Crucial Factors for the Application of Functional Nanoporous Carbon-Based Materials in Energy and Environmental Applications
Abstract
:1. The Family of Functional Nanoporous Carbon-Based Materials
2. Synthesis and Structure of Functional Inorganic Nanoporous Carbon-Based Materials
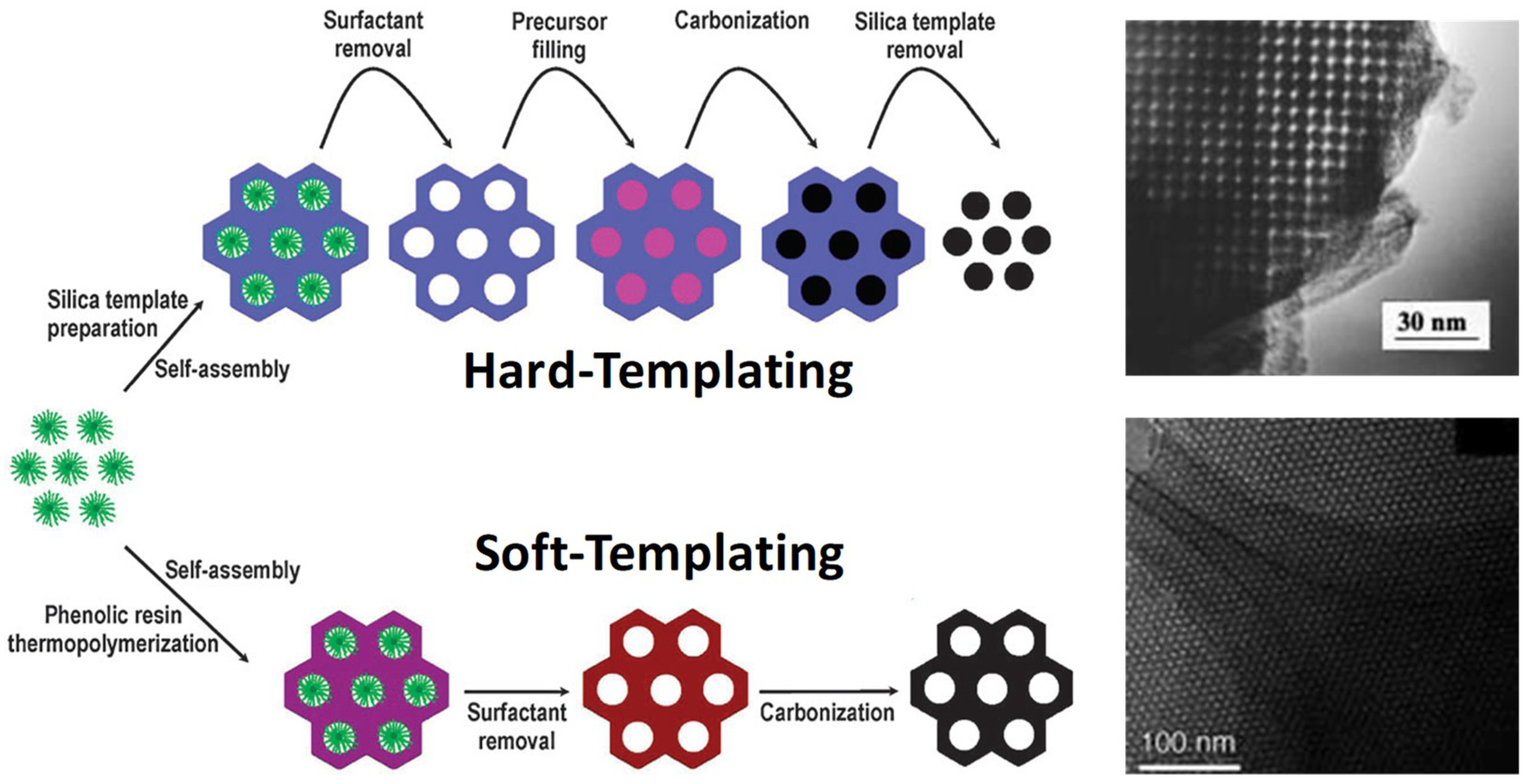
2.1. Synthesis of Functional Carbon-Based Materials with Different Pore Structures
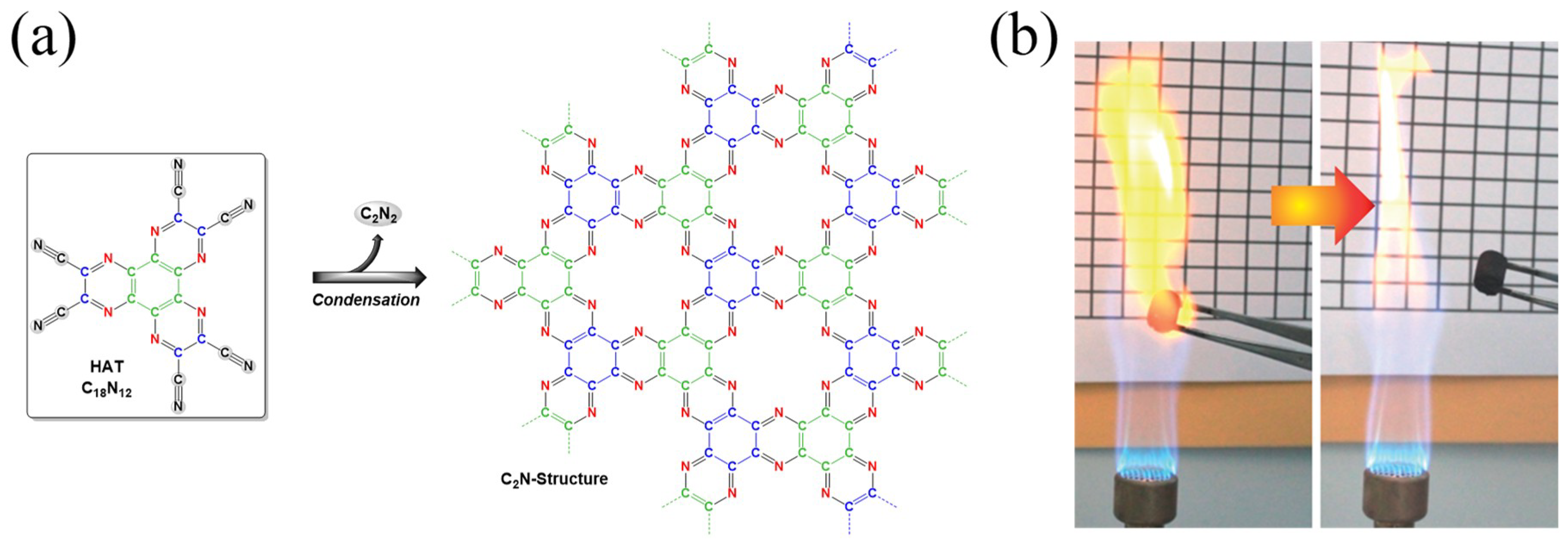
2.2. Controlling Atomic Construction and Introducing Chemical Functionality into Nanoporous Carbon-Based Materials
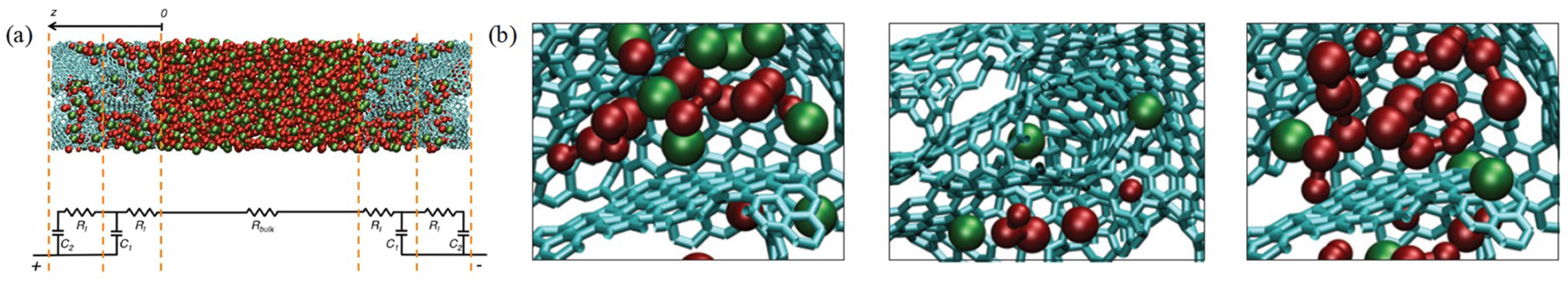
2.3. Characterization and Modelling of the Structure of Nanoporous Carbon-Based Materials
3. Energy and Environmental Applications of Functional Nanoporous Carbon-Based Materials
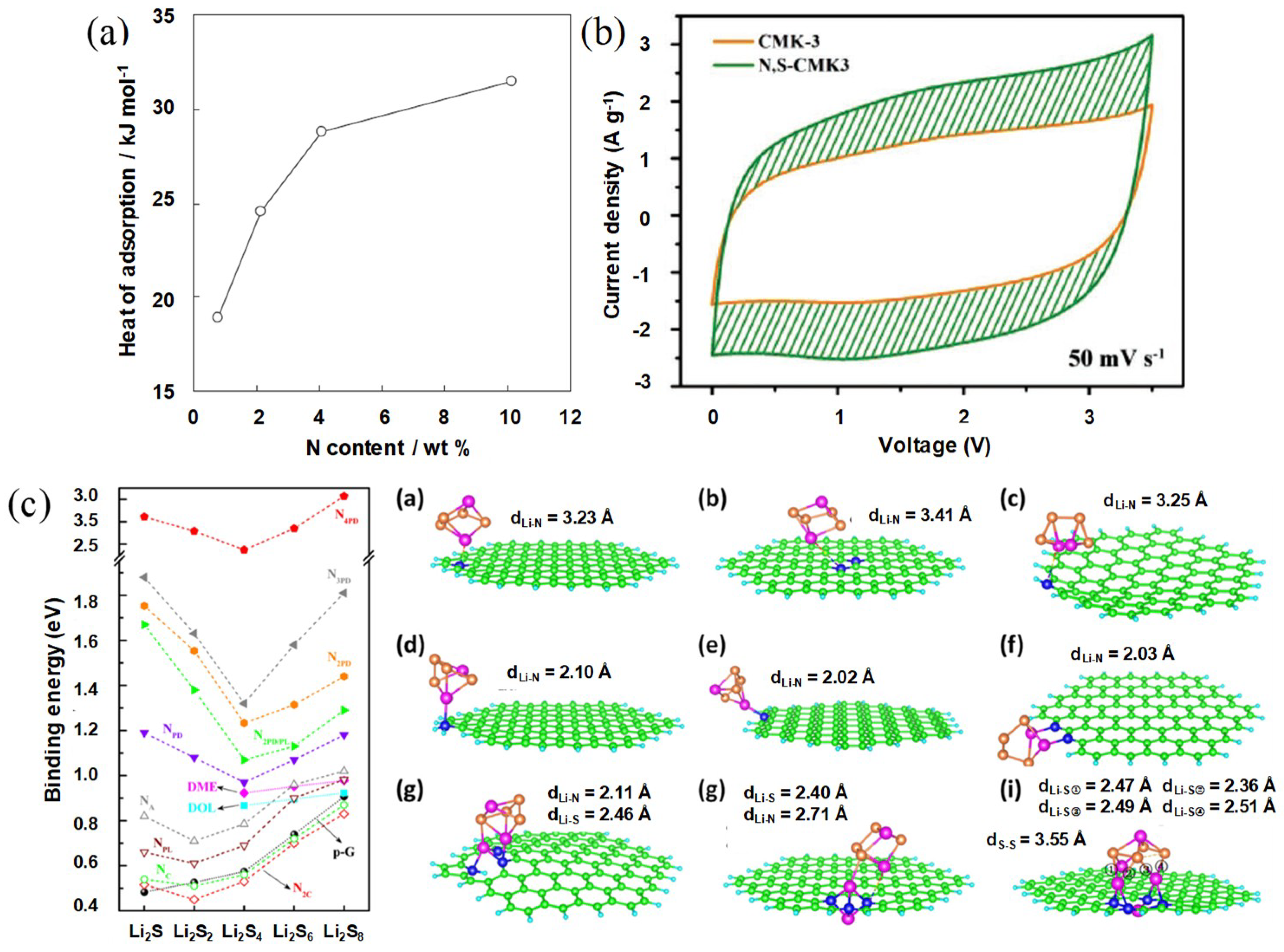
3.1. The Importance of Carbon-Guest Interactions
3.2. Where Will the Field Go?
4. Special Issue of “C” on Functional Nanoporous Carbon-Based Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roston, E. The Carbon Age: How Life’s Core Element Has Become Civilization’s Greatest Threat; Walker & Company: New York, NY, USA, 2008. [Google Scholar]
- Rosa, R.N. The role of synthetic fuels for a carbon neutral economy. C 2017, 3, 11. [Google Scholar] [CrossRef]
- Andreoli, E. Materials and processes for carbon dioxide capture and utilisation. C 2017, 3, 16. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Presser, V. (Eds.) Carbon Nanomaterials; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Tascón, J.M.D. (Ed.) Novel Carbon Adsorbents; Elsevier: Oxford, UK, 2012; ISBN 9780080977454. [Google Scholar]
- Serp, P.; Figueiredo, J.L. (Eds.) Carbon Materials for Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-17885-0. [Google Scholar]
- Krüger, A. (Ed.) Carbon Materials and Nanotechnology; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Feng, X. (Ed.) Nanocarbons for Advanced Energy Storage; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chang, D.W.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Mokaya, R. Templated nanoscale porous carbons. Nanoscale 2010, 2, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.C.; Llobet, A.; Yeon, S.H.; Fischer, J.E.; Shi, Y.; Gogotsi, Y.; Gubbins, K.E. Modeling the structural evolution of carbide-derived carbons using quenched molecular dynamics. Carbon 2010, 48, 1116–1123. [Google Scholar] [CrossRef]
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Chaoui, N.; Trunk, M.; Dawson, R.; Schmidt, J.; Thomas, A. Trends and challenges for microporous polymers. Chem. Soc. Rev. 2017, 46, 3302–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal-organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Sakaushi, K.; Antonietti, M. Carbon- and nitrogen-based porous solids: A recently emerging class of materials. Bull. Chem. Soc. Jpn. 2015, 88, 386–398. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of porous carbon materials with designed pore architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef]
- Lopez-Salas, N.; del Monte, F.; Tamayo, A.; Fierro, J.L.; De Lacey, A.L.; Ferrer, M.L.; Gutierrez, M.C. Sulfur-doped carbons prepared from eutectic mixtures containing hydroxymethylthiophene as metal-free oxygen reduction catalysts. ChemSusChem 2014, 7, 3347–3355. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Kim, J.H.; Kim, M.-S.; Yu, J.-S. Hierarchical nanostructured carbons with meso-macroporosity: Design, characterization, and applications. Acc. Chem. Res. 2013, 46, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Borchardt, L.; Thommes, M.; Cychosz, K.A.; Senkovska, I.; Klein, N.; Frind, R.; Leistner, M.; Presser, V.; Gogotsi, Y.; et al. Carbide-derived carbon monoliths with hierarchical pore architectures. Angew. Chem. Int. Ed. 2012, 51, 7577–7580. [Google Scholar] [CrossRef] [PubMed]
