A Library of Doped-Graphene Images via Transmission Electron Microscopy
Abstract
1. Introduction
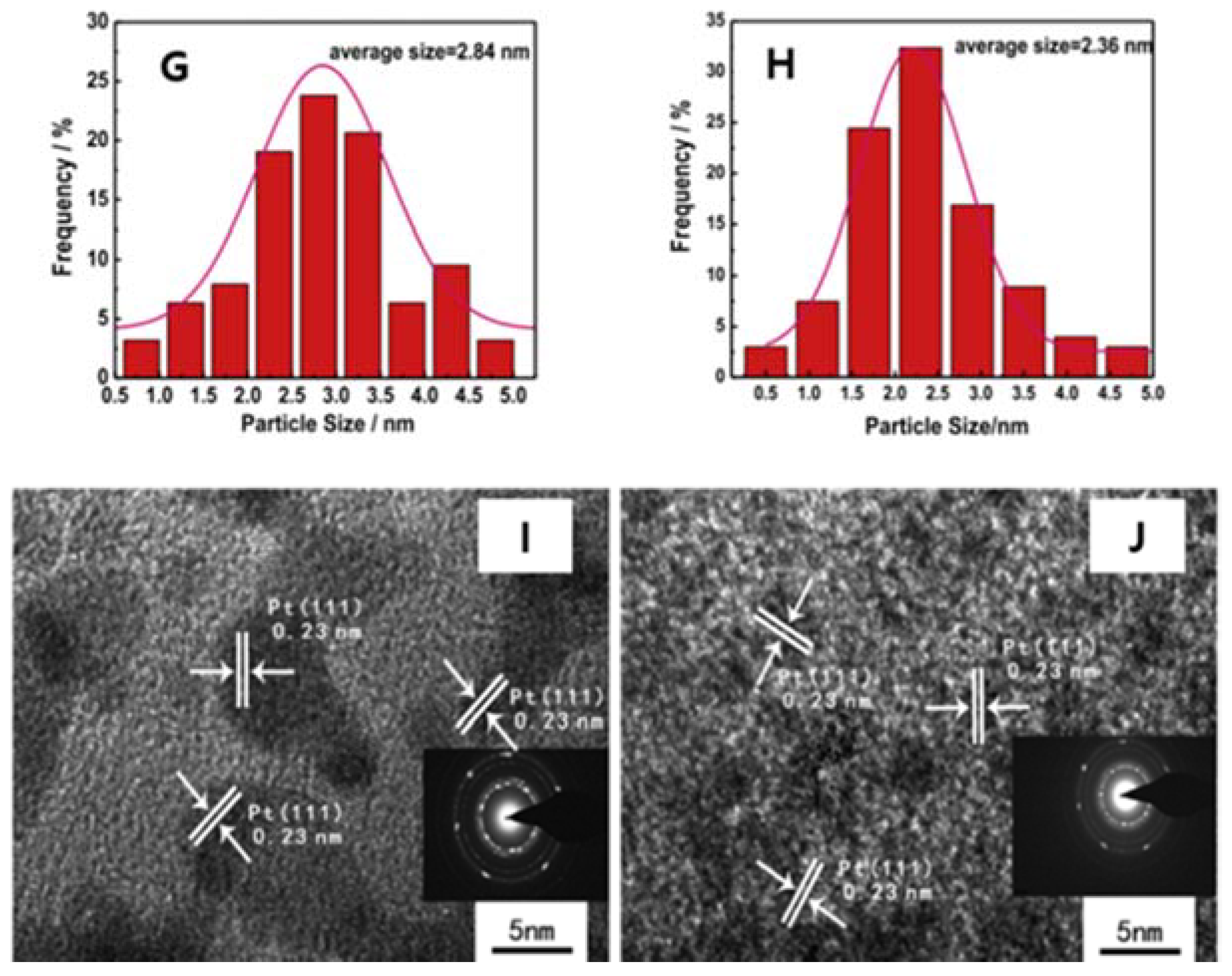
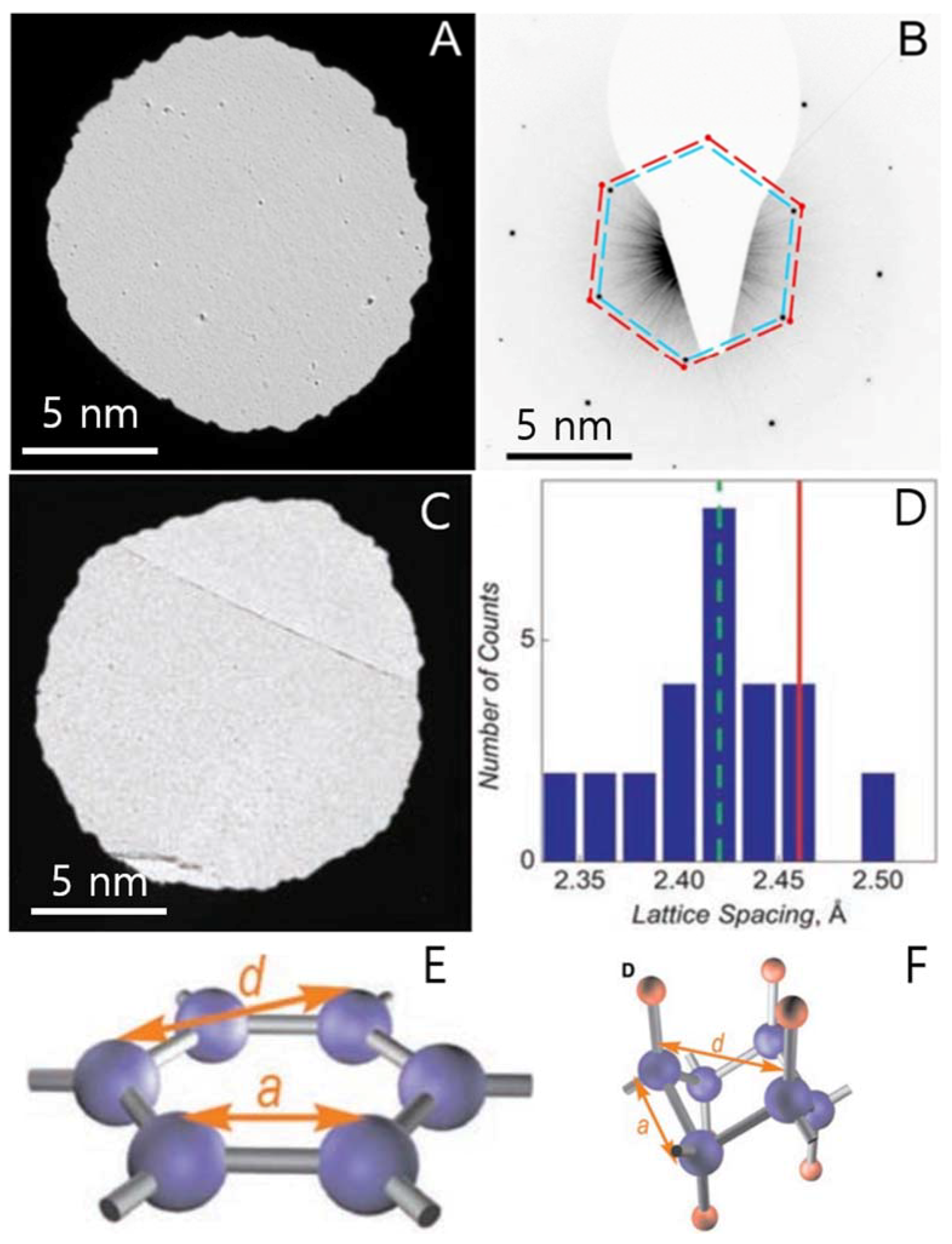
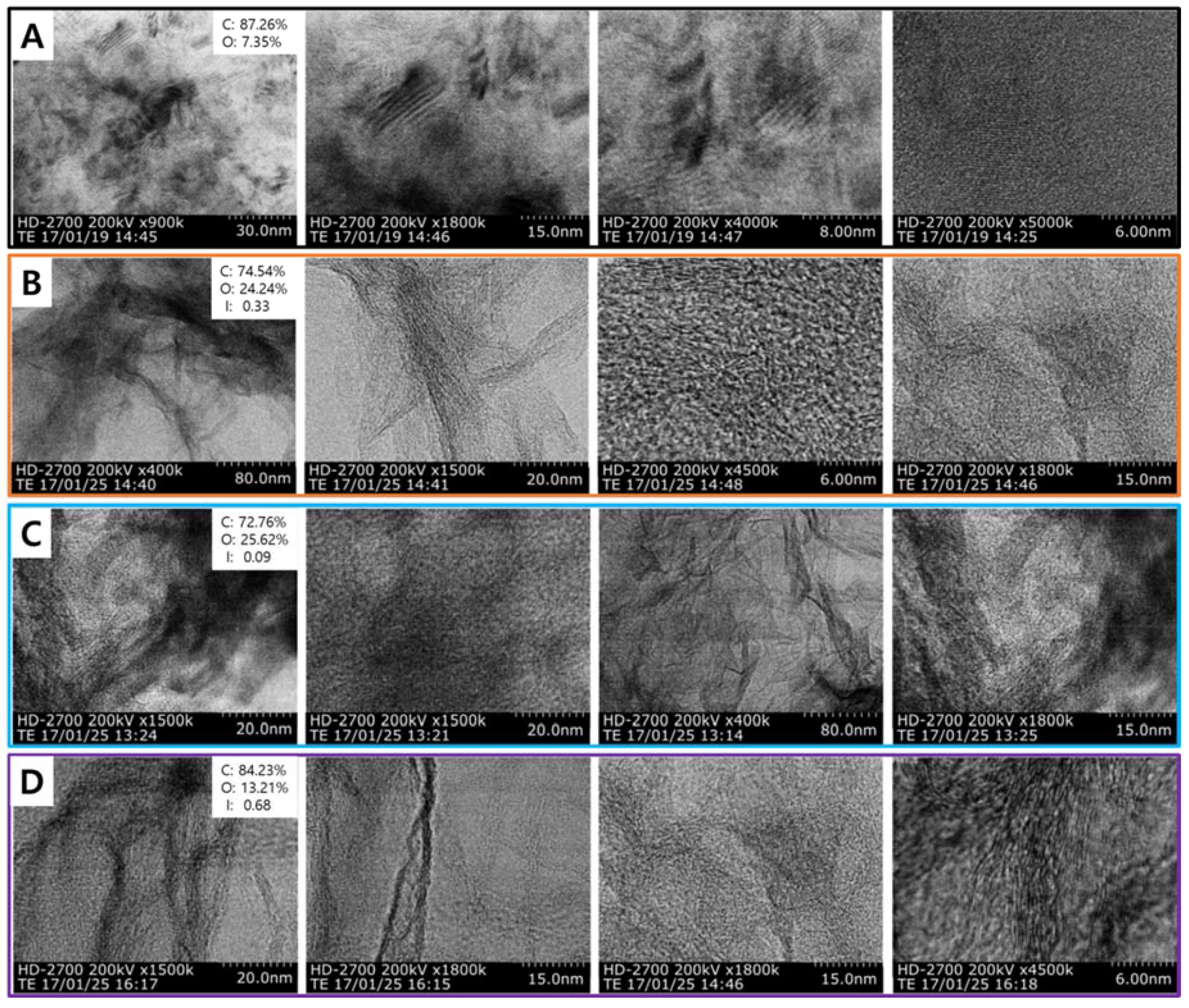
2. TEM Observations of Doped-Graphenes
3. Applications of Doped-Graphenes
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4587–5062. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Gupta, J.A.; Guitierrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F.; Halperin, E.J.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, P.; Prato, M. Grand challenges for nanoscience and nanotechnology. ACS Nano 2015, 9, 6637–6640. [Google Scholar]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Guo, B.; Fang, L.; Zhang, B.; Gong, J.R. Graphene doping: A review. Insciences J. 2011, 1, 80–89. [Google Scholar] [CrossRef]
- Pham, V.P.; Jang, H.S.; Whang, D.; Choi, J.Y. Direct growth of graphene on rigid and flexible substrates: Progress, applications and challenges. Chem. Soc. Rev. 2017, 46, 6276–6300. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.P. Chemical vapor deposited graphene synthesis with same-oriented hexagonal domains. Eng. Press 2018, 1, 39–42. [Google Scholar]
- Pham, V.P. How can the nanomaterial surfaces be highly cleaned? Edelweiss Appl. Sci. Technol. 2018, 2, 184–186. [Google Scholar]
- Pham, V.P. Layer-by-layer thinning of 2D materials. Edelweiss Appl. Sci. Technol. 2018, 2, 36–37. [Google Scholar]
- Pham, V.P. Cleaning of graphene surfaces by low pressure air plasma. R. Soc. Open Sci. 2018, 5, 172395. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.Y.; Zhang, L.; Wang, X.R.; Diankov, G.; Dai, H.J. Narrow graphene nanoribbons from carbon nanotubes. Nature 2009, 458, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.P.; Yeom, G.Y. Recent advances in doping on molybdenum disulphide: Industrial applications and future prospects. Adv. Mater. 2016, 28, 9024–9059. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, L.; Li, Y.; Wang, H.; Ouyang, Y.; Guo, J.; Dai, H. Controlled chlorine plasma reaction for noninvasive graphene doping. J. Am. Chem. Soc. 2011, 133, 19668–19671. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.P.; Kim, K.N.; Jeon, M.H.; Kim, K.S.; Yeom, G.Y. Cyclic chlorine trap-doping for transparent, conductive, thermally stable and damage-free graphene. Nanoscale 2014, 6, 15301–15308. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.P.; Nguyen, M.T.; Park, J.W.; Kwak, S.S.; Nguyen, D.H.T.; Mun, M.K.; Phan, H.D.; Kim, D.S.; Kim, K.H.; Lee, J.; et al. Chlorine-trapped CVD bilayer graphene for resistive pressure sensor with high detection limit and high sensitivity. 2D Mater. 2017, 4, 025049. [Google Scholar] [CrossRef]
- Pham, V.P.; Kim, K.H.; Jeon, M.H.; Lee, S.H.; Kim, K.N.; Yeom, G.Y. Low damage pre-doping on CVD graphene/Cu using a chlorine inductively coupled plasma. Carbon 2015, 95, 664–671. [Google Scholar] [CrossRef]
- Pham, V.P.; Mishra, A.; Yeom, G.Y. The enhancement of Hall mobility and conductivity of CVD graphene through radical doping and vacuum annealing. RSC Adv. 2017, 7, 16104–16108. [Google Scholar] [CrossRef]
- Pham, V.P.; Kim, D.S.; Kim, K.S.; Park, J.W.; Yang, K.C.; Lee, S.H.; Kim, K.N.; Yeom, G.Y. Low energy BCl3 plasma doping of few-layer graphene. Sci. Adv. Mater. 2016, 8, 884–890. [Google Scholar] [CrossRef]
- Kim, K.N.; Pham, V.P.; Yeom, G.Y. Chlorine radical doping of a few layer graphene with low damage. ECS J. Solid State Sci. Technol. 2015, 4, N5095–N5097. [Google Scholar] [CrossRef][Green Version]
