Carbon Dot Nanozymes in Orthopedic Disease Treatment: Comprehensive Overview, Perspectives and Challenges
Abstract
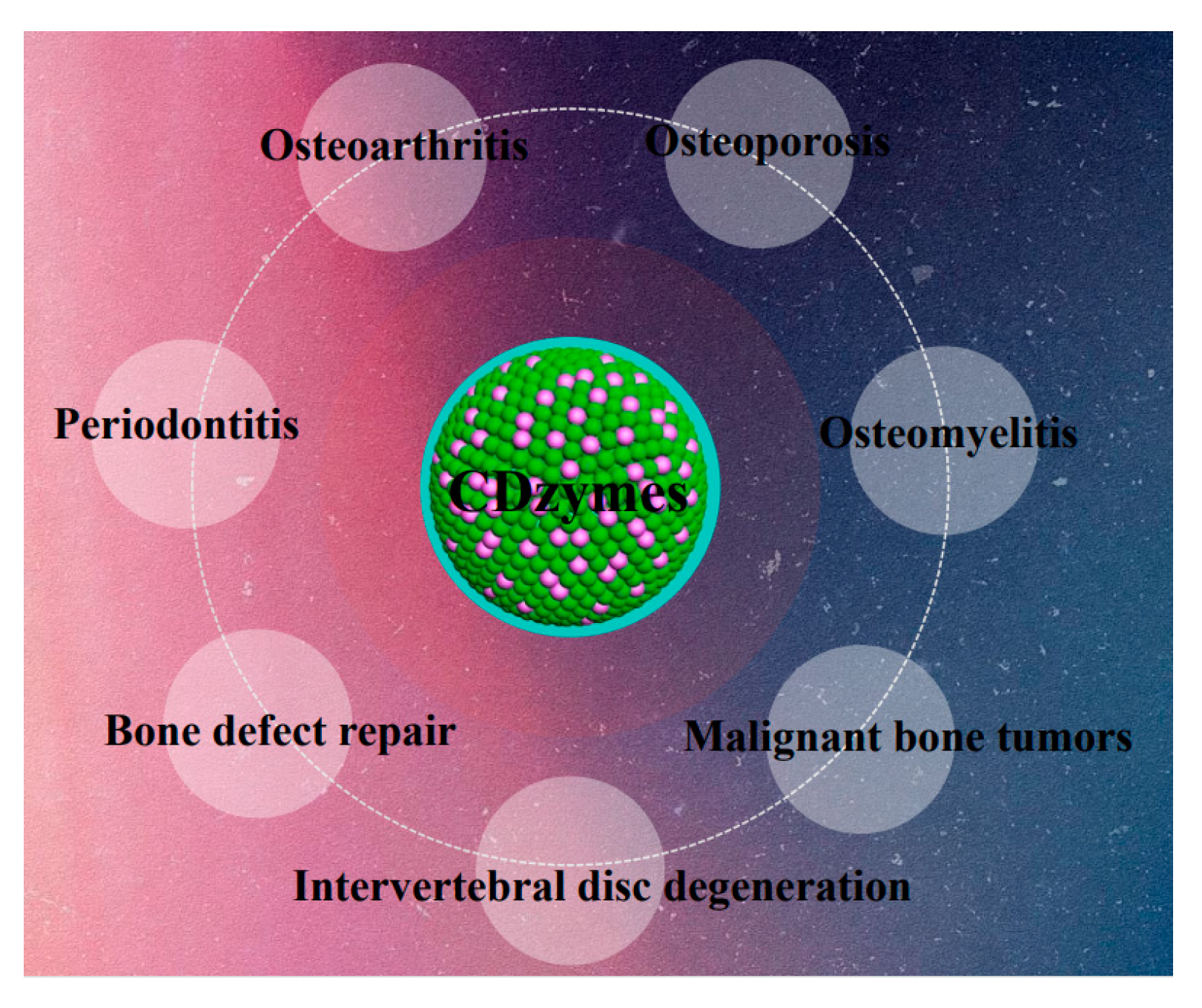
1. Introduction
2. Preparation Methods for CDzymes
2.1. Top-Down Approach
2.2. Bottom-Up Approach
3. Performance Regulation of CDzymes
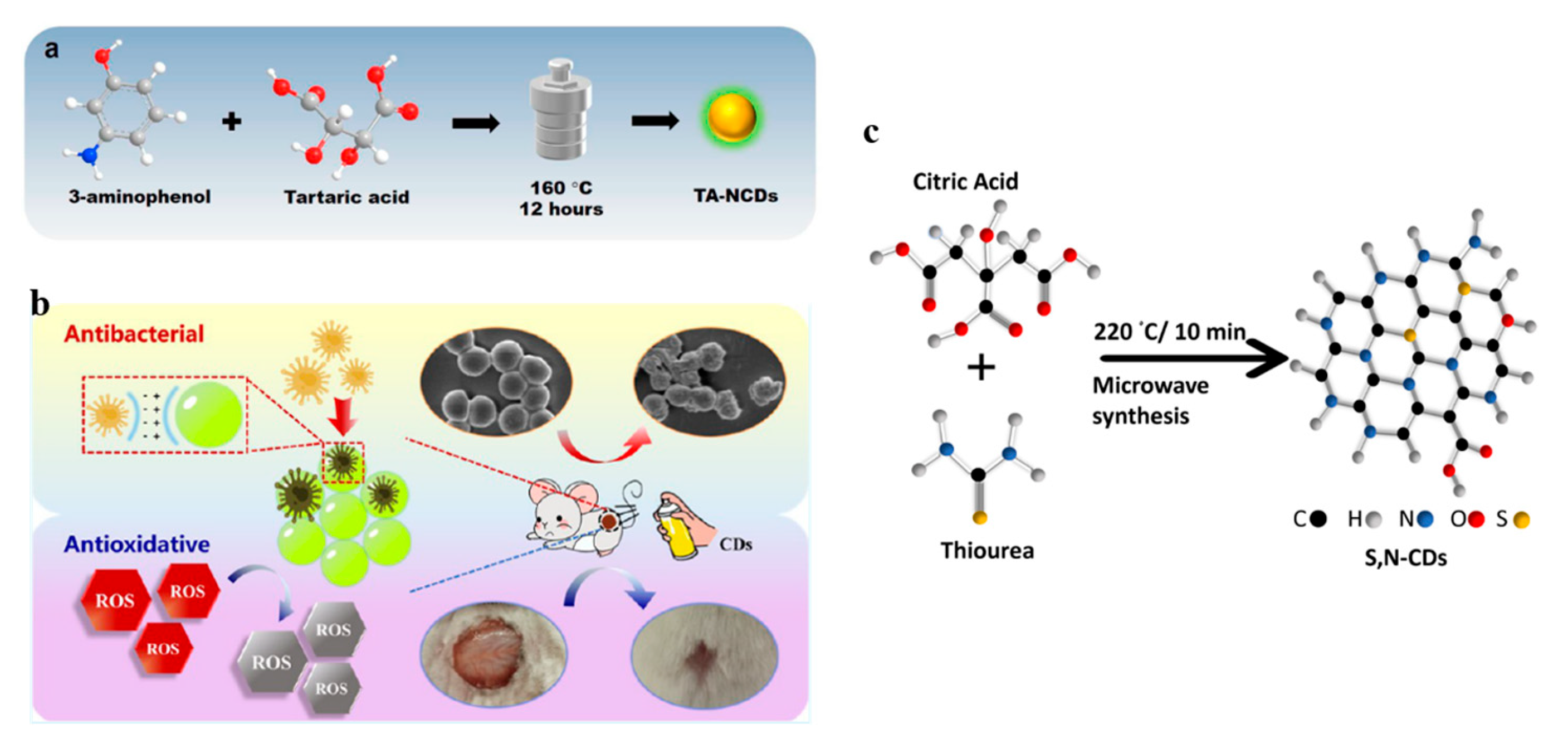
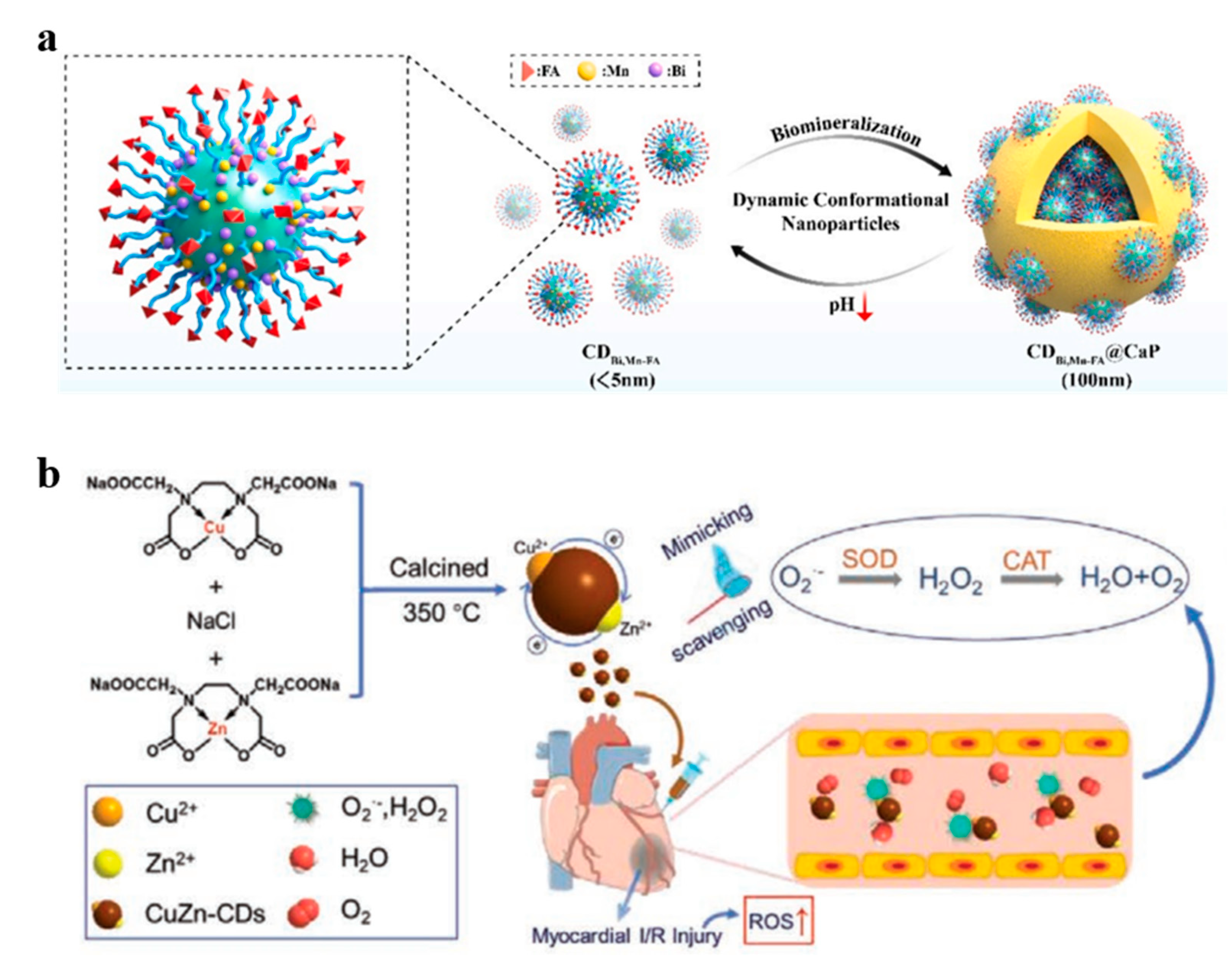
3.1. Element Doping
3.2. Surface Modification
3.3. Combined with Other Materials
3.4. Changes in Catalytic Conditions
4. Applications of CDzymes in the Treatment of Orthopedic Diseases
4.1. Osteoarthritis
4.2. Osteoporosis
4.3. Osteomyelitis
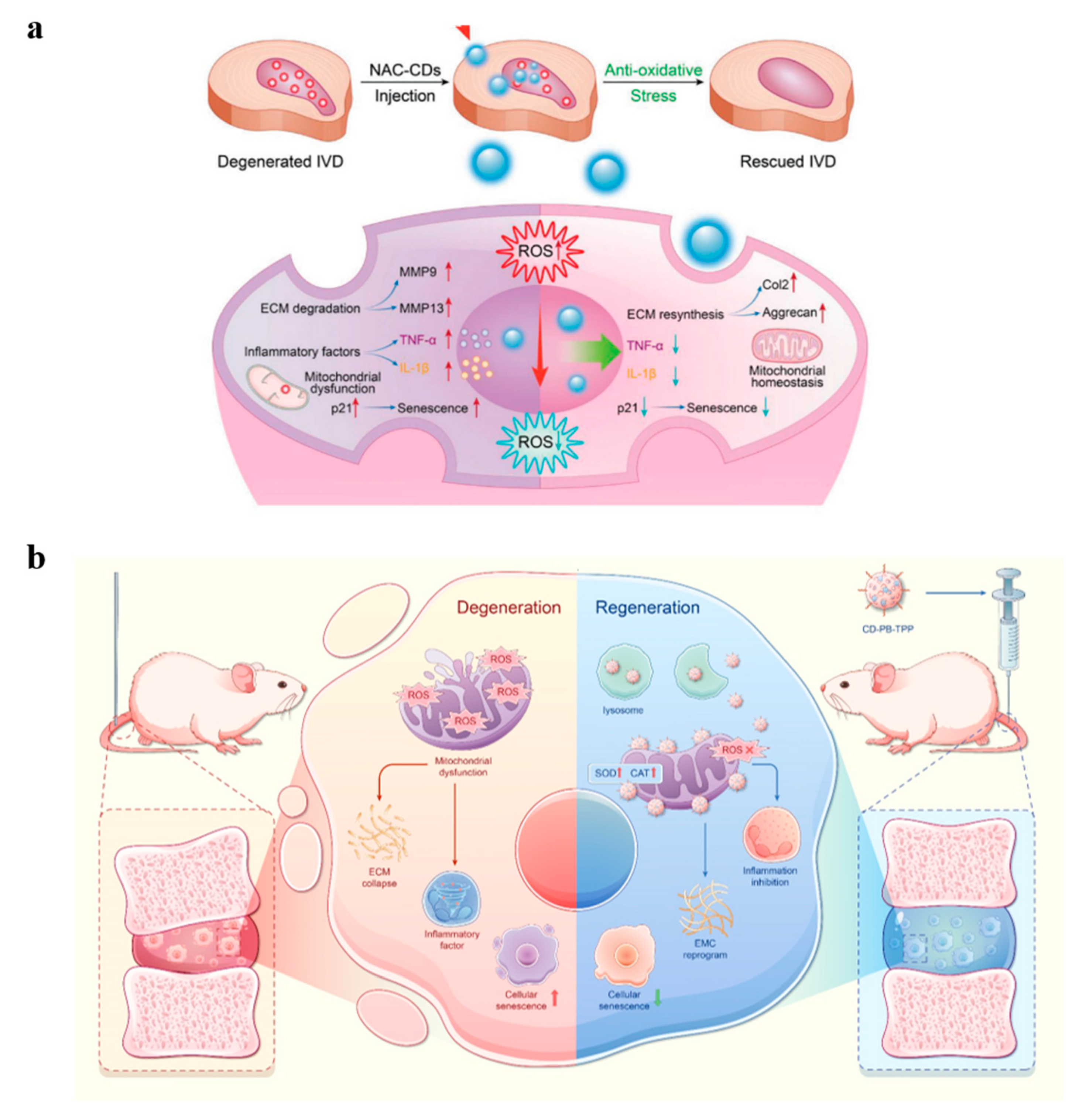
4.4. Intervertebral Disc Degeneration
4.5. Malignant Bone Tumors
4.6. Bone Defect Repair
4.7. Periodontitis
5. Opportunities and Challenges
6. Outlook
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| POD | Peroxidase |
| CDs | Carbon dots |
| CDzymes | Carbon dot nanozymes |
| RONs | Nitrogen species |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| TMB | 3,3,5,5′-tetramethylbenzidine |
| NIR | Near-infrared |
| FA | Folic acid |
| EPR | Enhanced permeability and retention |
| GPx | Glutathione peroxidase |
| OA | Osteoarthritis |
| OP | Osteoporosis |
| OVX | Ovariectomized |
| IVDD | Intervertebral disc degeneration |
| OM | Osteomyelitis |
| PB | Prussian blue |
| TPP | Triphenylphosphine |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| HO-1 | Heme oxygenase-1 |
References
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, A.; Gao, N.; Li, K.; Ren, J.; Qu, X. Deciphering a Nanocarbon-Based Artificial Peroxidase: Chemical Identification of the Catalytically Active and Substrate-Binding Sites on Graphene Quantum Dots. Angew. Chem. Int. Ed. Engl. 2015, 54, 7176–7180. [Google Scholar] [CrossRef]
- Qin, T.; Ma, S.; Miao, X.; Tang, Y.; Huangfu, D.; Wang, J.; Jiang, J.; Xu, N.; Yin, Y.; Chen, S.; et al. Mucosal Vaccination for Influenza Protection Enhanced by Catalytic Immune-Adjuvant. Adv. Sci. 2020, 7, 2000771. [Google Scholar] [CrossRef]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 2017, 7, 3207–3227. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Du, M.; Cui, C.; Bai, Z.; Liu, Y.; Sun, L.; Qin, D.; Zhou, J.; Wu, X.; et al. Stimuli-Responsive New Horizons for Biomedical Applications: Metal–Organic Framework-Based Nanozymes. Small Struct. 2024, 5, 2400029. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Liu, D.; Ma, W.; Dramou, P.; He, H. Nanozymes based on metal-organic frameworks: Construction and prospects. TrAC-Trend Anal. Chem. 2020, 133, 116080. [Google Scholar] [CrossRef]
- Gao, L.; Wei, H.; Dong, S.; Yan, X. Nanozymes. Adv. Mater. 2024, 36, 2305249. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Wu, Y.; Ding, H.; Feng, K.; Shen, Y.; Zhang, Y.; Gu, N. Multienzyme-Like Nanozymes: Regulation, Rational Design, and Application. Adv. Mater. 2023, 36, e2211210. [Google Scholar] [CrossRef]
