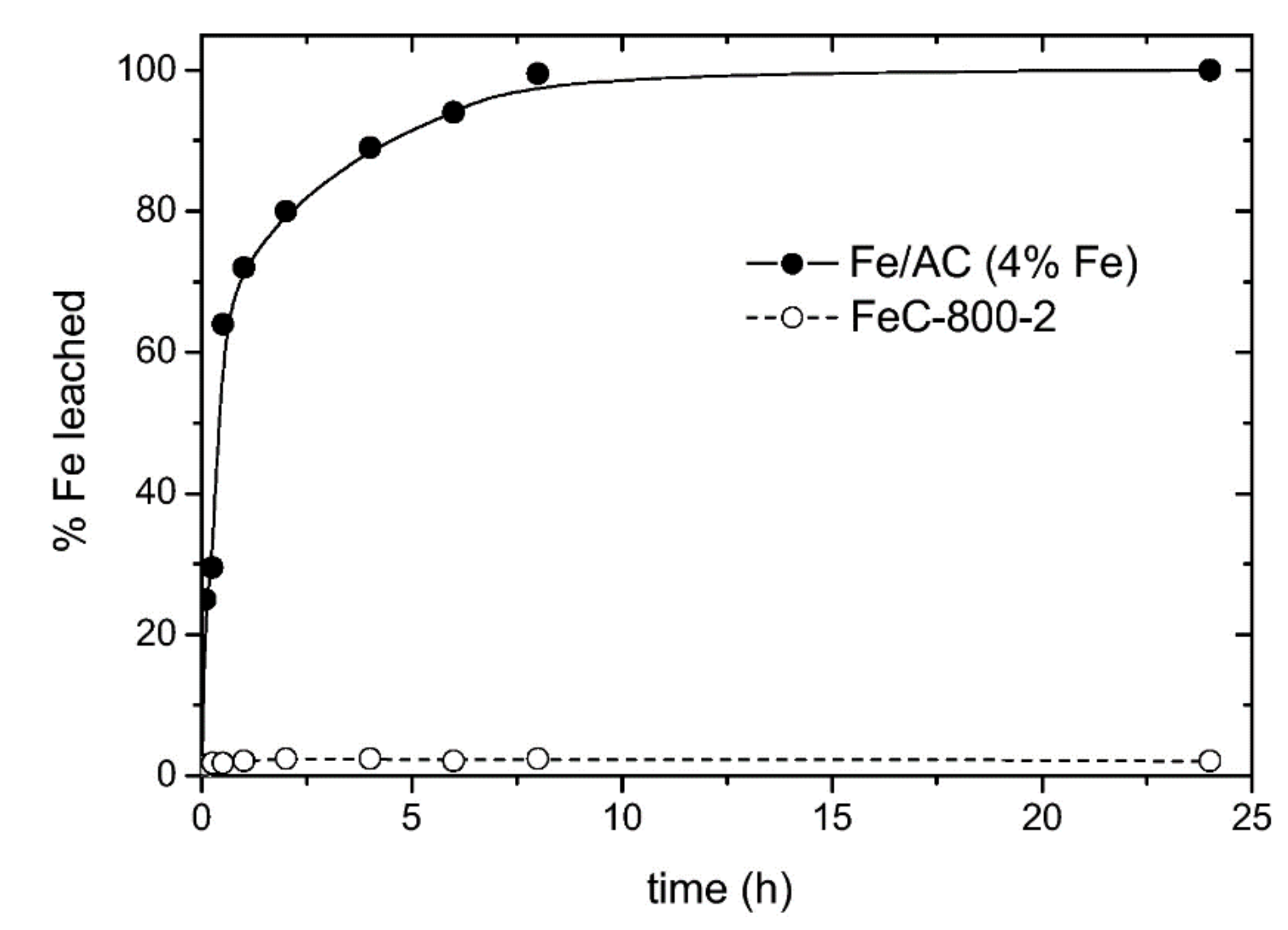
| Carbon Precursor | Type of Contact | R * | Tact * (°C) | tact * (h) | Ref. |
|---|---|---|---|---|---|
| Waste cotton | In solution | 1.62 | 400–700 | 1.0 | [44] |
| Waste cotton | In solution | 0.5–2.5 | 300–800 | 1.0–2.0 | [45] |
| Lignin | In solution | 1.0 | 500–850 | 2.0–6.0 | [56] |
| Sewage sludge | In solution | -- | 750 | 0.5 | [57] |
| Biomass waste | In solution | -- | 800 | 6.0 | [58] |
| Waste cotton | In solution | 1.0 | 400 | 1.0 | [59] |
| Eucalyptus sawdust | In solution | 2.0 | 700 | 1.25 | [60] |
| Lotus stem | In solution | 4.0 | 700 | 1.5 | [61] |
| Sawdust | In solution | 0.5–2.0 | 500–800 | 1.0 | [62] |
| Coconut shell | In solution | 1.0–3.0 | 700 | 1.5 | [48] |
| Date pits | In solution | 1.5 | 700 | 1.0 | [63,64] |
| Arundo donax | In solution | 1.65 | 700 | 1.0 | [65] |
| Coffee grounds | In solution | 1.0 | 900 | 1.0 | [66] |
| Coffee husks | In solution | 1.0 | 280 | 3.0 | [67] |
| Grape seeds | Solid mixing | 2.0–4.0 | 500 | 2.0 | [68] |
| Chestnut waste | Solid mixing | 0.5 | 220–800 | 1.0 | [69] |
| Sewage sludge | Solid mixing | 0.5–3.0 | 750 | 2.0 | [70] |
| Alfalfa leaves | Solid mixing | 3.0 | 900 | 2.0 | [71] |
| Tara gum | Solid mixing | 0.5–3.0 | 400–1000 | 2.0 | [72] |
| Lignin | Solid mixing | 3.0 | 800 | 2.0 | [73] |
| Oily sludge | Solid mixing | 1.0–3.0 | 500–700 | 1.0 | [74] |
| Carbon Precursor | R | Tact (°C) | SBET (m2·g−1) | SEXT (m2·g−1) | Vmic (cm3·g−1) | VTotal (cm3·g−1) | Smic/SBET (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Waste cotton | 1.5 | 700 | 942 | 124 | 0.33 | 0.64 | 87.3 | [45] |
| Lignin | 1.0 | 800 | 818 | 31 | 0.35 | 0.37 | 96.2 | [56] |
| Biomass waste | n.p. | 800 | 600 | n.p. | n.p. | n.p. | n.p. | [58] |