- Lv, R.; Chen, G.; Li, Q.; McCreary, A.; Botello-Mendez, A.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-Lopez, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, L.; Li, Z.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Zhu, H. Boron doping of graphene for graphene–silicon p-n junction solar cells. Adv. Energy Mater. 2012, 2, 425–429. [Google Scholar] [CrossRef]
- Sheng, Z.H.; Gao, H.L.; Bao, W.J.; Wang, F.B.; Xia, X.H. Synthesis of boron doped graphene for oxygen reduction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Gunes, F.; Shin, H.J.; Biswas, C.; Han, G.H.; Kim, E.S.; Chae, S.J.; Choi, J.Y.; Lee, Y.H. Layer-by-layer doping of few-layer graphene film. ACS Nano 2010, 4, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kim, K.K.; Reina, A.; Hofmann, M.; Li, L.J.; Kong, J. Work function engineering of graphene electrode via chemical doping. ACS Nano 2010, 4, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, D.H.; Lee, HS.; Jang, C.W.; Kim, J.M.; Seo, S.W.; Kim, S.; Choi, S.H. Enhancement of efficiency in graphene/porous silicon solar cells by co-doping graphene with gold nanoparticles and bis(trifluoromethansulfonyl)-amide. J. Mater. Chem. C 2017, 5, 9005–9011. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Thema, F.T.; Manikandan, E.; Maaza, M. Nano TiO2-doped graphene oxide (GO) wrinkle nanosheets by wet-chemical method. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 7129, 722. [Google Scholar]
- Deng, D.; Chen, X.; Yu, L.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; Si, R.; et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Yu, S.H.; Chung, D.Y.; Jin, A.; Park, J.H.; Sung, Y.E.; Piao, Y. Single source precursor-based solvothermal synthesis of heteroatom-doped graphene and its energy storage and conversion applications. Sci. Rep. 2014, 4, 5639. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Donnell, K.O.; Sun, H.; Wang, Y.; Wang, S. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Zhang, F.; Liu, S.; Wang, J.; Yin, D.; Wang, L. SnO2 nanocrystals anchored on N-doped graphene for high-performance lithium storage. Chem. Commun. 2015, 51, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, N.; Kang, D.J. Molybdenum disulphide nanosheets interconnected nitrogen-doped reduced graphene oxide hydrogel: A high performance heterostructure for lithium-ion batteries. Electrochim. Acta 2016, 193, 128–136. [Google Scholar] [CrossRef]
- She, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.L.; Leung, M.K.H. Oxygen reduction reaction mechanism of nitrogen-doped graphene derived from ionic liquid. Energy Procedia 2017, 142, 1319–1326. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Baek, J.B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalysts for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites as basal planes. Nano Lett. 2011, 11, 2472–2477. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Li, Q.; Mendez, A.R.B.; Hayashi, T.; Wang, B.; Berkdemir, A.; Hao, Q.; Elias, A.L.; Silva, R.C.; Gutierrez, H.R.; et al. Nitrogen-doped graphene: Beyond single substitution and enhanced molecular sensing. Sci. Rep. 2012, 2, 586. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Santos, M.C.D.; Carvalho, B.R.; Lv, R.; Li, Q.; Fujisawa, K.; Pimenta, M.A.; Terrones, M. Ultrasensitive molecular sensor using N-doped graphene through enhanced Raman scattering. Sci. Adv. 2016, 2, e1600322. