Nano-Functionalized Magnetic Carbon Composite for Purification of Man-Made Polluted Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Magnetoresponsive TEG–Bentonite Composite
2.3. Characterization Methods
2.4. Study of the Sorption Activity of the TEG–Bentonite Composite
2.4.1. Model Solution—Simulant of Radioactively Contaminated Water of Nuclear Power Plants
- Stable isotopes of radionuclides—cesium (10.2 mg/dm3), strontium (10.9 mg/dm3), cobalt (4.2 mg/dm3), and manganese (2.4 mg/dm3); they were used as nitrates (KhimLaborReaktiv, Brovary, Kyiv region, Ukraine).
- Organic substances—oxalic acid (65 mg/dm3); citric acid (10 mg/dm3); the decontamination surfactant “SHCHIT K” (Shield in Ukrainian, 180 mg/dm3, “Energokhim”, Kyiv, Ukraine), which is used for decontamination of workwear, equipment, and premises at nuclear power plants in Ukraine; sodium salt of ethylenediaminetetraacetic acid (KhimLaborReaktiv, 100 mg/dm3); shampoo/soap (150 mg/dm3); universal washing powder «Lotus» (10 mg/dm3); and oil (200 mg/dm3).
- Inorganic substances—boric acid (1200 mg/dm3), sodium hydroxide (1040 mg/dm3), potassium hydroxide (90 mg/dm3), and nitric acid (400 mg/dm3), KhimLaborReaktiv, Brovary, Kyiv region, Ukraine.
2.4.2. Radioactively Contaminated Water
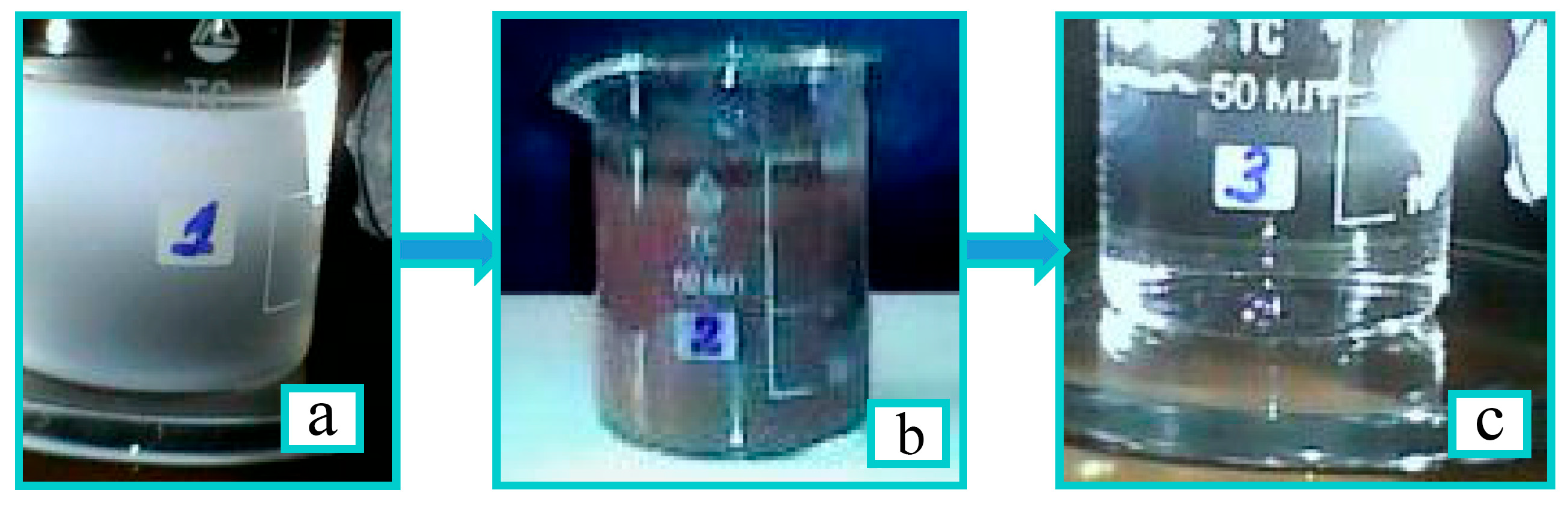
2.4.3. Study of Sorption Properties of TEG–Bentonite Composite
2.4.4. Further Purification of Filtrate
3. Results and Discussion
3.1. Characterization of the Nano-Functionalized Composite Based on Bentonite and Magnetically Responsive TEG
3.2. Study of Sorption Properties of the Obtained TEG–Bentonite Composite
3.2.1. Purification of NPP Radioactive Wastewater Simulant
3.2.2. Purification of a Sample of Radioactively Contaminated Water (RCW)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strokal, V.; Kuiper, E.J.; Bak, M.P.; Vriend, P.; Wang, M.; van Wijnen, J.; Strokal, M. Future microplastics in the black Sea: River exports and reduction options for zero pollution. Mar. Pollut. Bull. 2022, 178, 113633. [Google Scholar] [CrossRef] [PubMed]
- Kivva, S.; Zheleznyak, M.; Bezhenar, R.; Pylypenko, O.; Sorokin, M.; Demydenko, A.; Kanivets, V.; Laptev, G.; Votsekhovich, O.; Boyko, V.; et al. Modeling of major environmental risks for the Kyiv city, Ukraine from the Dnieper river waters-inundation of coastal areas and contamination by the radionuclides deposited in bottom sediments after the Chornobyl accident. In Proceedings of the EGU General Assembly 2021, Online, 19–30 April 2021. EGU21-13038. [Google Scholar] [CrossRef]
- Shumilova, O.; Sukhodolov, A.; Osadcha, N.; Oreshchenko, A.; Constantinescu, G.; Afanasyev, S.; Koken, M.; Osadchyi, V.; Rhoads, B.; Tockner, K.; et al. Environmental effects of the Kakhovka Dam destruction by warfare in Ukraine. Science 2025, 387, 1181–1186. [Google Scholar] [CrossRef]
