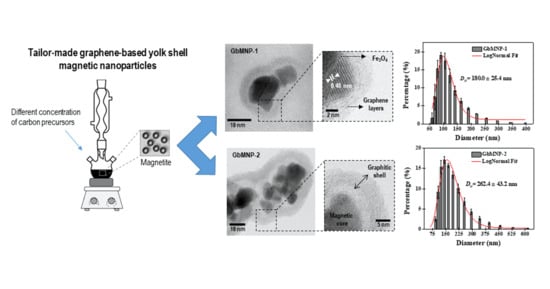
A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
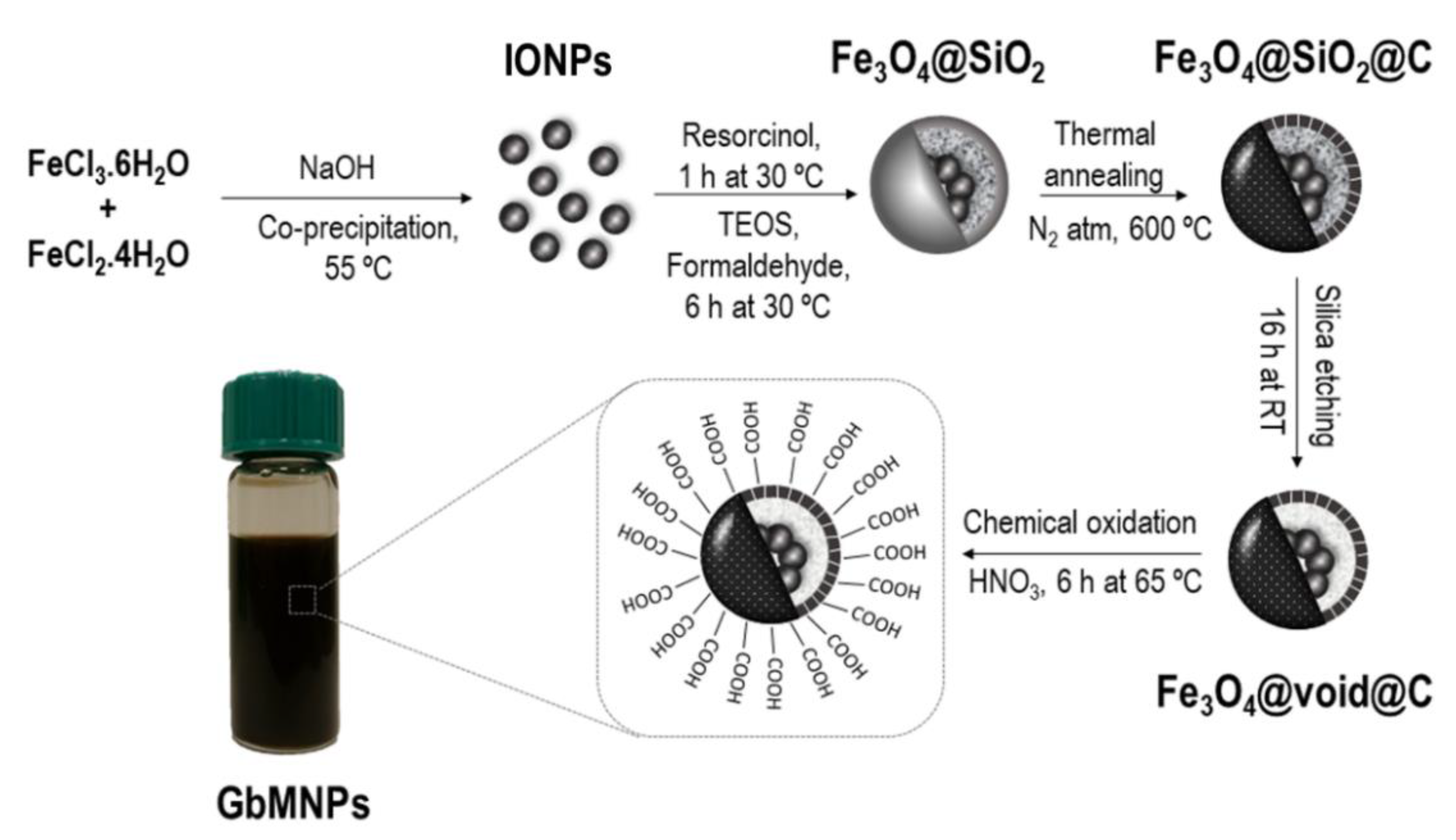
2.2. Synthesis of GbMNPs
2.3. Colloidal Stabilization of GbMNPs
2.4. Characterization of GbMNPs
2.5. Drug Loading Studies
2.6. In Vitro pH-Dependent Drug Release and Kinetics Studies
2.7. In Vitro Biostudies
2.7.1. Cell Culture
2.7.2. Biocompatibility and Cellular Drug-Delivery Assay
3. Results and Discussion
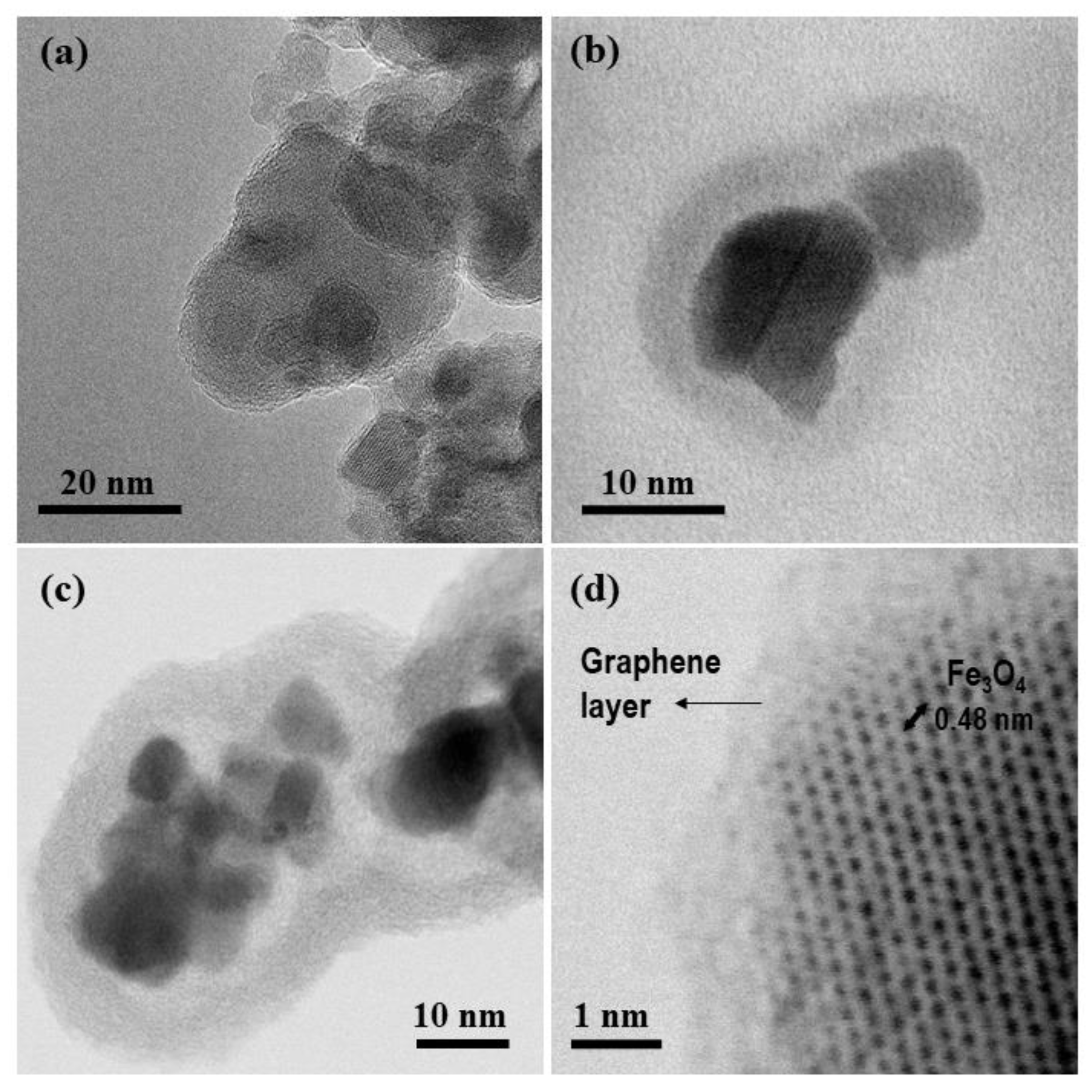
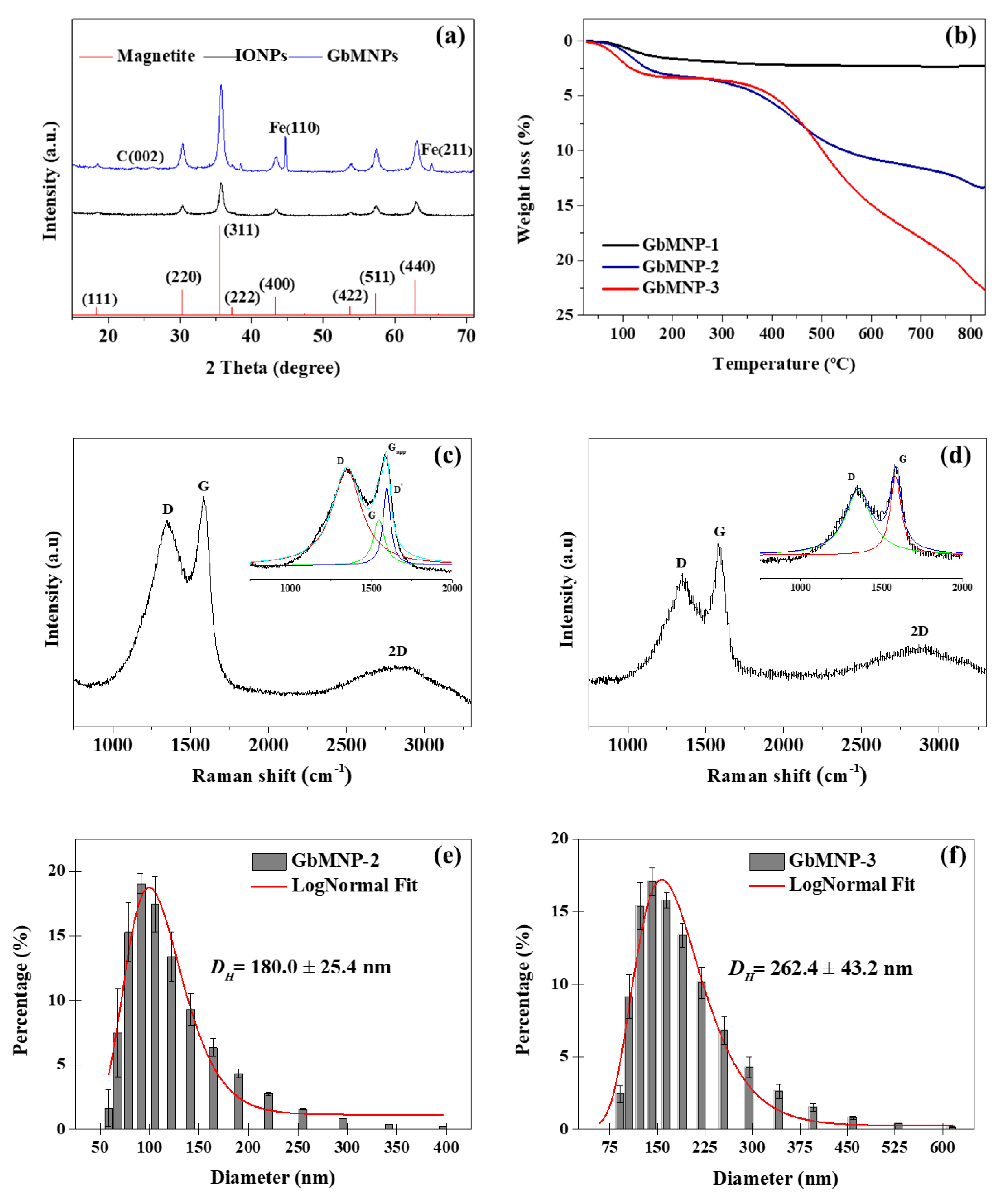
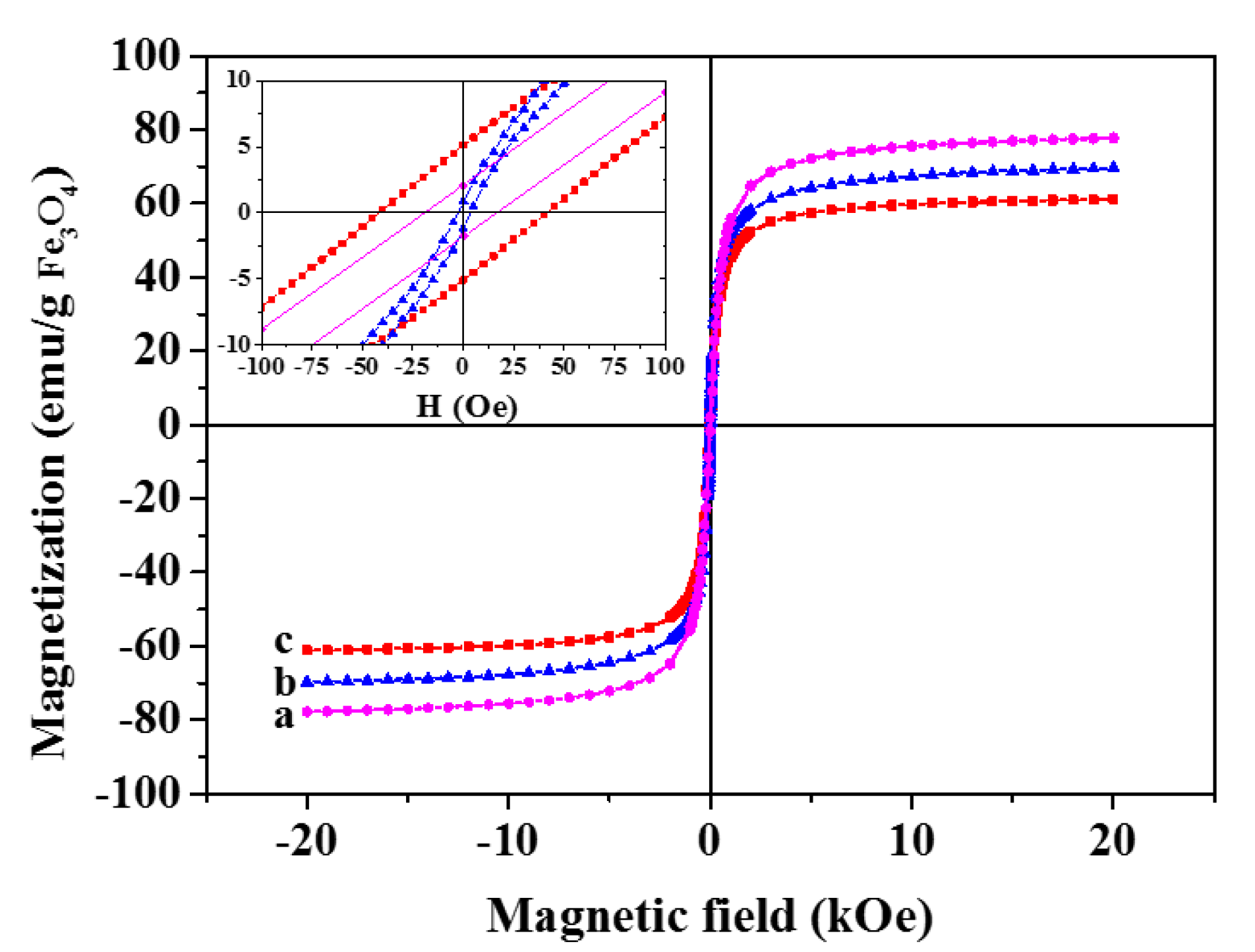
3.1. Synthesis and Characterization of the GbMNPs
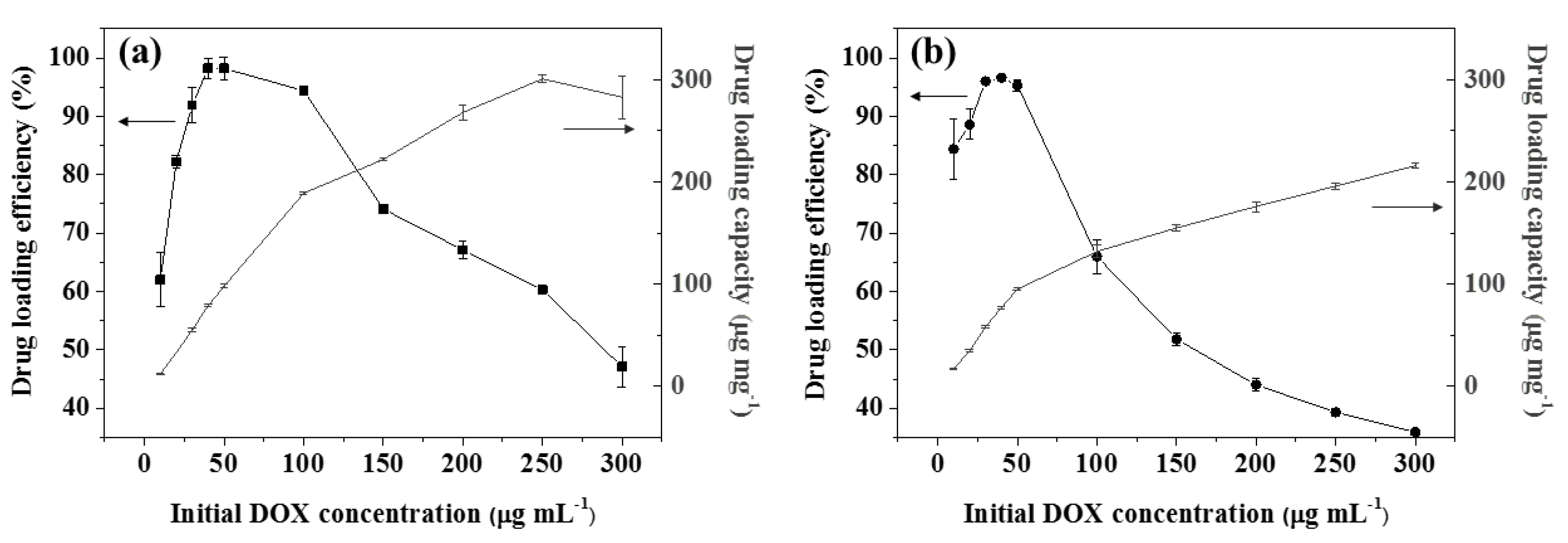
3.2. DOX Loading Studies
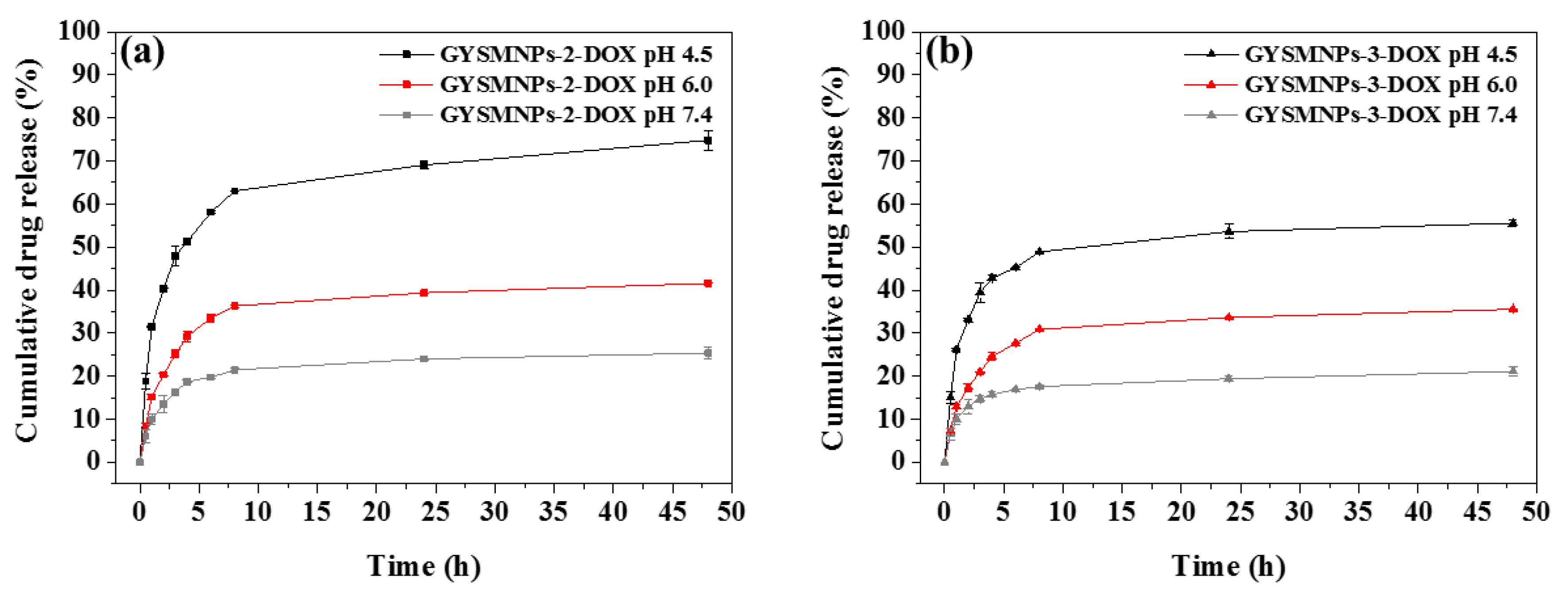
3.3. In Vitro pH-Dependent Drug Release and Kinetics Studies
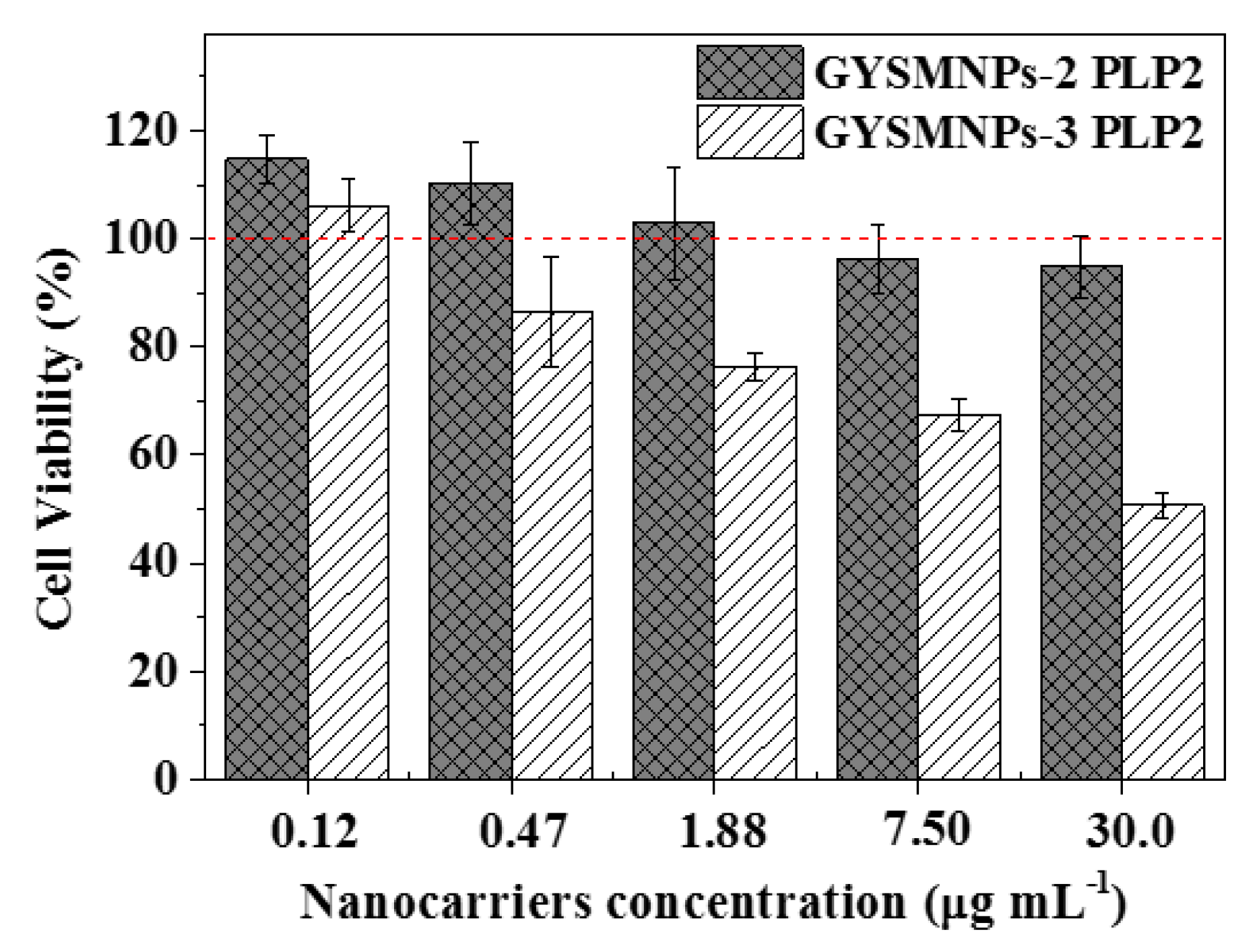
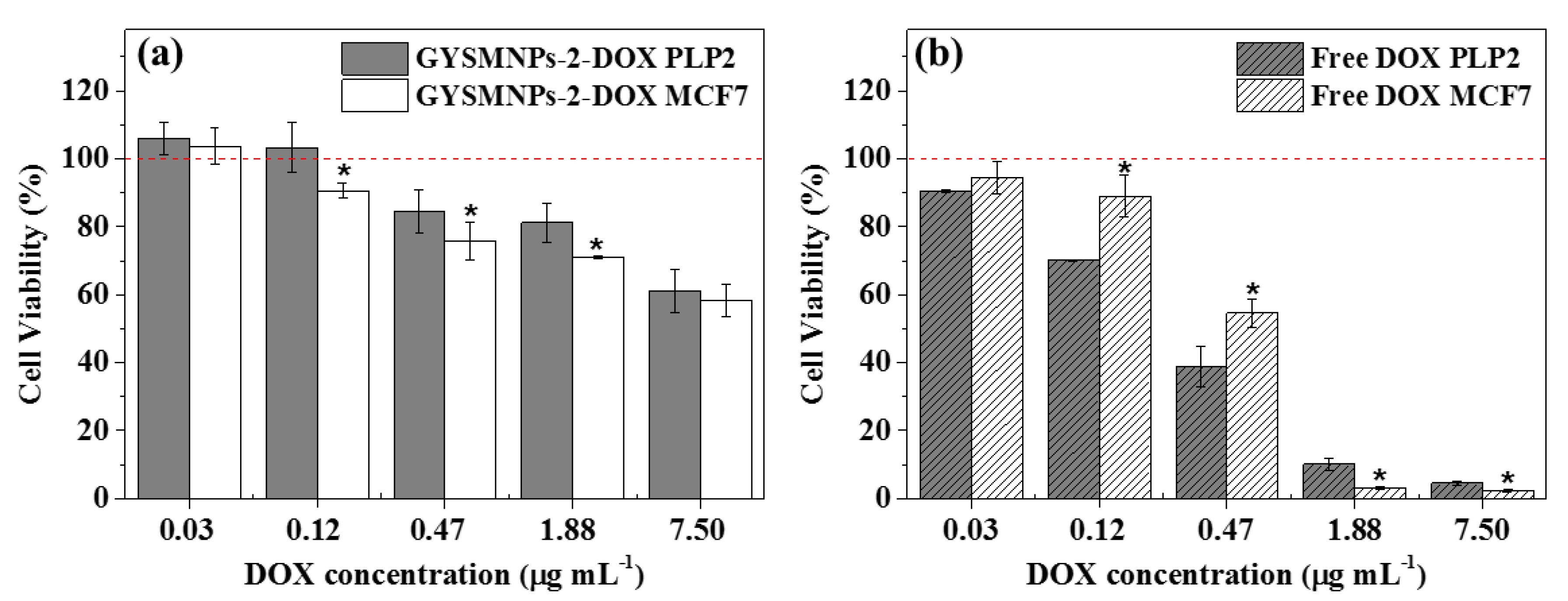
3.4. In Vitro Biocompatibility and Cellular Drug-Delivery Assay
4. Conclusions
Author contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Schadt, M.J.; Lim, I.I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.-Y.; Luo, J.; Zhong, C.-J. Fabrication of Magnetic Core@Shell Fe Oxide@Au Nanoparticles for Interfacial Bioactivity and Bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef] [PubMed]