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.; Han, Y. Microporous carbonaceous adsorbents for CO2 separation via selective adsorption. RSC Adv. 2015, 5, 30310–30330. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano 2012, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Yu, L.; Ania, C.O.; Titirici, M.-M. Supercapacitive behavior of two glucose-derived microporous carbons: Direct pyrolysis versus hydrothermal carbonization. ChemElectroChem 2014, 1, 2138–2145. [Google Scholar] [CrossRef]
- Grätz, S.; Beyer, D.; Tkachova, V.; Hellmann, S.; Berger, R.; Feng, X.; Borchardt, L. The mechanochemical scholl reaction—A solvent-free and versatile graphitization tool. Chem. Commun. 2018, 54, 5307–5310. [Google Scholar]
- Schneidermann, C.; Jackel, N.; Oswald, S.; Giebeler, L.; Presser, V.; Borchardt, L. Solvent-free mechanochemical synthesis of nitrogen-doped nanoporous carbon for electrochemical energy storage. ChemSusChem 2017, 10, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Casco, M.E.; Badaczewski, F.; Grätz, S.; Tolosa, A.; Presser, V.; Smarsly, B.M.; Borchardt, L. Mechanochemical synthesis of porous carbon at room temperature with a highly ordered sp2 microstructure. Carbon 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Borchardt, L.; Zhu, Q.-L.; Casco, M.E.; Berger, R.; Zhuang, X.; Kaskel, S.; Feng, X.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610. [Google Scholar] [CrossRef]
- Fuchs, I.; Fechler, N.; Antonietti, M.; Mastai, Y. Enantioselective nanoporous carbon based on chiral ionic liquids. Angew. Chem. Int. Ed. 2016, 55, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Fechler, N.; Zussblatt, N.P.; Rothe, R.; Schlogl, R.; Willinger, M.G.; Chmelka, B.F.; Antonietti, M. Eutectic syntheses of graphitic carbon with high pyrazinic nitrogen content. Adv. Mater. 2016, 28, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.S.; Adelhelm, P.; Smarsly, B.M.; Hore, S.; Antonietti, M.; Maier, J. Synthesis of hierarchically porous carbon monoliths with highly ordered microstructure and their application in rechargeable lithium batteries with high-rate capability. Adv. Funct. Mater. 2007, 17, 1873–1878. [Google Scholar] [CrossRef]
- Oschatz, M.; Zeiger, M.; Jäckel, N.; Strubel, P.; Borchardt, L.; Reinhold, R.; Nickel, W.; Eckert, J.; Presser, V.; Kaskel, S. Emulsion soft templating of carbide-derived carbon nanospheres with controllable porosity for capacitive electrochemical energy storage. J. Mater. Chem. A 2015, 3, 17983–17990. [Google Scholar] [CrossRef] [Green Version]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent progress in soft-templating of porous carbon materials. Soft Matter 2012, 8, 10801–10812. [Google Scholar] [CrossRef]
- Oschatz, M.; Kockrick, E.; Rose, M.; Borchardt, L.; Klein, N.; Senkovska, I.; Freudenberg, T.; Korenblit, Y.; Yushin, G.; Kaskel, S. A cubic ordered, mesoporous carbide-derived carbon for gas and energy storage applications. Carbon 2010, 48, 3987–3992. [Google Scholar] [CrossRef]
- Oschatz, M.; Nickel, W.; Thommes, M.; Cychosz, K.A.; Leistner, M.; Adam, M.; Mondin, G.; Strubel, P.; Borchardt, L.; Kaskel, S. Evolution of porosity in carbide-derived carbon aerogels. J. Mater. Chem. A 2014, 2, 18472–18479. [Google Scholar] [CrossRef]