- Zub, L.; Prokopuk, M.; Netsvetov, M.; Gudkov, D. Does long-term radiation exposure in Chornobyl impact the reproductive structures of Nuphar lutea (Linn´e) Smith? Environ. Pollut. 2024, 363, 125067. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, S.; Sarma, J.; Rani Das, A. Chapter 15. Radiological contaminants in water: Pollution, health risk, and treatment. In Contamination of Water; Elsevier: Amsterdam, The Netherlands, 2021; pp. 217–236. [Google Scholar] [CrossRef]
- Abdel Rahman, R.O.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Gossard, A.; Lilin, A.; Faure, S. Gels, coatings and foams for radioactive surface decontamination: State of the art and challenges for the nuclear industry. Prog. Nucl. Energy 2022, 149, 104255. [Google Scholar] [CrossRef]
- Rudenko, L.I.; Khan, V.E.; Kashkovskyi, V.I.; Dzhuzha, O.V. Purification of drain water and distillation residue from organic compounds, transuranic elements, and uranium at the Chornobyl NPP. Sci. Innov. 2014, 10, 16–25. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. IScience 2023, 26, 106061. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Z.; Meng, X.; Li, J.; Chen, H.; Yu, T.; Xu, M. A preliminary study on the “hitchhiking” of radionuclides on microplastics: A new threat to the marine environment from compound pollution. Toxics 2025, 13, 429. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Z.; Li, J.; Huang, F.; Zhu, X.; Gao, F.; Yu, T.; Hu, M. Marine microplastics fuel long-range transport of radioactive nuclides: A review. Mar. Pollut. Bull. 2025, 221, 118540. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, B.; Xin, X.; Lee, K.; Chen, B. Microplastic and oil pollution in oceans: Interactions and environmental impacts. Sci. Total Environ. 2022, 838, 156142. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, X.; Lin, H.; Yu, G.; Shen, L.; Yu, W.; Li, B.; Leihong, Z.; Ying, M. Membrane technology for microplastic removal: Microplastic occurrence, challenges, and innovations of process and materials. Chem. Eng. J. 2025, 520, 166183. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A. Tailored nanofibrous polyimide-based membranes for highly effective oil spill cleanup in marine ecosystems. Chemosphere 2024, 368, 143730. [Google Scholar] [CrossRef]
- de Rosset, A.; Torres-Mendieta, R.; Pasternak, G.; Yalcinkaya, F. Synergistic effects of natural biosurfactant and metal oxides modification on PVDF nanofiber filters for efficient microplastic and oil removal. Process Saf. Environ. Prot. 2020, 194, 997–1009. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Feicht, P.; Biskupek, J.; Gorelik, T.E.; Renner, J.; Halbig, C.E.; Maranska, M.; Puchtler, F.; Kaiser, U.; Eigler, S. Brodie’s or Hummers’ method: Oxidation conditions determine the structure of graphene oxide. Chem. Eur. J. 2019, 25, 8955–8959. [Google Scholar] [CrossRef]
- Kadoshnikov, V.M.; Melnychenko, T.I.; Arkhipenko, O.M.; Tutskyi, D.H.; Komarov, V.O.; Bulavin, L.A.; Zabulonov, Y.L. A composite magnetosensitive sorbent based on the expanded graphite for the clean-up of oil spills: Synthesis and structural properties. C 2023, 9, 39. [Google Scholar] [CrossRef]