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.D.; Lagoute, J.; Mouhoub, O.; Joucken, F.; Repain, V.; Chacon, C.; Bellec, A.; Girard, Y.; Rousset, S. Electronic interaction between nitrogen-doped graphene and phorphyrin molecules. ACS Nano 2014, 8, 9403–9409. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Lee, K.U.; Lee, J.W.; Ahn, B.T.; Woo, S.I. Electrochemical oxygen reduction on nitrogen doped graphene sheets in acid media. Electrochem. Commun. 2010, 12, 1052–1055. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Q.; Chen, E.; Zhu, H.; Fang, L.; Gong, J.R. Controllable N-doping of graphene. Nano Lett. 2010, 10, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.C.; Choi, K.S.; Kim, B.J.; Lee, J.L. Work-function decrease of graphene using alkali metal carbonates. J. Phys. Chem. C 2012, 116, 26586–26591. [Google Scholar] [CrossRef]
- Song, Y.; Fang, W.; Hsu, A.L.; Kong, J. Iron (III) chloride doping of CVD graphene. Nanotechnology 2014, 25, 395701. [Google Scholar] [CrossRef] [PubMed]
- Jie, W.; Hao, J. Graphene-based hybrid structures combined with functional materials of ferroelectrics and semiconductors. Nanoscale 2014, 6, 6346–6362. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, D.; Lee, Y.; Jeon, D.Y. Work-function engineering of graphene anode by bis(trifluoromethanesulfonyl)amide doping for efficient polymer light-emitting diodes. Adv. Funct. Mater. 2013, 23, 5049–5065. [Google Scholar] [CrossRef]
- Tongay, S.; Berke, K.; Lemaitre, M.; Nasrollahi, Z.; Tanner, D.B.; Hebard, A.F.; Appleton, B.R. Stable hole doping of graphene for low electrical resistance and high optical transparency. Nanotechnology 2011, 22, 425701. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhu, H.; Wang, K.; Wei, J.; Shu, Q.; Guo, N.; Wu, D. Graphene/silicon nanowire schottky junction for enhanced light harvesting. ACS Appl. Mater. Interfaces 2011, 3, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Lv, R.; Huang, Z.H.; Chen, S.; Zhang, Z.; Gan, X.; Jia, Y.; Li, X.; Wang, K.; Wu, D.; Kang, F. Enhanced efficiency of graphene/silicon heterojunction solar cells by molecular doping. J. Mater. Chem. A 2013, 1, 5736–5740. [Google Scholar] [CrossRef]
- Feng, T.; Xie, D.; Lin, Y.; Zang, Y.; Ren, T.; Song, R.; Zhao, H.; Tian, H.; Li, X.; Zhu, H.; Liu, L. Graphene based Schottky junction solar cells on patterned silicon-pillar-array substrate. Appl. Phys. Lett. 2011, 99, 233505. [Google Scholar] [CrossRef]
- Chen, W.; Chen, S.; Qi, D.C.; Gao, X.Y.; Wee, A.T.S. Surface transfer p-type doping of epitaxial graphene. J. Am. Chem. Soc. 2007, 129, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H.; Jones, R.; Goss, J.P.; Briddon, P.R. P-type doping of graphene with F4-TCNQ. J. Phys. Condens. Matter 2009, 21, 402001. [Google Scholar] [CrossRef] [PubMed]
- Coletti, C.; Riedl, C.; Lee, D.S.; Krauss, B.; Patthey, L.; Klitzing, K.V.; Smet, J.H.; Starke, U. Charge neutrality and band-gap tuning of epitaxial graphene on SiC by molecular doping. Phys. Rev. B 2010, 81, 235401. [Google Scholar] [CrossRef]
- Dong, X.; Fu, D.; Fang, W.; Shi, Y.; Chen, P.; Li, L.J. Doping single-layer graphene with aromatic molecules. Small 2009, 5, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Tan, P.H.; Zhang, J.; Liu, J. Charge transfer and optical phonon mixing in few-layer graphene chemically doped with sulfuric acid. Phys. Rev. B 2010, 82, 245423. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, J.M.; Jang, C.W.; Kim, J.H.; Kim, S.; Choi, S.H. Effect of layer number and metal-chloride dopant on multiple layers of graphene/porous Si solar cells. J. Appl. Phys. 2018, 123, 123101. [Google Scholar] [CrossRef]
