Plasma Oxidation Printing into DLC and Graphite for Surface Functionalization
Abstract
:1. Introduction
2. Experimental Procedure
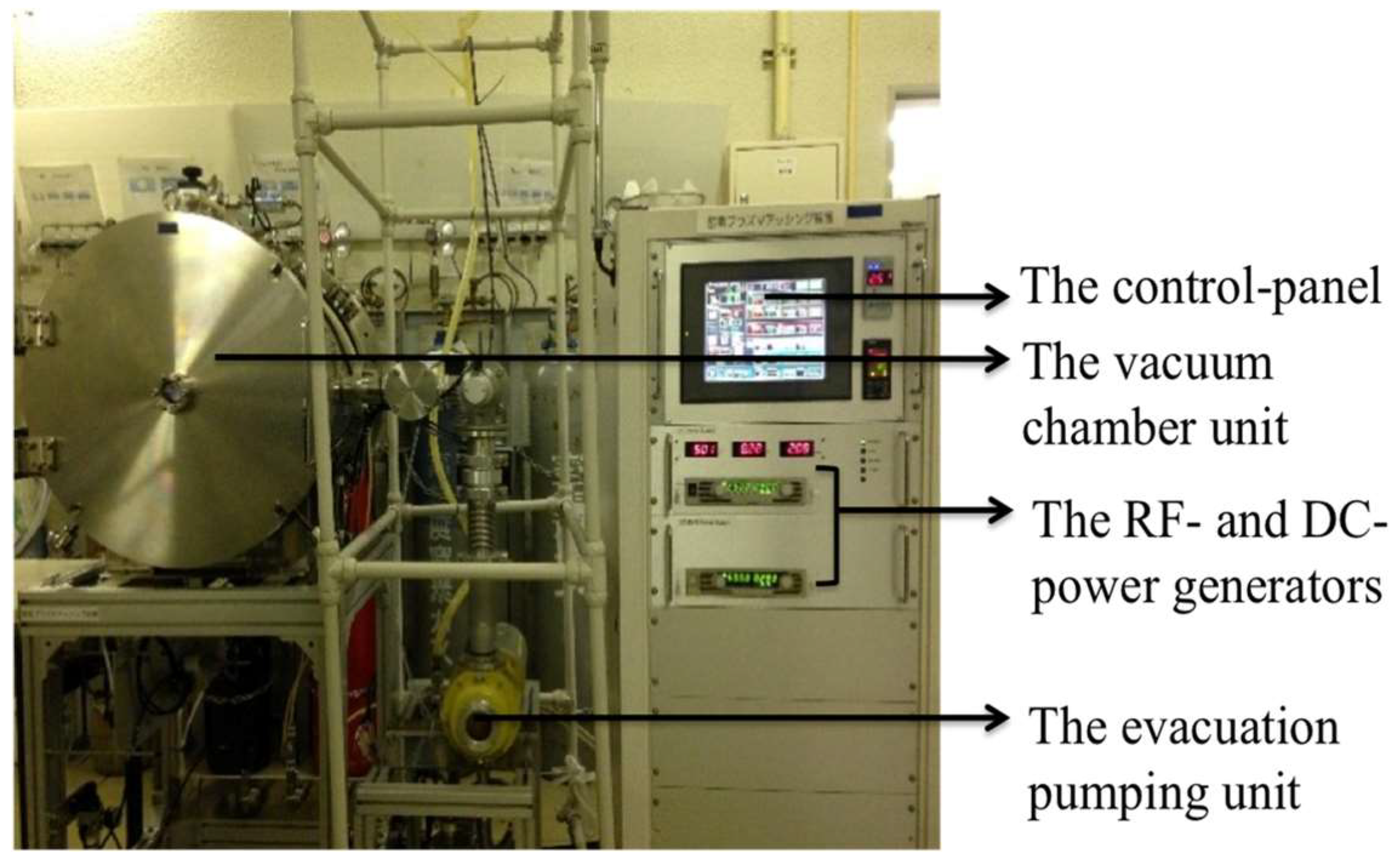
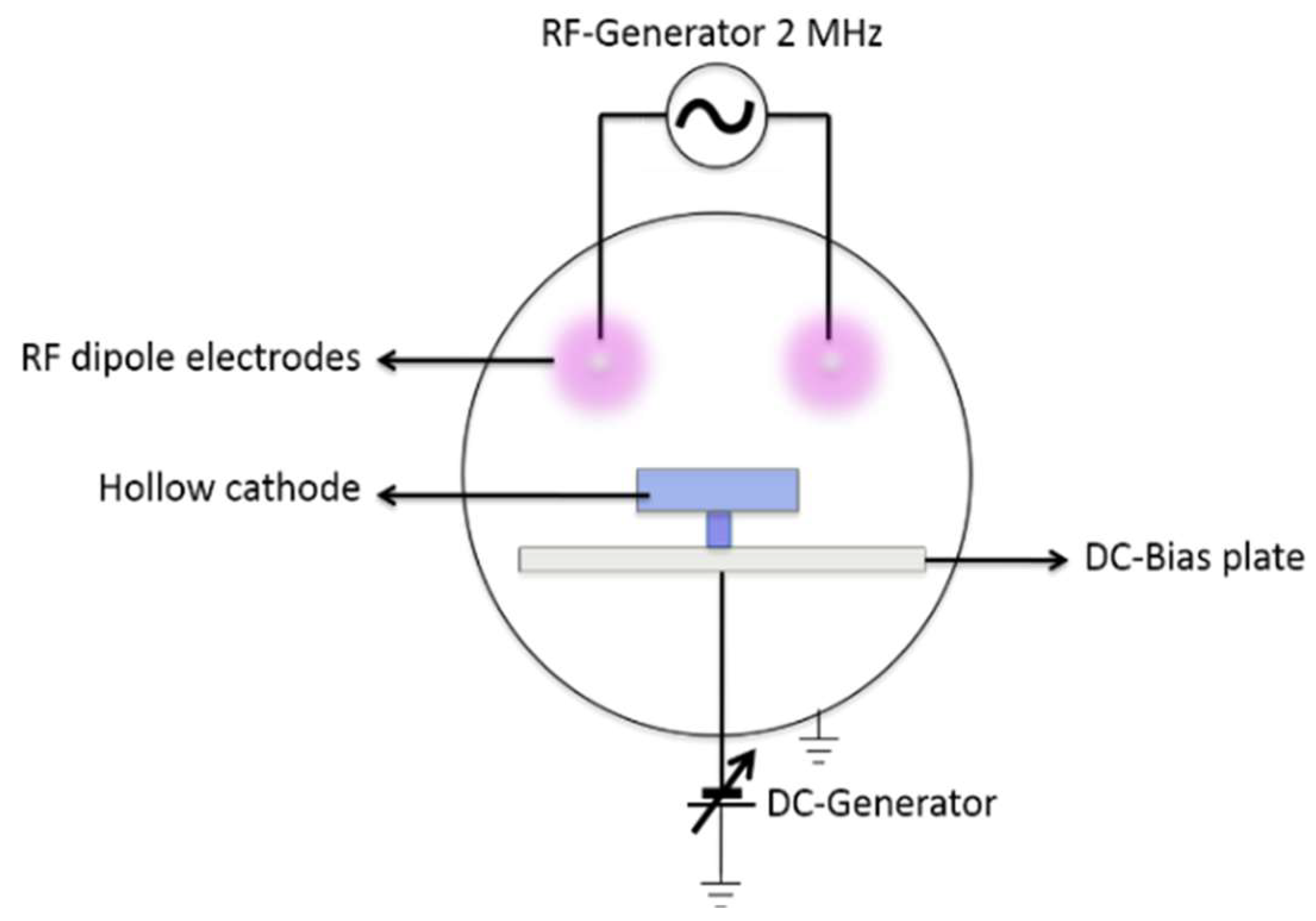
2.1. Plasma Oxidation Etching System
2.2. Preparation of Thick DLC-Coated Substrate and Thick Graphite
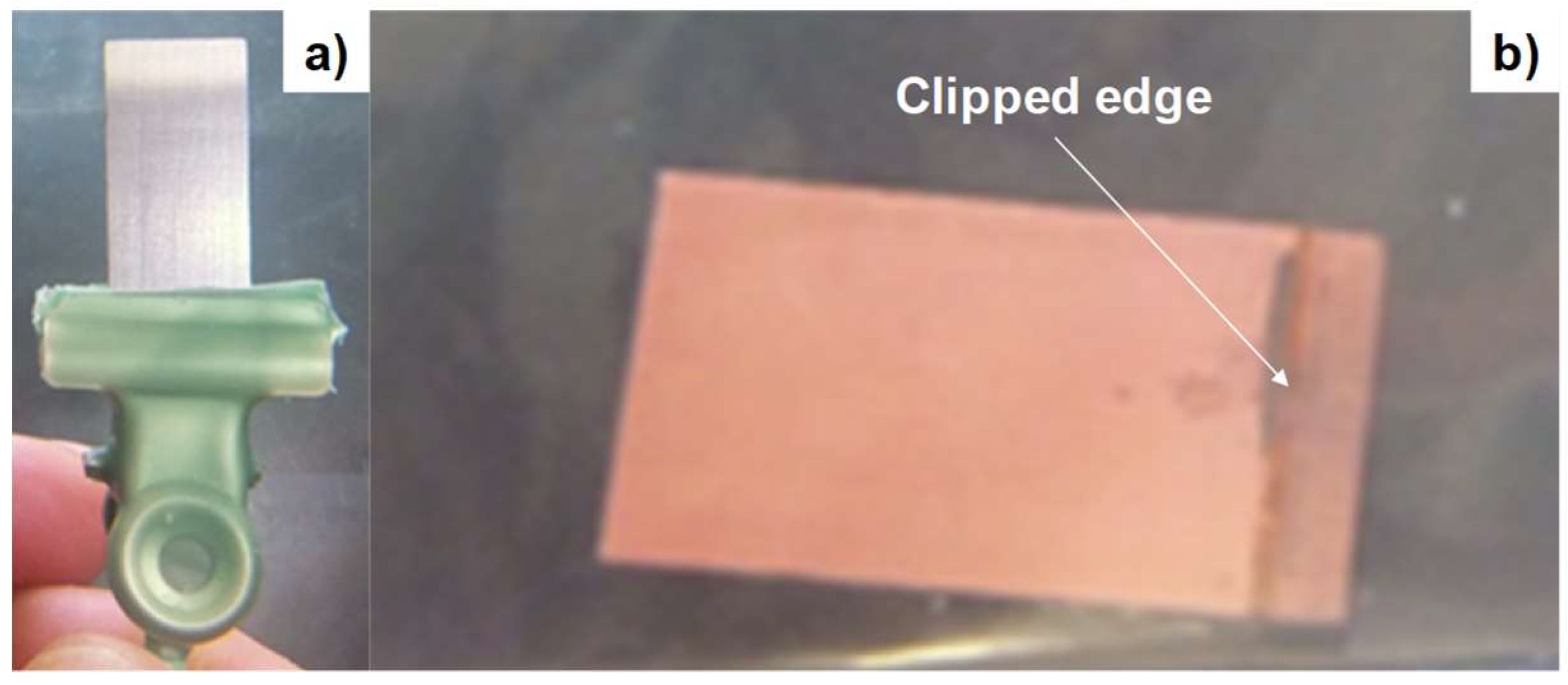
2.3. Measurement and Observation
3. Results
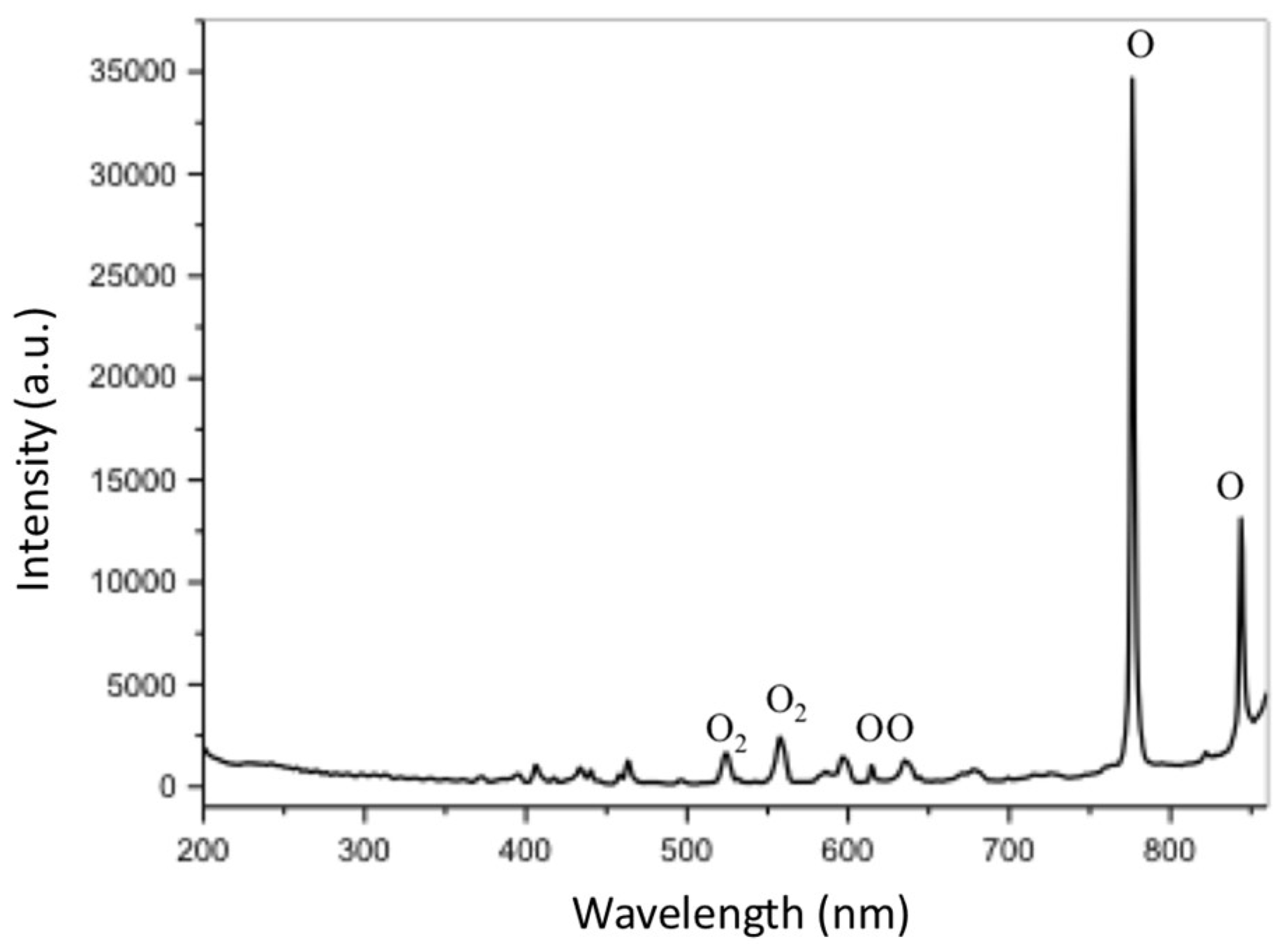
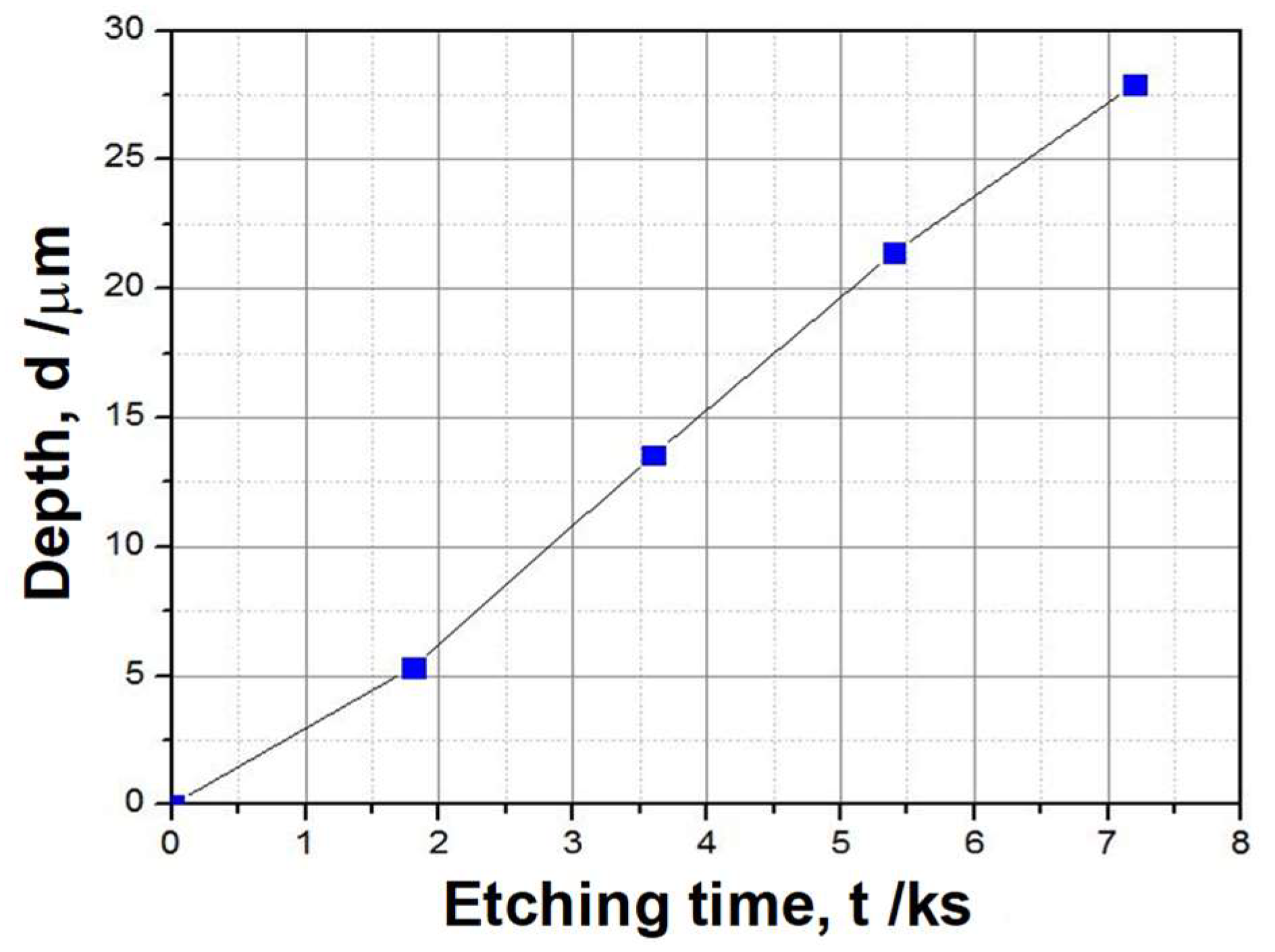
3.1. Plasma Oxidation Process
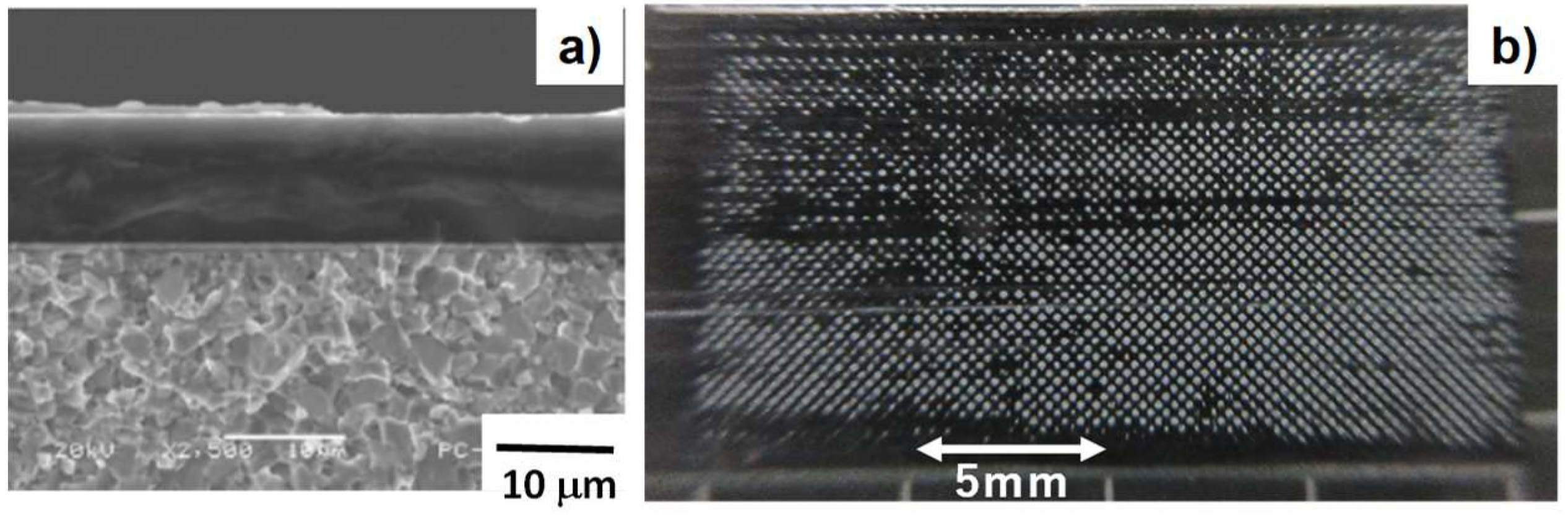