- Lopez-Cantu, D.; González-González, R.; Melchor-Martínez, E.; Martínez, S.; Araújo, R.; Parra-Arroyo, L.; Sosa-Hernández, J.; Parra-Saldívar, R.; Iqbal, H. Enzyme-mimicking capacities of carbon-dots nanozymes: Properties, catalytic mechanism, and applications—A review. Int. J. Biol. Macromol. 2022, 194, 676–687. [Google Scholar] [CrossRef]
- Li, J.; Fu, C.; Feng, B.; Liu, Q.; Gu, J.; Khan, M.N.; Sun, L.; Wu, H.; Wu, H. Polyacrylic Acid-Coated Selenium-Doped Carbon Dots Inhibit Ferroptosis to Alleviate Chemotherapy-Associated Acute Kidney Injury. Adv. Sci. 2024, 11, 2400527. [Google Scholar] [CrossRef]
- Liu, C.; Fan, W.; Cheng, W.X.; Gu, Y.; Chen, Y.; Zhou, W.; Yu, X.F.; Chen, M.; Zhu, M.; Fan, K.; et al. Red Emissive Carbon Dot Superoxide Dismutase Nanozyme for Bioimaging and Ameliorating Acute Lung Injury. Adv. Funct. Mater. 2023, 33, 2213856. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Chu, D.; Huang, X.; Shi, Y.; Zhao, Y.; Qu, H.; Li, D.; Xu, Z.; Wang, X.; et al. Targeted reversal of iron deposition by highly active manganese-doped carbon dots in MRI-guided treatment of Parkinson‘s disease. Carbon 2025, 237, 120152. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, L.; Li, X.; Zheng, J.; Chen, Q.; Yang, Z.; Cheng, M.; Chen, Y.; Wu, Y.; Zhang, W.; et al. Green functional carbon dots derived from herbal medicine ameliorate blood—Brain barrier permeability following traumatic brain injury. Nano Res. 2022, 15, 9274–9285. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Liu, Y.; Wu, H.; Zhang, Q.; Han, J.; Liu, J.; Zhang, C. Carbon Dots from Lycium barbarum Attenuate Radiation-Induced Bone Injury by Inhibiting Senescence via METTL3/Clip3 in an m6A-Dependent Manner. ACS Appl. Mater. Interfaces 2023, 15, 20726–20741. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, M.; Luo, Q.; Zhang, Y.; Liu, T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Lyu, Z.; Tieu, P.; Wang, M.; Feng, Z.; Pan, X.; Zhou, Y.; Niu, X.; Du, D.; et al. Single-Atomic Iron Doped Carbon Dots with Both Photoluminescence and Oxidase-Like Activity. Small 2022, 18, 2203001. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Liu, Z.; Ren, J.; Qu, X. Specific Oxygenated Groups Enriched Graphene Quantum Dots as Highly Efficient Enzyme Mimics. Small 2018, 14, 1703710. [Google Scholar] [CrossRef]
- Gao, F.; Liu, J.; Gong, P.; Yang, Y.; Jiang, Y. Carbon dots as potential antioxidants for the scavenging of multi-reactive oxygen and nitrogen species. Chem. Eng. J. 2023, 462, 142338. [Google Scholar] [CrossRef]
- Dong, C.; Wang, S.; Ma, M.; Wei, P.; Chen, Y.; Wu, A.; Zha, Z.; Bi, H. Inhibition of oxidative stress in vivo through enzyme-like activity of carbon dots. Appl. Mater. Today 2021, 25, 101178. [Google Scholar] [CrossRef]
- Kong, B.; Yang, T.; Cheng, F.; Qian, Y.; Li, C.; Zhan, L.; Li, Y.; Zou, H.; Huang, C. Carbon dots as nanocatalytic medicine for anti-inflammation therapy. J. Colloid Interface Sci. 2022, 611, 545–553. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Sun, C.; Xia, J.; Jiao, Y.; Xu, H. Selenium-Doped Carbon Quantum Dots (Se-CQDs) for Free Radical Scavenging. Angew. Chem. Int. Ed. Engl. 2017, 56, 9910–9914. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Jain, A.; Kumar, A.; Sarraf, S.; Basu, A.K.; Raje, C.I.; Saha, A. Solvent-Free Synthesis of S,N-Doped Carbon Dots for Extended Visible-Light-Induced Oxidase-Mimicking Activities and Antimicrobial Applications. ChemPlusChem 2023, 88, e202300125. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.; Chen, B.; Wang, X.; Xia, Q.; Ge, Z.; Wang, Y.; Li, X. Selenium-doped two-photon fluorescent carbon nanodots for in-situ free radical scavenging in mitochondria. J. Colloid Interface Sci. 2020, 567, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, W.; Ma, Y.; Cheng, L.; Zhang, L.; Liu, Q.; Chen, J.; Zhao, Y.; Tu, K.; Zhang, M.; et al. Integrating Pt nanoparticles with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. Nano Today 2023, 49, 101768. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Lan, J.-Y.; Jin, J.-C.; Dong, P.; Jiang, F.-L.; Liu, Y. Highly Photoluminescent Nitrogen-Doped Carbon Nanodots and Their Protective Effects against Oxidative Stress on Cells. ACS Appl. Mater. Interfaces 2015, 7, 28346–28352. [Google Scholar] [CrossRef]
- Rizzo, C.; Arcudi, F.; Đorđević, L.; Dintcheva, N.T.; Noto, R.; D’Anna, F.; Prato, M. Nitrogen-Doped Carbon Nanodots-Ionogels: Preparation, Characterization, and Radical Scavenging Activity. ACS Nano 2018, 12, 1296–1305. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Wei, K.; Zhao, Y.; Nie, H.; Ma, Y.; Zhou, Y.; Huang, H.; Liu, Y.; Shao, M.; et al. Pyrrolic nitrogen dominated the carbon dot mimic oxidase activity. Carbon 2021, 179, 692–700. [Google Scholar] [CrossRef]
- Qu, X.; Gao, C.; Fu, L.; Chu, Y.; Wang, J.; Qiu, H.; Chen, J. Positively Charged Carbon Dots with Antibacterial and Antioxidant Dual Activities for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 18608–18619. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Lu, X.; Wu, P.; Liu, J. Manganese as a Catalytic Mediator for Photo-oxidation and Breaking the pH Limitation of Nanozymes. Nano Lett. 2019, 19, 3214–3220. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, X.; Ma, T.; Huang, Y.; Yan, C.; Du, Y. Multifunctional cerium doped carbon dots nanoplatform and its applications for wound healing. Chem. Eng. J. 2021, 423, 130301. [Google Scholar] [CrossRef]
- Dehvari, K.; Chiu, S.-H.; Lin, J.-S.; Girma, W.M.; Ling, Y.-C.; Chang, J.-Y. Heteroatom doped carbon dots with nanoenzyme like properties as theranostic platforms for free radical scavenging, imaging, and chemotherapy. Acta Biomater. 2020, 114, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Ge, J.; Liu, W.; Zheng, X.; Chen, S.; Wen, Y.; Zhang, H.; Wang, P. A Magnetofluorescent Carbon Dot Assembly as an Acidic H2O2-Driven Oxygenerator to Regulate Tumor Hypoxia for Simultaneous Bimodal Imaging and Enhanced Photodynamic Therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Jiang, P.; Wang, G.; Wu, X.; Zhao, H.; Zhang, C. Superior peroxidase mimetic activity of carbon dots–Pt nanocomposites relies on synergistic effects. New J. Chem. 2015, 39, 4141–4146. [Google Scholar] [CrossRef]