- Coetzee, D.; Rojviroon, T.; Niamlang, S.; Militký, J.; Wiener, J.; Večerník, J.; Melicheríková, J.; Müllerová, J. Effects of expanded graphite’s structural and elemental characteristics on its oil and heavy metal sorption properties. Sci. Rep. 2024, 14, 13716. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, T.; Chen, H.; Hu, Y.; Tian, F.; Lin, Q. Petroleum residue-based ultrathin-wall graphitized mesoporous carbon and its high-efficiency adsorption mechanism. Diam. Relat. Mater. 2024, 148, 111497. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hof, F.; Bauer, W.; Hirsch, A. Sulfur species in graphene oxide. Chem. Eur. J. 2013, 19, 9490–9496. [Google Scholar] [CrossRef]
- Amirov, R.R.; Shayimova, J.; Nasirova, Z.; Solodov, A.; Dimiev, A.M. Analysis of competitive binding of several metal cations by graphene oxide reveals the quantity and spatial distribution of carboxyl groups on its surface. Phys. Chem. Chem. Phys. 2018, 20, 2320–2329. [Google Scholar] [CrossRef]
- Schiffman, P.; Southard, R.J. Cation exchange capacity of layer silicates and palagonitized glass in mafic volcanic rocks: A comparative study of bulk extraction and in situ techniques. Clays Clay Min. 1996, 44, 624–634. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Sarwar, M.T.; Huaming Yang, H. Recent progress in radionuclides adsorption by bentonite-based materials as ideal adsorbents and buffer/backfill materials. Appl. Clay Sci. 2023, 232, 106796. [Google Scholar] [CrossRef]
- Uddin, F. Ch 1: Montmorillonite: An introduction to properties and utilization. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; Zoveidavianpoor, M., Ed.; IntechOpen: London, UK, 2018; pp. 3–23. ISBN 978-1-78923-729-0. [Google Scholar] [CrossRef]
- İnan, S. Inorganic ion exchangers for strontium removal from radioactive waste: A review. J. Radioanal. Nucl. Chem. 2022, 331, 1137–1154. [Google Scholar] [CrossRef]
- Saleh, A.S.; Afolabi, O.O. Enhancement and modelling of caesium and strontium adsorption behaviour on natural and activated bentonite. Environ. Technol. Innov. 2025, 37, 103937. [Google Scholar] [CrossRef]
- Muslim, W.A.; Al-Nasri, S.K.; Albayati, T.M.; Salih, I.K. Investigation of bentonite clay minerals as a natural adsorbents for Cs-137 real radioactive wastewater treatment. Desalination Water Treat. 2024, 317, 100121. [Google Scholar] [CrossRef]
- Khodakov, G.S. Fizikaizmel’Cheniya (Physics of Comminution in Russian); Nauka Publ.: Moscow, Russia, 1972; UDK 532.6. [Google Scholar]
- Zabashta, Y.F.; Kovalchuk, V.I.; Svechnikova, O.S.; Bulavin, L.A. Electrocapillary properties of hydrogels. Ukr. J. Phys. 2022, 67, 658–662. [Google Scholar] [CrossRef]
- Esmaeili, E.; Rounaghi, S.A.; Eckert, J. Mechanochemical synthesis of rosin-modified montmorillonite: A breakthrough approach to the next generation of OMMT/rubber nanocomposites. Nanomaterials 2021, 11, 1974. [Google Scholar] [CrossRef]
