Photoelectrochemical Response of WO3/Nanoporous Carbon Anodes for Photocatalytic Water Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Electrodes
2.3. Photoelectrochemical Measurements
2.4. Characterization Techniques
3. Results and Discussion
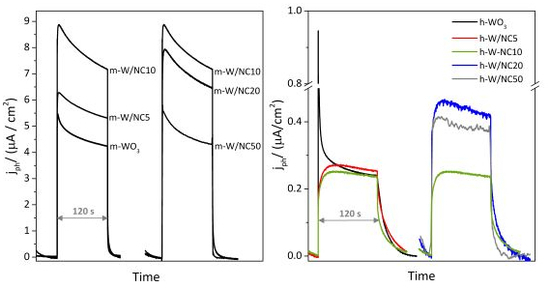
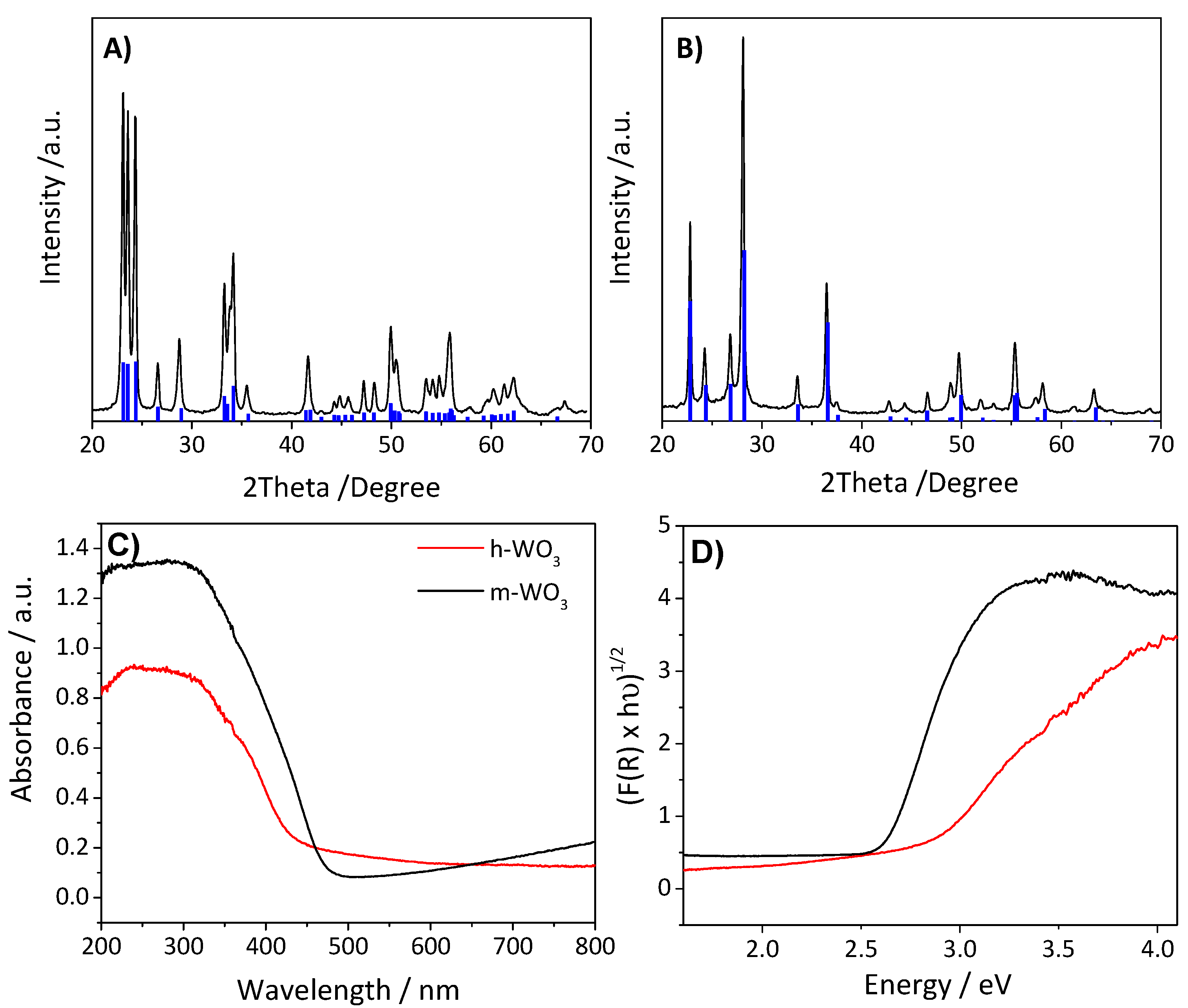
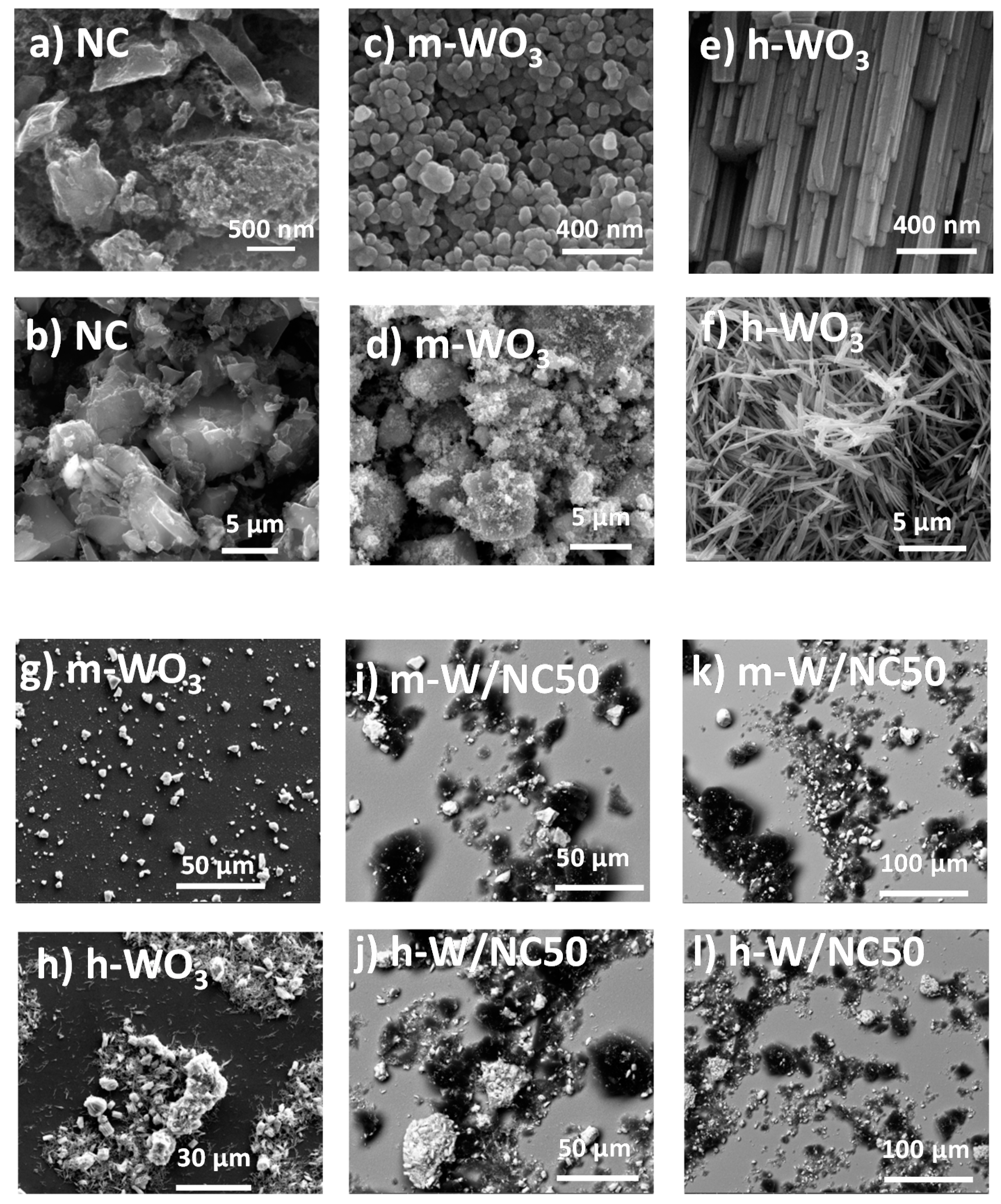
3.1. Characterization of the Materials
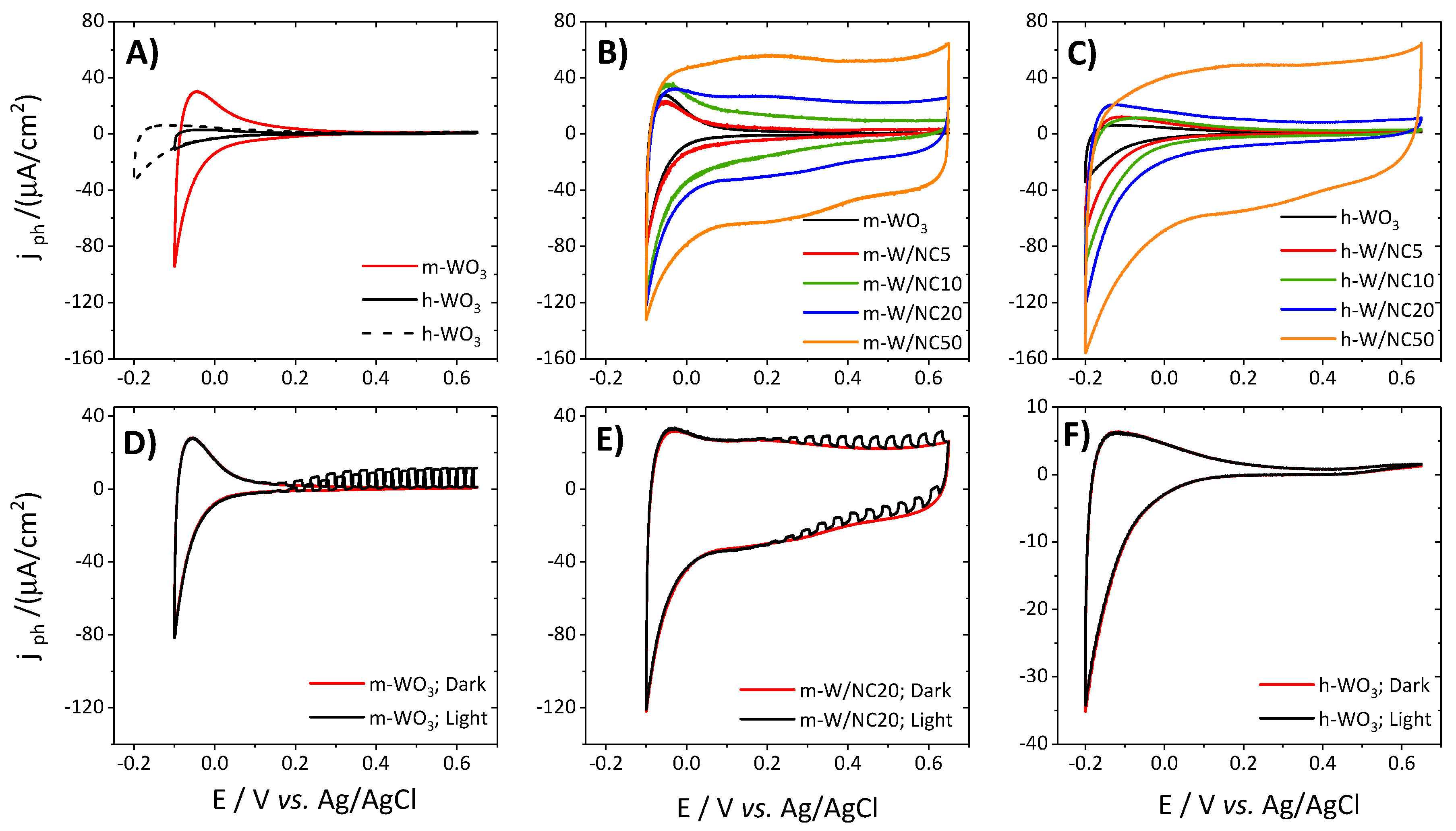
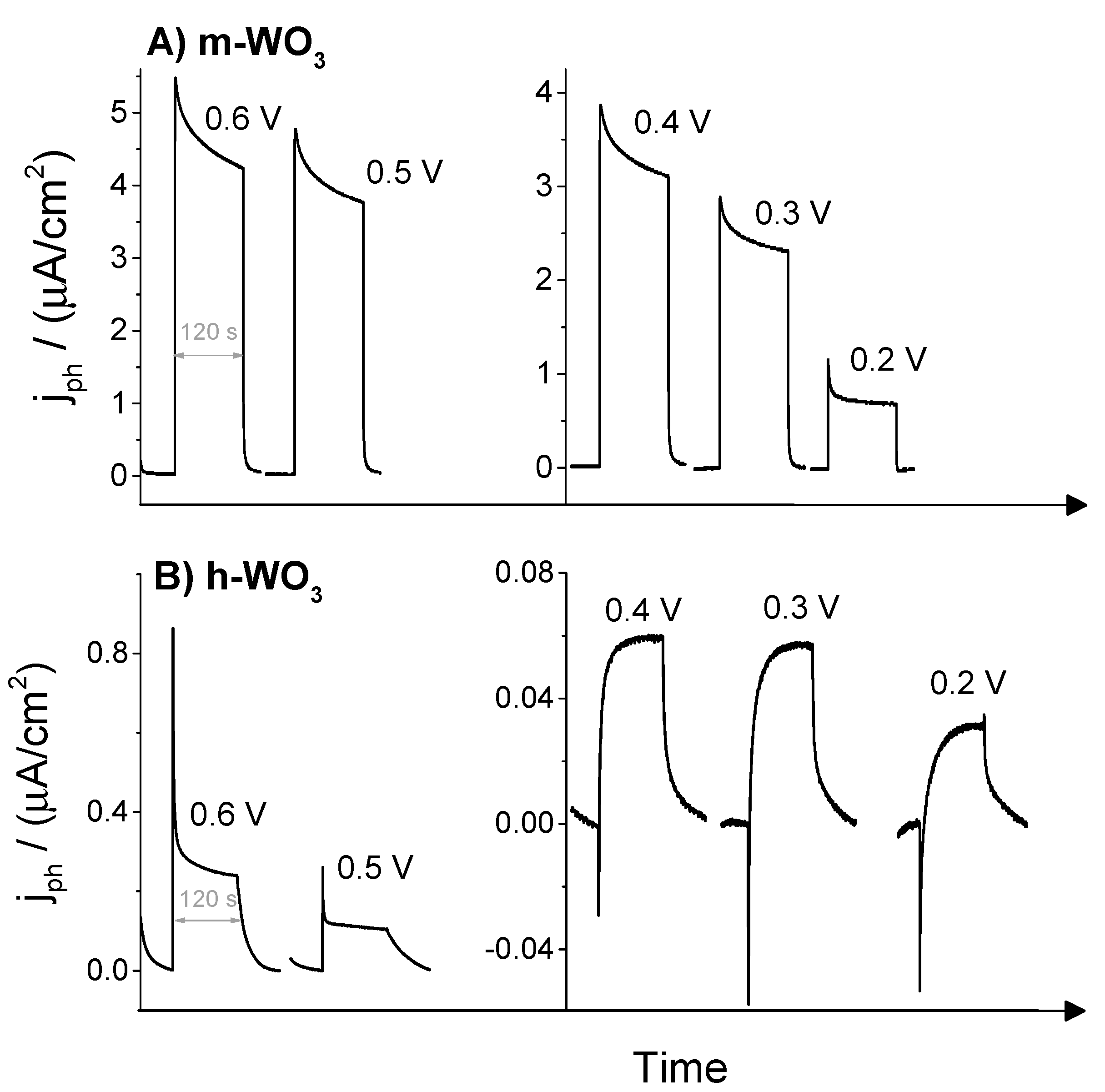
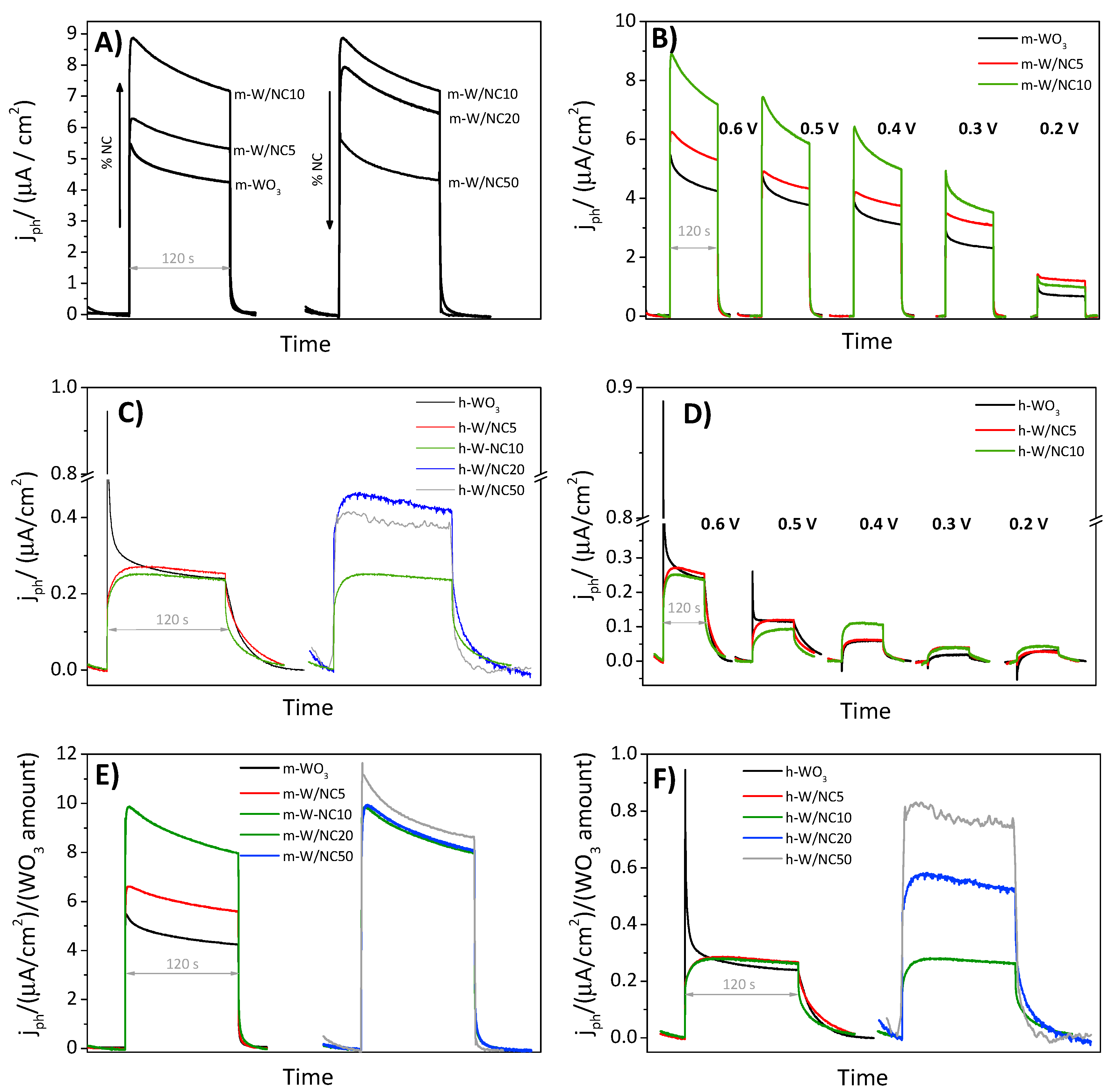
3.2. Photoelectrochemical Response of the Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Botella, P.; García-González, E.; Dejoz, A.; López Nieto, J.M.; Vázquez, M.I.; González-Cablet, J. Selective oxidative dehydrogenation of ethane on MoWTeNbO mixed metal oxide catalysts. J. Catal. 2004, 225, 428–438. [Google Scholar] [CrossRef]
- Abdelouabad, A.L.B.; Roullet, M.; Brun, M.; Burrows, A.; Kiely, C.J.; Volta, J.C.; Abon, M. Surface alteration of (VO)(2)P2O7 by alpha-Sb2O4 as a route to control then-butane selective oxidation. Appl. Catal. A Gen. 2001, 210, 121–136. [Google Scholar] [CrossRef]
- Chabi, S.; Papadantonakis, K.M.; Lewis, N.S.; Freud, M.S. Membranes for artificial photosynthesis. Energy Environ. Sci. 2017, 10, 1320–1338. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Fan, W.; Ma, W.; Liang, Z.; Shi, J.; Liao, S.; Li, C. Photoassisted oxygen reduction reaction in H2-O2 fuel cells. Angew. Chem. Int. Ed. 2016, 55, 14748–14751. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Bignozzi, C.A.; Caramori, S.; Cristino, V.; Argazzi, R.; Meda, L.; Tacca, A. Nanostructured photoelectrodes based on WO3: Applications to photooxidation of aqueous electrolytes. Chem. Soc. Rev. 2013, 42, 2228–2246. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Wang, Q. Nanostructure-based WO3 photoanodes for photoelectrochemical water splitting. Phys. Chem. Phys. 2012, 14, 7894–7911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Song, G.; Kim, C.W.; Kang, Y.S. Fabrication of (001)-Oriented monoclinic WO3 films on FTO substrates. Nanoscale 2013, 5, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wygant, B.R.; Mabayoje, O.; Lin, J.; Kawashima, K.; Kim, J.H.; Li, W.; Li, J.; Mullins, C.B. Interface engineering and its effect on WO3-based photoanode and tandem cell. ACS Appl. Mater. Interfaces 2018, 10, 12639–12650. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, M.; Chacón, C.; Palacios-González, E.; Rodríguez-Gattorno, G.; Oskam, G. Photoelectrochemical water oxidation at electrophoretically deposited WO3 films as a function of cristal structure and morphology. Electrochim. Acta 2014, 140, 320–331. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, A.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Liu, Z.; Yu, A.; Jiang, X. Advances on tungsten oxide based photochromic materials: strategies to improve their photochromic properties. J. Mater. Chem. C. 2018, 6, 191–212. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Saukko, S.; Mizsei, J.; Tóth, A.; Madarász, J.; Pokol, G. Gas sensing selectivity of hexagonal and monoclinic WO3 to H2S. Solid States Sci. 2010, 12, 1857–1860. [Google Scholar] [CrossRef]
- Wang, N.; Wang, D.; Li, M.; Shi, J.; Li, C. Photoelectrochemical water oxidation on photoanodes fabricated with hexagonal nanoflower and nanoblock WO3. Nanoscale 2014, 6, 2061–2066. [Google Scholar] [CrossRef] [PubMed]
