Individual Gas Molecules Detection Using Zinc Oxide–Graphene Hybrid Nanosensor: A DFT Study
Abstract
1. Introduction
2. Computational Methods
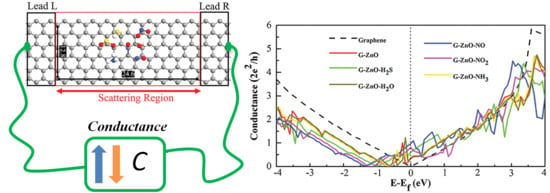
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.; Jiang, D.; Katsnelson, M.; Grigorieva, I.; Dubonos, S.; Firsov, A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.; Morozov, S.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Yin, Z.; Zhang, H. Graphene-based electronic sensors. Chem. Sci. 2012, 3, 1764–1772. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J. Adsorption of molecular oxygen on doped graphene: Atomic, electronic, and magnetic properties. Phys. Rev. B 2010, 81, 165414. [Google Scholar] [CrossRef]
- Nasehnia, F.; Seifi, M. Adsorption of molecular oxygen on VIIIB transition metal-doped graphene: A DFT study. Mod. Phys. Lett. B 2014, 28, 1450237. [Google Scholar] [CrossRef]
- Borisova, D.; Antonov, V.; Proykova, A. Hydrogen sulfide adsorption on a defective graphene. Int. J. Quantum Chem. 2013, 113, 786–791. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Wang, R.; Hao, L.; Song, D.; Li, Z. DFT study of hydrogen adsorption on Eu-decorated single-and double-sided graphene. Phys. Status Solidi B 2014, 251, 229–234. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.; Kim, H.-Y.; Ahn, J.; Park, Y.-M.; Lee, K.-Y.; Kim, J. Graphene-based nitrogen dioxide gas sensors. Curr. Appl. Phys. 2010, 10, 1002–1004. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Harutyunyan, A.R. Sub-ppt gas detection with pristine graphene. Appl. Phys. Lett. 2012, 101, 053119. [Google Scholar] [CrossRef]
- Some, S.; Xu, Y.; Kim, Y.; Yoon, Y.; Qin, H.; Kulkarni, A.; Kim, T.; Lee, H. Highly sensitive and selective gas sensor using hydrophilic and hydrophobic graphenes. Sci. Rep. 2013, 3, 1868. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A.; Khatami, S. Selective H2S Gas Sensing with a Graphene/n-Si Schottky Diode. IEEE Sens. J. 2014, 14, 4104–4108. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Hafiz, S.M.; Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuators B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Hu, N.; Wang, Y.; Chai, J.; Gao, R.; Yang, Z.; Kong, E.S.-W.; Zhang, Y. Gas sensor based on p-phenylenediamine reduced graphene oxide. Sens. Actuators B Chem. 2012, 163, 107–114. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Cho, H.Y.; Na, H.G.; Lee, B.C.; Kim, S.S.; Kim, H.W. Improvement of gas sensing behavior in reduced graphene oxides by electron-beam irradiation. Sens. Actuators B Chem. 2014, 203, 143–149. [Google Scholar] [CrossRef]
- Topsakal, M.; Aktürk, E.; Sevinçli, H.; Ciraci, S. First-principles approach to monitoring the band gap and magnetic state of a graphene nanoribbon via its vacancies. Phys. Rev. B 2008, 78, 235435. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Z.; Guo, W. Electronic and magnetic properties of zigzag edge graphene nanoribbons with Stone–Wales defects. Phys. Lett. A 2009, 373, 3354–3358. [Google Scholar] [CrossRef]
- Lopez-Bezanilla, A.; Zhou, W.; Idrobo, J.-C. Electronic and Quantum Transport Properties of Atomically Identified Si Point Defects in Graphene. J. Phys. Chem. Lett. 2014, 5, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, S.M.; Monshi, M.M.; Torres, I.; Calizo, I. Edge functionalization and doping effects on the stability, electronic and magnetic properties of silicene nanoribbons. RSC Adv. 2016, 6, 17046–17058. [Google Scholar] [CrossRef]
- Rad, A.S. First principles study of Al-doped graphene as nanostructure adsorbent for NO2 and N2O: DFT calculations. Appl. Surf. Sci. 2015, 357, 1217–1224. [Google Scholar] [CrossRef]
- Tit, N.; Said, K.; Mahmoud, N.M.; Kouser, S.; Yamani, Z.H. Ab-initio investigation of adsorption of CO and CO2 molecules on graphene: Role of intrinsic defects on gas sensing. Appl. Surf. Sci. 2017, 394, 219–230. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.-M.; Xu, K.-W.; Ji, V. A first-principles study on gas sensing properties of graphene and Pd-doped graphene. Appl. Surf. Sci. 2015, 343, 121–127. [Google Scholar] [CrossRef]