- Li, C.; Zeng, J.; Guo, D.; Liu, L.; Xiong, L.; Luo, X.; Hu, Z.; Wu, F. Cobalt-Doped Carbon Quantum Dots with Peroxidase-Mimetic Activity for Ascorbic Acid Detection through Both Fluorometric and Colorimetric Methods. ACS Appl. Mater. Interfaces 2021, 13, 49453–49461. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Sun, D.; Kong, L.; Liu, Y.; Yu, D.; Song, K.; Ni, S.; Jiang, W.; Zhan, J. Cu-O4 Single Atom Carbon Dots Nanozymes for Efficient Self-Adaptive Bacteria-Infected Wound Therapy. Adv. Funct. Mater. 2025, 2509879. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Tu, H.; Feng, J.; Fan, X.; Mi, Z.; Han, H.; Ma, X.; Liang, Q.; Zhang, Z.; et al. Bismuth/manganese-doped carbon dots in calcium phosphate matrix: Dynamic conformational nanoparticles enhancing tumor accumulation, deep penetration, and radiosensitivity. Chem. Eng. J. 2025, 504, 158683. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, T.; Wang, X.; Zhang, Q.; Huang, S.; Zhang, L.; Zhang, L.; Zhu, W.; Wang, Y.; Wu, M.; et al. Cu Zn Dopants Boost Electron Transfer of Carbon Dots for Antioxidation. Small 2021, 17, 2102178. [Google Scholar] [CrossRef]
- Das, S.; Ngashangva, L.; Mog, H.; Gogoi, S.; Goswami, P. An insight into the mechanism of peroxidase-like activity of carbon dots. Opt. Mater. 2021, 115, 111017. [Google Scholar] [CrossRef]
- Shi, J.; Yin, T.; Shen, W. Effect of Surface Modification on the Peroxidase-like Behaviors of Carbon Dots. Colloids Surf. B 2019, 178, 163–169. [Google Scholar] [CrossRef]
- Bu, W.; Shi, Y.; Huang, X.; Wu, S.; Jiang, L.; Pan, C.; Li, D.; Xu, Z.; Wang, H.; Chen, H.; et al. Rescue of nucleus pulposus cells from an oxidative stress microenvironment via glutathione-derived carbon dots to alleviate intervertebral disc degeneration. J. Nanobiotechnol. 2024, 22, 412. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, L.; Ma, S.; Ding, L.; Zhang, W.; Xu, Z.; Li, D.; Gao, L. Self-Assembled Multiple-Enzyme Composites for Enhanced Synergistic Cancer Starving–Catalytic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20191–20201. [Google Scholar] [CrossRef]
- Luo, T.; Yang, H.; Wang, R.; Pu, Y.; Cai, Z.; Zhao, Y.; Bi, Q.; Lu, J.; Jin, R.; Nie, Y.; et al. Bifunctional Cascading Nanozymes Based on Carbon Dots Promotes Photodynamic Therapy by Regulating Hypoxia and Glycolysis. ACS Nano 2023, 17, 16715–16730. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Wei, J.; Jiang, C.; Liu, Z.; Yang, B.; Zhao, B.; Song, W. SERS monitoring of photoinduced-enhanced oxidative stress amplifier on Au@carbon dots for tumor catalytic therapy. Light-Sci. Appl. 2022, 11, 286. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Dong, X.; Sun, Y. A multi-target theranostic nano-composite against Alzheimer‘s disease fabricated by conjugating carbon dots and triple-functionalized human serum albumin. Acta Biomater. 2022, 148, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, Y.; Wu, S.; Hou, X.; Zheng, C.; Wu, P.; Liu, J. Self-photo-oxidation for extending visible light absorption of carbon dots and oxidase-like activity. Carbon 2021, 182, 537–544. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Tian, X.; Ma, X.; Wen, T.; Wamer, W.G.; Chen, C.; Chai, Z.; Yin, J.-J. Crossover between Anti- and Pro-oxidant Activities of Graphene Quantum Dots in the Absence or Presence of Light. ACS Nano 2016, 10, 8690–8699. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.-M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Primers 2025, 11, 10. [Google Scholar] [CrossRef]