- Robinson, I.; Tung, L.D.; Maenosono, S.; Wälti, C.; Thanh, N.T.K. Synthesis of core-shell gold coated magnetic nanoparticles and their interaction with thiolated DNA. Nanoscale 2010, 2, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Chao, J.; Song, C.; Liu, B.; Zhu, D.; Sun, Y.; Yang, B.; Zhang, Q.; Chen, Y.; et al. Synthesis of magnetic core-branched Au shell nanostructures and their application in cancer-related miRNA detection via SERS. Sci. China Mater. 2017, 60, 1129–1144. [Google Scholar] [CrossRef]
- Jovanovic, A.V.; Flint, J.A.; Varshney, M.; Morey, T.E.; Dennis, D.M.; Duran, R.S. Surface Modification of Silica Core-Shell Nanocapsules: Biomedical Implications. Biomacromolecules 2006, 7, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, C.; Wang, F.; Xil, Z.; Wang, Z.; Deng, Y.; Hel, N. Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J. Nanosci. Nanotechnol. 2012, 12, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Superparamagnetic Iron Oxide (Fe3O4) Nanoparticles Coated with PEG/PEI for Biomedical Applications: A Facile and Scalable Preparation Route Based on the Cathodic Electrochemical Deposition Method. Adv. Phys. Chem. 2017, 2017, 9437487. [Google Scholar] [CrossRef]
- Medeiros, S.F.; Santos, A.M.; Fessi, H.; Elaissari, A. Stimuli-responsive magnetic particles for biomedical applications. Int. J. Pharm. 2011, 403, 139–161. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, S.; Du, X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon 2016, 101, 135–142. [Google Scholar] [CrossRef]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly Hydrophilic Luminescent Magnetic Mesoporous Carbon Nanospheres for Controlled Release of Anticancer Drug and Multimodal Imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Zhang, Y.N.; Zhang, W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug Discov. Today 2017, 22, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Zhang, Y.S.; Khademhosseini, A. Boosting clinical translation of nanomedicine. Nanomedicine 2016, 11, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, J.; Chen, D.; Wu, Y.; Zhang, W.; Zheng, F.; Cao, J.; Ma, H.; Liu, Y. Yolk-shell hybrid nanoparticles with magnetic and pH-sensitive properties for controlled anticancer drug delivery. Nanoscale 2013, 5, 11718–11724. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Scudieri, C.; Polichetti, M.; Ciambelli, P. Electrochemical Applications of Magnetic Core-Shell Graphene-Coated FeCo Nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 3157–3166. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Bañobre-López, M.; Gallo, J.; Tavares, P.B.; Silva, A.M.T.; Lima, R.; Gomes, H.T. Haemocompatibility of iron oxide nanoparticles synthesized for theranostic applications: A high-sensitivity microfluidic tool. J. Nanopart. Res. 2016, 18, 1–17. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Baldi, G.; Doumett, S.; Garcia-Hevia, L.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; et al. Multifunctional graphene-based magnetic nanocarriers for combined hyperthermia and dual stimuli-responsive drug delivery. Mater. Sci. Eng. C 2018, 93, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Liu, Y.-X.; Yan, X.-Y.; Yong, G.-P.; Xu, Y.-P.; Liu, S.-M. One-pot synthesis of yolk-shell mesoporous carbon spheres with high magnetisation. J. Mater. Chem. A 2014, 2, 9600–9606. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Frontistis, Z.; Mantzavinos, D.; Venieri, D.; Antonopoulou, M.; Konstantinou, I.; Silva, A.M.T.; Faria, J.L.; Gomes, H.T. Magnetic carbon xerogels for the catalytic wet peroxide oxidation of sulfamethoxazole in environmentally relevant water matrices. Appl. Catal. B Environ. 2016, 199, 170–186. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, B.; Barros, L.; Calhelha, R.C.; Heleno, S.; Alves, M.J.; Walcott, S.; Bittova, M.; Kuban, V.; Ferreira, I.C.F.R. Bioactive properties and phenolic profile of Momordica charantia L. medicinal plant growing wild in Trinidad and Tobago. Ind. Crops Prod. 2017, 95, 365–373. [Google Scholar] [CrossRef]