- Oschatz, M.; Boukhalfa, S.; Nickel, W.; Hofmann, J.P.; Fischer, C.; Yushin, G.; Kaskel, S. Carbide-derived carbon aerogels with tunable pore structure as versatile electrode material in high power supercapacitors. Carbon 2017, 113, 283–291. [Google Scholar] [CrossRef]
- Oschatz, M.; Borchardt, L.; Pinkert, K.; Thieme, S.; Lohe, M.R.; Hoffmann, C.; Benusch, M.; Wisser, F.M.; Ziegler, C.; Giebeler, L.; et al. Hierarchical carbide-derived carbon foams with advanced mesostructure as a versatile electrochemical energy-storage material. Adv. Energy Mater. 2014, 4, 1300645. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, K.; Hyeon, T. Fabrication of novel mesocellular carbon foams with uniform ultralarge mesopores. J. Am. Chem. Soc. 2001, 123, 5146–5147. [Google Scholar] [CrossRef] [PubMed]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q.; et al. The green chemistree: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Lim, S.; Suh, K.; Kim, Y.; Yoon, M.; Park, H.; Dybtsev, D.N.; Kim, K. Porous carbon materials with a controllable surface area synthesized from metal-organic frameworks. Chem. Commun. 2012, 48, 7447–7449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Liu, B.; Lan, Y.Q.; Kuratani, K.; Akita, T.; Shioyama, H.; Zong, F.; Xu, Q. From metal-organic framework to nanoporous carbon: Toward a very high surface area and hydrogen uptake. J. Am. Chem. Soc. 2011, 133, 11854–11857. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Tang, J.; Salunkhe, R.R.; Liu, J.; Torad, N.L.; Imura, M.; Furukawa, S.; Yamauchi, Y. Thermal conversion of core-shell metal-organic frameworks: A new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 2015, 137, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Krause, S.; Krans, N.A.; Hernandez Mejia, C.; Kaskel, S.; de Jong, K.P. Influence of precursor porosity on sodium and sulfur promoted iron/carbon fischer-tropsch catalysts derived from metal-organic frameworks. Chem. Commun. 2017, 53, 10204–10207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal-organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Wang, G.H.; Zhang, W.P.; Zhang, T.; Wang, A.Q.; Schuth, F.; Bongard, H.J.; Lu, A.H. Structurally designed synthesis of mechanically stable poly (benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J. Am. Chem. Soc. 2011, 133, 11378–11388. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-derived carbons—From porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Nikitin, A.; Ye, H.; Zhou, W.; Fischer, J.E.; Yi, B.; Foley, H.C.; Barsoum, M.W. Nanoporous carbide-derived carbon with tunable pore size. Nat. Mater. 2003, 2, 591–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jänes, A.; Thomberg, T.; Kurig, H.; Lust, E. Nanoscale fine-tuning of porosity of carbide-derived carbon prepared from molybdenum carbide. Carbon 2009, 47, 23–29. [Google Scholar] [CrossRef]
- Dash, R.; Chmiola, J.; Yushin, G.; Gogotsi, Y.; Laudisio, G.; Singer, J.; Fischer, J.; Kucheyev, S. Titanium carbide derived nanoporous carbon for energy-related applications. Carbon 2006, 44, 2489–2497. [Google Scholar] [CrossRef] [Green Version]
- Futamura, R.; Iiyama, T.; Takasaki, Y.; Gogotsi, Y.; Biggs, M.J.; Salanne, M.; Segalini, J.; Simon, P.; Kaneko, K. Partial breaking of the coulombic ordering of ionic liquids confined in carbon nanopores. Nat. Mater. 2017, 16, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Silvestre-Albero, A.; Rico-Francés, S.; Rodríguez-Reinoso, F.; Kern, A.M.; Klumpp, M.; Etzold, B.J.M.; Silvestre-Albero, J. High selectivity of TiC-CDC for CO2/N2 separation. Carbon 2013, 59, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Oschatz, M.; Hoffmann, H.C.; Pallmann, J.; Schaber, J.; Borchardt, L.; Nickel, W.; Senkovska, I.; Rico-Francés, S.; Silvestre-Albero, J.; Kaskel, S.; et al. Structural characterization of micro- and mesoporous carbon materials using in situ high pressure 129Xe nmr spectroscopy. Chem. Mater. 2014, 26, 3280–3288. [Google Scholar] [CrossRef]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef]