- Kwon, K.C.; Kim, B.J.; Lee, J.L.; Kim, S.Y. Effects of anions in Au complexes on doping and degradation of graphene. J. Mater. Chem. C 2013, 1, 2463–2469. [Google Scholar] [CrossRef]
- Kwon, K.C.; Choi, K.S.; Kim, S.Y. Increased work function in few-layer graphene sheets via metal chloride doping. Adv. Funct. Mater. 2012, 22, 4724–4731. [Google Scholar] [CrossRef]
- Feng, T.; Xie, D.; Wang, D.; Wen, L.; Wu, M. Electron-doping of graphene-based devices by hydrazine. J. Appl. Phys. 2014, 116, 224511. [Google Scholar] [CrossRef]
- Lee, I.Y.; Park, H.Y.; Park, J.H.; Lee, J.; Jung, W.S.; Yu, H.Y.; Kim, S.W.; Kim, G.H.; Park, J.H. Hydrazine-based n-type doping process to modulate Dirac point of graphene and its application to complementary inverter. Org. Electron. 2013, 14, 1586–1590. [Google Scholar] [CrossRef]
- Wei, P.; Liu, N.; Lee, H.R.; Adijanto, E.; Ci, L.; Naab, B.D.; Zhong, J.Q.; Park, J.; Chen, W.; Cui, Y.; et al. Tuning the Dirac point in CVD-grown graphene through solution processed n-type doping with 2-(2-methoxyphenyl)-1,3-dimethyl-2,3-dihydro-1H-benzoimidazole. Nano Lett. 2013, 13, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Santoso, I.; Wang, R.; Xie, L.F.; Mao, H.Y.; Huang, H.; Wang, Y.Z.; Gao, X.Y.; Chen, Z.K.; Ma, D.; et al. Surface transfer hole doping of epitaxial graphene using MoO3 thin film. Appl. Phys. Lett. 2010, 96, 213104. [Google Scholar] [CrossRef]
- D’Arsie, L.; Esconjauregui, S.; Weatherup, R.; Guo, Y.; Bhardwaj, S.; Centeno, A.; Zurutuza, A.; Cepek, C.; Robertson, J. Stability of graphene doping with MoO3 and I2. Appl. Phys. Lett. 2014, 105, 103103. [Google Scholar] [CrossRef]
- Han, C.; Lin, J.; Xiang, D.; Wang, C.; Wang, L.; Chen, W. Improving chemical vapor deposition graphene conductivity using molybdenum trioxide: An in-situ field effect transistor study. Appl. Phys. Lett. 2013, 103, 263117. [Google Scholar] [CrossRef]
- Xie, L.; Wang, X.; Mao, H.; Wang, R.; Ding, M.; Wang, Y.; Ozyilmaz, B.; Loh, K.P.; Wee, A.T.S.; Ariando; et al. Electrical measurement of non-destructively p-type doped graphene using molybdenum trioxide. Appl. Phys. Lett. 2011, 99, 012112. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, H.; Qiu, C.; Yang, H.; Yu, F.; Sun, L. Layer-dependent fluorination and doping of graphene via plasma treatment. Nanotechnology 2012, 23, 115706. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, E.W.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nature 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Siegel, D.A.; Fedorov, A.V.; Lanzara, A. Metal insulator transition in epitaxial graphene induced by molecular doping. Phys. Rev. Lett. 2008, 101, 086402. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Jang, C.; Adam, S.; Fuhrer, M.S.; Williams, E.D.; Ishigami, M. Charged-impurity scattering in graphene. Nat. Phys. 2008, 4, 377–381. [Google Scholar] [CrossRef]
- Bostwick, A.; Ohta, T.; Seyller, T.; Horn, K.; Rotenberg, E. Quasiparticle dynamics in graphene. Nat. Phys. 2007, 3, 36–40. [Google Scholar] [CrossRef]
- Jung, N.; Kim, N.; Jockusch, S.; Turro, N.J.; Kim, P.; Brus, L. Charge transfer chemical doping of few layer graphenes: Charges distribution and band gap formation. Nano Lett. 2009, 9, 4133–4137. [Google Scholar] [CrossRef] [PubMed]