- Bharath, G.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Mangalaraj, D.; Ponpandian, N. Solvent-free mechanochemical synthesis of graphene oxide and Fe3O4-reduced graphene oxide nanocomposites for sensitive detection of nitrite. J. Mater. Chem. A 2015, 3, 15529–15539. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, N.; Wei, Y.; Zhang, G. An in situ gelatin-assisted hydrothermal synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic performance under ultraviolet and visible light. RSC Adv. 2014, 4, 7933–7943. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Z.; Xu, F.; Thummavichai, K.; Chen, H.; Xia, Y.; Zhu, Y. A generic method to synthesise graphitic carbon coated nanoparticles in large scale and their derivative polymer nanocomposites. Sci. Rep. 2017, 7, 11829. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ryu, S.Y.; Kim, J.; Jun, Y. A study of TiO2/carbon black composition as counter electrode materials for dye-sensitized solar cells. Nanoscale Res. Lett. 2013, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Silva, A.M.T.; Tavares, P.B.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Hybrid magnetic graphitic nanocomposites for catalytic wet peroxide oxidation applications. Catal. Today 2017, 280, 184–191. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Li, W.; Li, J.; Yu, H.; Liu, L.; Wu, G.; Yang, T.; Luo, L. Preparation of boron nitride nanosheet-coated carbon fibres and their enhanced antioxidant and microwave-absorbing properties. RSC Adv. 2018, 8, 17944–17949. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183. [Google Scholar] [CrossRef]