- Zera, E.; Nickel, W.; Hao, G.P.; Vanzetti, L.; Kaskel, S.; Sorarù, G.D. Nitrogen doped carbide derived carbon aerogels by chlorine etching of a sicn aerogel. J. Mater. Chem. A 2016, 4, 4525–4533. [Google Scholar] [CrossRef]
- Pérez, C.R.; Yeon, S.-H.; Ségalini, J.; Presser, V.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Structure and electrochemical performance of carbide-derived carbon nanopowders. Adv. Funct. Mater. 2013, 23, 1081–1089. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Oschatz, M.; Borchardt, L.; Senkovska, I.; Klein, N.; Leistner, M.; Kaskel, S. Carbon dioxide activated carbide-derived carbon monoliths as high performance adsorbents. Carbon 2013, 56, 139–145. [Google Scholar] [CrossRef]
- Osswald, S.; Portet, C.; Gogotsi, Y.; Laudisio, G.; Singer, J.P.; Fischer, J.E.; Sokolov, V.V.; Kukushkina, J.A.; Kravchik, A.E. Porosity control in nanoporous carbide-derived carbon by oxidation in air and carbon dioxide. J. Solid State Chem. 2009, 182, 1733–1741. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Lozano-Castelló, D.; Titirici, M.M.; Zhao, L.; Yu, L.; Morallón, E.; Cazorla-Amoros, D. Electrochemical behaviour of activated carbons obtained via hydrothermal carbonization. J. Mater. Chem. A 2015, 3, 15558–15567. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Heerwig, A.; Lohe, M.R.; Oschatz, M.; Borchardt, L.; Kaskel, S. Fungi-based porous carbons for CO2 adsorption and separation. J. Mater. Chem. 2012, 22, 13911–13913. [Google Scholar] [CrossRef]
- Lu, A.H.; Schüth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, D. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Yang, H.; Li, Z.; Yu, C.; Tu, B.; Zhao, D. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Lee, J.T.; Kim, H.; Nickel, W.; Borchardt, L.; Cho, W.I.; Ziegler, C.; Kaskel, S.; Yushin, G. Micro- and mesoporous carbide-derived carbon prepared by a sacrificial template method in high performance lithium sulfur battery cathodes. J. Mater. Chem. A 2014, 2, 17649–17654. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Heil, T.; Presser, V.; Walczak, R.; Antonietti, M.; Oschatz, M. Ordered mesoporous carbons with high micropore content and tunable structure prepared by combined hard and salt templating as electrode materials in electric double-layer capacitors. Adv. Sustain. Syst. 2018, 2, 1700128. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Lohe, M.; Presser, V.; Gogotsi, Y.; Kaskel, S. Ordered mesoporous carbide-derived carbons prepared by soft templating. Carbon 2012, 50, 3987–3994. [Google Scholar] [CrossRef]
- Ma, T.Y.; Liu, L.; Yuan, Z.Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Fechler, N.; Fellinger, T.P.; Antonietti, M. “Salt templating”: A simple and sustainable pathway toward highly porous functional carbons from ionic liquids. Adv. Mater. 2013, 25, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Antonietti, M.; Oschatz, M. Toward the experimental understanding of the energy storage mechanism and ion dynamics in ionic liquid based supercapacitors. Adv. Energy Mater. 2018, 8, 1800026. [Google Scholar] [CrossRef]