- Gupta, S.; Sabarou, H.; Zhong, Y.; Singh, P. Phase evolution and electrochemical performance of iron doped lanthanum strontium chromite in oxidizing and reducing atmosphere. Int. J. Hydrogen Energy 2017, 42, 6262–6271. [Google Scholar] [CrossRef]
- Chawla, N.; Chamaani, A.; Safa, M.; El-Zahab, B. Palladium-Filled Carbon Nanotubes Cathode for Improved Electrolyte Stability and Cyclability Performance of Li-O2 Batteries. J. Electrochem. Soc. 2017, 164, A6303–A6307. [Google Scholar] [CrossRef]
- Basu, S.; Hazra, S.K. Graphene–Noble Metal Nano-Composites and Applications for Hydrogen Sensors. C 2017, 3, 29. [Google Scholar] [CrossRef]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of single-and multilayer MoS2 film-based field-effect transistors for sensing NO at room temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of Flexible MoS2 Thin-Film Transistor Arrays for Practical Gas-Sensing Applications. Small 2012, 8, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuators B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a superior gas sensor: Selective adsorption and distinct IV response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C. Black phosphorus gas sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef] [PubMed]
- Mahabal, M.S.; Deshpande, M.D.; Hussain, T.; Ahuja, R. Sensing Characteristics of a Graphene-like Boron Carbide Monolayer towards Selected Toxic Gases. Chemphyschem 2015, 16, 3511–3517. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, S.M.; Monshi, M.; Torres, I.; Zeidi, S.; Calizo, I. DFT study of adsorption behavior of NO, CO, NO2, and NH3 molecules on graphene-like BC3: A search for highly sensitive molecular sensor. Appl. Surf. Sci. 2018, 427, 326–333. [Google Scholar] [CrossRef]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.-S.; Xia, J.-B.; Li, J. Photoresponsive and gas sensing field-effect transistors based on multilayer WS2 nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Lee, K.; Morrish, R.; Berner, N.C.; McEvoy, N.; Wolden, C.A.; Duesberg, G.S. Plasma assisted synthesis of WS2 for gas sensing applications. Chem. Phys. Lett. 2014, 615, 6–10. [Google Scholar] [CrossRef]
- Hu, W.; Xia, N.; Wu, X.; Li, Z.; Yang, J. Silicene as a highly sensitive molecule sensor for NH3, NO and NO2. Phys. Chem. Chem. Phys. 2014, 16, 6957–6962. [Google Scholar] [CrossRef] [PubMed]
- Chandiramouli, R.; Srivastava, A.; Nagarajan, V. NO adsorption studies on silicene nanosheet: DFT investigation. Appl. Surf. Sci. 2015, 351, 662–672. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Monshi, M.M.; Calizo, I. A theoretical study of gas adsorption on silicene nanoribbons and its application in a highly sensitive molecule sensor. RSC Adv. 2016, 6, 94417–94428. [Google Scholar] [CrossRef]
- Monshi, M.M.; Aghaei, S.M.; Calizo, I. Doping and defect-induced germanene: A superior media for sensing H2S, SO2, and CO2 gas molecules. Surf. Sci. 2017, 665, 96–102. [Google Scholar] [CrossRef]
- Rai, P.; Kwak, W.-K.; Yu, Y.-T. Solvothermal synthesis of ZnO nanostructures and their morphology-dependent gas-sensing properties. ACS Appl. Mater. Interfaces 2013, 5, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Kim, S.-W.; Lee, D.-H.; Moon, S.-E.; Kim, K.-C.; Maiti, A. SnO2 nanoslab as NO2 sensor: Identification of the NO2 sensing mechanism on a SnO2 surface. ACS Appl. Mater. Interfaces 2013, 6, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.; Han, D.; Gu, F.; Guo, G. Porous ZnO polygonal nanoflakes: Synthesis, use in high-sensitivity NO2 gas sensor, and proposed mechanism of gas sensing. J. Phys. Chem. C 2011, 115, 12763–12773. [Google Scholar] [CrossRef]
- Rossinyol, E.; Prim, A.; Pellicer, E.; Arbiol, J.; Hernández-Ramírez, F.; Peiró, F.; Cornet, A.; Morante, J.R.; Solovyov, L.A.; Tian, B. Synthesis and Characterization of Chromium-Doped Mesoporous Tungsten Oxide for Gas Sensing Applications. Adv. Funct. Mater. 2007, 17, 1801–1806. [Google Scholar] [CrossRef]