- Mohan, P.; Rapoport, N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Rahmani, F.; Nouranian, S. Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems. J. Mater. Chem. B 2016, 4, 7441–7451. [Google Scholar] [CrossRef]
- Al-Nahain, A.; Lee, S.Y.; In, I.; Lee, K.D.; Park, S.Y. Triggered pH/redox responsive release of doxorubicin from prepared highly stable graphene with thiol grafted Pluronic. Int. J. Pharm. 2013, 450, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of oxygen functionalities on the synthesis of photocatalytically active graphene-TiO2 composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Adnan, A.; Lam, R.; Chen, H.; Lee, J.; Schaffer, D.J.; Barnard, A.S.; Schatz, G.C.; Ho, D.; Liu, W.K. Atomistic Simulation and Measurement of pH Dependent Cancer Therapeutic Interactions with Nanodiamond Carrier. Mol. Pharm. 2011, 8, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials—An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Zhai, G. Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C 2016, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Kumeria, T.; Maher, S.; Wang, Y.; Kaur, G.; Wang, L.; Erkelens, M.; Forward, P.; Lambert, M.F.; Evdokiou, A.; Losic, D. Naturally Derived Iron Oxide Nanowires from Bacteria for Magnetically Triggered Drug Release and Cancer Hyperthermia in 2D and 3D Culture Environments: Bacteria Biofilm to Potent Cancer Therapeutic. Biomacromolecules 2016, 17, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
| Material | Magnetic Core (g) | Resorcinol (g) | Formaldehyde (mL) | TEOS (mL) | Hollow Thickness (nm) a | Carbon-Shell Thickness (nm) a |
|---|---|---|---|---|---|---|
| GbMNP-1 | 0.25 | 0.05 | 0.075 | 0.10 | Not detected | 1.41 ± 0.44 |
| GbMNP-2 | 0.25 | 0.10 | 0.150 | 0.21 | 0.70 ± 0.30 | 3.55 ± 1.27 |
| GbMNP-3 | 0.25 | 0.20 | 0.300 | 0.41 | 2.07 ± 0.92 | 7.07 ± 1.88 |
| Material | SBET (m2·g−1) | Smeso (m2·g−1) | Vmicro (cm3·g−1) | Vtotal (cm3·g−1) | Vmicro/Vtotal | daverage (nm) |
|---|---|---|---|---|---|---|
| GbMNP-2 | 156 | 123 | 0.013 | 0.318 | 0.041 | 8.2 |
| GbMNP-3 | 245 | 160 | 0.035 | 0.333 | 0.105 | 5.4 |
| Sample | Ms (emu·g−1 IONPs) | Hc (Oe) | Mr (emu·g−1 IONPs) |
|---|---|---|---|
| IONPs | 77.7 | 18.33 | 1.94 |
| GbMNP-2 | 69.8 | 3.54 | 1.16 |
| GbMNP-3 | 61.2 | 41.33 | 5.08 |
| Sample | pH | Zero-Order | First-Order | Hixson-Crowell | Higuchi | Korsmeyer–Peppas | |
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | ||
| GbMNP-2 | 7.4 | 0.45 | 0.37 | 0.47 | 0.72 | 0.85 | 0.30 |
| 6.0 | 0.46 | 0.35 | 0.50 | 0.73 | 0.84 | 0.33 | |
| 4.5 | 0.47 | 0.39 | 0.58 | 0.73 | 0.96 | 0.44 | |