- Farmer, D.B.; Mojarad, R.G.; Perebeinos, V.; Lin, Y.M.; Tulevski, G.S.; Tsang, J.C.; Avouris, P. Chemical doping and lectron-hole conduction asymmetry in graphene devices. Nano Lett. 2009, 9, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, Z.; Gou, X. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, Y.; Wang, J.; Liu, H.; Liu, X.; Wang, L.; Liu, X.; Xue, W.; Ma, N. Electrochemical synthesis quantum dots for free radical scavenging. Phys. Chem. Chem. Phys. 2017, 19, 11631–11638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wei, Z.; Gou, X.; Xu, W. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv. 2013, 3, 9978–9984. [Google Scholar] [CrossRef]
- An, M.; Du, C.; Du, L.; Sun, Y.; Wang, Y.; Chen, C.; Han, G. Phosphorus-doped graphene support to enhance electrocatalysis of methanol oxidation reaction on platinum nanoparticles. Chem. Phys. Lett. 2017, 687, 1–8. [Google Scholar] [CrossRef]
- Marinoiu, A.; Raceanu, M.; Carcadea, E.; Varlam, M.; Balan, D.; Ebrasu, D.I.; Stefanescu, I.; Enachescu, M. Iodine-doped graphene for enhanced electrocatalytic oxygen reduction reaction in proton exchange membrane fuel cell applications. J. Electrochem. Energy Convers. Storage 2017, 14, 031001. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Q.; Yang, C.; Quhe, R.; Guan, P.; Lu, P. Crown oxygen-doping graphene with embedded main-group metal atoms. Eur. Phys. J. B 2018, 91, 46. [Google Scholar] [CrossRef]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; Magna, A.L.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Agnello, S. Effect of air on oxygen p-doped graphene on SiO2. Phys. Status Solidi A 2016, 213, 2341–2344. [Google Scholar] [CrossRef]
- Yan, H.J.; Xu, B.; Shi, S.Q.; Ouyang, C.Y. First-principles study of the oxygen adsorption and dissociation on graphene and nitrogen doped graphene for Li-air batteries. J. Appl. Phys. 2012, 112, 104316. [Google Scholar] [CrossRef]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2013, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, Y.Y.; Matson, D.W.; Li, J.H.; Lin, Y.H. Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 2010, 4, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhang, L.; Yoon, Y.; Weber, P.K.; Wang, H.; Guo, J.; Dai, H. N-doping of graphene through electrothermal reactions with ammonia. Science 2009, 324, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, J.; Wang, L.; Wang, D.; Ding, F.; Tao, X.; Chen, W. Manageable N-doped graphene for high performance oxygen reduction reaction. Sci. Rep. 2013, 3, 2771. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shi, Q.; Li, Y.; Lai, Z.; Yu, H.; Wang, H.; Peng, F. Nitrogen-doped graphene-supported cobalt carbonnitride@oxide core-shell nanoparticles as a non-noble metal electrocatalyst for an oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 1142. [Google Scholar] [CrossRef]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; Magna, A.L.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Agnello, S. Graphene p-type doping and stability by thermal treatments in molecular oxygen controlled atmosphere. J. Phys. Chem. C 2015, 119, 22718–22723. [Google Scholar] [CrossRef]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; Magna, A.L.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Pignataro, B.; Scopelliti, M.; et al. Substrate and atmosphere influence on oxygen p-doped graphene. Carbon 2016, 107, 696–704. [Google Scholar] [CrossRef]
