Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries
Abstract
:1. Introduction
2. Precursors and Yield
3. Microstructure and Texture
4. Performances
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 88th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Goodenough, J.B. Rechargeable batteries: Challenges old and new. J. Solid State Electrochem. 2012, 16, 2019–2029. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, Y.; Fu, Z.W. Electrochemical properties of room temperature sodium–air batteries with non-aqueous electrolyte. Electrochem. Commun. 2012, 16, 22–25. [Google Scholar] [CrossRef]
- Kummer, J.T.; Weber, N. Battery Having a Molten Alkali Metal Anode and Molten Sulfur Cathode. U.S. Patent 3,413,150, 26 November 1968. [Google Scholar]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Bullis, K. Sodium-Ion Cells for Cheap Energy Storage; MIT Technology Review: Cambridge, MA, USA, 2009. [Google Scholar]
- Pan, H.; Hu, Y.S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Liu, Y.; Merinov, B.V.; Goddard, W.A. Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals. Proc. Natl. Acad. Sci. USA 2016, 113, 3735–3739. [Google Scholar] [CrossRef] [PubMed]
- Nita, C.; Bensafia, M.; Vaulot, C.; Delmotte, L.; Matei Ghimbeu, C. Insights on the synthesis mechanism of green phenolic resin derived porous carbons via a salt-soft templating approach. Carbon 2016, 109, 227–238. [Google Scholar] [CrossRef]
- Kumpf, R.; Dougherty, D. A mechanism for ion selectivity in potasium channels: Computational studies of cation-pi interactions. Science 1993, 261, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium ion batteries. Grand challenges in energy storage. Adv. Funct. Mater. 2013, 23, 917–1089. [Google Scholar] [CrossRef]
- Asher, R.C. A lamellar compound of sodium and graphite. J. Inorg. Nucl. Chem. 1959, 10, 238–249. [Google Scholar] [CrossRef]
- Ge, P.; Fouletier, M. Electrochemical intercalation of sodium in graphite. Solid State Ion. 1988, 30, 1172–1175. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. The mechanisms of lithium and sodium insertion in carbon materials. J. Electrochem. Soc. 2001, 148, A803–A811. [Google Scholar] [CrossRef]
- Ponrouch, A.; Dedryvère, R.; Monti, D.; Demet, A.E.; Ateba Mba, J.M.; Croguennec, L.; Masquelier, C.; Johansson, P.; Palacín, M.R. Towards high energy density sodium ion batteries through electrolyte optimization. Energy Environ. Sci. 2013, 6, 2361–2369. [Google Scholar] [CrossRef]