- Oschatz, M.; Boukhalfa, S.; Nickel, W.; Lee, J.T.; Klosz, S.; Borchardt, L.; Eychmüller, A.; Yushin, G.; Kaskel, S. Kroll-carbons based on silica and alumina templates as high-rate electrode materials in electrochemical double-layer capacitors. J. Mater. Chem. A 2014, 2, 5131–5139. [Google Scholar] [CrossRef]
- Nickel, W.; Oschatz, M.; Rico-Frances, S.; Klosz, S.; Biemelt, T.; Mondin, G.; Eychmüller, A.; Silvestre-Albero, J.; Kaskel, S. Synthesis of ordered mesoporous carbon materials by dry etching. Chem. Eur. J. 2015, 21, 14753–14757. [Google Scholar] [CrossRef] [PubMed]
- Walczak, R.; Kurpil, B.; Savateev, A.; Heil, T.; Schmidt, J.; Qin, Q.; Antonietti, M.; Oschatz, M. Template- and metal-free synthesis of nitrogen-rich nanoporous “noble” carbon materials by direct pyrolysis of a preorganized hexaazatriphenylene precursor. Angew. Chem. Int. Ed. 2018, 57, 10765–10770. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Brun, N.; Budarin, V.L.; Clark, J.H.; Titirici, M.M. Always look on the “light” side of life: Sustainable carbon aerogels. ChemSusChem 2014, 7, 670–689. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ritter, J.A. Carbonization and activation of sol-gel derived carbon xerogels. Carbon 2000, 38, 849–861. [Google Scholar] [CrossRef]
- Oschatz, M.; Pré, P.; Dörfler, S.; Nickel, W.; Beaunier, P.; Rouzaud, J.-N.; Fischer, C.; Brunner, E.; Kaskel, S. Nanostructure characterization of carbide-derived carbons by morphological analysis of transmission electron microscopy images combined with physisorption and raman spectroscopy. Carbon 2016, 105, 314–322. [Google Scholar] [CrossRef]
- Faber, K.; Badaczewski, F.; Oschatz, M.; Mondin, G.; Nickel, W.; Kaskel, S.; Smarsly, B.M. In-depth investigation of the carbon microstructure of silicon carbide-derived carbons by wide-angle x-ray scattering. J. Phys. Chem. C 2014, 118, 15705–15715. [Google Scholar] [CrossRef]
- Mangarella, M.C.; Ewbank, J.L.; Dutzer, M.R.; Alamgir, F.M.; Walton, K.S. Synthesis of embedded iron nanoparticles in Fe3C-derived carbons. Carbon 2014, 79, 74–84. [Google Scholar] [CrossRef]
- Oschatz, M.; van Deelen, T.W.; Weber, J.L.; Lamme, W.S.; Wang, G.; Goderis, B.; Verkinderen, O.; Dugulan, A.I.; de Jong, K.P. Effects of calcination and activation conditions on ordered mesoporous carbon supported iron catalysts for production of lower olefins from synthesis gas. Catal. Sci. Technol. 2016, 6, 8464–8473. [Google Scholar] [CrossRef] [Green Version]
- Antonietti, M.; Oschatz, M. The concept of “noble, heteroatom-doped carbons,” their directed synthesis by electronic band control of carbonization, and applications in catalysis and energy materials. Adv. Mater. 2018, 30, 1706836. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fechler, N.; Bandosz, T.J. Chemically heterogeneous nitrogen sites of various reactivity in porous carbons provide high stability of CO2 electroreduction catalysts. Appl. Catal. B Environ. 2018, 234, 1–9. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.P.; Varela, A.S.; Sinev, I.; Bon, V.; Roldan Cuenya, B.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Hofmann, J.P.; van Deelen, T.W.; Lamme, W.S.; Krans, N.A.; Hensen, E.J.; de Jong, K.P. Effects of the functionalization of the ordered mesoporous carbon support surface on iron catalysts for the fischer-tropsch synthesis of lower olefins. ChemCatChem 2017, 9, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Lama, S.M.G.; Schmidt, J.; Malik, A.; Walczak, R.; Silva, D.V.; Völkel, A.; Oschatz, M. Modification of salt-templated carbon surface chemistry for efficient oxidation of glucose with supported gold catalysts. ChemCatChem 2018, 10, 2458–2465. [Google Scholar] [CrossRef]