- Piazza, A.; Giannazzo, F.; Buscarino, G.; Fisichella, G.; Magna, A.L.; Roccaforte, F.; Cannas, M.; Gelardi, F.M.; Agnello, S. In-situ monitoring by Raman spectroscopy of the thermal doping of graphene and MoS2 in O2-cotrolled atmosphere. Beilstein J. Nanotechnol. 2017, 8, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Armano, A.; Buscarino, G.; Cannas, M.; Gelardi, F.M.; Giannazzo, F.; Schiliro, E.; Agnello, S. Monolayer graphene doping and strain dynamics induced by thermal treatments in controlled atmosphere. Carbon 2018, 127, 270–279. [Google Scholar] [CrossRef]
- Marconcini, P.; Cresti, A.; Roche, S. Effect of the channel length on the transport characteristics of transistors based on boron-doped graphene ribbons. Materials 2018, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.; Lopez, N.P.; Elias, A.L.; Fujisawa, K.; Carozo, V.; Feng, S.; Lv, R.; Santos, M.C.D.; Terrones, M.; Araujo, P.T. Probing the interaction of noble gases with pristine and nitrogen-doped graphene through Raman spectroscopy. Phys. Rev. B 2018, 97, 195419. [Google Scholar] [CrossRef]
- Feng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-doped graphene via solvothermal synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar]
- Baskin, Y.; Meyer, L. Lattice constants of graphite at low temperatures. Phys Rev. 1955, 100, 544. [Google Scholar] [CrossRef]
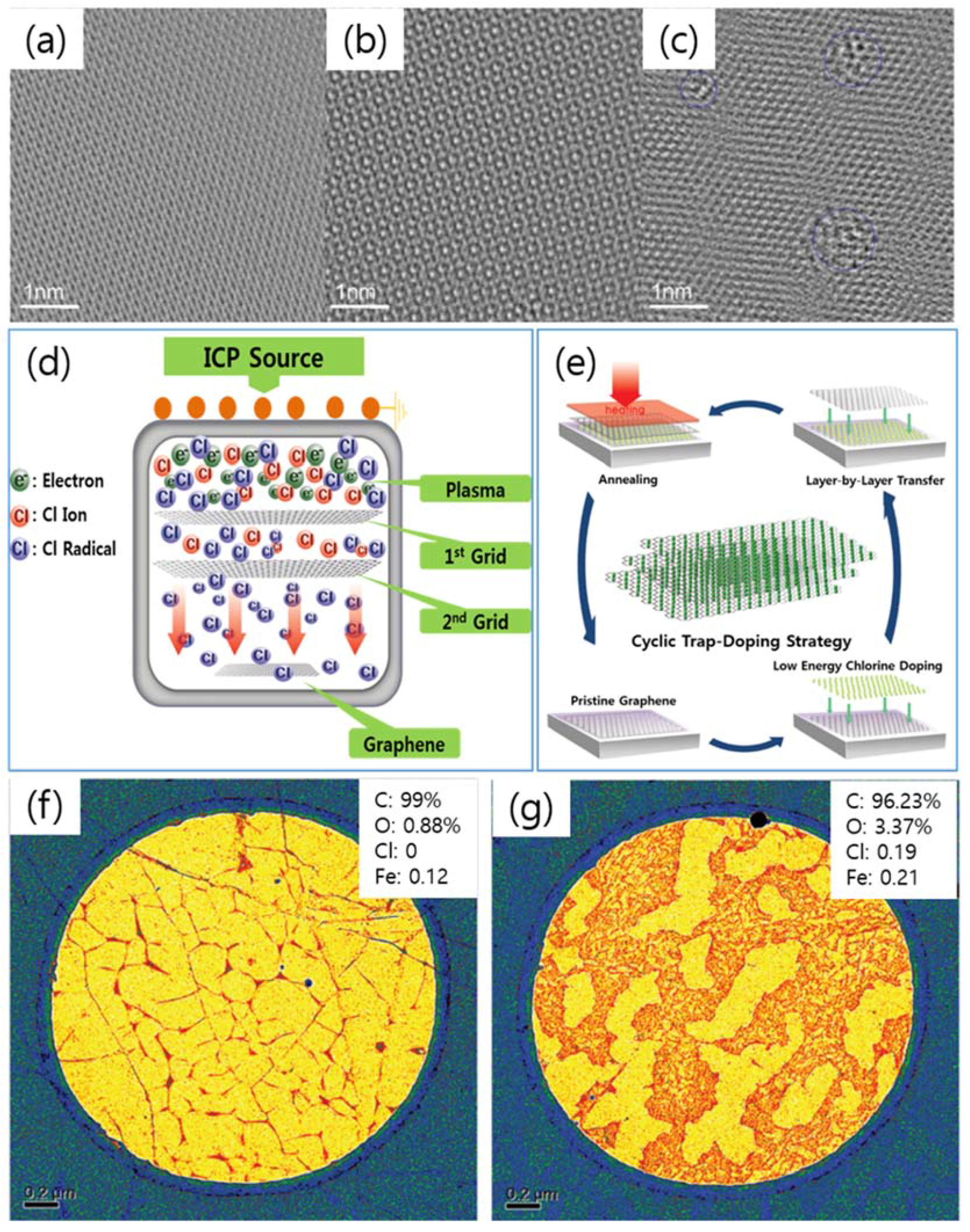
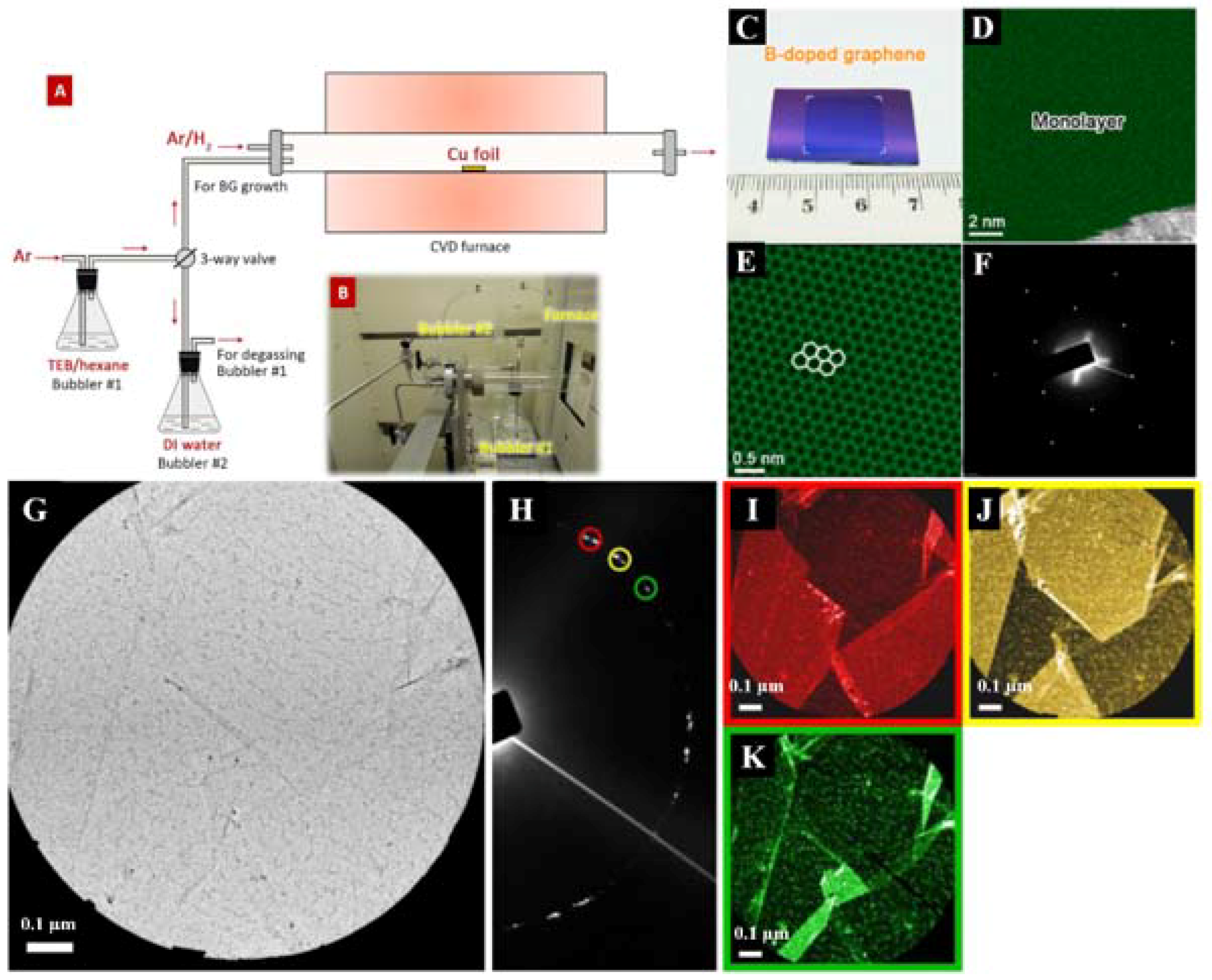
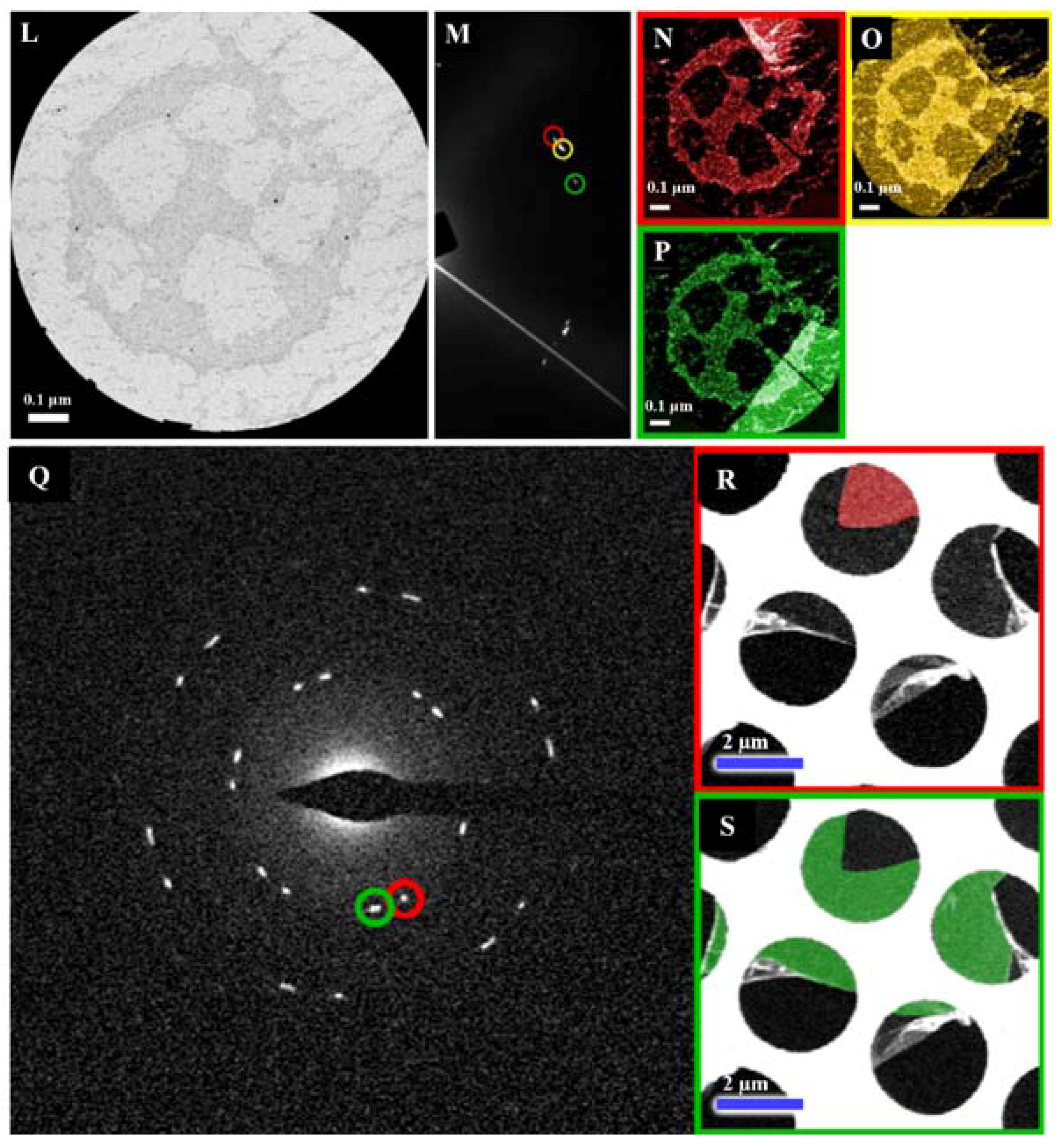
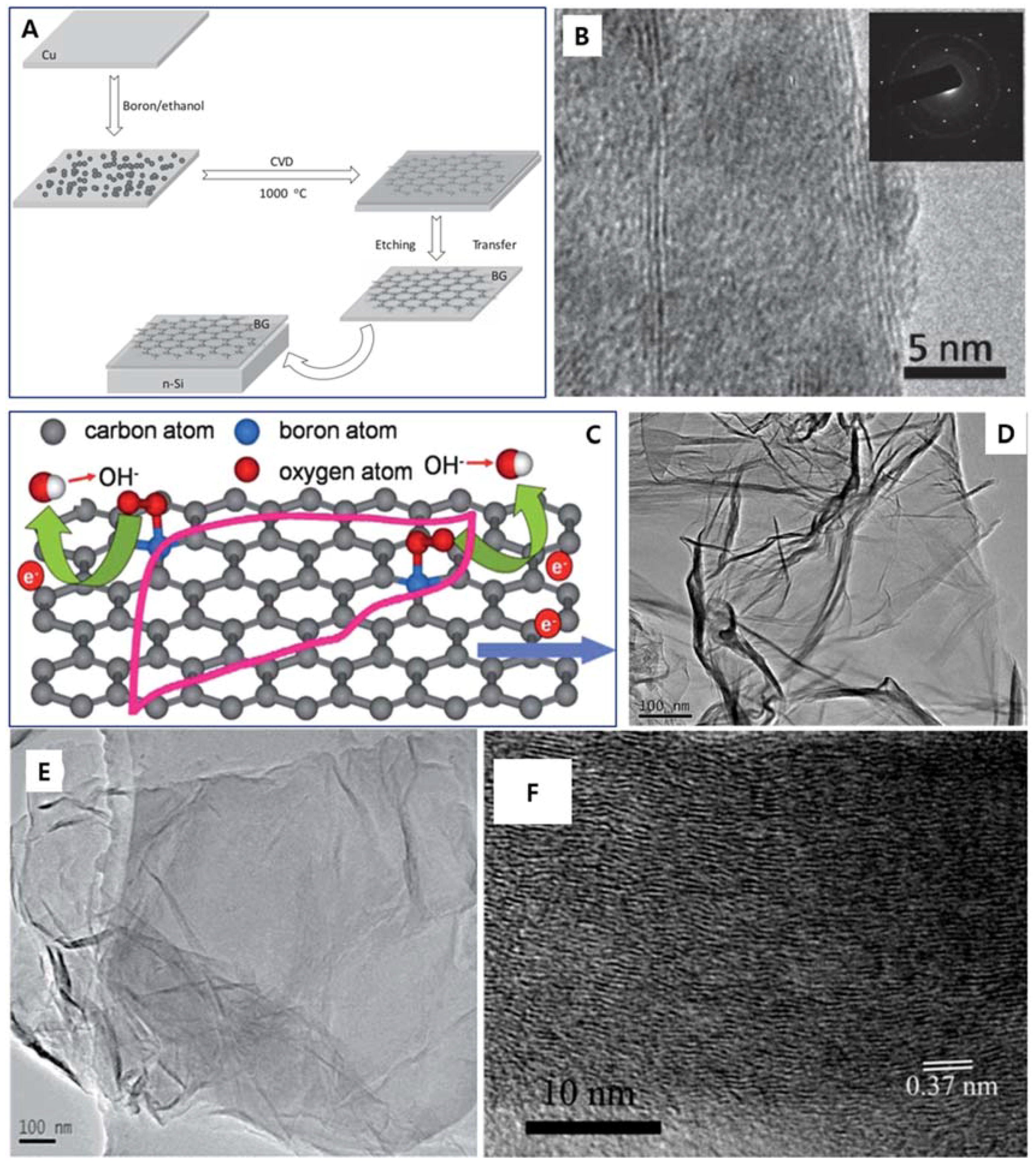
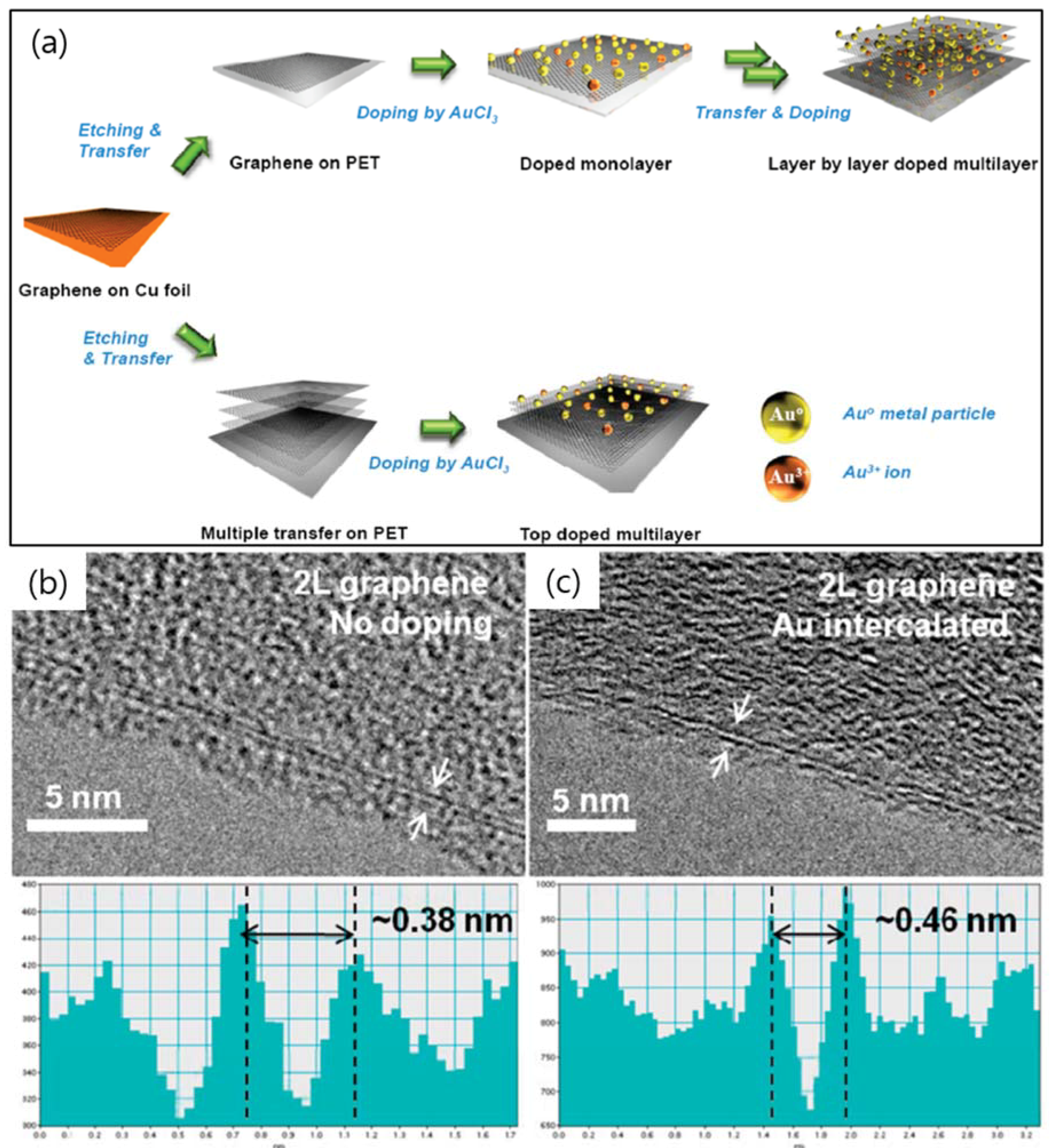
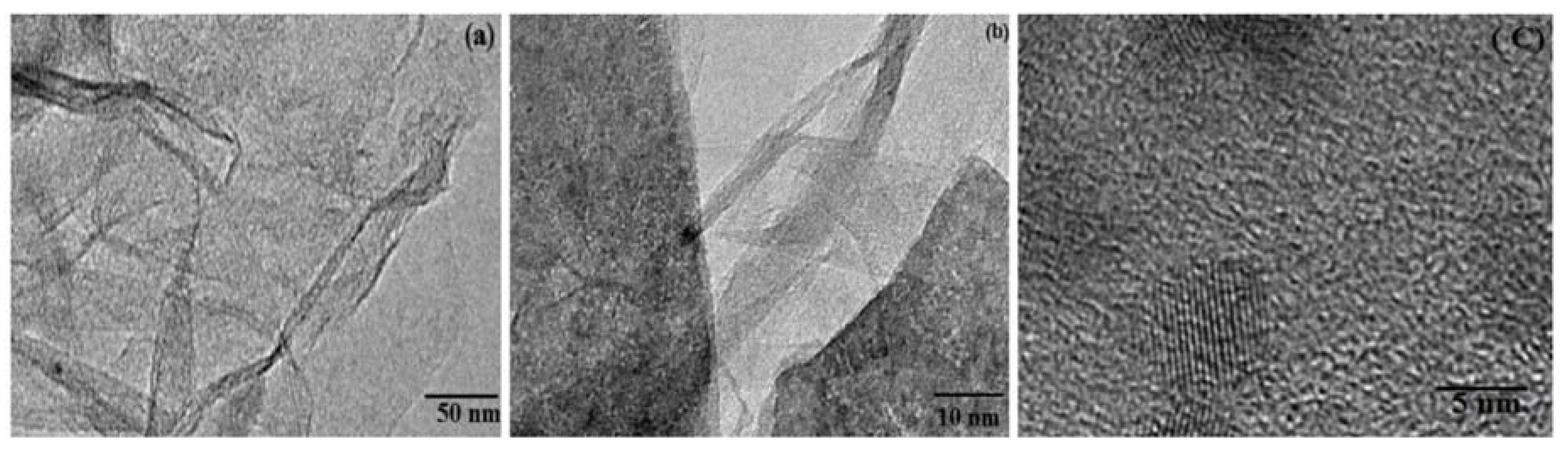
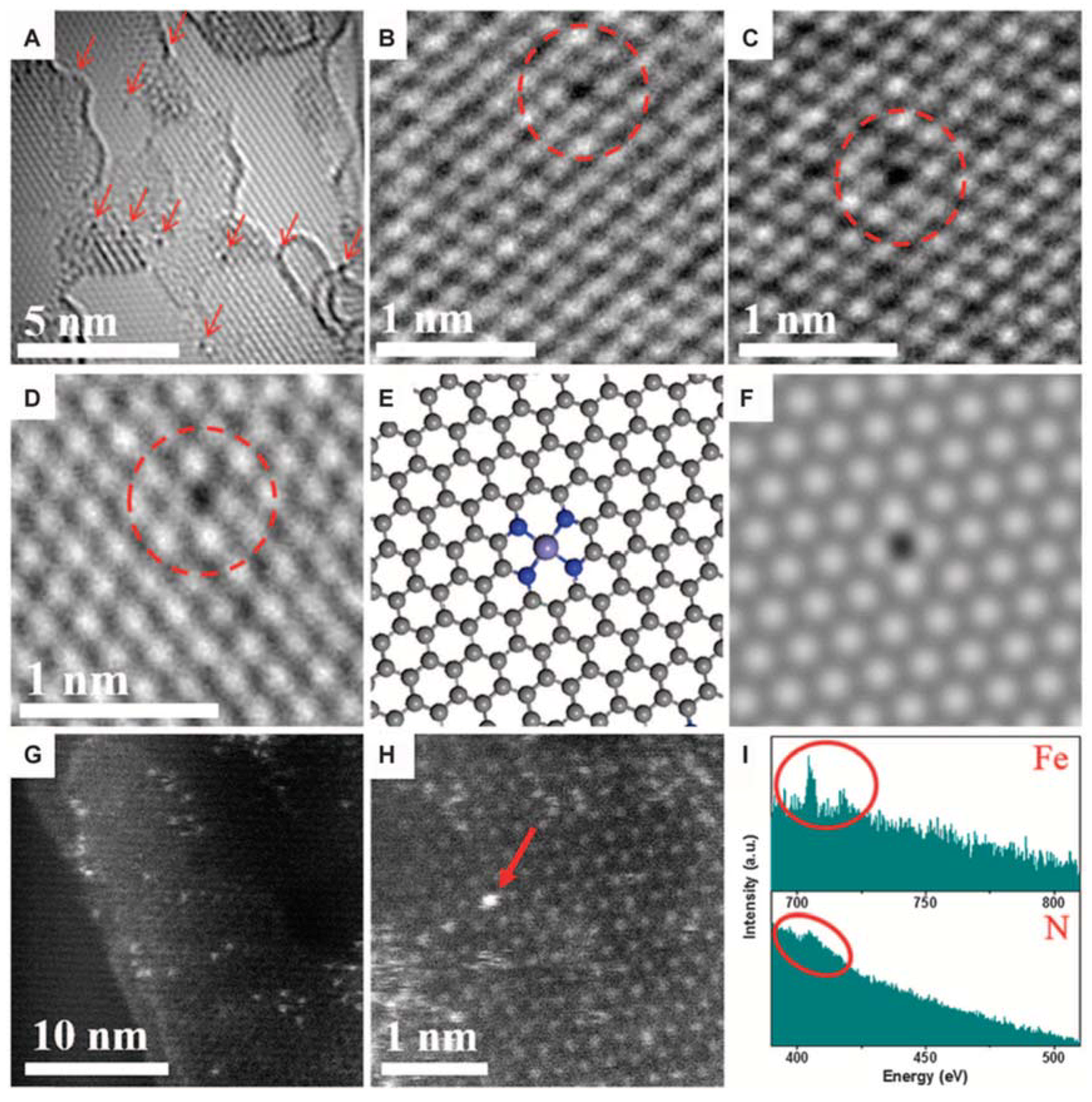
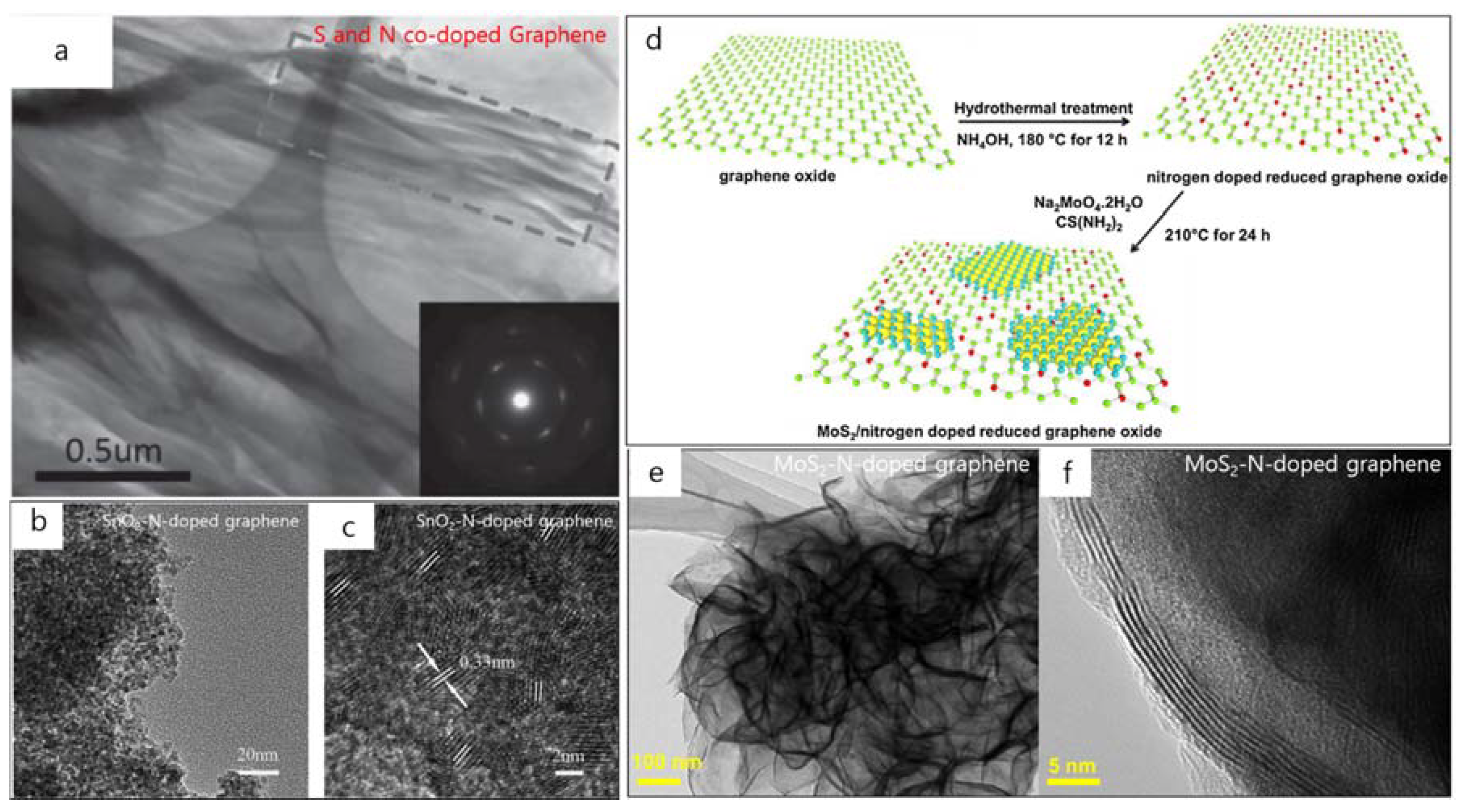
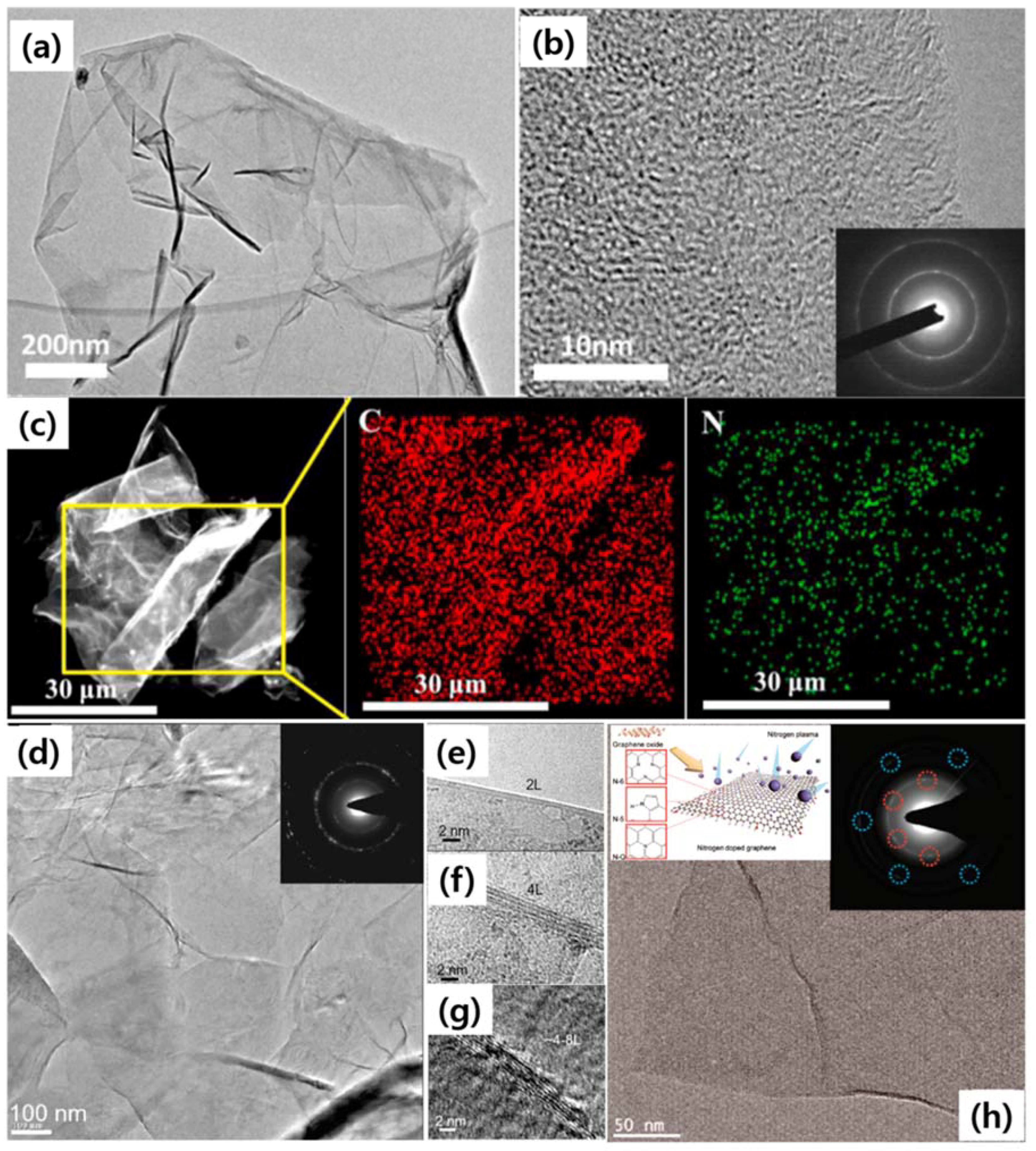

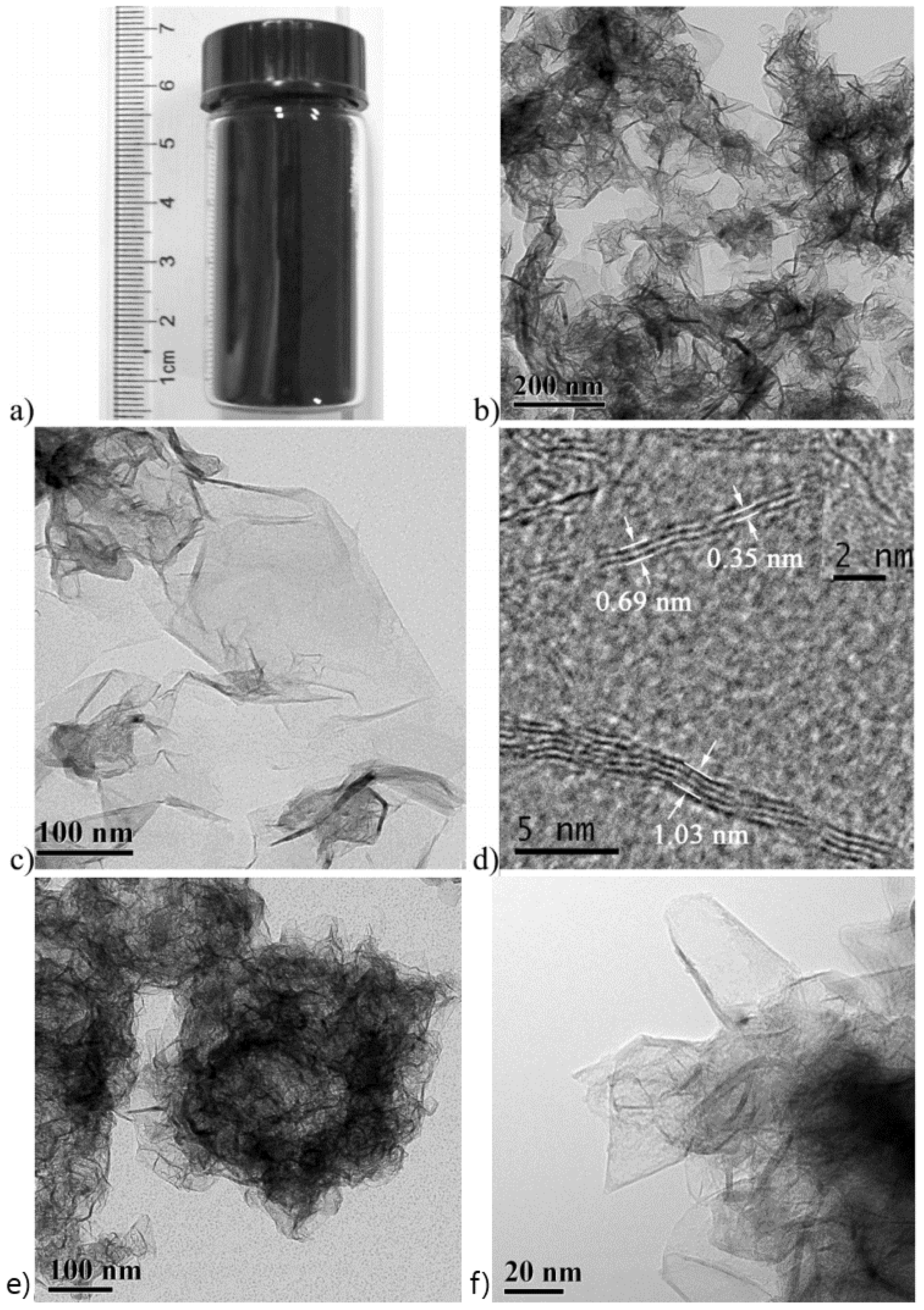
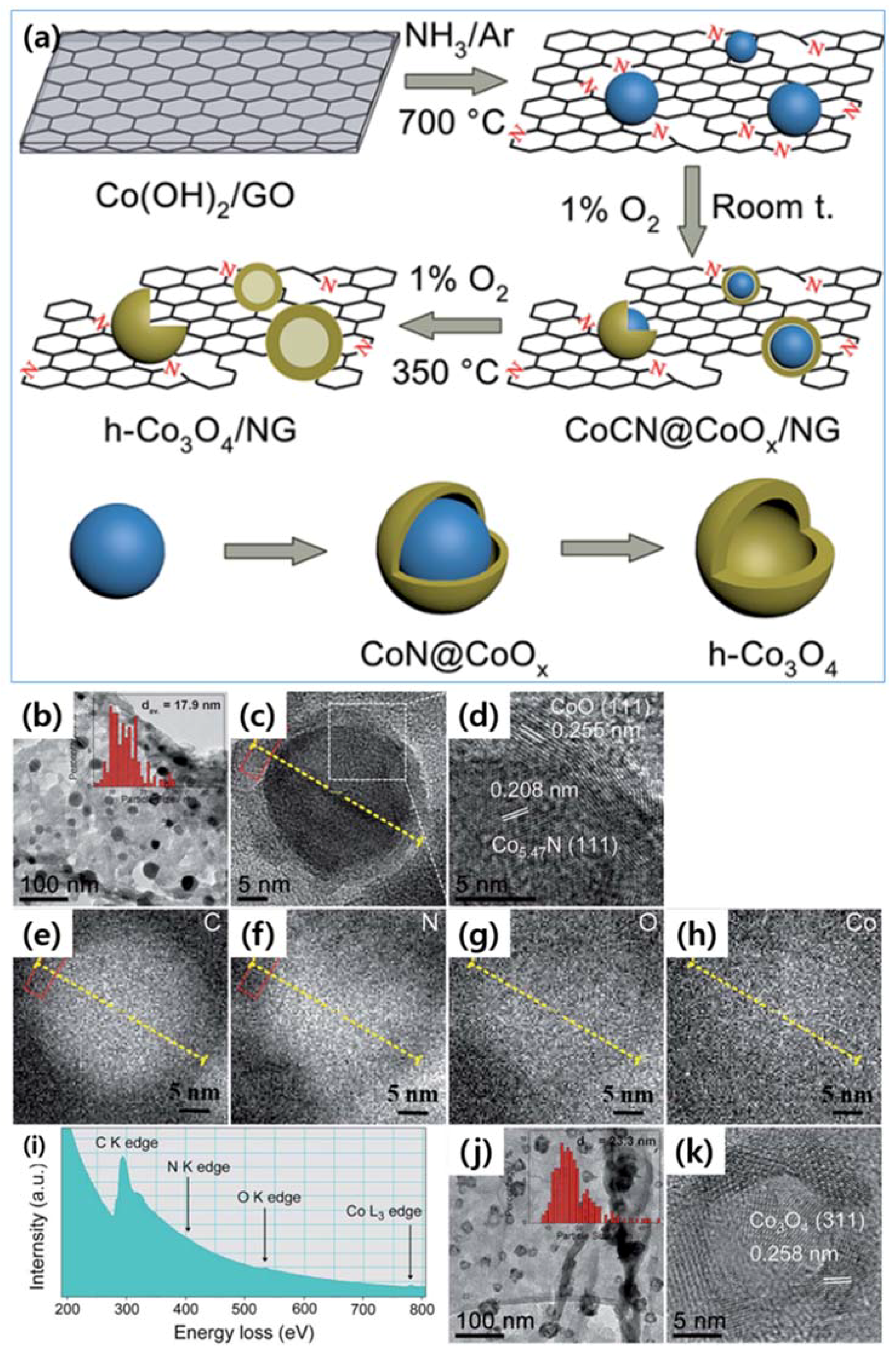
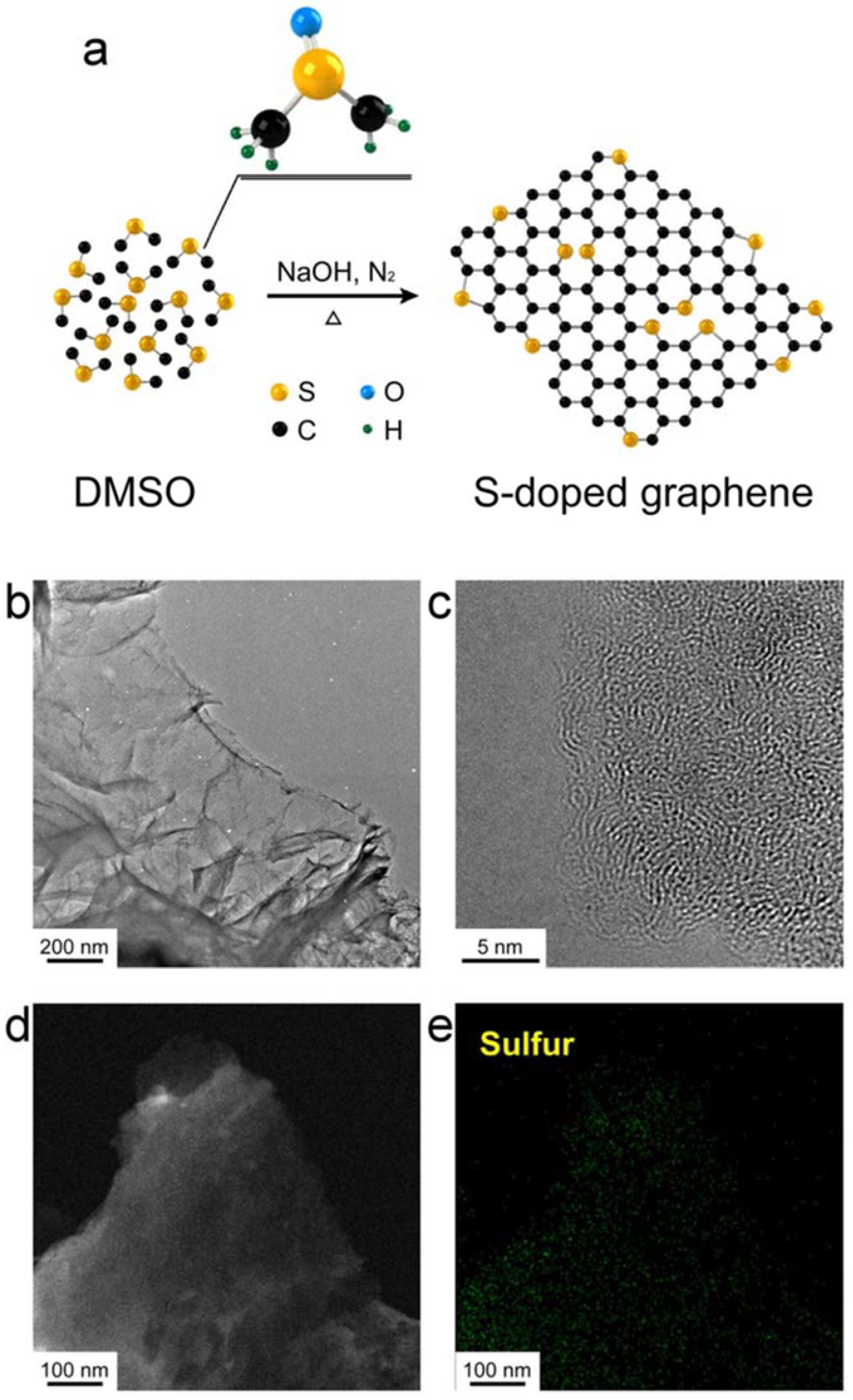
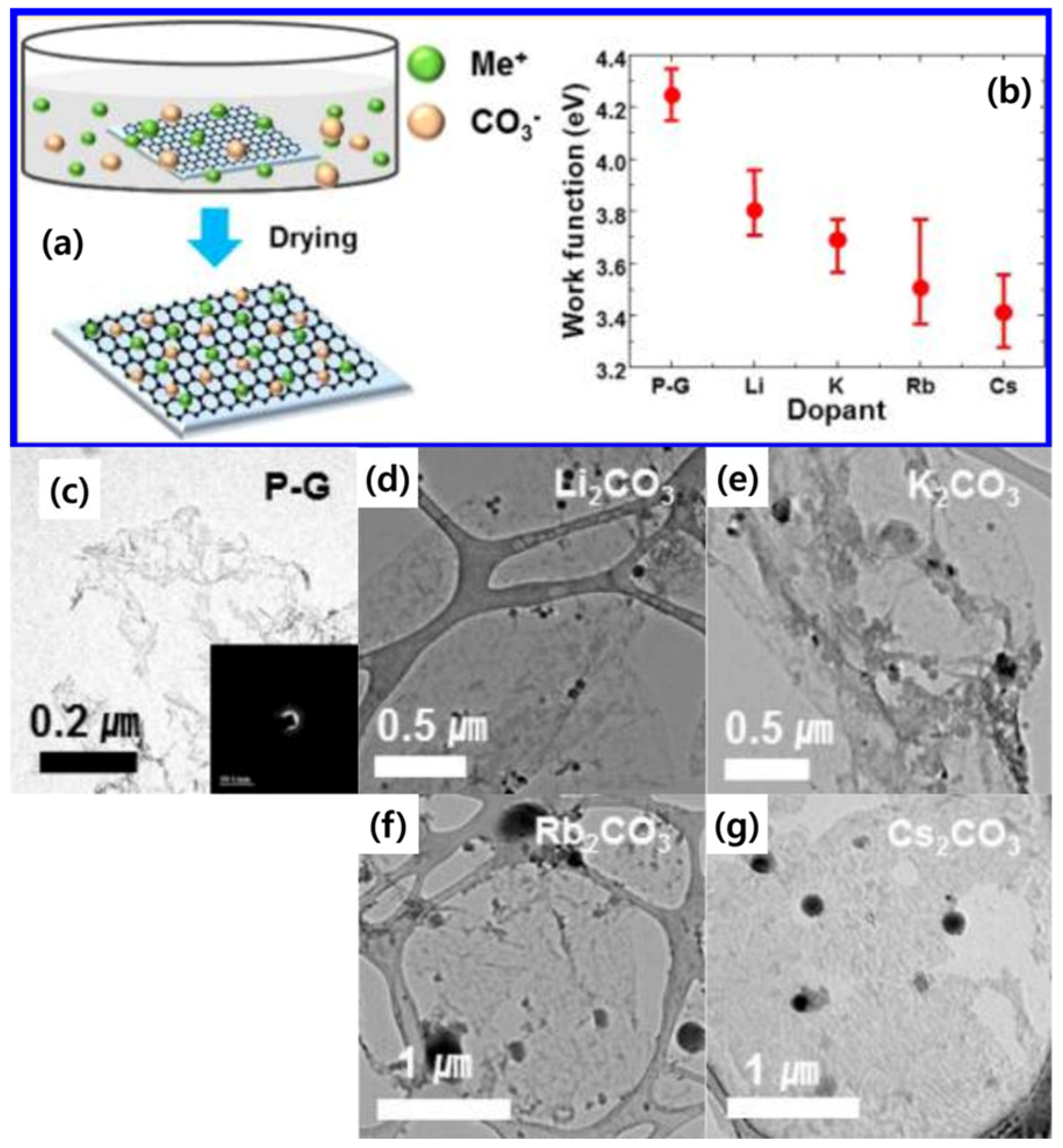
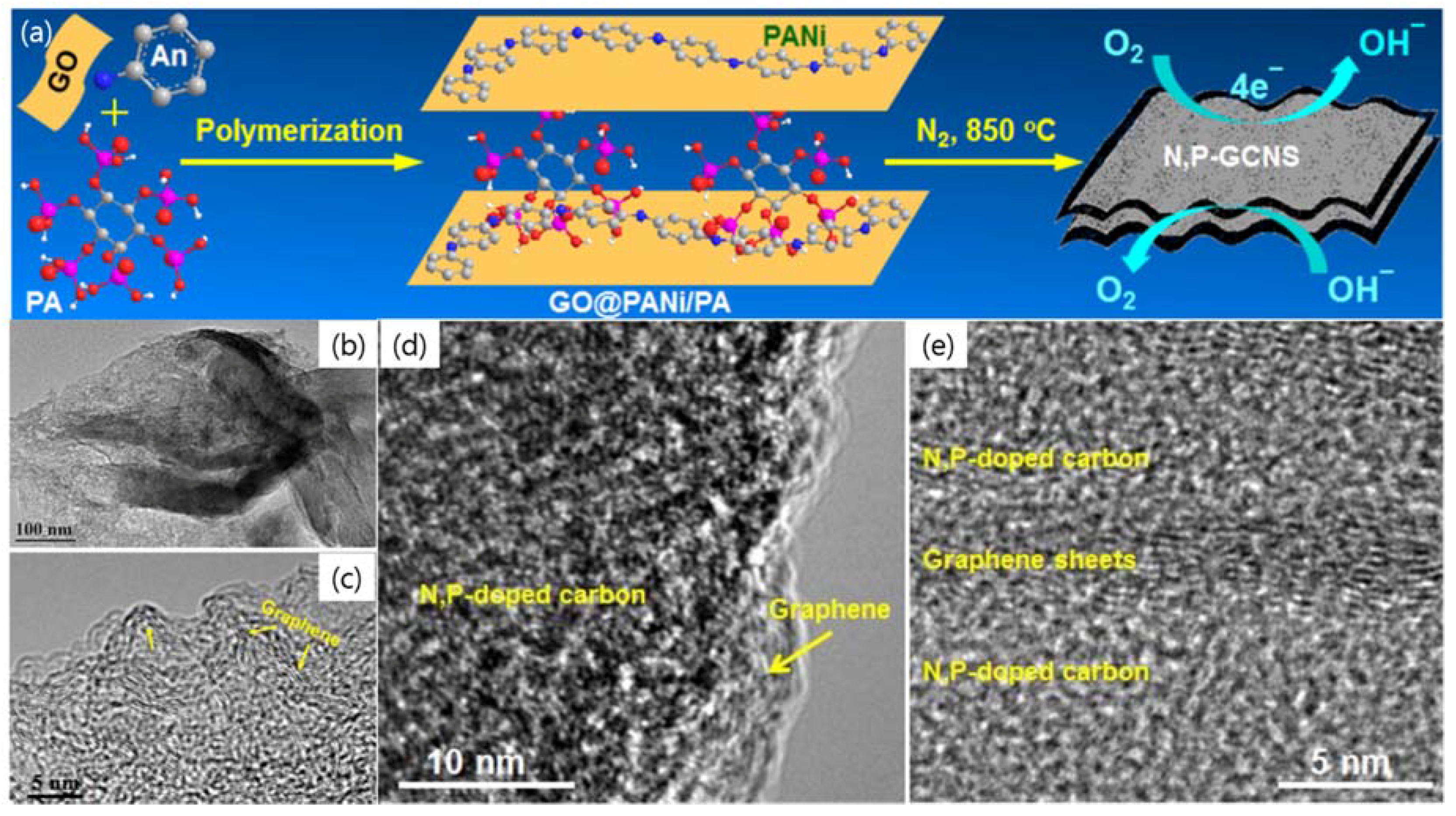
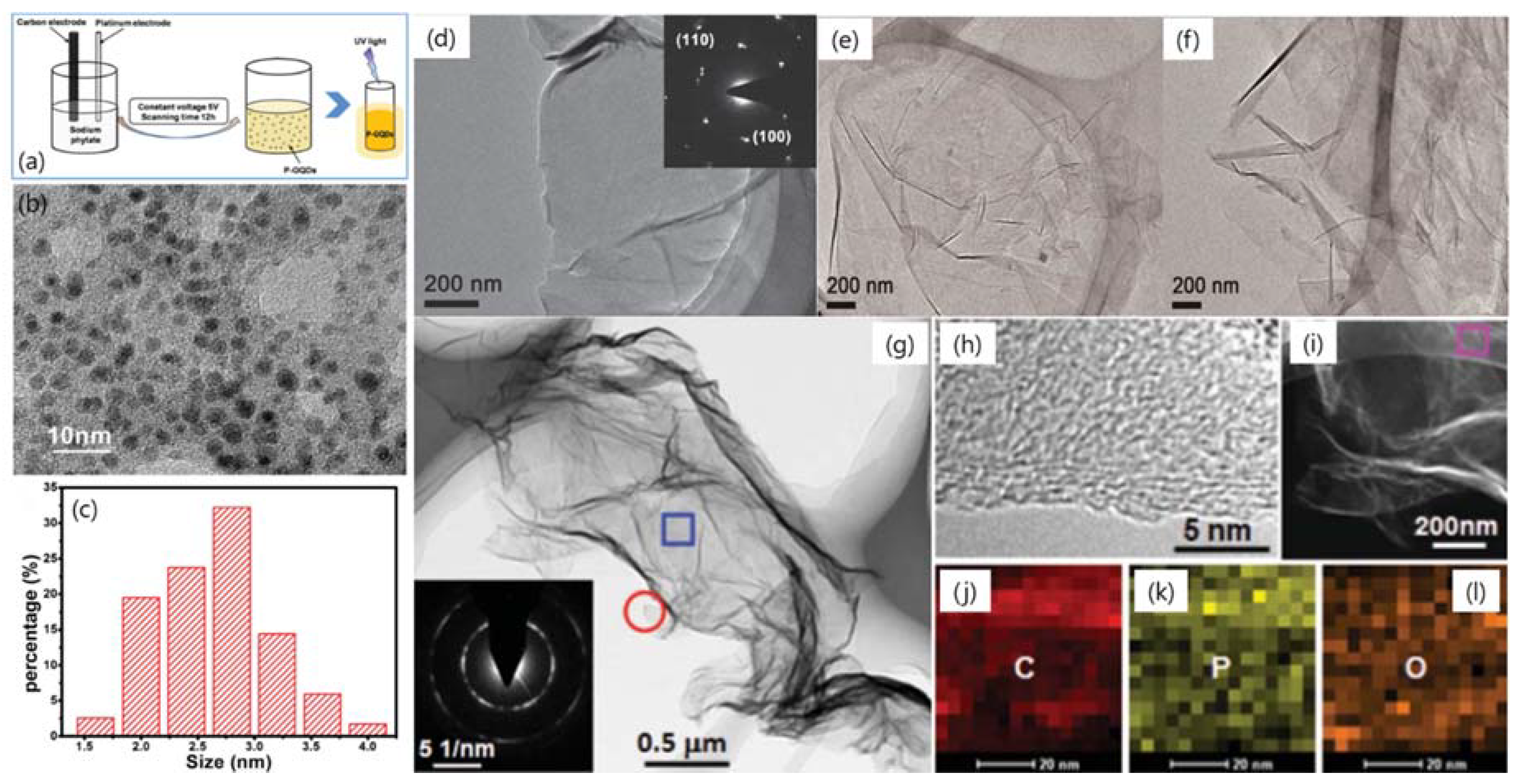
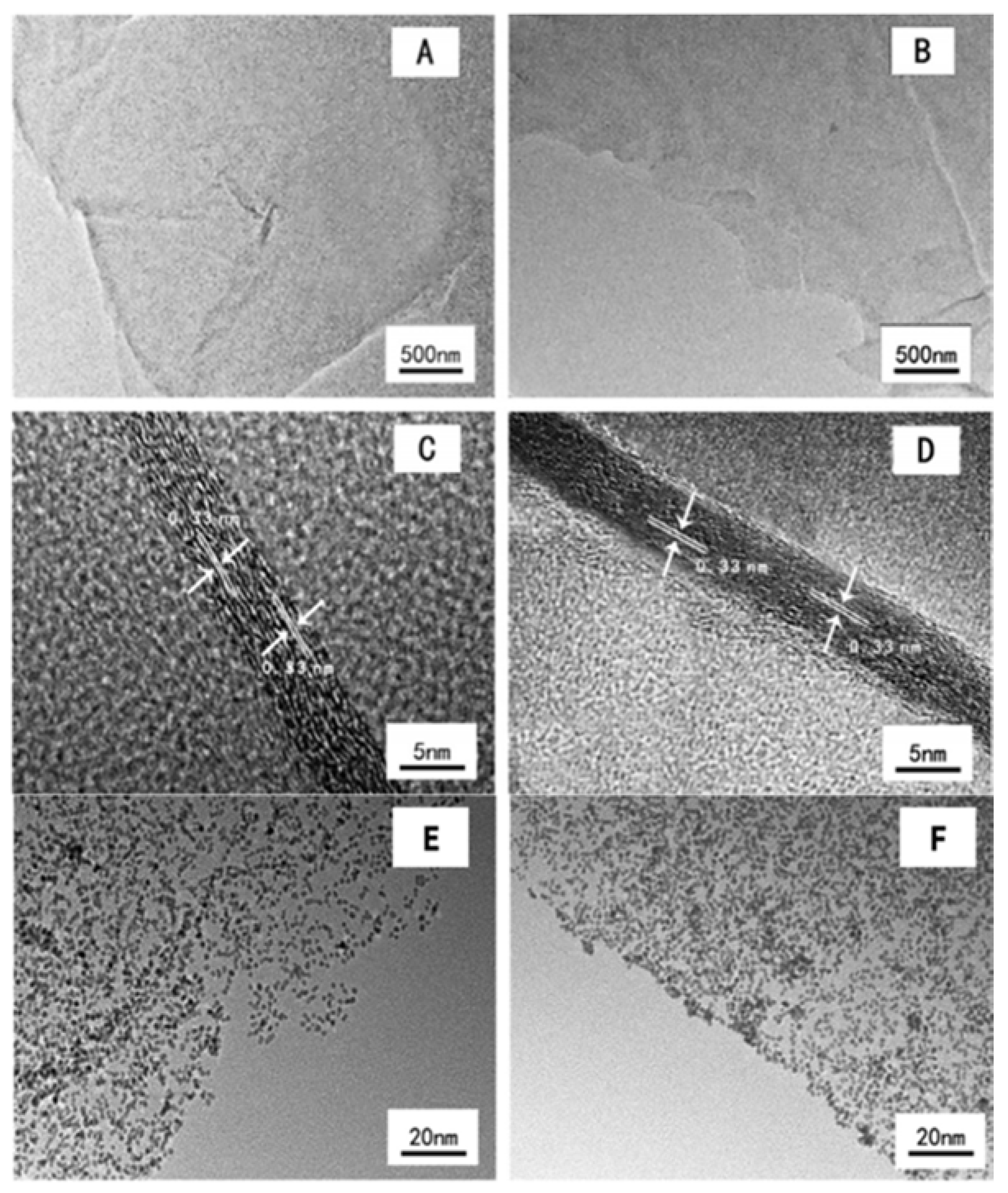
| Dopant | Doping Type | Strategies | Applications of Doped-Graphene | Results | Ref. |
|---|---|---|---|---|---|
| Cl | p | Plasma doping | NA | High-damage plasma | [18] |
| Damage-free plasma dry doping | NA | Damage-free plasma | [19,20,21,22,23,24] | ||
| HNO3 | p | Wet chemical doping | NA | NA | [46] |
| Wet chemical doping | Heterojunction solar cell | Power conversion efficiency (5.47%) | [51] | ||
| Wet chemical doping | Schottky junction solar cell | Short-circuit current density (14.58 mA/cm2), Open-circuit voltage (466.86 mV), Fill factor (0.29), Energy conversion efficiency (3.55%) | [52] | ||
| AuCl3 | p | Wet chemical doping | NA | %T (85%), Rs (54 Ω/sq) | [28] |
| Photovoltaic diodes | Power conversion efficiency (0.08%) | [29] | |||
| NA | NA | [46] | |||
| NA | Rs (300 Ω/sq) | [59] | |||
| NA | %T (94.5%), Rs (500 Ω/sq), Work function (5 eV) | [60] | |||
| SOCl2 | p | Wet chemical doping | Schottky junction solar cell | Short-circuit current density (11.24 mA/cm2), Open-circuit voltage (503 mV), Fill factor (0.506), Energy conversion efficiency (2.86%) | [50] |
| Heterojunction solar cell | Power conversion efficiency (5.95%) | [51] | |||
| S | n | Wet chemical doping | Energy storage and conversion | novel | [33] |
| S–N | n | Solvothermal wet doping | Metal-free catalyst for green remediation of organic pollutants in water | Novel metal-free catalyst | [34] |
| TiO2 | p | Wet chemical doping | NA | NA | [31] |
| B | p | Thermal annealing | Gas sensors | Novel sensing for NO2 and NH3 gases | [25] |