- Franklin, R.E. Crystallite growth in graphitizing and non-graphitizing carbons. Proc. R. Soc. Lond. Ser. A 1951, 209, 196–218. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shi, Y.; Zhao, D. Supramolecular aggregates as templates: Ordered mesoporous polymers and carbons. Chem. Mater. 2008, 20, 932–945. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yin, Y.-X.; Guo, Y.-G.; Wan, L.Y. A Sandwich-Like Hierarchically Porous Carbon/Graphene Composite as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1301584. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Banks, C.E.; Jing, M.; Zhang, Y.; Ji, X. Carbon Quantum Dots and Their Derivative 3D Porous Carbon Frameworks for Sodium-Ion Batteries with Ultralong Cycle Life. Adv. Mater. 2015, 27, 7861–7866. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Shao, L.; Zhang, Y.; Zou, G.; Chen, J.; Ji, X. Large-Area Carbon Nanosheets Doped with Phosphorus: A High-Performance Anode Material for Sodium-Ion Batteries. Adv. Sci. 2016, 1600243–1600254. [Google Scholar] [CrossRef]
- Peled, E.; Eshkenazi, V.; Rosenberg, Y. Study of lithium insertion in hard carbon made from cotton wool. J. Power Sources 1998, 76, 153–158. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Ouyang, Y.; Liu, L.; Wang, Y. Two-dimensional hierarchical porous carbon composites derived from corn stalks for electrode materials with high performance. Electrochim. Acta 2016, 214, 119–128. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.; Yin, S.; Guo, Z.; Wang, S.; Feng, C. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surf. Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Yang, T.; Qian, T.; Wang, M.; Shen, X.; Xu, N.; Sun, Z.; Yan, C. A Sustainable Route from Biomass Byproduct Okara to High Content Nitrogen-Doped Carbon Sheets for Efficient Sodium Ion Batteries. Adv. Mater. 2016, 28, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Prabakar, S.J.R.; Jeong, J.; Pyo, M. Nanoporous hard carbon anodes for improved electrochemical performance in sodium ion batteries. Electrochim. Acta 2015, 161, 23–31. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Wu, X.; Yu, J.; Wang, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. Amorphous monodispersed hard carbon microspherules derived from biomass as a high performance negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Bommier, C.; Luo, W.; Gao, W.