- Nukui, Y.; Srinivasan, N.; Shoji, S.; Atarashi, D.; Sakai, E.; Miyauchi, M. Vertically aligned hexagonal WO3 nanotree electrode for photoelectrochemica water oxidation. Chem. Phys. Lett. 2015, 306–311. [Google Scholar] [CrossRef]
- Ham, D.J.; Phuruangrant, A.; Thongtem, S.; Lee, J.S. Hydrothermal synthesis of monoclinic WO3 nanoplates and nanorods used as an electrocatalyst for hydrogen evolution reactions from water. Chem. Eng. J. 2010, 165, 365–369. [Google Scholar] [CrossRef]
- He, Q.; Zhou, F.; Zhan, S.; Huang, N.; Tian, Y. Photoassited oxygen reduction reaction on mpg-C3N4: The effects of elements doping on the performance of ORR. Appl. Surf. Sci. 2018, 430, 325–334. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 2390. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.F.; Chen, S.J.; Yeh, C.S.; Teng, H. Tuning the electronic structure of graphite oxide through ammonia treatment for photocatalytic generation of H2 and O2 from water splitting. J. Phys. Chem. C 2013, 117, 6516–6524. [Google Scholar] [CrossRef]
- Xu, Y.; Kraft, M.; Xu, R. Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem. Soc. Rev. 2016, 45, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Ania, C.O.; Seredych, M.; Rodriguez Castellon, E.; Bandosz, T.J. Visible light driven photoelectrochemical water splitting on metal free nanoporous carbon promoted by chromophoric functional groups. Carbon 2014, 79, 432–441. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Velo-Gala, I.; Rodriguez-Castellón, E.; Ania, C.O. Surface modification of a nanoporous carbon photoanode upon irradiation. Molecules 2016, 21, 1611. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, C.; Zhang, M.; Lin, L.; Ye, X.; Lin, S.; Markus Antonietti, M.; Wang, X. Carbon-Doped BN nanosheets for metal-free photoredox catalysis. Nat. Commun. 2015, 6, 7698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zang, S.; Wang, X. Layered Co(OH)2 deposited polymeric carbon nitrides for photocatalytic water oxidation. ACS Catal. 2015, 5, 941–947. [Google Scholar] [CrossRef]
- Leary, R.; Westwood, A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 2011, 49, 741–772. [Google Scholar] [CrossRef]
- Faria, J.L.; Wang, W. Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons: New York, NY, USA, 2009; Chapter 13; pp. 481–506. [Google Scholar]
- Matos, J.; Laine, J.; Herrmann, M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl. Catal. B Environ. 1998, 18, 281–291. [Google Scholar] [CrossRef]
- Haro, M.; Velasco, L.F.; Ania, C.O. Carbon-Mediated photoinduced reactions as a key factor in the photocatalytic performance of C/TiO2. Catal. Sci. Technol. 2012, 2, 2264–2272. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic carbon nitride polymers toward sustainable photoredox catalysis. Angew. Chem. Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Velasco, L.F.; Fonseca, I.M.; Parra, J.B.; Lima, J.C.; Ania, C.O. Photochemical behaviour of activated carbons under UV irradiation. Carbon 2012, 50, 249–258. [Google Scholar] [CrossRef]
- Velasco, L.F.; Gomis-Berenguer, A.; Lima, J.C.; Ania, C.O. Tuning the surface chemistry of nanoporous carbons for an enhanced nanoconfined photochemical activity. ChemCatChem 2015, 7, 3012–3319. [Google Scholar] [CrossRef]