| p-n junction solar cell | Power conversion efficiency (3.4%) | [26] | |||
| Thermal annealing | Fuel cells | Novel electrocatalytic activity, long-term stability, O-reduction reactions, and good tolerance to poisons | [27] | ||
| NA | Transistor | Improved switching performance | [94] | ||
| TFSA | p | Wet chemical doping | NA | NA | [46] |
| Polymer Light Emitting Diodes (PLEDs) | %T (88%), Rs (90 Ω/sq), current efficiency (9.6 cd/A), power efficiency (10.5 lm/W) | [48] | |||
| NA | Rs (129 Ω/sq) | [49] | |||
| FeN4 | n | High-energy ball milling synthesis | Catalyst | High catalytic activity | [32] |
| FeCl3 | p | Wet chemical doping | NA | Rs (72 Ω/sq) for doped- single-layer graphene Rs (42 Ω/sq) of doped-four-layer graphene | [46] |
| HCl | p | Wet chemical doping | Heterojunction solar cell | Power conversion efficiency (4.93%) | [51] |
| Au(OH)3 | p | Wet chemical doping | NA | Rs (820 Ω/sq) | [59] |
| Au2S | Rs (600 Ω/sq) | ||||
| AuBr3 | Rs (530 Ω/sq) | ||||
| Au NPs/TFSA | p | Wet chemical doping | Solar cell | Power conversion efficiency (10.69%) | [30] |
| H2O2 | n | Wet chemical doping | Heterojunction solar cell | Power conversion efficiency (5.12%) | [51] |
| CoCN@CoOx/N | n | Wet chemical doping | Catalyst | High activity, good selectivity, and durability | [89] |
| SnO2/N | n | Microwave-assisted solvothermal | Li storage | Excellent Li storage capability and high durability | [35] |
| MoS2/N | n | Hydrothermal | Li-ion battery | High capacity (1140 mA/hg) at current density (100 mA/g), Energy density (890 Wh/kg), Power density (130 W/kg) | [36] |
| N | n | Wet chemical doping | Energy storage and conversion | Good Li-ion storage and O-reduction reaction catalytic activity | [33] |
| Plasma doping | Ultracapacitor | Capacitance (280 F/g), novel cycle life (>200,000), and high-power capability | [39] | ||
| Pyrolysis | Catalyst | High O-reduction reaction | [43] | ||
| Thermal annealing in APCVD | Organic molecular sensing | Novel probing of Rhodamine (RhB) molecules | [40] | ||
| Thermal annealing in APCVD | Ultrasensitive molecular sensor | Novel sensing of RhB, crystal violet (CRV), and methylene blue (MB) molecules | [41] | ||
| Pyrolysis | Catalyst | High O-reduction reaction | [37] | ||
| Thermal annealing in CVD | Fuel cells | High O-reduction reactions, long-term stability, tolerance to crossover and poison | [38] | ||
| Plasma doping | NA | NA | [42] | ||
| Annealing at 1100 °C | Back-gate FET | Mobility (6000 cm2/Vs) | [44] | ||
| Plasma doping | Biosensor | High electrocatalytic activity, Novel glucose biosensing with low concentration (0.01 mM) | [86] | ||
| Electrothermal annealing | FET | Highly edge functionalization of GNRs by N2 species | [87] | ||
| Wet chemical doping | Catalyst | Good electrocatalytic activity, long term stability, and tolerance to crossover effect | [88] | ||
| Soft thermal doping | NA | NA | [92,94] | ||
| Solvothermal doping | Fuel cell | Enhanced catalytic activity in O-reduction reaction | [96] | ||
| Thermal annealing in APCVD | NA | NA | [95] | ||
| IrCl3 | p | Wet chemical doping | NA | Rs (500 Ω/sq), Work function (4.9 eV) | [60] |
| MoCl3 | %T (86.2%), Rs (720 Ω/sq), Work function (4.8 eV) | ||||
| OsCl3 | Rs (700 Ω/sq), Work function (4.68 eV) | ||||
| PdCl2 | Rs (520 Ω/sq), Work function (5 eV) | ||||
| RhCl3 | Rs (620 Ω/sq), Work function (5.14 eV) | ||||
| N2H4 | n | Wet chemical doping | 1. Back-gate FET 2. G/p-Si heterojunction | 1. Good controllable doping effect 2. Increased Schottky junction barrier between graphene and Si | [61] |