| GbMNP-3 | 7.4 | 0.41 | 0.39 | 0.43 | 0.67 | 0.85 | 0.23 |
| 6.0 | 0.48 | 0.37 | 0.51 | 0.74 | 0.85 | 0.33 | |
| 4.5 | 0.40 | 0.34 | 0.46 | 0.66 | 0.81 | 0.26 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.O.; Baldi, G.; Doumett, S.; Gallo, J.; Bañobre-López, M.; Dražić, G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Lima, R.; Silva, A.M.T.; et al. A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C 2018, 4, 55. https://doi.org/10.3390/c4040055
Rodrigues RO, Baldi G, Doumett S, Gallo J, Bañobre-López M, Dražić G, Calhelha RC, Ferreira ICFR, Lima R, Silva AMT, et al. A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C. 2018; 4(4):55. https://doi.org/10.3390/c4040055
Chicago/Turabian StyleRodrigues, Raquel O., Giovanni Baldi, Saer Doumett, Juan Gallo, Manuel Bañobre-López, Goran Dražić, Ricardo C. Calhelha, Isabel C. F. R. Ferreira, Rui Lima, Adrián M. T. Silva, and et al. 2018. "A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine" C 4, no. 4: 55. https://doi.org/10.3390/c4040055
APA StyleRodrigues, R. O., Baldi, G., Doumett, S., Gallo, J., Bañobre-López, M., Dražić, G., Calhelha, R. C., Ferreira, I. C. F. R., Lima, R., Silva, A. M. T., & Gomes, H. T. (2018). A Tailor-Made Protocol to Synthesize Yolk-Shell Graphene-Based Magnetic Nanoparticles for Nanomedicine. C, 4(4), 55. https://doi.org/10.3390/c4040055