- Wang, J.; Khoo, E.; See Lee, P.; Ma, J. Synthesis, assembly, and electrochromic properties of uniform crystalline WO3 nanorods. J. Phys. Chem. C 2008, 112, 14306–14312. [Google Scholar] [CrossRef]
- Velasco, L.F.; Lima, J.C.; Ania, C.O. Visible-light photochemical activity of nanoporous carbons under monochromatic light. Angew. Chem. Int. Ed. 2014, 53, 4146–4148. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Pan, L.; Ren, Z.; Yang, Y.; Xiao, Y.; Li, Z. Nanostructured WO3 films synthesized on mica substrate with novel photochromic properties. J. Alloys Compd. 2016, 657, 450–456. [Google Scholar] [CrossRef]
- Ramkumar, S.; Rajarajan, G. Effect of Fe doping on structural, optical and photocatalytic activity of WO3 nanostructured thin films. J. Mater. Sci. Mater. Eelctron. 2016, 27, 1847–1853. [Google Scholar] [CrossRef]
- Nosaka, Y.; Takahashi, S.; Sakamoto, H.; Nosaka, A.Y. Reaction mechanism of Cu(II)-grafted visible-light responsive TiO2 and WO3 photocatalysts studied by means of ESR spectroscopy and chemiluminescence photometry. J. Phys. Chem. C 2011, 115, 21283–21290. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Tungsten-Based nanomaterials (WO3 & Bi2WO6): Modifications related to charge carrier transfer mechanisms and photocatalytic applications. Appl. Surf. Sci. 2015, 355, 939–958. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Hwang, Y.J.; Chae, S.Y.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Araña, J.; Doña-Rodriguez, J.M.; Tello Rendón, E.; Garriga i Cabo, C.; González-Diaz, C.; Herrera-Melián, J.A.; Pérez-Peñ, A.J.; Colón, G.; Navío, J.A. TiO2 activation by using activated carbon as a support: Part II. Photoreactivity FTIR study. Appl. Catal. B Environ. 2003, 44, 153–160. [Google Scholar] [CrossRef]