- Yang, B.; Yao, H.; Yang, J.; Chen, C.; Shi, J. Construction of a two-dimensional artificial antioxidase for nanocatalytic rheumatoid arthritis treatment. Nat. Commun. 2022, 13, 1988. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, S.; Sun, Z.; Li, J.; Zhang, H.; Liu, W.; Ruan, J.; Chen, S.; Gao, C.; Fan, C. The ROS-responsive scavenger with intrinsic antioxidant capability and enhanced immunomodulatory effects for cartilage protection and osteoarthritis remission. Appl. Mater. Today 2022, 26, 101366. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Q.; Qin, X.; Liu, Z.; Li, Z.; Zhong, X.; Xia, L.; He, J.; Fang, B. Carbon dots derived from folic acid attenuates osteoarthritis by protecting chondrocytes through NF-κB/MAPK pathway and reprogramming macrophages. J. Nanobiotechnol. 2022, 20, 469. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, J.; Xing, X.; Liu, H.; Zhou, Z.; Dong, H. Red emissive carbon dots Self-Cascading antioxidant nanozymes combine with anti-miRNA-155 for accurate monitoring and ameliorating rheumatoid arthritis. Chem. Eng. J. 2024, 481, 148523. [Google Scholar] [CrossRef]
- He, Q.; Li, R.; Yang, H.; Li, B.; Zhang, L. Inflammation-Targeting Multienzyme Activity Carbon Dots Loaded with Methotrexate for Synergistic Immunotherapy in Rheumatoid Arthritis. Small 2025, 21, 2412491. [Google Scholar] [CrossRef]
- Reid, I.R.; Billington, E.O. Drug therapy for osteoporosis in older adults. Lancet 2022, 399, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Tu, J.; Zhang, Z.; Czuczejko, J. Ferroptosis: A New Regulatory Mechanism in Osteoporosis. Oxid. Med. Cell Longev. 2022, 2022, 2634431. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Li, B.; Tan, H.; Zhang, C.; Liang, H.; Na, H.; Chen, S.; Liu, C.; Zhao, L. Alendronate-Decorated Nanoparticles as Bone-Targeted Alendronate Carriers for Potential Osteoporosis Treatment. ACS Appl. Bio Mater. 2021, 4, 4907–4916. [Google Scholar] [CrossRef]
- Skjødt, M.K.; Frost, M.; Abrahamsen, B. Side effects of drugs for osteoporosis and metastatic bone disease. Br. J. Clin. Pharmacol. 2018, 85, 1063–1071. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Huang, X.; Wang, J.; Zhang, W.; Zhang, S.; Ren, J.; Liu, L.; Zhan, Y.; Zhang, B.; et al. Alendronate carbon dots targeting bone immune microenvironment for the treatment of osteoporosis. Chem. Eng. J. 2024, 494, 152209. [Google Scholar] [CrossRef]
- Juma, T.; Liang, G.-H.; Jiao, Y.; Ma, Y.-Y.; Yu, B.-X.; Guo, Y.-M.; Yang, X.; Liu, H.; Meng, Z.-C.; Wang, R.; et al. Anti-osteoporotic effects and good biocompatibility of novel bioactive carbon quantum dots in vitro and in ovariectomized mice. iScience 2025, 28, 111700. [Google Scholar] [CrossRef]