-Y.; Greaney, A.; Ma, S.; Ji, X. Predicting capacity of hard carbon anodes in sodium-ion batteries using porosity measurements. Carbon 2014, 76, 165–174. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Li, L.; Peng, S.; Zhang, L.; Srinivasan, M.; Ramakrishna, S. Preparation of nitrogen- and phosphorous co-doped carbon microspheres and their superior performance as anode in sodium-ion batteries. Carbon 2016, 99, 556–563. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef] [PubMed]
- Lotfabad, E.M.; Kalisvaart, P.; Kohandehghan, A.; Karpuzovc, D.; Mitlin, D. Origin of non-SEI related coulombic efficiency loss in carbons tested against Na and Li. J. Mater. Chem. A 2014, 2, 19685–19695. [Google Scholar] [CrossRef]
- Hong, K.-L.; Qie, L.; Zeng, R.; Yi, Z.-Q.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.-J.; Zhang, W.-X.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Xu, B. Facile synthesis of high performance hard carbon anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 20560–20566. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Shi, J.; Mao, N.; Chen, S.; Liu, W. Biomass derived hierarchical porous carbons as high-performance anodes for sodium-ion batteries. Electrochim. Acta 2016, 188, 103–110. [Google Scholar] [CrossRef]
- Luo, W.; Schardt, J.; Bommier, C.; Wang, B.; Razink, J.; Simonsen, J.; Ji, X. Carbon nanofibers derived from cellulose nanofibers as a long-life anode material for rechargeable sodium-ion batteries. J. Mater. Chem. A 2013, 1, 10662–10666. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, H.; Luo, W.; Wan, J.; Zhou, L.; Dai, J.; Zhao, B.; Han, X.; Fu, K.; Hu, L. Chemically Crushed Wood Cellulose Fiber towards High- Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23291–23296. [Google Scholar] [CrossRef] [PubMed]
- Simone, V.; Boulineau, A.; de Geyer, A.; Rouchon, D.; Simonin, L.; Martinet, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Jin, J.; Yu, B.-J.; Shi, Z.-Q.; Wang, C.-Y.; Chong, C.-B. Lignin-based electrospun carbon nanofibrous webs as free-standing and binder-free electrodes for sodium ion batteries. J. Power Source 2014, 272, 800–807. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.-S.; Li, H.; Chen, L.; Huang, X. A superior low-cost amorphous carbon anode made from pitch and lignin for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 96–104. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, K.-Y.; Lee, B.; Cho, S.Y.; Park, Y.-U.; Hong, S.J.; Kim, B.H.; Gwon, H.; Kim, H.; Lee, S.; et al. Sodium-Ion Storage in Pyroprotein-Based Carbon Nanoplates. Adv. Mater. 2015, 27, 6914–6921. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Savage, P.E.; Deng, D. Trash to Treasure: From Harmful Algal Blooms to High-Performance Electrodes for Sodium-Ion Batteries. Environ. Sci. Technol. 2015, 49, 12543–12550. [Google Scholar] [CrossRef] [PubMed]
- Emaga, T.H.; Robert, C.; Ronkart, S.N.; Wathelet, B.; Paquot, M. Dietary Fibre Components and Pectin Chemical Features of Peels during Ripening in Banana and Plantain Varieties. Bioresour. Technol. 2008, 99, 4346–4354. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M. A Direct Synthesis of Mesoporous Carbons with Bicontinuous Pore Morphology from Crude Plant Material by Hydrothermal Carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Puncha-arnon, S.; Puttanlek, C.; Rungsardthong, V.; Wetprasit, N. Enrichment of rice noodles with fibre-rich fractions derived from cassava pulp and pomelo peel. Int. J. Food Sci. Technol. 2014, 49, 2348–2355. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.J.; Tang, K.; Song, K.P.; Aken, P.A.V.; Yu, Y.; Maier, J. Nitrogen doped porous carbon fibres as anode materials for sodium ion batteries with excellent rate performance. Nanoscale 2014, 6, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Qie, L.; Yuan, L.X.; Zhang, W.X.; Hu, X.L.; Huang, Y.H. Functionalized N-doped interconnected carbon nanofibers as an anode material for sodium-ion storage with excellent performance. Carbon 2013, 55, 328–334. [Google Scholar] [CrossRef]
- Wang, H.G.; Wu, Z.; Meng, F.L.; Ma, D.L.; Huang, X.L.; Wang, L.M.; Zhang, X.B. Nitrogen-doped porous carbon nanosheets as low-cost, high-performance anode material for sodium-ion batteries. ChemSusChem 2013, 6, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Wang, M.; Wickramaratne, N.P.; Jaroniec, M.; Dou, S.Q.; Dai, L.M. High-performance sodium ion batteries based on a 3D anode from nitrogen-doped graphene foams. Adv. Mater. 2015, 27, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Yu, C.; Zhang, X.; Qin, W.; Lu, T.; Hu, B.; Li, H.; Pan, L. Nitrogen-doped carbon microspheres derived from oatmeal as high capacity and superior long life anode material for sodium ion battery. Electrochim. Acta 2016, 191, 385–391. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, B.; Du, Y.; Li, Y.; Zhou, X.; Dai, Z.; Bao, J. Fluorine-Doped Carbon Particles Derived from Lotus Petioles as High-Performance Anode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2015, 119, 21336–21344. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Jeguirim, M.; Guizani, C.; Limousy, L.; Dupont, C.; Gadiou, R. Thermogravimetric study on the influence of structural, textural andchemical properties of biomass chars on CO2 gasification reactivity. Energy 2015, 88, 703–710. [Google Scholar] [CrossRef]
- Kraiem, N.; Lajili, M.; Limousy, L.; Said, R.; Jeguirim, M. Energy recovery from Tunisian agri-food wastes: Evaluation of combustion performance and emissions characteristics of green pellets prepared from tomato residues and grape marc. Energy 2016, 107, 409–418. [Google Scholar] [CrossRef]
- Dahn, J.R.; Sleigh, A.K.; Shi, H.; Reimers, J.N.; Zhong, Q.; Way, B.M. Dependence of the electrochemical intercalation of lithium in carbons on the crystal-structure of the carbon. Electrochim. Acta 1993, 38, 1179–1191. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.S.; Zheng, T.; Dahn, J.R. Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 1996, 34, 193–200. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Z.-Q.; Wang, C.-Y. Electrochemical Performance of Electrospun carbon nanofibers as free-standing and binder-free anodes for Sodium-Ion and Lithium-Ion Batteries. Electrochim. Acta 2014, 141, 302–310. [Google Scholar] [CrossRef]
- Dahn, J.R.; Xing, W.; Gao, Y. The “falling cards model” for the structure of microporous carbons. Carbon 1997, 35, 825–830. [Google Scholar] [CrossRef]
- Irisarri, E.; Ponrouch, A.; Palacin, M.R. Review—Hard Carbon Negative Electrode Materials for Sodium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2476–A2482. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Handbook of Battery Materials, 2nd ed.; Daniel, C., Besenhard, J.O., Eds.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gibaud, A.; Xue, J.S.; Dahn, J.R. A small angle X-ray scattering study of carbons made from pyrolyzed sugar. Carbon 1996, 34, 499–503. [Google Scholar] [CrossRef]