- Carmona, R.J.; Velasco, L.F.; Hidalgo, M.C.; Navío, J.A.; Ania, C.O. Boosting the visible-light photoactivity of Bi2WO6 using acidic carbon additives. Appl. Catal. A Gen. 2015, 505, 467–477. [Google Scholar] [CrossRef]
- Bedja, I.; Hotchandani, S.; Kamat, P.V. Photoelectrochemistry of quantized WO3 colloids. Electron storage, electrochromic, and photoelectrochromic effects. J. Phys. Chem. 1993, 97, 11064–11070. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromic tungsten oxide films: Review of progress 1993–1998. Sol. Energy Mater. Sol. Cells 2000, 60, 201–262. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photocatalysis at semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Celorrio, V.; Bradley, K.; Weber, O.J.; Hall, S.R.; Fermín, D.J. Photoelectrochemical Properties of LaFeO3 Nanoparticles. ChemElectroChem 2014, 1, 1667–1671. [Google Scholar] [CrossRef]
- Peter, L.M.; Ponomarev, W.A.; Fermín, D.J. Intensity-Modulated photocurrent spectroscopy: Reconciliation of phenomenological analysis with multistep electron transfer mechanisms. J. Electroanal. Chem. 1997, 427, 79–96. [Google Scholar] [CrossRef]
- Li, S.; Shelar, D.P.; Hou, C.-C.; Chen, Q.-Q.; Deng, P.; Chen, Y. WO3 nanospheres with improved catalytic activity for visible light induced cross dehydrogenative coupling reactions. J. Photochem. Photobiol. A 2018, 363, 44–50. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent progress in photoelectrochemical water splitting activity of WO3 photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Han, X.; Xu, D.; An, L.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. WO3/g-C3N4 two-dimensional composites for visible-light driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2018, 43, 2264–2272. [Google Scholar] [CrossRef]