- Epifani, M.; Díaz, R.; Arbiol, J.; Comini, E.; Sergent, N.; Pagnier, T.; Siciliano, P.; Faglia, G.; Morante, J.R. Nanocrystalline Metal Oxides from the Injection of Metal Oxide Sols in Coordinating Solutions: Synthesis, Characterization, Thermal Stabilization, Device Processing, and Gas-Sensing Properties. Adv. Funct. Mater. 2006, 16, 1488–1498. [Google Scholar] [CrossRef]
- Hazra, S.K.; Basu, S. Graphene-Oxide Nano Composites for Chemical Sensor Applications. C 2016, 2, 12. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature. Sens. Actuators B Chem. 2012, 173, 139–147. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B Chem. 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Singh, G.; Choudhary, A.; Haranath, D.; Joshi, A.G.; Singh, N.; Singh, S.; Pasricha, R. ZnO decorated luminescent graphene as a potential gas sensor at room temperature. Carbon 2012, 50, 385–394. [Google Scholar] [CrossRef]
- Jammula, R.K.; Srikanth, V.V.; Hazra, B.K.; Srinath, S. ZnO nanoparticles’ decorated reduced-graphene oxide: Easy synthesis, unique polarization behavior, and ionic conductivity. Mater. Des. 2016, 110, 311–316. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.; Abdala, A.; Mittal, V. In situ formed graphene/ZnO nanostructured composites for low temperature hydrogen sulfide removal from natural gas. RSC Adv. 2016, 6, 81142–81150. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Manesh, E.; Vaezzadeh, M.; Saeidi, M. Theoretical study on electronic structure, and electrical conductance at room temperature of Cu2O–GS nanosensors and detection of H2S gas. Comput. Mater. Sci. 2015, 97, 181–185. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The synthesis and properties of ZnO–graphene nano hybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Wang, X.; Yu, J.; Wang, J.; Tang, Z.; Akbar, S. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B Chem. 2013, 188, 902–908. [Google Scholar] [CrossRef]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Khadem, S.J.; Abdi, Y.; Darbari, S.; Ostovari, F. Investigating the effect of gas absorption on the electromechanical and electrochemical behavior of graphene/ZnO structure, suitable for highly selective and sensitive gas sensors. Curr. Appl. Phys. 2014, 14, 1498–1503. [Google Scholar] [CrossRef]
- Monshi, M.M.; Aghaei, S.M.; Calizo, I. Band gap opening and optical absorption enhancement in graphene using ZnO nanocluster. Phys. Lett. A 2018, 382, 1171–1175. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, N.; Xia, B. Facile fabrication of ZnO nanocrystalline-modified graphene hybrid nanocomposite toward methane gas sensing application. J. Mater. Sci. Mater. Electron. 2015, 26, 5937–5945. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Xu, J.-L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined formation of ultrathin ZnO nanorods/reduced graphene oxide mesoporous nanocomposites for high-performance room-temperature NO2 sensors. ACS Appl. Mater. Interfaces 2016, 8, 35454–35463. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zeng, D.; Li, H.; Xie, C. Room temperature formaldehyde sensors with enhanced performance, fast response and recovery based on zinc oxide quantum dots/graphene nanocomposites. Nanoscale 2012, 4, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Zhang, Z.; Zhao, X.; Liu, F.; Wang, K.; Xie, H. High sensitive formaldehyde graphene gas sensor modified by atomic layer deposition zinc oxide films. Appl. Phys. Lett. 2014, 105, 033107. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Xia, B. Layer-by-layer self-assembly of zinc oxide/graphene oxide hybrid toward ultrasensitive humidity sensing. IEEE Electron Device Lett. 2016, 37, 916–919. [Google Scholar] [CrossRef]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 2001, 63, 245407. [Google Scholar] [CrossRef]
- Brandbyge, M.; Mozos, J.-L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef]
- Atomistix ToolKit (ATK), Version 2017.2, QuantumWise Simulator A/S. Available online: www.quantumwise.com (accessed on 9 August 2018).