- Qin, Q.; Heil, T.; Antonietti, M.; Oschatz, M. Single-site gold catalysts on hierarchical n-doped porous noble carbon for enhanced electrochemical reduction of nitrogen. Small Methods 2018, 1800202. [Google Scholar] [CrossRef]
- Men, Y.; Siebenbürger, M.; Qiu, X.; Antonietti, M.; Yuan, J. Low fractions of ionic liquid or poly (ionic liquid) can activate polysaccharide biomass into shaped, flexible and fire-retardant porous carbons. J. Mater. Chem. A 2013, 1, 11887–11893. [Google Scholar] [CrossRef]
- Nishihara, H.; Kyotani, T. Zeolite-templated carbons—three-dimensional microporous graphene frameworks. Chem. Commun. 2018, 54, 5648–5673. [Google Scholar] [CrossRef] [PubMed]
- Péan, C.; Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Daffos, B.; Salanne, M.; Simon, P. On the dynamics of charging in nanoporous carbon-based supercapacitors. ACS Nano 2014, 8, 1576–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlet, C.; Pean, C.; Rotenberg, B.; Madden, P.A.; Daffos, B.; Taberna, P.L.; Simon, P.; Salanne, M. Highly confined ions store charge more efficiently in supercapacitors. Nat. Commun. 2013, 4, 2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, Y.; Kim, H.J. Nanoporous carbon supercapacitors in an ionic liquid: A computer simulation study. ACS Nano 2010, 4, 2345–2355. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Olivier, J.P. Improving the models used for calculating the size distribution of micropore volume of activated carbons from adsorption data. Carbon 1998, 36, 1469–1472. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2d-nldft adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Silvestre-Albero, J.; Silvestre-Albero, A.; Rodríguez-Reinoso, F.; Thommes, M. Physical characterization of activated carbons with narrow microporosity by nitrogen (77.4 K), carbon dioxide (273 K) and argon (87.3 K) adsorption in combination with immersion calorimetry. Carbon 2012, 50, 3128–3133. [Google Scholar] [CrossRef]
- Osswald, S.; Chmiola, J.; Gogotsi, Y. Structural evolution of carbide-derived carbons upon vacuum annealing. Carbon 2012, 50, 4880–4886. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Pré, P.; Huchet, G.; Jeulin, D.; Rouzaud, J.-N.; Sennour, M.; Thorel, A. A new approach to characterize the nanostructure of activated carbons from mathematical morphology applied to high resolution transmission electron microscopy images. Carbon 2013, 52, 239–258. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: Catalysis beyond electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- López-Salas, N.; Gutiérrez, M.C.; Ania, C.O.; Muñoz-Márquez, M.A.; Luisa Ferrer, M.; Monte, F.D. Nitrogen-doped carbons prepared from eutectic mixtures as metal-free oxygen reduction catalysts. J. Mater. Chem. A 2016, 4, 478–488. [Google Scholar] [CrossRef] [Green Version]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Antonietti, M.; Garcia, H. Active sites on graphene-based materials as metal-free catalysts. Chem. Soc. Rev. 2017, 46, 4501–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef]
- Hirst, E.A.; Taylor, A.; Mokaya, R. A simple flash carbonization route for conversion of biomass to porous carbons with high CO2 storage capacity. J. Mater. Chem. A 2018, 6, 12393–12403. [Google Scholar] [CrossRef]
- Hao, G.-P.; Li, W.-C.; Wang, S.; Zhang, S.; Lu, A.-H. Tubular structured ordered mesoporous carbon as an efficient sorbent for the removal of dyes from aqueous solutions. Carbon 2010, 48, 3330–3339. [Google Scholar] [CrossRef]