- Yu, L.; Li, X.; He, M.; Wang, Q.; Chen, C.; Li, F.; Li, B.; Li, L. Antioxidant Carboxymethyl Chitosan Carbon Dots with Calcium Doping Achieve Ultra-Low Calcium Concentration for Iron-Induced Osteoporosis Treatment by Effectively Enhancing Calcium Bioavailability in Zebrafish. Antioxidants 2023, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Veis, D.J.; Cassat, J.E. Infectious Osteomyelitis: Marrying Bone Biology and Microbiology to Shed New Light on a Persistent Clinical Challenge. J. Bone Miner. Res. 2020, 36, 636–643. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Luo, Y.; Ma, X.; Luo, L.; Liang, L.; Deng, T.; Qiao, Y.; Ye, F.; Liao, H. A carbon dot nanozyme hydrogel enhances pulp regeneration activity by regulating oxidative stress in dental pulpitis. J. Nanobiotechnol. 2024, 22, 537. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, H.; Yang, X.; Qiao, L.; Rao, X.; Zhou, R. Poly-Lysine-Derived Carbon Quantum Dots Promote the Repair of Bone Defects in Osteomyelitis Through Antibacterial and Osteogenic Effects. Int. J. Nanomed. 2025, 20, 7199–7214. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Lyu, F.-J.; Cui, H.; Pan, H.; Mc Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Eisenstein, S.M.; Balain, B.; Roberts, S. Current Treatment Options for Intervertebral Disc Pathologies. Cartilage 2020, 11, 143–151. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Guo, J.; Li, J.; Zhang, P.; Yang, H.; Rong, K.; Zhou, T.; Fu, J.; Zhao, J. Polydopamine Nanoparticles Targeting Ferroptosis Mitigate Intervertebral Disc Degeneration Via Reactive Oxygen Species Depletion, Iron Ions Chelation, and GPX4 Ubiquitination Suppression. Adv. Sci. 2023, 10, 2207216. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Y.; Jiang, L.; Bu, W.; Zhang, K.; Lin, W.; Pan, C.; Xu, Z.; Du, J.; Chen, H.; et al. N-Acetylcysteine-Derived Carbon Dots for Free Radical Scavenging in Intervertebral Disc Degeneration. Adv. Healthc. Mater. 2023, 12, 2300533. [Google Scholar] [CrossRef]
- Shi, Y.; Bu, W.; Chu, D.; Lin, W.; Li, K.; Huang, X.; Wang, X.; Wu, Y.; Wu, S.; Li, D.; et al. Rescuing Nucleus Pulposus Cells from ROS Toxic Microenvironment via Mitochondria-Targeted Carbon Dot-Supported Prussian Blue to Alleviate Intervertebral Disc Degeneration. Adv. Healthc. Mater. 2024, 13, 2303206. [Google Scholar] [CrossRef]
- Bădilă, A.; Rădulescu, D.; Niculescu, A.; Grumezescu, A.; Rădulescu, M.; Rădulescu, A. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, N.; Cornel, E.J.; Cai, H.; Xue, S.; Xi, H.; Fan, Z.; He, S.; Du, J. Bone-targeting polymer vesicles for simultaneous imaging and effective malignant bone tumor treatment. Biomaterials 2021, 269, 120345. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Qu, H.; Huang, X.; Shi, Y.; Li, K.; Lin, W.; Xu, Z.; Li, D.; Chen, H.; Gao, L.; et al. Manganese Amplifies Photoinduced ROS in Toluidine Blue Carbon Dots to Boost MRI Guided Chemo/Photodynamic Therapy. Small 2023, 20, 2304968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jiang, X.; Zhou, X.; Tan, W.; Luo, H.; Lei, S.; Yang, Y. GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: Therapeutic strategies and recent advances. Biomater. Res. 2023, 27, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, H.; Zhang, C.; Xu, S.; Zhang, F.; Wei, L.; Zhu, F.; Chen, Y.; Chen, Y.; Huang, Y.; et al. In situ activation of flexible magnetoelectric membrane enhances bone defect repair. Nat. Commun. 