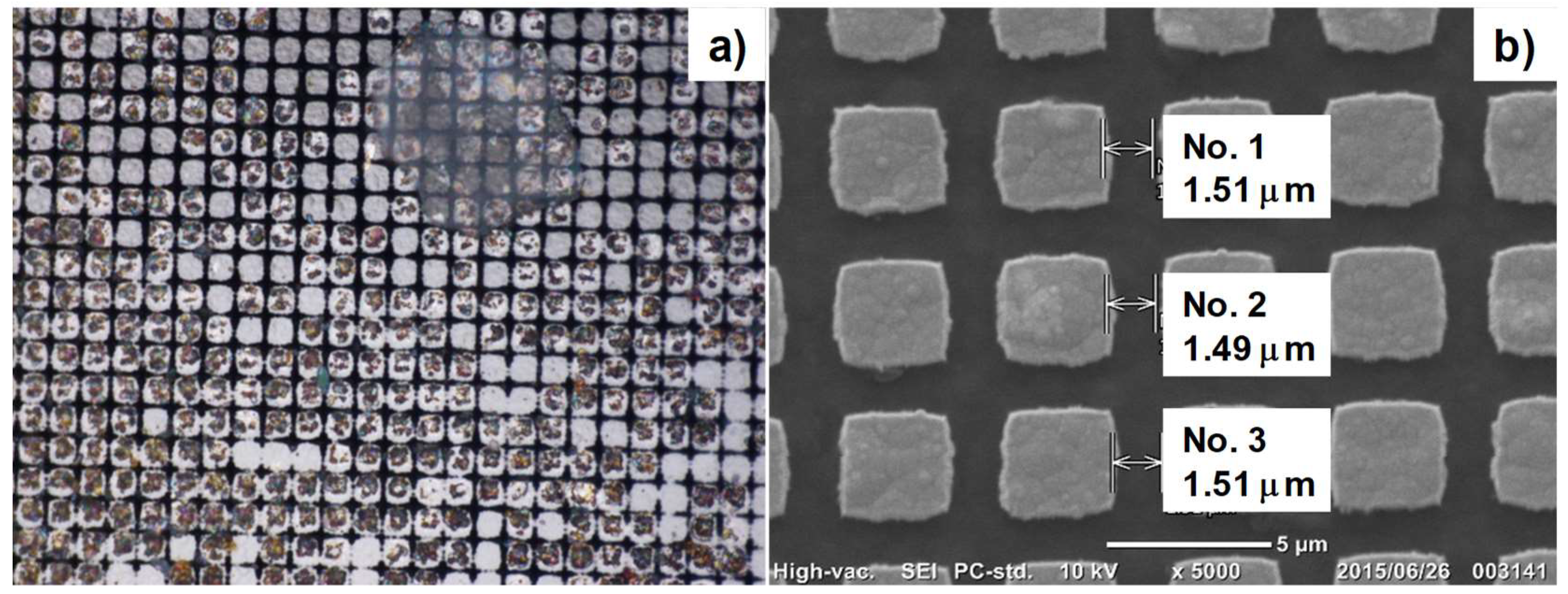
3.2. Shaping to DLC-Punch Array and DLC-Nozzle Array
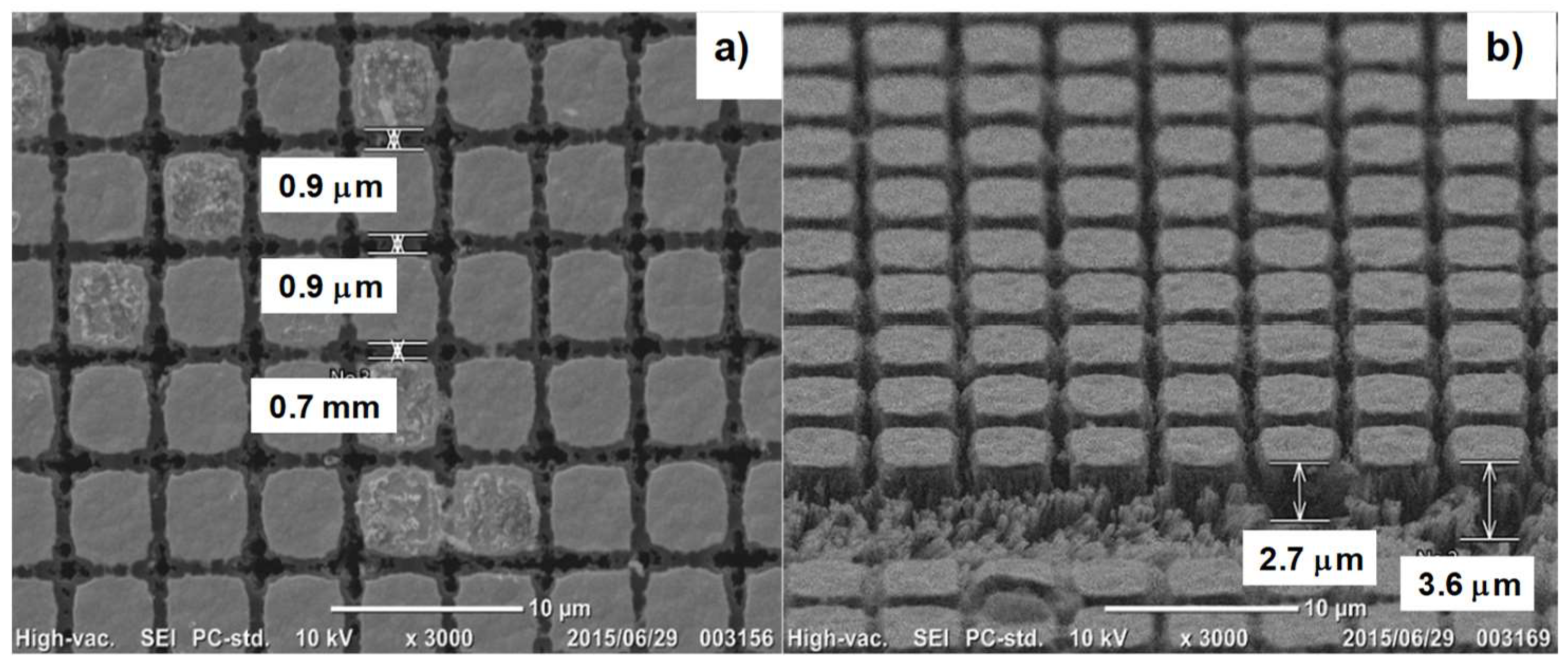
3.3. Selective Plasma Oxidation of VAG
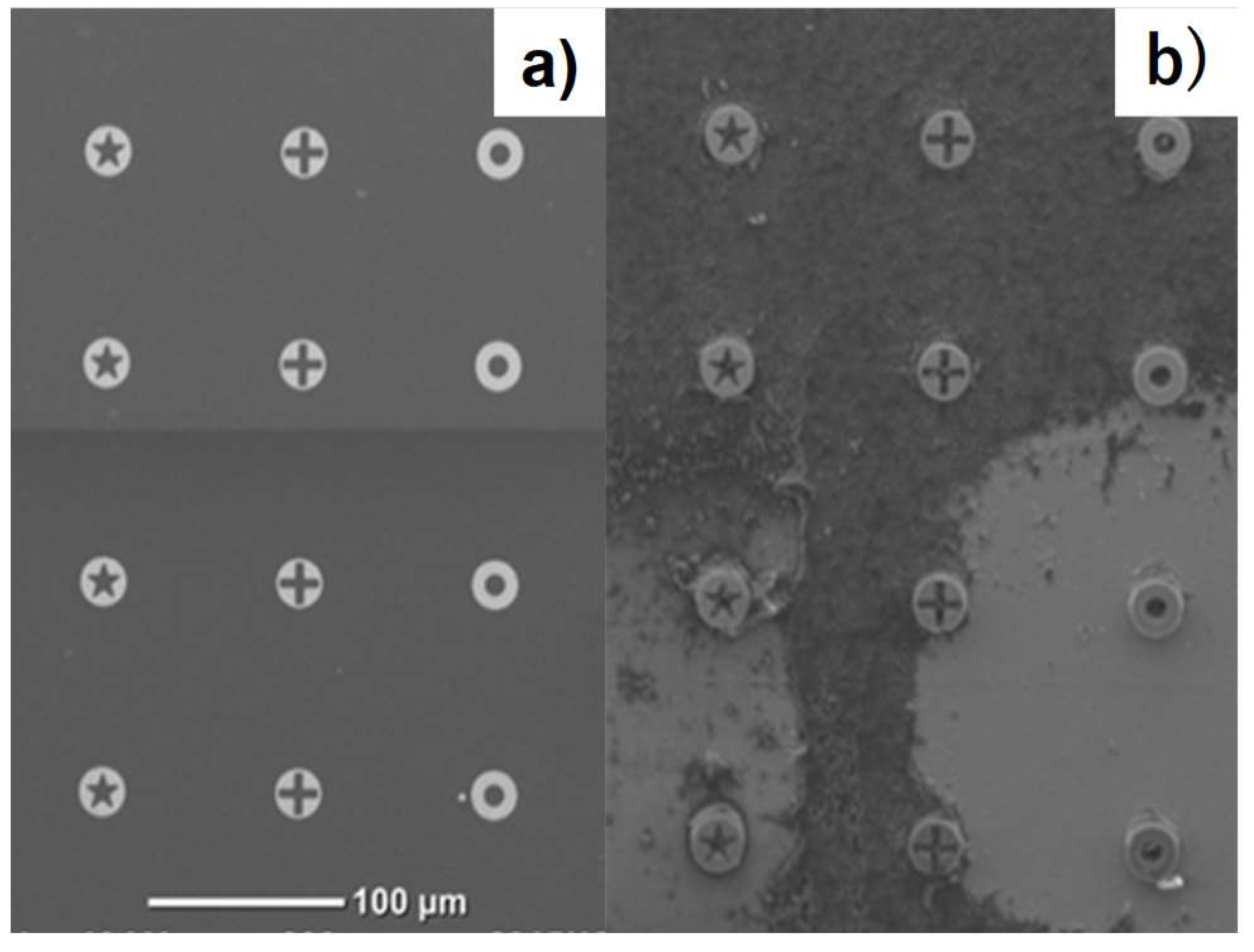
3.4. Surface Activation of VAG
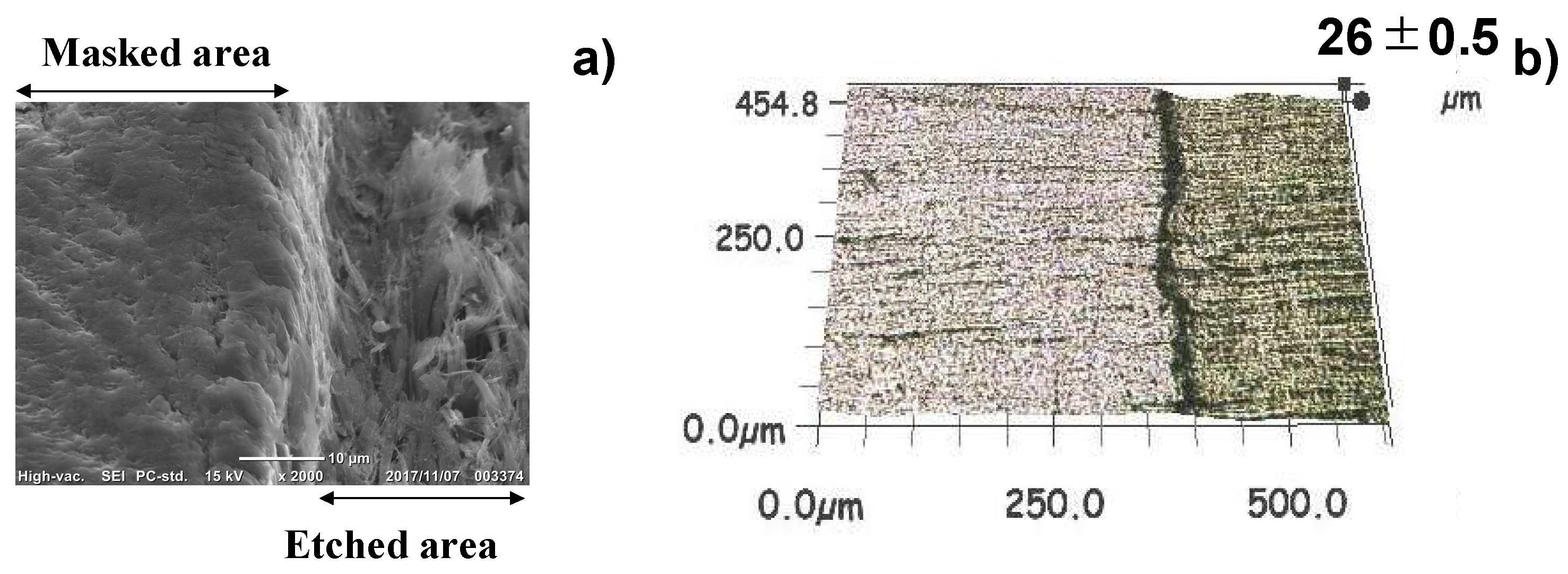
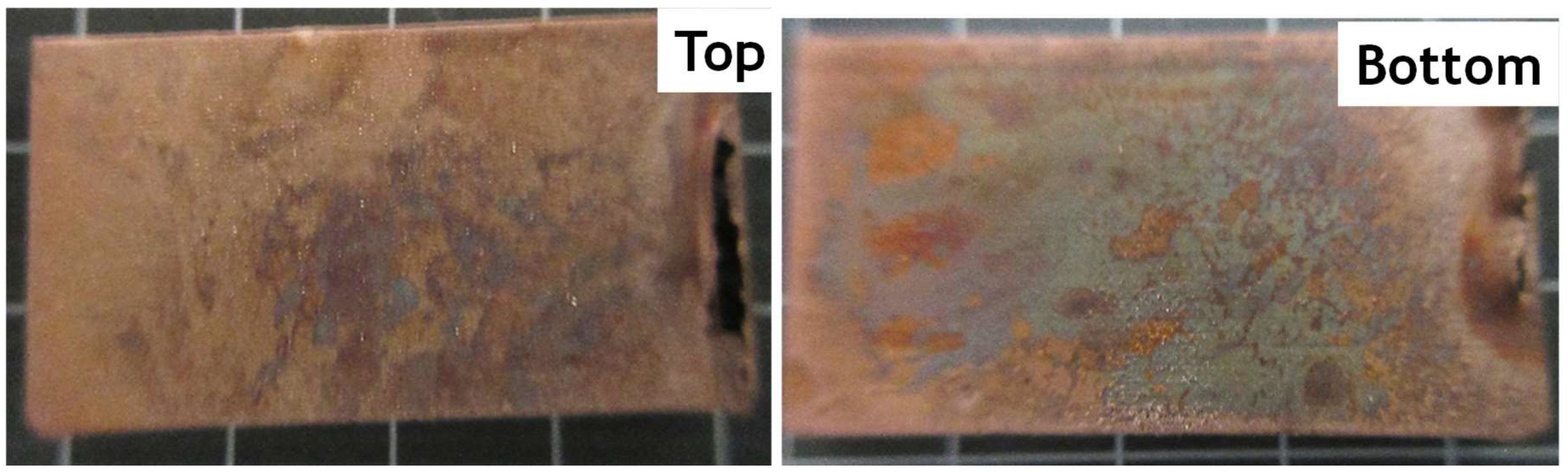
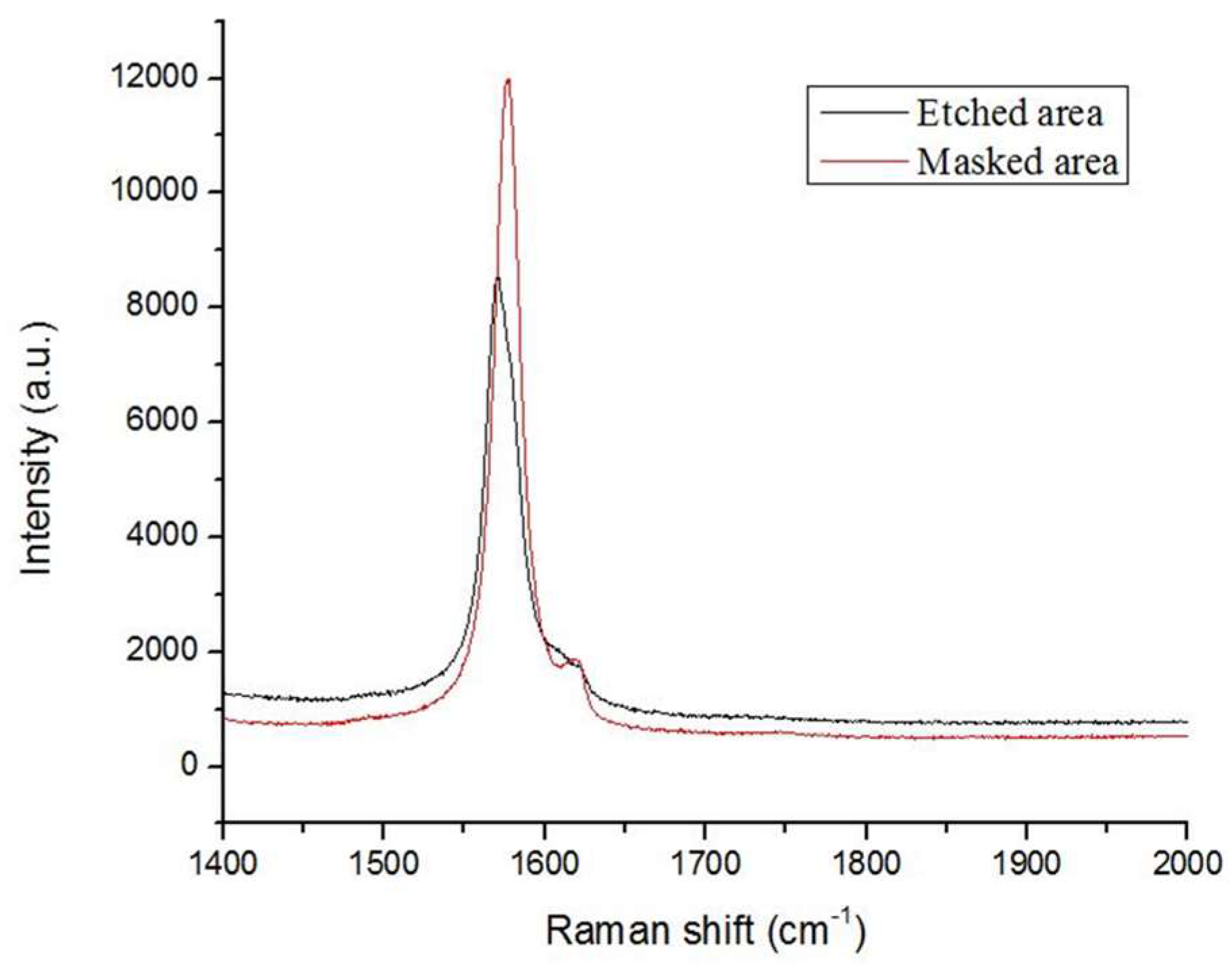
3.5. Quality Check of Etched VAG
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fugallo, G.; Epellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal conductivity of graphene