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Mück-Lichtenfeld, C.; Antony, J. Noncovalent interactions between graphene sheets and in multishell (hyper) fullerenes. J. Phys. Chem. C 2007, 111, 11199–11207. [Google Scholar] [CrossRef]
- Pyykkö, P.; Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1–118. Chem. A Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, B.; Xu, Z. Adsorption properties of polyvinyl-alcohol-grafted particles toward genistein driven by hydrogen-bond interaction. J. Phys. Chem. B 2013, 117, 5730–5736. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhao, Y.-P.; Li, L.; Wang, T. Ab initio study of ZnO-based gas-sensing mechanisms: Surface reconstruction and charge transfer. J. Phys. Chem. C 2009, 113, 6107–6113. [Google Scholar] [CrossRef]
- Casarin, M.; Tondello, E.; Vittadini, A. A LCAO-LDF study of CO and NH3 chemisorption on ZnO (0001). Surf. Sci. 1994, 307, 1182–1187. [Google Scholar] [CrossRef]
- An, W.; Wu, X.; Zeng, X.C. Adsorption of O2, H2, CO, NH3, and NO2 on ZnO nanotube: A density functional theory study. J. Phys. Chem. C 2008, 112, 5747–5755. [Google Scholar] [CrossRef]
- Spencer, M.J.; Yarovsky, I. ZnO nanostructures for gas sensing: Interaction of NO2, NO, O, and N with the ZnO (1010) surface. J. Phys. Chem. C 2010, 114, 10881–10893. [Google Scholar] [CrossRef]
- Breedon, M.; Spencer, M.; Yarovsky, I. Adsorption of NO2 on oxygen deficient ZnO (2110) for gas sensing applications: A DFT study. J. Phys. Chem. C 2010, 114, 16603–16610. [Google Scholar] [CrossRef]
- Ma, J.; Michaelides, A.; Alfe, D.; Schimka, L.; Kresse, G.; Wang, E. Adsorption and diffusion of water on graphene from first principles. Phys. Rev. B 2011, 84, 033402. [Google Scholar] [CrossRef]
- Ye, H.; Chen, G.; Niu, H.; Zhu, Y.; Shao, L.; Qiao, Z. Structures and mechanisms of water adsorption on ZnO (0001) and GaN (0001) surface. J. Phys. Chem. C 2013, 117, 15976–15983. [Google Scholar] [CrossRef]
- Meyer, B.; Rabaa, H.; Marx, D. Water adsorption on ZnO (1010): From single molecules to partially dissociated monolayers. Phys. Chem. Chem. Phys. 2006, 8, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Goclon, J.; Meyer, B. The interaction of H2S with the ZnO (1010) surface. Phys. Chem. Chem. Phys. 2013, 15, 8373–8382. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Zhang, R.; Han, P.; Wang, B. DFT study on the sulfurization mechanism during the desulfurization of H2S on the ZnO desulfurizer. Fuel Process. Technol. 2013, 106, 222–230. [Google Scholar] [CrossRef]
- Ganji, M.D.; Sharifi, N.; Ardjmand, M.; Ahangari, M.G. Pt-decorated graphene as superior media for H2S adsorption: A first-principles study. Appl. Surf. Sci. 2012, 261, 697–704. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Maiti, A. Adsorption and decomposition of H2S on MgO (100), NiMgO (100), and ZnO (0001) surfaces: A first-principles density functional study. J. Phys. Chem. B 2000, 104, 3630–3638. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Yue, L.-J.; Gong, F.-L.; Li, F.; Zhang, H.-L.; Chen, J.-L. Highly enhanced H2S gas sensing and magnetic performances of metal doped hexagonal ZnO monolayer. Vacuum 2017, 141, 109–115. [Google Scholar] [CrossRef]
- Song, H.S.; Park, M.G.; Kwon, S.J.; Yi, K.B.; Croiset, E.; Chen, Z.; Nam, S.C. Hydrogen sulfide adsorption on nano-sized zinc oxide/reduced graphite oxide composite at ambient condition. Appl. Surf. Sci. 2013, 276, 646–652. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Zhang, X. Novel 3D graphene aerogel–ZnO composites as efficient detection for NO2 at room temperature. Sens. Actuators B Chem. 2015, 211, 220–226. [Google Scholar] [CrossRef]
- Song, H.S.; Park, M.G.; Ahn, W.; Lim, S.N.; Yi, K.B.; Croiset, E.; Chen, Z.; Nam, S.C. Enhanced adsorption of hydrogen sulfide and regeneration ability on the composites of zinc oxide with reduced graphite oxide. Chem. Eng. J. 2014, 253, 264–273. [Google Scholar] [CrossRef]
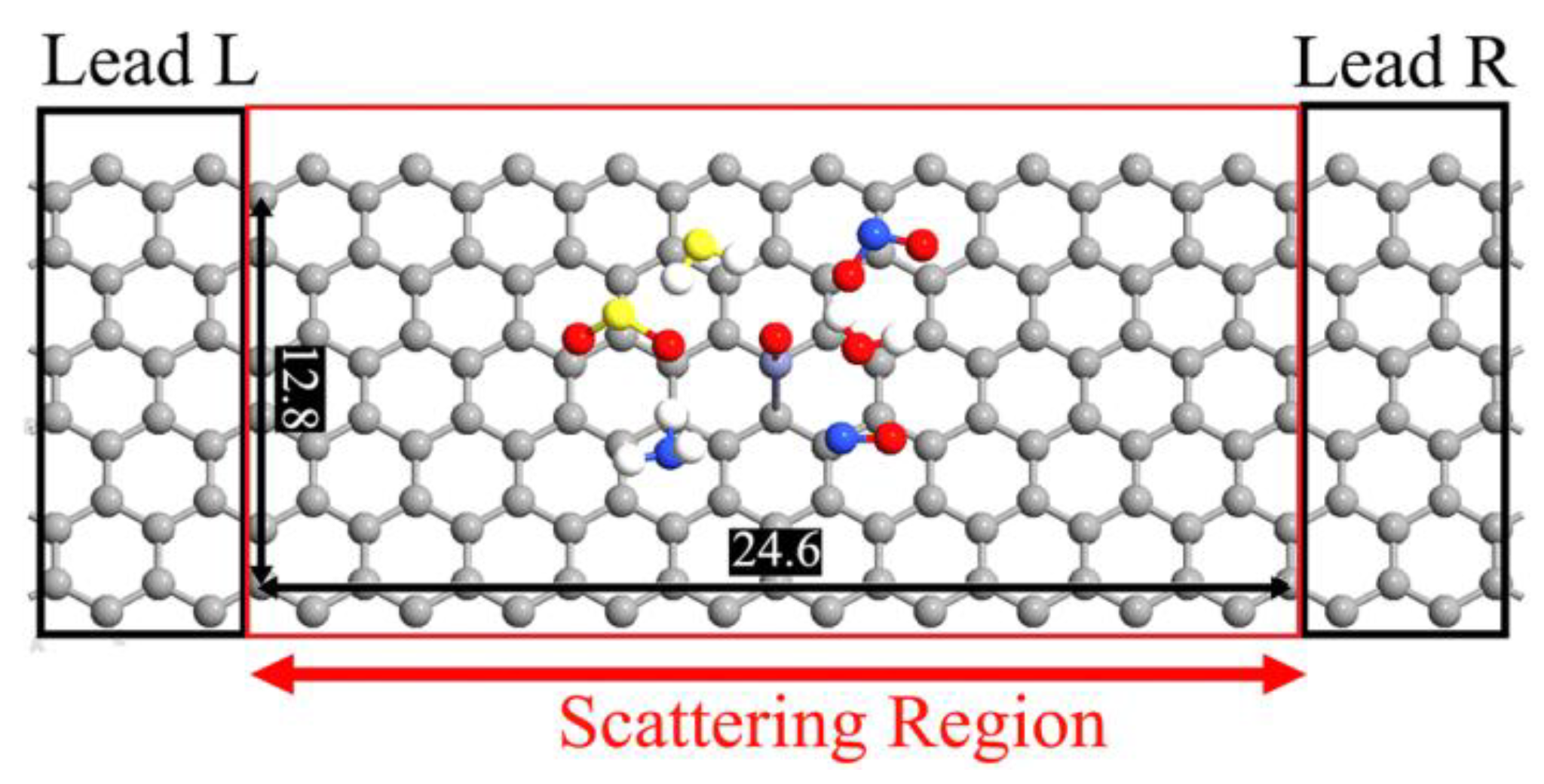
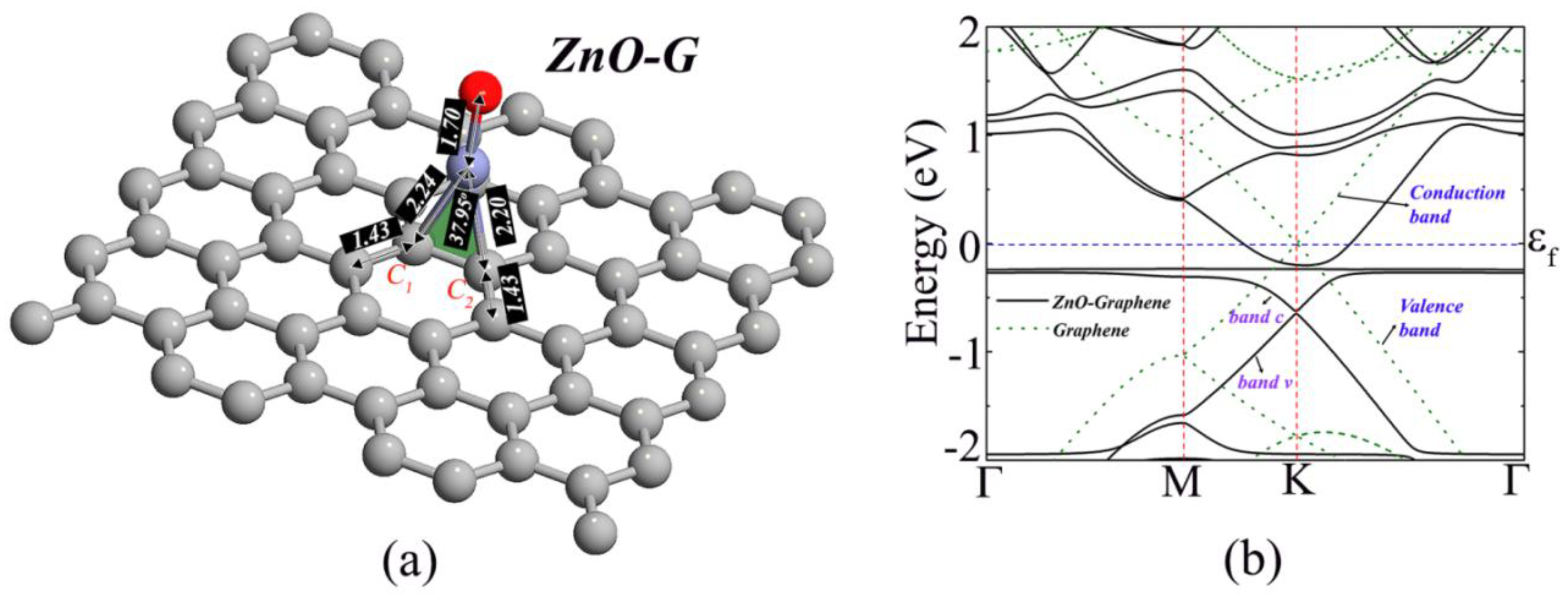
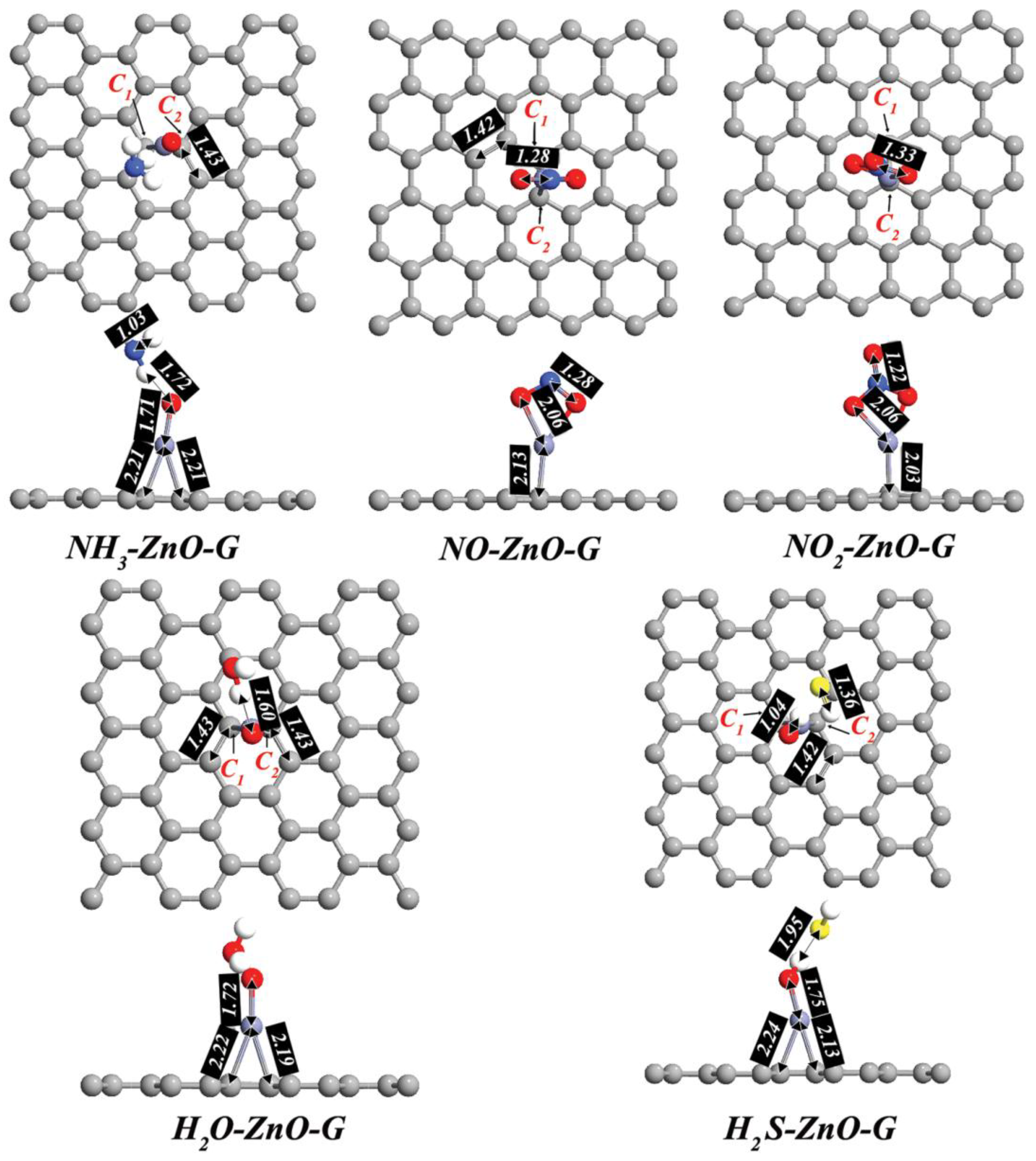
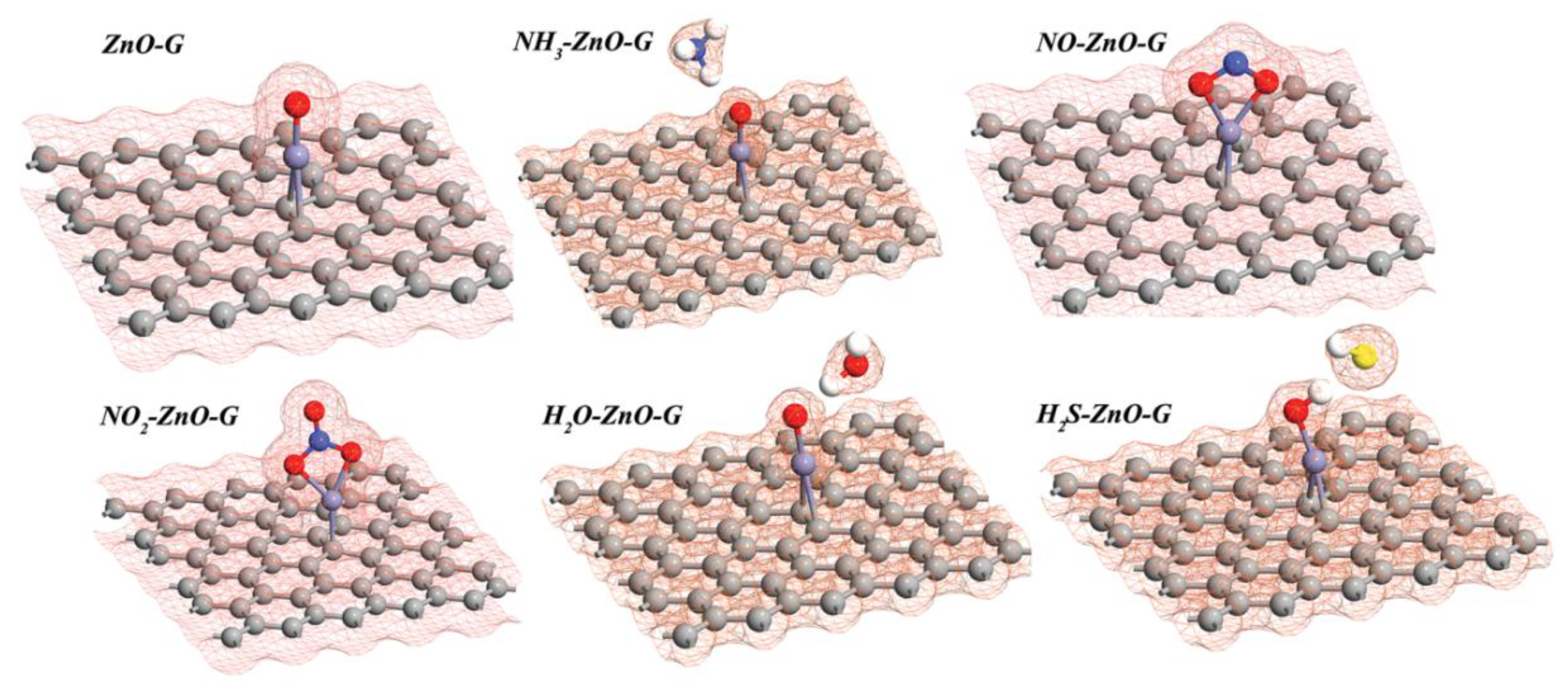
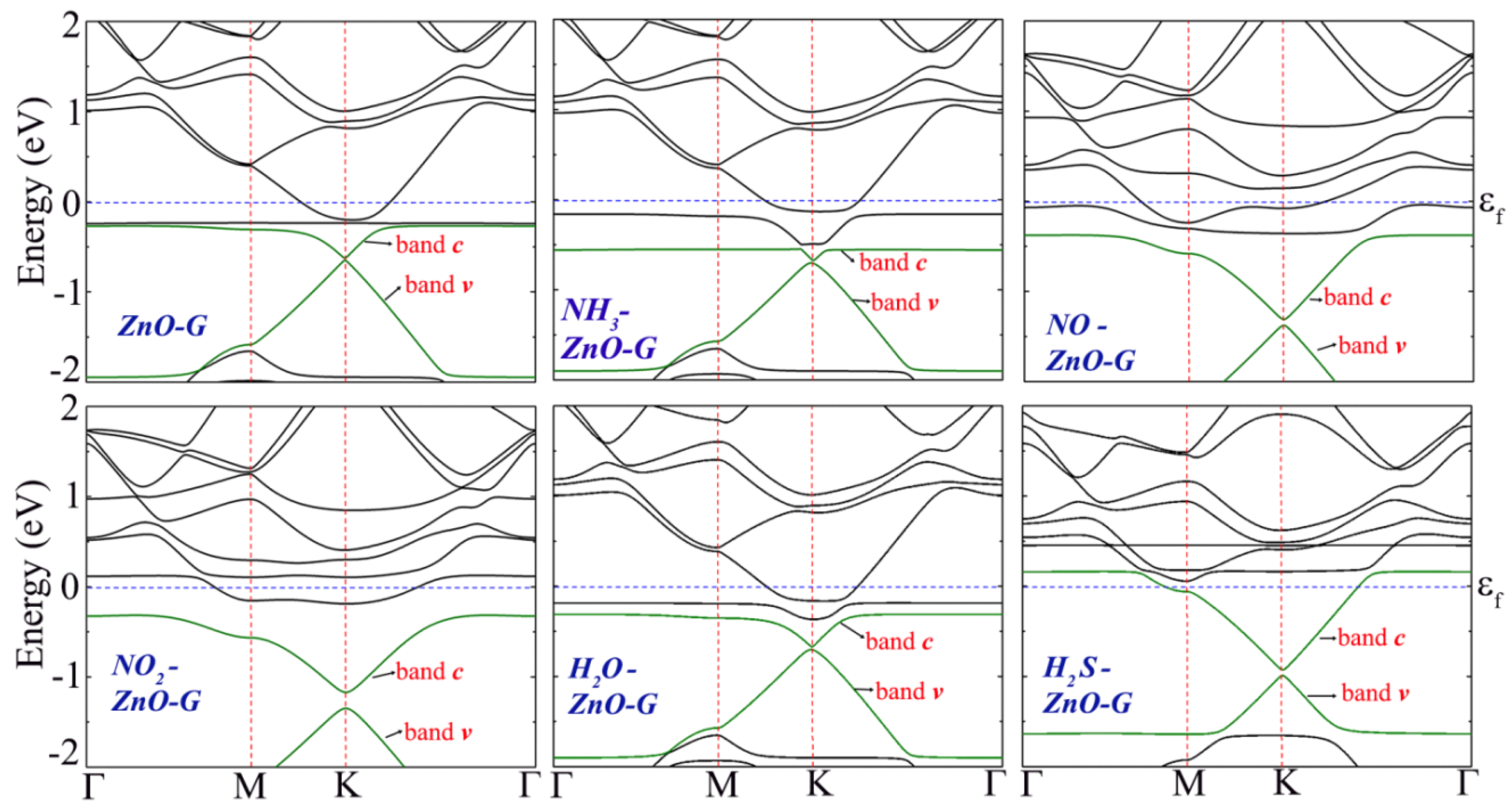

| System | Ead (eV) | D (Å) | Q (e) | S (%) |
|---|---|---|---|---|
| NH3-ZnO-G | −0.55 | 1.72 | +0.056 | 11.8 |
| NO-ZnO-G | −2.21 | 1.28 | +0.155 | 188.2 |
| NO2-ZnO-G | −1.85 | 1.33 | +0.141 | 141.1 |