2023, 14, 4091. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Sun, H.; Yu, M.; Wang, H.; Meng, Q.; Li, Z.; Liu, D.; Bai, J.; Liu, G.; et al. Transformation of arginine into zero-dimensional nanomaterial endows the material with antibacterial and osteoinductive activity. Sci. Adv. 2023, 9, 8645. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.; Yu, S.; Su, Y.; Guo, H.; Jiang, H.; He, H.; Hu, J.; Liu, Y.; Yao, L.; et al. “Small Dots, Multiple Functions”: Unveiling the Veil of a Bioactive Quercetin Carbon Dot and Its Multifaceted Antibacterial and Osteogenesis Mechanisms for Infectious Bone Defect Repair. Adv. Funct. Mater. 2025, 2507840. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current concepts in the pathogenesis of periodontitis: From symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef]
- Yang, B.; Pang, X.; Li, Z.; Chen, Z.; Wang, Y. Immunomodulation in the Treatment of Periodontitis: Progress and Perspectives. Front. Immunol. 2021, 12, 781378. [Google Scholar] [CrossRef]
- Xin, X.; Liu, J.; Liu, X.; Xin, Y.; Hou, Y.; Xiang, X.; Deng, Y.; Yang, B.; Yu, W. Melatonin-Derived Carbon Dots with Free Radical Scavenging Property for Effective Periodontitis Treatment via the Nrf2/HO-1 Pathway. ACS Nano 2024, 18, 8307–8324. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.; Xu, X.; Wei, X.; Yang, X.; Li, X.; Wang, B.; Xu, Y.; Li, L.; Yang, Z. Copper Doped Carbon Dots for Addressing Bacterial Biofilm Formation, Wound Infection, and Tooth Staining. ACS Nano 2022, 6, 9479–9497. [Google Scholar] [CrossRef]
- Gao, W.; He, J.; Chen, L.; Meng, X.; Ma, Y.; Cheng, L.; Tu, K.; Gao, X.; Liu, C.; Zhang, M.; et al. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef]
- Cui, X.; Wan, X.; Chang, X.; Bao, L.; Wu, J.; Tan, Z.; Chen, J.; Li, J.; Gao, X.; Ke, P.; et al. A new capacity of gut microbiota: Fermentation of engineered inorganic carbon nanomaterials into endogenous organic metabolites. Proc. Natl. Acad. Sci. USA 2023, 120, e2218739120. [Google Scholar] [CrossRef]

| Synthesis Methods | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Top-Down | In situ pyrulysis | High yield, cheap raw materials | Ultra-high temperature, easy to aggregate | [16] |
| Oxidation reflux | High purity and high yield | Strong acid and strong alkali environment | [17] | |
| Bottom-Up | Hydrothermal | Easy control of both size and shape, high purity | High pressure and high heating temperature | [21] |
| Microwave | Reaction conditions environmentally friendly | Low yield | [22] |
| Precursor | Synthesis | Enzyme-Like Activity | Application | Ref |
|---|---|---|---|---|
| Metal doping | ||||
| NaFeEDTA | In situ pyrulysis | OXD (Vmax = 10.4 nMs−1, Km = 168 μM) | Phosphate monitoring | [16] |
| Mn, Mo | In situ pyrulysis | CAT | Photodynamic therapy | [42] |
| Cu, Zn chelated EDTA | In situ pyrulysis | CAT, SOD | Ischemia–reperfusion injury | [37] |