and graphite: Collective excitations and mean free paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Bartley, G.K.; Johnson, C.L.; Kelly, J.E.; Kuczynski, J.K.; Motschman, D.R.; Sinha, K.; Splittstoesser, K.A.; Tofil, T.J. Horizontally and Vertically Aligned Graphite Nanofibers Thermal Interface Material for Use in Chip Stacks. U.S. Patent US9111899B2, 13 September 2012. (Application granted, 18 August 2015). [Google Scholar]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ren, Y.J.; Bai, S.L. Vertically aligned graphene tape/epoxy composites as heat dissipating materials. Int. J. Heat Mass Transf. 2018, 118, 510–517. [Google Scholar] [CrossRef]
- Aizawa, T. Development of micro-manufacturing by controlled plasma technologies. J. Jpn Soc. Technol. Plast. 2017, 58, 1064–1068. [Google Scholar] [CrossRef]
- Aizawa, T.; Fukuda, T. Oxygen plasma etching of diamond-like carbon coated mold-die for micro-texturing. Surf. Coat. Technol. 2013, 215, 364–368. [Google Scholar] [CrossRef]
- Aizawa, T.; Inohara, T. Micro-texturing onto glassy carbon substrates by multi-axially controlled pico-second laser machining. In Proceedings of the 7th International Conference on MicroManufacturing, Chicago, IL, USA, 11–14 March 2012; pp. 66–73. [Google Scholar]
- Bardos, L. Radio frequency hollow cathodes for the plasma processing technology. Surf. Coat. Technol. 1996, 86–87, 648–656. [Google Scholar] [CrossRef]
- Aizawa, T.; Wasa, K.; Tamagaki, H. A DLC-punch array to fabricate the micro-textured aluminum sheet for boiling heat transfer control. Micromachines 2018, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Ikhsani, R.N.; Aizawa, T.; Santjojo, D.J.D.H.; Yunata, E.E.; Sakti, S.P. Micro