| H2O-ZnO-G | −0.32 | 1.60 | +0.031 | 6.4 |
| H2S-ZnO-G | −1.01 | 1.04 | +0.121 | 76.47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, I.; Mehdi Aghaei, S.; Rabiei Baboukani, A.; Wang, C.; Bhansali, S. Individual Gas Molecules Detection Using Zinc Oxide–Graphene Hybrid Nanosensor: A DFT Study. C 2018, 4, 44. https://doi.org/10.3390/c4030044
Torres I, Mehdi Aghaei S, Rabiei Baboukani A, Wang C, Bhansali S. Individual Gas Molecules Detection Using Zinc Oxide–Graphene Hybrid Nanosensor: A DFT Study. C. 2018; 4(3):44. https://doi.org/10.3390/c4030044
Chicago/Turabian StyleTorres, Ingrid, Sadegh Mehdi Aghaei, Amin Rabiei Baboukani, Chunlei Wang, and Shekhar Bhansali. 2018. "Individual Gas Molecules Detection Using Zinc Oxide–Graphene Hybrid Nanosensor: A DFT Study" C 4, no. 3: 44. https://doi.org/10.3390/c4030044
APA StyleTorres, I., Mehdi Aghaei, S., Rabiei Baboukani, A., Wang, C., & Bhansali, S. (2018). Individual Gas Molecules Detection Using Zinc Oxide–Graphene Hybrid Nanosensor: A DFT Study. C, 4(3), 44. https://doi.org/10.3390/c4030044