| Waste cotton | 1.0 | 400 | 504 | 151 | 0.17 | n.p. | 72.0 | [59] |
| Eucalyptus sawdust | 2.0 | 700 | 645 | n.p. | 0.28 | 0.44 | n.p. | [60] |
| Lotus stem | 4.0 | 700 | 374 | n.p. | n.p. | 0.20 | n.p. | [61] |
| Date pits | 1.5 | 700 | 780 | n.p. | 0.47 | 0.57 | n.p. | [63,64] |
| Arundo donax | 1.65 | 700 | 927 | 106 | 0.36 | 0.51 | 88.6 | [65] |
| Coffee grounds | 1.0 | 900 | 846 | n.p. | 0.21 | n.p. | n.p. | [66] |
| Coffee husks | 1.0 | 280 | 965 | n.p. | 0.53 | 0.65 | n.p. | [67] |
| Grape seeds | 3.0 | 500 | 417 | 54 | 0.17 | 0.19 | 86.8 | [68] |
| Chestnut waste | 0.5 | 800 | 568 | n.p. | n.p | 0.29 | n.p. | [69] |
| Sewage sludge | 3.0 | 750 | 836 | 148 | 0.33 | 0.62 | 82.3 | [70] |
| Alfalfa leaves | 3.0 | 900 | 773 | n.p. | n.p. | n.p. | n.p. | [71] |
| Tara gum | 2.0 | 800 | 1680 | 143 | 0.75 | 0.99 | 91.0 | [72] |
| Lignin | 3.0 | 800 | 951 | 34 | 0.44 | 0.53 | 96.4 | [73] |
| Oily sludge | 2.0 | 700 | 683 | 254 | 0.20 | 0.68 | 62.7 | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on Activated Carbons by Chemical Activation with FeCl3. C 2020, 6, 21. https://doi.org/10.3390/c6020021
Bedia J, Peñas-Garzón M, Gómez-Avilés A, Rodriguez JJ, Belver C. Review on Activated Carbons by Chemical Activation with FeCl3. C. 2020; 6(2):21. https://doi.org/10.3390/c6020021
Chicago/Turabian StyleBedia, Jorge, Manuel Peñas-Garzón, Almudena Gómez-Avilés, Juan J. Rodriguez, and Carolina Belver. 2020. "Review on Activated Carbons by Chemical Activation with FeCl3" C 6, no. 2: 21. https://doi.org/10.3390/c6020021
APA StyleBedia, J., Peñas-Garzón, M., Gómez-Avilés, A., Rodriguez, J. J., & Belver, C. (2020). Review on Activated Carbons by Chemical Activation with FeCl3. C, 6(2), 21. https://doi.org/10.3390/c6020021