texturing into the graphite part for joining and packaging. In Proceedings of the 12th SEATUC Symposium, Yogyakarta, Indonesia, 12–13 March 2018; pp. 127–132. [Google Scholar]
- Gao, S.; Fan, M.J.; Wang, G.P. Copper plating on the carbon fibers by double pulse. Appl. Mech. Mater. 2011, 148–149, 928–931. [Google Scholar] [CrossRef]
- Davami, K.; Shaygam, M.; Kheirabi, N. Synthesis and characterization of carbon nano-walls on different substrates by radio frequency plasma enhanced chemical vapor deposition. Carbon 2014, 72, 372–380. [Google Scholar] [CrossRef]
- Aizawa, T.; Mizushima, K.; Redationo, T.N.; Yang, M. Micro-imprinting onto DLC and CNT coatings via high density oxygen plasma etching. In Proceedings of the 8th International Conference Micromanufacturing (ICOMM 2013), Victoria, BC, Canada, 25–28 March 2013; pp. 459–466. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aizawa, T.; Wasa, K.; Nogami, Y. Plasma Oxidation Printing into DLC and Graphite for Surface Functionalization. C 2019, 5, 11. https://doi.org/10.3390/c5010011
Aizawa T, Wasa K, Nogami Y. Plasma Oxidation Printing into DLC and Graphite for Surface Functionalization. C. 2019; 5(1):11. https://doi.org/10.3390/c5010011
Chicago/Turabian StyleAizawa, Tatsuhiko, Kenji Wasa, and Yoshiro Nogami. 2019. "Plasma Oxidation Printing into DLC and Graphite for Surface Functionalization" C 5, no. 1: 11. https://doi.org/10.3390/c5010011
APA StyleAizawa, T., Wasa, K., & Nogami, Y. (2019). Plasma Oxidation Printing into DLC and Graphite for Surface Functionalization. C, 5(1), 11. https://doi.org/10.3390/c5010011