- Xing, W.; Xue, J.S.; Dahn, J.R. Optimizing pyrolysis of sugar carbons for use as anode materials in lithium-ion batteries. J. Electrochem. Soc. 1996, 143, 3046–3052. [Google Scholar] [CrossRef]
- Buiel, E.R.; George, A.E.; Dahn, J.R. Model of micropore closure in hard carbon prepared from sucrose. Carbon 1999, 37, 1399–1407. [Google Scholar] [CrossRef]
- Luo, W.; Bommier, C.; Jian, Z.; Li, X.; Carter, R.; Vail, S.; Lu, Y.; Lee, J.-J.; Ji, X. Low-Surface-Area Hard Carbon Anode for Na-Ion Batteries via Graphene Oxide as a Dehydration Agent. ACS Appl. Mater. Interfaces 2015, 7, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.V.S.N.; Kumar, N.A.; Zhao, X.S. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, F.; Tarascon, J.-M.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Zhang, B.; Matei Ghimbeu, C.; Laberty, C.; Vix-Guterl, C.; Tarascon, J.-M. Correlation between Microstructure and Na Storage Behavior in Hard Carbon. Adv. Energy Mater. 2016, 6, 1501588. [Google Scholar] [CrossRef]
- Bold, H.C. Morphology of Plants; Joanna Cotler Books: New York, NY, USA, 1967; pp. 225–229. [Google Scholar]
- Li, H.; Shen, F.; Luo, W.; Dai, J.; Han, X.; Chen, Y.; Yao, J.; Zhu, H.; Fu, K.; Hitz, E.; et al. Carbonized-leaf Membrane with Anisotropic Surfaces for Sodium-ion Battery. ACS Appl. Mater. Interfaces 2016, 8, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.; Carton, B.; Herold, A.; Metrot, A. Insertion of sodium and potassium in some hard carbons. C. R. Acad. Sci. Paris Ser. C 1970, 271, 837–840. [Google Scholar]
- Alcantara, R.; Ortiz, G.F.; Lavela, P.; Tirado, J.L. EPR, NMR, and electrochemical studies of surface-modified carbon microbeads. Chem. Mater. 2006, 18, 2293–2301. [Google Scholar] [CrossRef]
- Gotoh, K.; Ishikawa, T.; Shimadzu, S.; Yabuuchi, N.; Komaba, S.; Takeda, K.; Goto, A.; Deguchi, K.; Ohki, S.; Hashi, K.; et al. NMR study for electrochemically inserted Na in hard carbon electrode of sodium ion battery. J. Power Sources 2013, 225, 137–140. [Google Scholar] [CrossRef]
- Ponrouch, A.; Goni, A.R.; Palacin, M.R. High capacity hard carbon anodes for sodium ion batteries in additive free electrolyte. Electrochem. Commun. 2013, 27, 85–88. [Google Scholar] [CrossRef]
- Larcher, D.; Mudalige, C.; Gharghouri, M.; Dahn, J.R. Electrochemical insertion of Li and irreversibility in disordered carbons prepared from oxygen and sulfur-containing pitches. Electrochim. Acta 1999, 44, 4069–4072. [Google Scholar] [CrossRef]