| Complementary graphene inverter with p- and n-channel FETs | Well-control of Dirac point in graphene | [62] | |||
| o-MeO-DMBI | n | Spin coating and inkjet printing | Complementary graphene inverter with p- and n-channel FETs | Low performance due to gapless of graphene, reduced work function (3.46 eV) | [63] |
| F4-TCNQ | p | Wet chemical doping | NA | Novel surface transfer doping | [53] |
| [54] | |||||
| [55] | |||||
| An–CH3 | n | Wet chemical doping | NA | Left Raman shift | [56] |
| Na–NH2 | n | ||||
| An–Br | p | Right Raman shift | |||
| TPA | p | ||||
| H2SO4 | p | Wet chemical doping | NA | A stable electron-acceptor dopant for graphene | [57] |
| Diazonium salt | p | Wet chemical doping | FET | Long-range scatterer on graphene | [74] |
| Poly(ethylene imine) | n | ||||
| MoO3 | p | Thermal evaporation in Knudsen cell | NA | NA | [64,66,67] |
| Wet chemical doping | NA | NA | [65] | ||
| Li2CO3 | n | Wet chemical doping | NA | Rs (2050 Ω/sq), Work function (3.8 eV) | [45] |
| K2CO3 | Rs (1750 Ω/sq), Work function (3.7 eV) | ||||
| Rb2CO3 | Rs (2520 Ω/sq), Work function (3.5 eV) | ||||
| Cs2CO3 | Rs (1500 Ω/sq), Work function (3.4 eV) | ||||
| NH3 | n | Annealing at 1100 °C | Back-gate FET | Mobility (6000 cm2/Vs) | [44] |
| CF4 | p | Plasma doping | NA | High-damage plasma | [18,68] |
| CHF3 | p | Plasma doping | NA | High-damage plasma | [68] |
| H2 | p | Plasma doping | NA | High-damage plasma | [18] |
| Dry doping | NA | NA | [75] | ||
| P | n | Wet chemical doping | Free radical scavenging | High scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals | [77] |
| 1. Electrocatalyst 2. Lithium-ion batteries | 1.High O-reduction reaction 2. Highly enhanced cycle and rate capabilities | [78] | |||
| Electrocatalyst | Good O-reduction reaction catalytic performance | [79] | |||
| Annealing | Electrocatalyst | The enhanced activity of electrochemical surface area | [80] | ||
| N2/P | n | Wet chemical doping | Bifunctional electrocatalyst | Good O-reduction and evolution | [76] |
| NO2 | n | Wet chemical doping | Gas sensor | Ultrasensitive with individual gas molecules | [69] |
| NA | NA | [70] | |||
| Br2 | p | Wet chemical doping | NA | NA | [73] |
| I2 | p | Wet chemical doping | NA | NA | [73] |
| Wet chemical doping | NA | NA | [65] | ||
| Wet chemical doping | Fuel cell | Good electrochemical performance | [81] | ||
| K | p | Dry doping | NA | NA | [71] |
| NA | [72] | ||||
| O2 | p | Dry doping | NA | NA | [82] |
| Thermal annealing | NA | NA | [83] | ||
| Dry doping | Li-ion batteries | Most adsorption energetically stable at bridge sites | [84] | ||
| Soft thermal doping | NA | NA | [91,92,93,94] | ||
| Air | p | Soft thermal doping | NA | NA | [92,94] |
| CO2 | p | Soft thermal doping | NA | NA | [92,94] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, P.V. A Library of Doped-Graphene Images via Transmission Electron Microscopy. C 2018, 4, 34. https://doi.org/10.3390/c4020034
Pham PV. A Library of Doped-Graphene Images via Transmission Electron Microscopy. C. 2018; 4(2):34. https://doi.org/10.3390/c4020034
Chicago/Turabian StylePham, Phuong Viet. 2018. "A Library of Doped-Graphene Images via Transmission Electron Microscopy" C 4, no. 2: 34. https://doi.org/10.3390/c4020034
APA StylePham, P. V. (2018). A Library of Doped-Graphene Images via Transmission Electron Microscopy. C, 4(2), 34. https://doi.org/10.3390/c4020034