- Yudina, T.F.; Ershova, T.V.; Beylina, N.Y.; Smirnov, N.N.; Bratkov, I.V.; Shchennikov, D.V. Mechanochemical activation of graphite materials. News of Higher Educational Institutions. Chemistry and Chemical Technology, 2012; p. 55, line 29–33. [Google Scholar]
- Smirnova, D.N.; Grishin, I.S.; Smirnov, N.N. Synthesis, structure and properties of bentonite-activated carbon composite. ChemChemTech 2024, 67, 59–66. [Google Scholar] [CrossRef]
- Yashkova, D.; Grishin, I.; Smirnov, N. Mechanism of tetracycline sorption on carbon-bentonite. Chem. Towards Technol. Step-By-Step 2024, 5, 54–60. [Google Scholar] [CrossRef]
- Nasiedkin, D.B.; Grebenyuk, A.G.; Babich, I.V.; Plyuto, Y.V.; Kartel, M.T. Experimental and theoretical study on expanded graphite oxidation. Surface 2015, 7, 126–136, UDK 543.573. [Google Scholar]
- Boichenko, S.; Lejda, K.; Mateichyk, V.; Topilnytskyi, P. Problems of Chemmotology. Theory and Practice of Rational Use of Traditional and Alternative Fuels & Lubricants: Monograph; Center of Educational Literature: Kyiv, Ukraine, 2017; pp. 136–141. ISBN 978-617-673-632-5. [Google Scholar]
- ISO 15705: 2002; Water Quality: Determination of the Chemical Oxygen Demand Index (ST-COD)-Small-Scale Sealed-Tube Method. International Standardisation Organisation: Geneva, Switzerland, 2002.
- Rudenko, L.I.; Gumenna, O.A.; Dzhuzha, O.V. Membrane methods of treatment of water, which contains polymeric substances and compounds of uranium, strontium, and sodium. Rep. Natl. Acad. Sci. Ukr. 2010, 6, 134–138. [Google Scholar]
- Zabulonov, Y.; Melnychenko, T.; Kadoshnikov, V.; Kuzenko, S.; Guzii, S.; Peer, I. New sorbents and their application for deactivation of liquid radioactive waste. In Liquid Radioactive Waste Treatment: Ukrainian Context; LWRT 2022; Lecture Notes in Civil Engineering; Zabulonov, Y., Peer, I., Zheleznyak, M., Eds.; Springer: Cham, Switzerland, 2024; Volume 469, pp. 126–136. [Google Scholar] [CrossRef]
- Toropov, A.S.; Satayeva, A.R.; Mikhalovsky, S.; Cundy, A.B. The use of composite ferrocyanide materials for treatment of high salinity liquid radioactive wastes rich in cesium isotopes. Radiochim. Acta 2014, 102, 911–917. [Google Scholar] [CrossRef]
- Sun, M.M.; Zhang, J.L.; Li, K.J.; Ren, S.; Wang, Z.M.; Jiang, C.H.; Li, H.T. Dissolution behaviors of various carbonaceous materials in liquid iron: Interaction between graphite and iron. JOM 2019, 12, 4305–4310. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Shukhina, K.; Khannanov, A. Mechanism of the graphene oxide formation: The role of water,“reversibility” of the oxidation, and mobility of the C–O bonds. Carbon 2020, 166, 1–14. [Google Scholar] [CrossRef]
- Zabulonov, Y.L.; Melnychenko, T.I.; Kadoshnikov, V.M.; Odukalets, L.A.; Kuzenko, S.V. A Method for Producing a Magnetically Sensitive Nanocomposite for the Purification of Technologically Contaminated and Radioactive Waters Containing Micro- and Nanoplastics and Oil Products. UA156277U; 2024; (In Ukrainian). Available online: https://sis.nipo.gov.ua/uk/search/detail/1801151/ (accessed on 18 August 2025).