- Fischer, C.; Oschatz, M.; Nickel, W.; Leistenschneider, D.; Kaskel, S.; Brunner, E. Bioinspired carbide-derived carbons with hierarchical pore structure for the adsorptive removal of mercury from aqueous solution. Chem. Commun. 2017, 53, 4845–4848. [Google Scholar] [CrossRef] [PubMed]
- Yachamaneni, S.; Yushin, G.; Yeon, S.H.; Gogotsi, Y.; Howell, C.; Sandeman, S.; Phillips, G.; Mikhalovsky, S. Mesoporous carbide-derived carbon for cytokine removal from blood plasma. Biomaterials 2010, 31, 4789–4794. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon materials for lithium sulfur batteries-ten critical questions. Chem. Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-doped porous carbons with exceptionally high CO2 selectivity for CO2 capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Lai, F.; Feng, J.; Yan, R.; Wang, G.-C.; Antonietti, M.; Oschatz, M. Breaking the limits of ionic liquid-based supercapacitors: Mesoporous carbon electrodes functionalized with manganese oxide nanosplotches for dense, stable, and wide-temperature energy storage. Adv. Funct. Mater. 2018, 28, 1801298. [Google Scholar] [CrossRef]
- Krüner, B.; Schreiber, A.; Tolosa, A.; Quade, A.; Badaczewski, F.; Pfaff, T.; Smarsly, B.M.; Presser, V. Nitrogen-containing novolac-derived carbon beads as electrode material for supercapacitors. Carbon 2018, 132, 220–231. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Polypyrrole-derived activated carbons for high-performance electrical double-layer capacitors with ionic liquid electrolyte. Adv. Funct. Mater. 2012, 22, 827–834. [Google Scholar] [CrossRef]
- Yin, L.-C.; Liang, J.; Zhou, G.-M.; Li, F.; Saito, R.; Cheng, H.-M. Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy 2016, 25, 203–210. [Google Scholar] [CrossRef]
- Hippauf, F.; Nickel, W.; Hao, G.-P.; Schwedtmann, K.; Giebeler, L.; Oswald, S.; Borchardt, L.; Doerfler, S.; Weigand, J.J.; Kaskel, S. The importance of pore size and surface polarity for polysulfide adsorption in lithium sulfur batteries. Adv. Mater. Interfaces 2016, 3, 1600508. [Google Scholar] [CrossRef]
- Song, J.; Gordin, M.L.; Xu, T.; Chen, S.; Yu, Z.; Sohn, H.; Lu, J.; Ren, Y.; Duan, Y.; Wang, D. Strong lithium polysulfide chemisorption on electroactive sites of nitrogen-doped carbon composites for high-performance lithium-sulfur battery cathodes. Angew. Chem. Int. Ed. 2015, 54, 4325–4329. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.; Paraknowitsch, J.; Schlogl, R. Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem 2010, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Antonietti, M. Metal nanoparticles at mesoporous n-doped carbons and carbon nitrides: Functional mott-schottky heterojunctions for catalysis. Chem. Soc. Rev. 2013, 42, 6593–6604. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, K.X.; Zhang, B.; Wang, H.H.; Yu, Q.Y.; Li, X.H.; Antonietti, M.; Chen, J.S. Activating cobalt nanoparticles via the mott-schottky effect in nitrogen-rich carbon shells for base-free aerobic oxidation of alcohols to esters. J. Am. Chem. Soc. 2017, 139, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Vile, G.; Albani, D.; Nachtegaal, M.; Chen, Z.; Dontsova, D.; Antonietti, M.; Lopez, N.; Perez-Ramirez, J. A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. 2015, 54, 11265–11269. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Foulston, R.; Mokaya, R. Superactivated carbide-derived carbons with high hydrogen storage capacity. Energy Environ. Sci. 2010, 3, 223–227. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigalov, S.; Levi, M.D.; Salitra, G.; Aurbach, D.; Jänes, A.; Lust, E.; Halalay, I.C. Selective adsorption of multivalent ions into tic-derived nanoporous carbon. Carbon 2012, 50, 3957–3960. [Google Scholar] [CrossRef]