- Beguin, F.; Chevallier, F.; Vix-Guterl, C.; Saadallah, S.; Bertagna, V.; Rouzaud, J.N.; Frackowiak, E. Correlation of the irreversible lithium capacity with the active surface area of modified carbons. Carbon 2005, 43, 2160–2167. [Google Scholar] [CrossRef]
- Beguin, F.; Chevallier, F.; Vix-Guterl, C.; Saadallah, S.; Bertagna, V.; Rouzaud, J.N.; Frackowiak, E. New Carbon Based Materials for Electrochemical Energy Storage Systems; Barsukov, E., Ed.; Springer: Dordrecht, The Netherlands, 2006; p. 231. [Google Scholar]
- Chen, T.; Pan, L.; Lu, T.; Fu, C.; Chua, D.H.C.; Sun, Z. Fast synthesis of carbon microspheres via a microwave-assisted reaction for sodium ion batteries. J. Mater. Chem. A 2014, 2, 1263–1267. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High capacity anode materials for rechargeable sodium-ion batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Yang, T.; Wang, G.; Tade, M.O. Mesoporous carbon with large pores as anode for Na-ion batteries. Chin. Sci. Bull. 2014, 59, 2186–2190. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium Ion Insertion in Hollow Carbon Nanowires for Battery Applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Environ. Sci. 2011, 4, 3342–3345. [Google Scholar] [CrossRef]
- Song, H.; Li, N.; Cui, H.; Wang, C. Enhanced storage capability and kinetic processes by pores- and hetero-atoms-riched carbon nanobubbles for lithium-ion and sodium-ion batteries anodes. Nano Energy 2014, 4, 81–87. [Google Scholar] [CrossRef]
- Tong, Y.C.; Zhang, X.Y.; Wang, Q.Y.; Xu, X.J. The adsorption mechanism of platinum on phosphorus-doped single walled carbon nanotube. Comput. Theor. Chem. 2015, 1059, 1–6. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Alonso, A.M.; Garcia, F.S.; Tascon, J.M.D. Synthetic carbons activated with phosphoric acid: II. Porous structure. Carbon 2002, 40, 1507–1519. [Google Scholar] [CrossRef]
- Shao, Y.-Y.; Xiao, J.; Wang, W.; Engelhard, M.; Chen, X.L.; Nie, Z.M.; Gu, M.; Saraf, L.V.; Exarhos, G.; Zhang, J.G.; et al. Surface-Driven Sodium Ion Energy Storage in Nanocellular Carbon Foams. Nano Lett. 2013, 13, 3909–3914. [Google Scholar] [CrossRef] [PubMed]
- Byon, H.R.; Gallant, B.M.; Lee, S.W.; Horn, Y.S. Role of Oxygen Functional Groups in Carbon Nanotube/Graphene Freestanding Electrodes for High Performance Lithium Batteries. Adv. Funct. Mater. 2013, 23, 1037–1045. [Google Scholar] [CrossRef]