- Determining the Mass Related Activity of Radionuclides. ÄQUIVAL/MASSAKT-01. Procedures Manual for Monitoring of Radioactive Substances in the Environment and of External Radiation. 2022. ISSN 1865-8725. Available online: https://www.bmuv.de/fileadmin/Daten_BMU/Download_PDF/Strahlenschutz/Messanleitungen_2022/aequival_massakt_v2022-03_en_bf.pdf (accessed on 20 August 2025).






| Fraction, μm | <1 | 1–10 | 10–100 | >100 |
| Sample, % | 20.26 | 13.46 | 62.70 | 3.58 |
| Processing Stage | Sorbent Used | Radionuclide | Activity, Bq/dm3 |
|---|---|---|---|
| Before (initial) | none | 137Cs | 3.3 × 107 |
| 90Sr | 4.9 × 106 | ||
| After stage 1 | TEG–bentonite composite | 137Cs | (7.50 ± 0.31) × 103 |
| 90Sr | (1.83 ± 0.28) × 103 | ||
| After stage 2 | Iron hydroxide with nickel–potassium ferrocyanide | 137Cs | (2.24 ± 0.48) × 102 |
| 90Sr | (2.11 ± 0.52) × 102 |
| Radionuclide | Initial Activity, Bq/dm3 | Specific Activity, Bq/g * | Initial Concentration, μg/dm3 | Concentration in RCW, μg/dm3 | |
|---|---|---|---|---|---|
| After First Treatment | After Second Treatment | ||||
| 137Cs | 3.3 × 107 | 3.2 × 1012 | 10 | 2.4 × 10−3 | 0.7 × 10−4 |
| 90Sr | 4.9 × 106 | 5.1 × 1012 | 0.96 | 0.36 × 10−3 | 0.4 × 10−4 |
| 154Eu | 2.4 × 103 | 1.0 × 1012 ** | 2.4 × 10−3 | not detected | not detected |
| 241Am | 2.2 × 104 | 1.27 × 1011 | 0.17 | not detected | not detected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnychenko, T.I.; Kadoshnikov, V.M.; Arkhipenko, O.M.; Nosenko, T.I.; Mashkina, I.V.; Odukalets, L.A.; Mikhalovsky, S.V.; Zabulonov, Y.L. Nano-Functionalized Magnetic Carbon Composite for Purification of Man-Made Polluted Waters. C 2025, 11, 77. https://doi.org/10.3390/c11040077
Melnychenko TI, Kadoshnikov VM, Arkhipenko OM, Nosenko TI, Mashkina IV, Odukalets LA, Mikhalovsky SV, Zabulonov YL. Nano-Functionalized Magnetic Carbon Composite for Purification of Man-Made Polluted Waters. C. 2025; 11(4):77. https://doi.org/10.3390/c11040077
Chicago/Turabian StyleMelnychenko, Tetyana I., Vadim M. Kadoshnikov, Oksana M. Arkhipenko, Tetiana I. Nosenko, Iryna V. Mashkina, Lyudmila A. Odukalets, Sergey V. Mikhalovsky, and Yuriy L. Zabulonov. 2025. "Nano-Functionalized Magnetic Carbon Composite for Purification of Man-Made Polluted Waters" C 11, no. 4: 77. https://doi.org/10.3390/c11040077
APA StyleMelnychenko, T. I., Kadoshnikov, V. M., Arkhipenko, O. M., Nosenko, T. I., Mashkina, I. V., Odukalets, L. A., Mikhalovsky, S. V., & Zabulonov, Y. L. (2025). Nano-Functionalized Magnetic Carbon Composite for Purification of Man-Made Polluted Waters. C, 11(4), 77. https://doi.org/10.3390/c11040077