- Kwong, W.L.; Nakaruk, A.; Koshy, P.; Sorrell, C.C. Photoelectrochemical properties of WO3 nanoparticulate thin prepared by carboxylic acid-assisted electrodeposition. Thin Solid Films 2013, 544, 191–196. [Google Scholar] [CrossRef]
- Peter, L.M. Dynamic aspects of semiconductor photoelectrochemistry. Chem. Rev. 1990, 90, 753–769. [Google Scholar] [CrossRef]
- Leempoel, P.; Fan, F.R.F.; Bard, A.J. Semiconductor electrodes. 50. Effect of mode of illumination and doping on photochemical behavior of phthalocyanine films. J. Phys. Chem. 1983, 87, 2948–2955. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, H.; Yang, Z.; Long, X.; Wang, Z.; Zhu, Z.; Qu, Y.; Yang, S. Origin of the different photoelectrochemical performance of mesoporous BiVO4 photoanodes between the BiVO4 and the FTO side Illumination. J. Phys. Chem. C 2015, 119, 23350–23357. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Gómez, R. A photoelectrochemical and spectroscopic study of phenol and catechol oxidation on titanium dioxide nanoporous electrodes. Electrochim. Acta 2010, 55, 4661–4668. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Celorrio, V.; Iniesta, J.; Fermin, D.J.; Ania, C.O. Nanoporous carbon/WO3 anodes for an enhanced water photooxidation. Carbon 2016, 108, 471–479. [Google Scholar] [CrossRef]
- Fu, L.; Xia, T.; Zheng, Y.; Yang, J.; Wang, A.; Wang, Z. Preparation of WO3-reduced graphene oxide nanocomposites with enhanced photocatalytic property. Ceram. Int. 2015, 41, 5903–5908. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Iniesta, J.; Moro, A.; Maurino, V.; Lima, J.C.; Ania, C.O. Boosting visible light conversion in the confined pore space of nanoporous carbons. Carbon 2016, 96, 98–104. [Google Scholar] [CrossRef]