| Se | Hydrothermal | Antioxidant | Free radical scavenging | [21] |
| Mn (II) phthalocyanine | Hydrothermal | CAT | Antitumor | [32] |
| lignosulfonate Cu | Hydrothermal | OXD, POD, SOD, CAT | Wounding healing | [35] |
| Bi | Hydrothermal | CAT | Antitumor | [36] |
| Se, Ce | Hydrothermal | SOD, GPx | OA | [52] |
| PB | Hydrothermal | SOD, CAT | IVDD | [70] |
| Mn, toluidine blue | Hydrothermal | POD | Malignant bone tumors | [73] |
| Guanidine, Cu | Hydrothermal | POD, CAT | Periodontitis | [82] |
| Biomass | ||||
| Scutellaria barbata and Herba Hedyotis diffusae | Hydrothermal | Antioxidant | Cigarette filter | [18] |
| Coffee | Hydrothermal | GSH oxidase | Antitumor Immunity | [15] |
| Quercetin | Hydrothermal | Antioxidant | Bone defect repair | [77] |
| Melatonin | Hydrothermal | Antioxidant | Periodontitis | [81] |
| Inorganic carbon | ||||
| Graphite plates | Liquid-phase pulse method | Antioxidant | OP | [60] |
| Multiwalled carbon nanotubes | Oxidation reflux | POD (Vmax = 7.755 × 10−8 Ms−1, Km = 1.363 × 10−1 mM) | Glucose detection | [17] |
| Graphite powder | Oxidation reflux | POD | Detection of H2O2 and glucose | [47] |
| N-doping | ||||
| p-Phenylenediamine | Hydrothermal | SOD, CAT | Anti-inflammation in liver | [20] |
| p-Phenylenediamine and Polyethyleneimine | Hydrothermal | Antioxidant | Wounding healing | [27] |
| o-Phenylenediamine | Hydrothermal | Antioxidant | OM | [63] |
| Tartaric acid and 3-aminophenol | Hydrothermal | OXD (Vmax = 121.95 × 10−8 Ms−1, Km = 0.61 mM) | Antitumor | [26] |
| L-glutamic acid, L-glutamine, succinic acid, citric acid, and glycine | In situ pyrulysis | POD | [38] | |
| Folic acid | Hydrothermal | SOD | OA | [51] |
| Hemin chloride and polyethyleneimine | Hydrothermal | SOD, CAT | OA | [53] |
| Carboxymethyl chitosan and acrylamide | Microwave | Antioxidant | OP | [61] |
| S-doping | ||||
| Thiourea | Hydrothermal | POD | [39] | |
| Glutathione- | Hydrothermal | SOD, CAT, GPx | IVDD | [40] |
| Glutathione | Hydrothermal | SOD | Alzheimer’s disease | [44] |
| N-Acetylcysteine | Hydrothermal | SOD (250 U mg−1), CAT, PGx | IVDD | [69] |
| Thiourea | Microwave | Photo-oxidase (Vmax = 4.66 × 10−8 Ms−1, Km = 1.18 mM) | Antibacterial | [28] |
| P-doping | ||||
| Alendronate | Hydrothermal | Antioxidant | OP | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H. Carbon Dot Nanozymes in Orthopedic Disease Treatment: Comprehensive Overview, Perspectives and Challenges. C 2025, 11, 58. https://doi.org/10.3390/c11030058
Wang H. Carbon Dot Nanozymes in Orthopedic Disease Treatment: Comprehensive Overview, Perspectives and Challenges. C. 2025; 11(3):58. https://doi.org/10.3390/c11030058
Chicago/Turabian StyleWang, Huihui. 2025. "Carbon Dot Nanozymes in Orthopedic Disease Treatment: Comprehensive Overview, Perspectives and Challenges" C 11, no. 3: 58. https://doi.org/10.3390/c11030058
APA StyleWang, H. (2025). Carbon Dot Nanozymes in Orthopedic Disease Treatment: Comprehensive Overview, Perspectives and Challenges. C, 11(3), 58. https://doi.org/10.3390/c11030058