- Heon, M.; Lofland, S.; Applegate, J.; Nolte, R.; Cortes, E.; Hettinger, J.D.; Taberna, P.-L.; Simon, P.; Huang, P.; Brunet, M.; et al. Continuous carbide-derived carbon films with high volumetric capacitance. Energy Environ. Sci. 2011, 4, 135–138. [Google Scholar] [CrossRef]
- Wuttke, S.; Medina, D.D.; Rotter, J.M.; Begum, S.; Stassin, T.; Ameloot, R.; Oschatz, M.; Tsotsalas, M. Bringing porous organic and carbon-based materials toward thin-film applications. Adv. Funct. Mater. 2018, 1801545. [Google Scholar] [CrossRef]
- Gao, Y.; Presser, V.; Zhang, L.; Niu, J.J.; McDonough, J.K.; Pérez, C.R.; Lin, H.; Fong, H.; Gogotsi, Y. High power supercapacitor electrodes based on flexible tic-cdc nano-felts. J. Power Sources 2012, 201, 368–375. [Google Scholar] [CrossRef]
- Rose, M.; Kockrick, E.; Senkovska, I.; Kaskel, S. High surface area carbide-derived carbon fibers produced by electrospinning of polycarbosilane precursors. Carbon 2010, 48, 403–407. [Google Scholar] [CrossRef]
- Borchardt, L.; Leistenschneider, D.; Haase, J.; Dvoyashkin, M. Revising the concept of pore hierarchy for ionic transport in carbon materials for supercapacitors. Adv. Energy Mater. 2018, 8, 1800892. [Google Scholar] [CrossRef]
- Schönherr, A. Boehm titration revisited (part i): Practical aspects for achieving a high precision in quantifying oxygen-containing surface groups on carbon materials. C 2018, 4, 21. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.; Scholz, P.; Adelhelm, P. Boehm titration revisited (part ii): A comparison of boehm titration with other analytical techniques on the quantification of oxygen-containing surface groups for a variety of carbon materials. C 2018, 4, 22. [Google Scholar] [CrossRef]
- Leistenschneider, D.; Zürbes, K.; Schneidermann, C.; Grätz, S.; Oswald, S.; Wegner, K.; Klemmed, B.; Giebeler, L.; Eychmüller, A.; Borchardt, L. Mechanochemical functionalization of carbon black at room temperature. C 2018, 4, 14. [Google Scholar] [CrossRef]
- Xing, R.; Zhou, Y.; Ma, R.; Liu, Q.; Luo, J.; Yang, M.; Wang, J. Nitrogen-doped hollow carbon spheres with embedded co nanoparticles as active non-noble-metal electrocatalysts for the oxygen reduction reaction. C 2018, 4, 11. [Google Scholar] [CrossRef]
- Seredych, M.; Mikhalovska, L.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of bovine serum albumin on carbon-based materials. C 2018, 4, 3. [Google Scholar] [CrossRef]
- Moo, J.; Omar, A.; Jaumann, T.; Oswald, S.; Balach, J.; Maletti, S.; Giebeler, L. One-pot synthesis of graphene-sulfur composites for li-s batteries: Influence of sulfur precursors. C 2017, 4, 2. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oschatz, M.; Walczak, R. Crucial Factors for the Application of Functional Nanoporous Carbon-Based Materials in Energy and Environmental Applications. C 2018, 4, 56. https://doi.org/10.3390/c4040056
Oschatz M, Walczak R. Crucial Factors for the Application of Functional Nanoporous Carbon-Based Materials in Energy and Environmental Applications. C. 2018; 4(4):56. https://doi.org/10.3390/c4040056
Chicago/Turabian StyleOschatz, Martin, and Ralf Walczak. 2018. "Crucial Factors for the Application of Functional Nanoporous Carbon-Based Materials in Energy and Environmental Applications" C 4, no. 4: 56. https://doi.org/10.3390/c4040056
APA StyleOschatz, M., & Walczak, R. (2018). Crucial Factors for the Application of Functional Nanoporous Carbon-Based Materials in Energy and Environmental Applications. C, 4(4), 56. https://doi.org/10.3390/c4040056