| h-WO3 | m-WO3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amount of Nanoporous Carbon (wt. %) | |||||||||||
| 0 | 5 | 10 | 20 | 50 | 0 | 5 | 10 | 20 | 50 | ||
| Potential (V vs. Ag/AgCl) | 0.6 V | 0.40 | 0.40 | 0.44 | 0.66 | 0.74 | 5.88 | 10.32 | 13.19 | 12.58 | 7.75 |
| 0.5 V | 0.30 | 0.27 | 0.38 | 0.60 | 0.51 | 5.38 | 8.95 | 10.71 | 9.97 | 6.22 | |
| 0.4 V | 0.08 | 0.12 | 0.24 | 0.47 | 0.27 | 5.72 | 7.08 | 9.32 | 9.60 | 4.97 | |
| 0.3 V | 0.04 | 0.09 | 0.18 | 0.36 | 0.14 | 3.41 | 6.16 | 6.72 | 5.31 | 2.77 | |
| 0.2 V | 0.02 | 0.01 | 0.17 | 0.11 | 0.09 | 1.32 | 2.63 | 1.97 | 1.26 | 0.55 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomis-Berenguer, A.; Iniesta, J.; Fermín, D.J.; Ania, C.O. Photoelectrochemical Response of WO3/Nanoporous Carbon Anodes for Photocatalytic Water Oxidation. C 2018, 4, 45. https://doi.org/10.3390/c4030045
Gomis-Berenguer A, Iniesta J, Fermín DJ, Ania CO. Photoelectrochemical Response of WO3/Nanoporous Carbon Anodes for Photocatalytic Water Oxidation. C. 2018; 4(3):45. https://doi.org/10.3390/c4030045
Chicago/Turabian StyleGomis-Berenguer, Alicia, Jesús Iniesta, David J. Fermín, and Conchi O. Ania. 2018. "Photoelectrochemical Response of WO3/Nanoporous Carbon Anodes for Photocatalytic Water Oxidation" C 4, no. 3: 45. https://doi.org/10.3390/c4030045
APA StyleGomis-Berenguer, A., Iniesta, J., Fermín, D. J., & Ania, C. O. (2018). Photoelectrochemical Response of WO3/Nanoporous Carbon Anodes for Photocatalytic Water Oxidation. C, 4(3), 45. https://doi.org/10.3390/c4030045