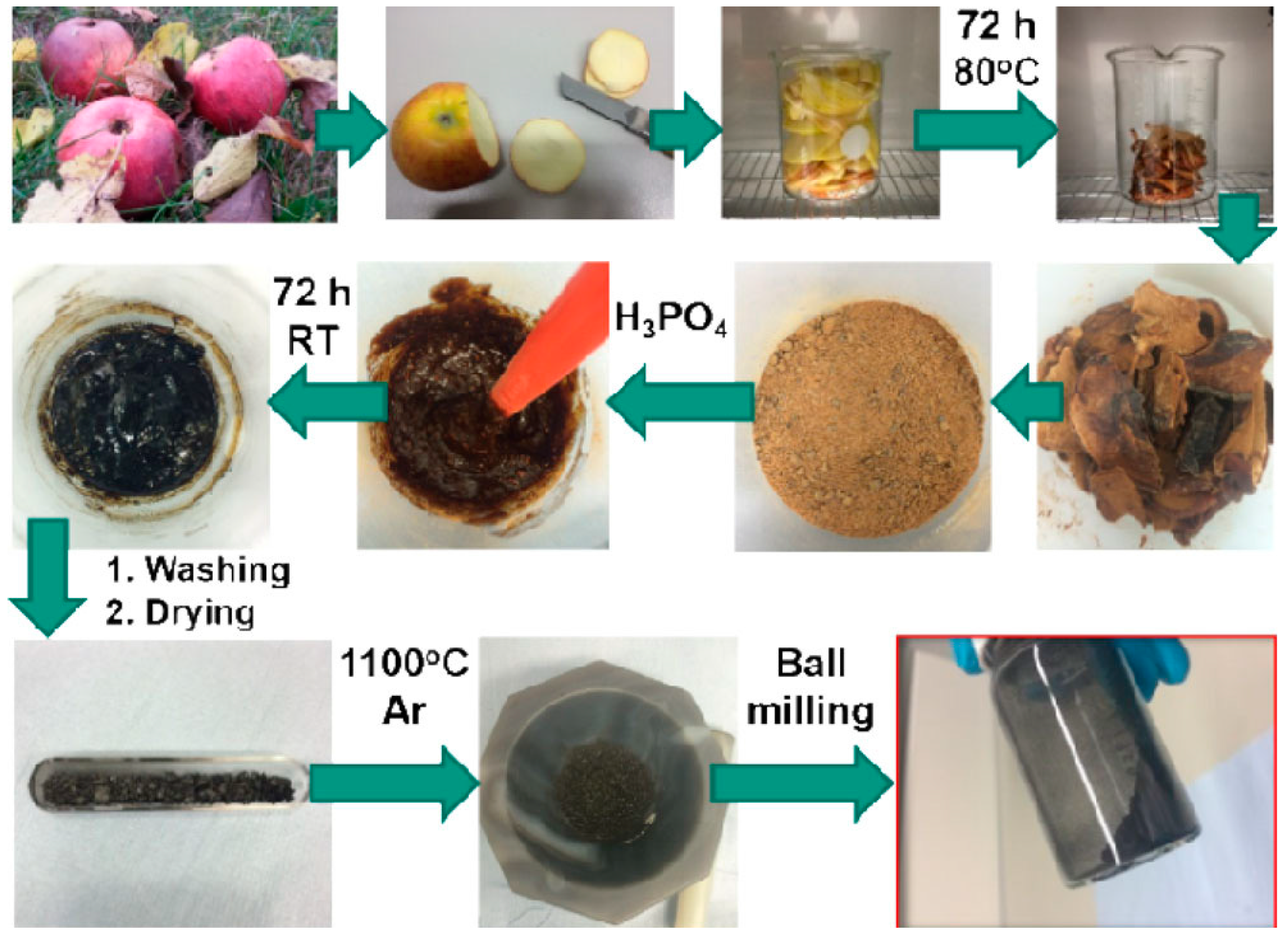
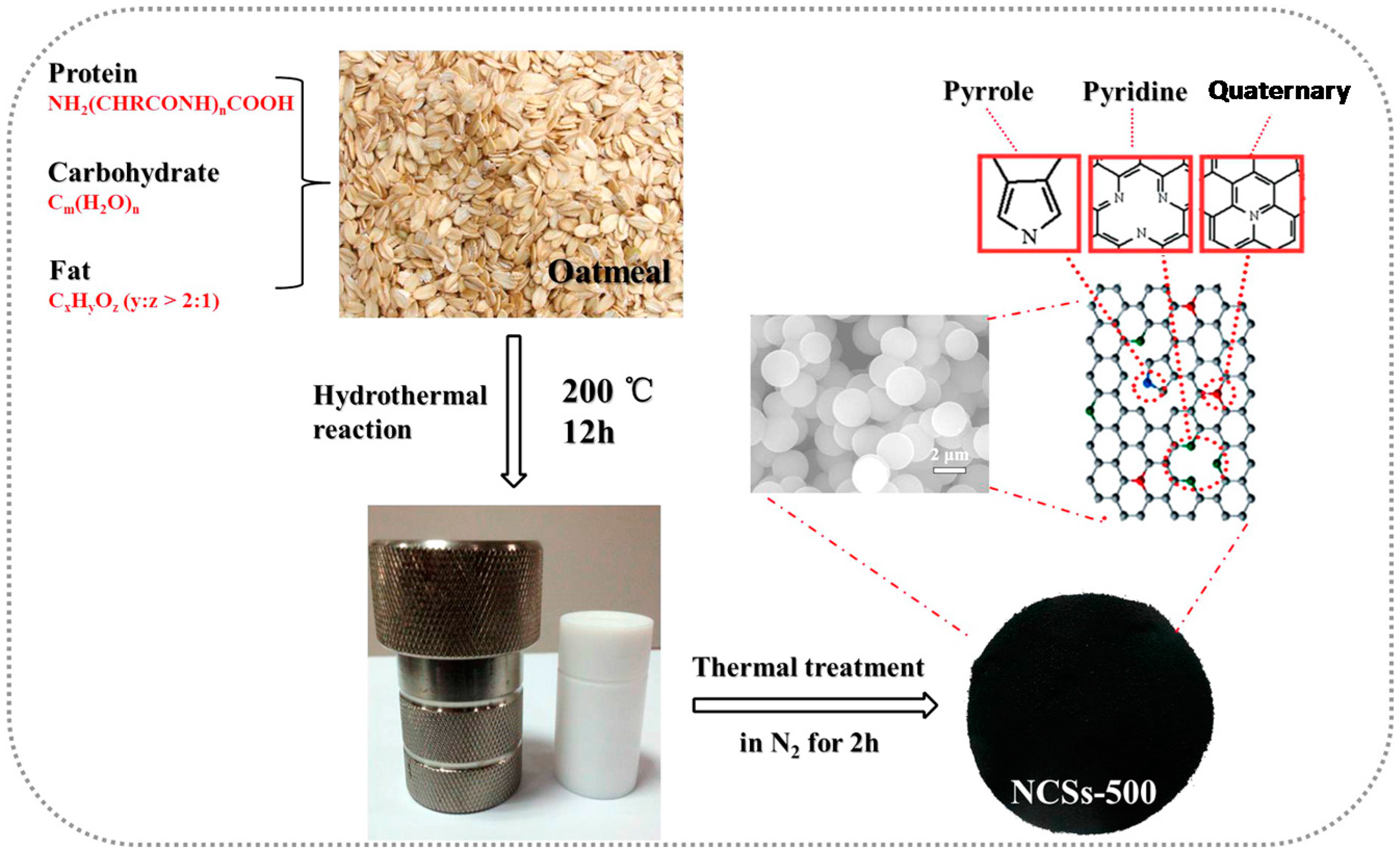
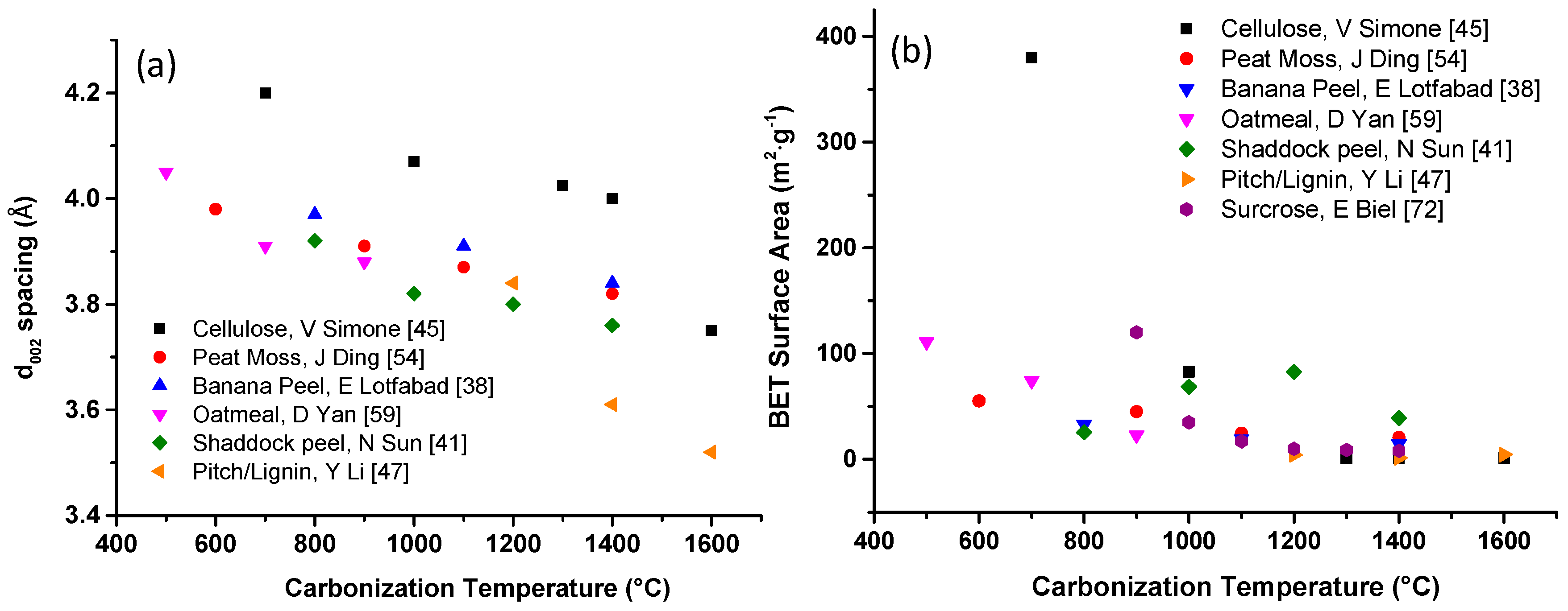
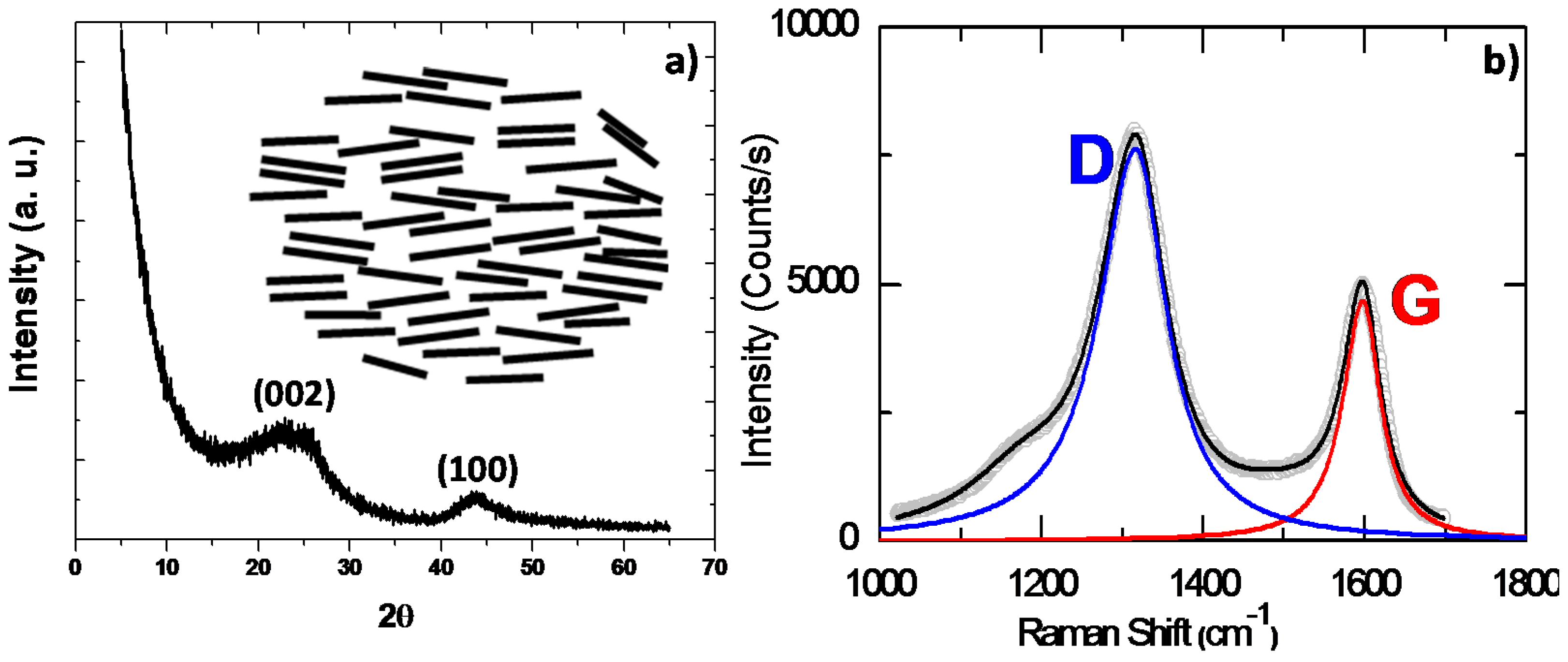
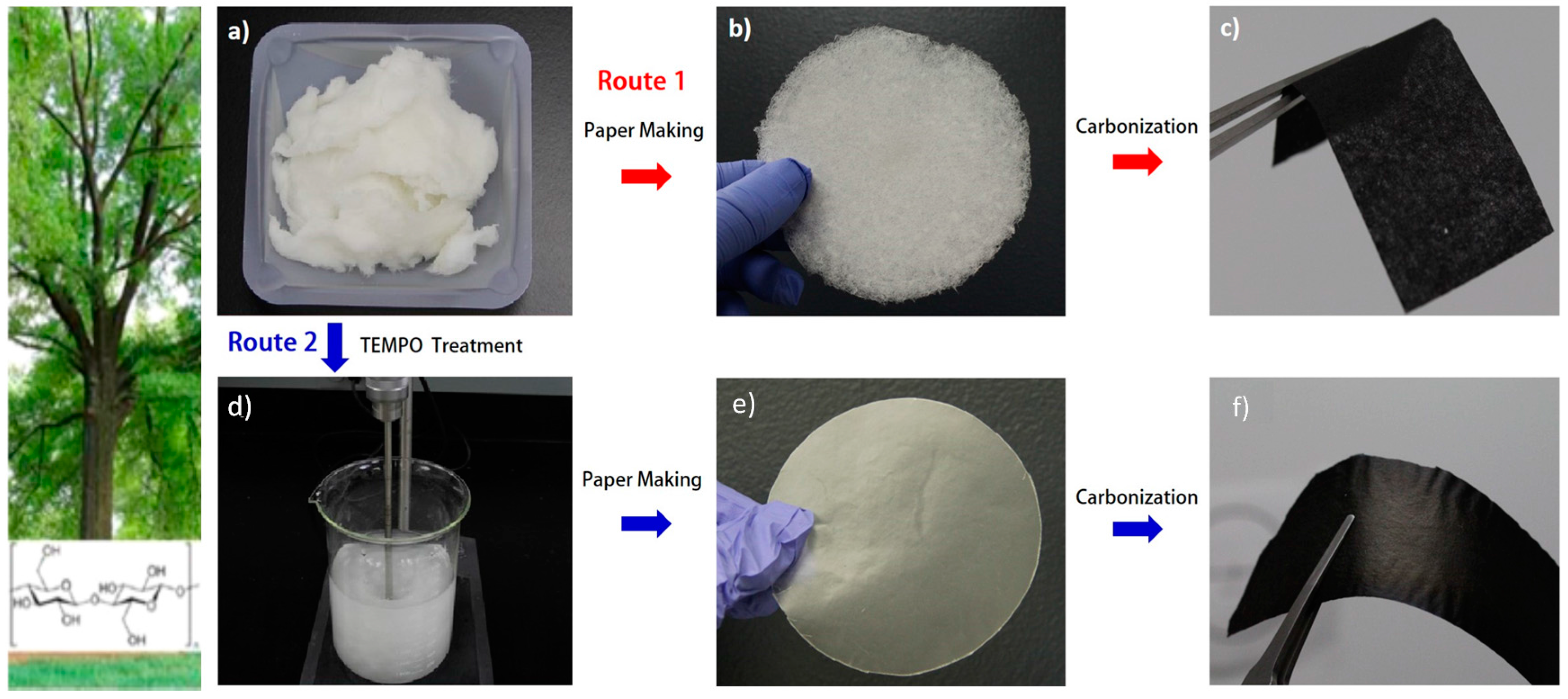
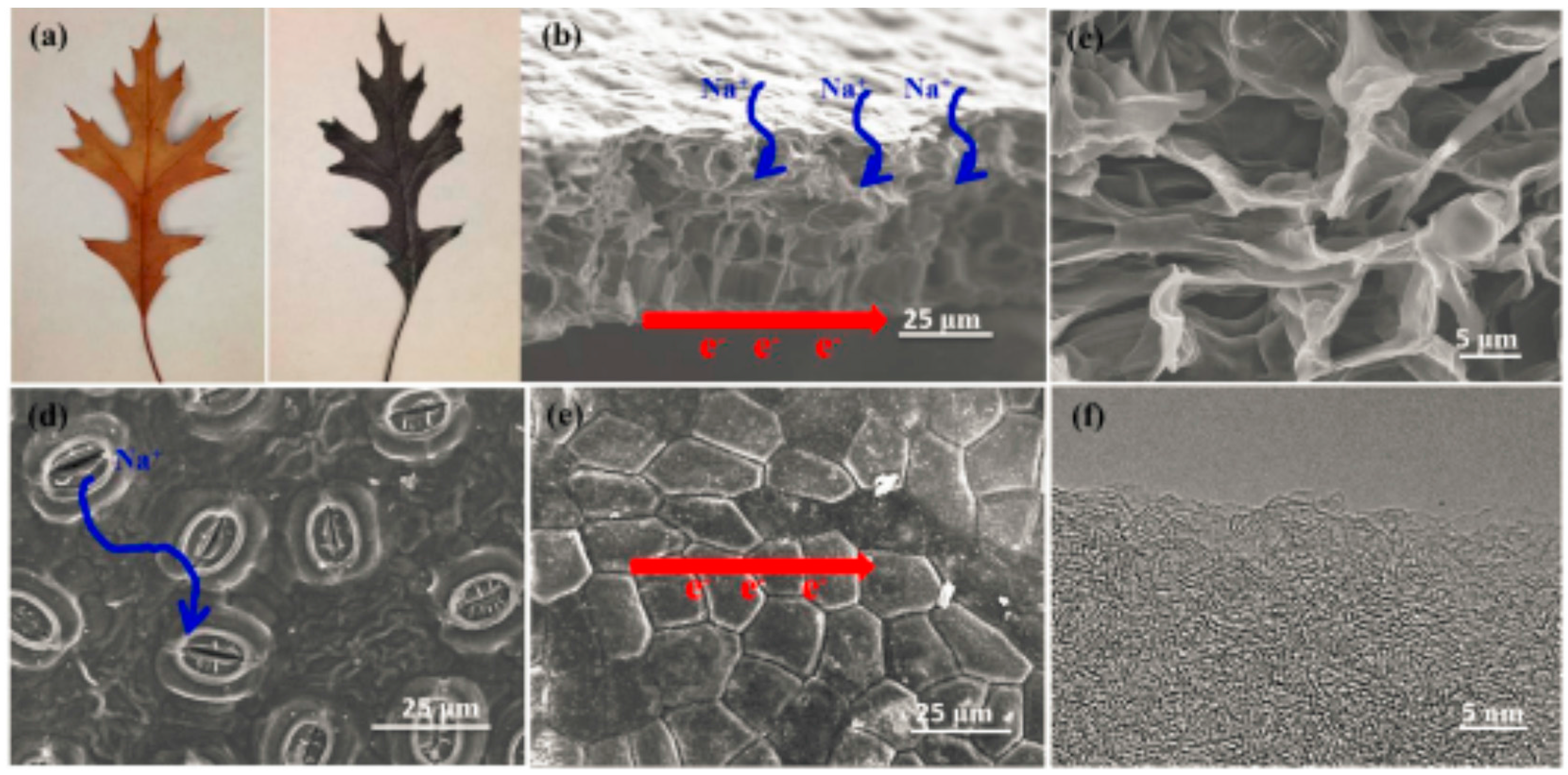
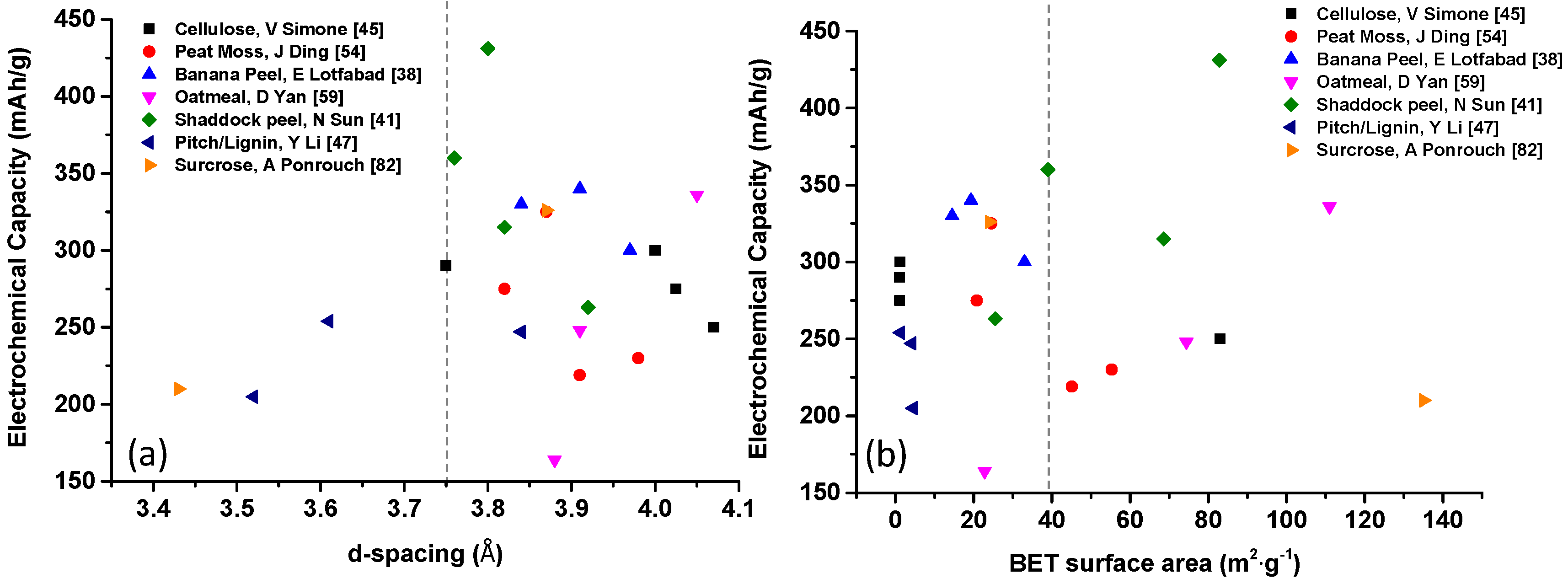
| Biomass Precursor | Annealing Conditions | SSA (m2∙g−1) | Columbic Efficiency (%) | Discharge Capacity (mAh∙g−1) | Current Density (mA∙g−1) | d-Spacing (Å) | Refs. |
|---|---|---|---|---|---|---|---|
| Bleached Softwood Pulp | 1000 °C/2 h/Ar | 586 156 (oxidized) | 25 72 | 252 260 | 20 | NA | [44] |
| Oak Leaves | 1000 °C/1 h/Ar | 161 | 75 | 360 | 10 | 3.60 | [78] |
| Peat Moss | 600–1400 °C/2 h | 78 to 54 369 to 92 (oxidized) | 44–64 | 215–298 | 50 | 3.82–3.99 | [54] |
| Banana Peel | 800–1400 °C/5 h | 33–14 217–60 (oxidized) | 61–73 | 300–355 | 50 | 3.86–3.97 | [38] |
| Oatmeal | 500–900 °C/2 h/N2 | 111–23 | - | 320–164 | 50 | 3.88–4.18 | [60] |
| Shaddock Peel | 800–1400 °C/2 h/N2 | 26–83 | - | 263–430 | 30 | 3.76–3.92 | [41] |
| Peanut Skin-Activated KOH | 800 °C/1 h/Ar | 1430–2500 | 29–34 | 174–431 | 100 | 3.70–3.90 | [42] |
| Harmful Algal Blooms | 700–900 °C/5 h/Ar | - | 46–52 | 158–231 | 20 | 3.60 | [49] |
| Pomelo Peel | 700 °C/2 h/N2 | 0.9 1272 (activated H3PO4) | 54 27 | 215 314 | 50 | NA | [40] |
| Pitch/Lignin | 1200–1600 °C/2 h/Ar | 1.3 to 35 | 60–82 | 205–254 | 30 | 3.52–3.84 | [47] |
| Cellulose | 700–1600 °C/1 h/Ar | 1.0 to 380 | 39–84 | 150–310 | 37.2 | 3.75–4.20 | [45] |
| Surcrose | 1100 °C/6 h/Ar | 24 135 (ball milled) | 61 41 | 326 210 | C/10 | 3.87 3.43 | [82] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górka, J.; Vix-Guterl, C.; Matei Ghimbeu, C. Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. C 2016, 2, 24. https://doi.org/10.3390/c2040024
Górka J, Vix-Guterl C, Matei Ghimbeu C. Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. C. 2016; 2(4):24. https://doi.org/10.3390/c2040024
Chicago/Turabian StyleGórka, Joanna, Cathie Vix-Guterl, and Camelia Matei Ghimbeu. 2016. "Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries" C 2, no. 4: 24. https://doi.org/10.3390/c2040024
APA StyleGórka, J., Vix-Guterl, C., & Matei Ghimbeu, C. (2016). Recent Progress in Design of Biomass-Derived Hard Carbons for Sodium Ion Batteries. C, 2(4), 24. https://doi.org/10.3390/c2040024








