Dual pH- and Temperature-Responsive Fluorescent Hybrid Materials Based on Carbon Dot-Grafted Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Hybrid Materials
2.2.1. Synthesis of CDs
2.2.2. Grafting of Chain Transfer Agents (CDs-DATC)
2.2.3. Synthesis of Acrylamido ATPE (Acr-ATPE)
2.2.4. Preparation of CDs Grafted with Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers (CDs-PNAT)
2.3. Characterizations
3. Results and Discussion
3.1. Structure Characterizations of the Hybrid Materials
3.1.1. FTIR Spectroscopy and 1H NMR Spectrum
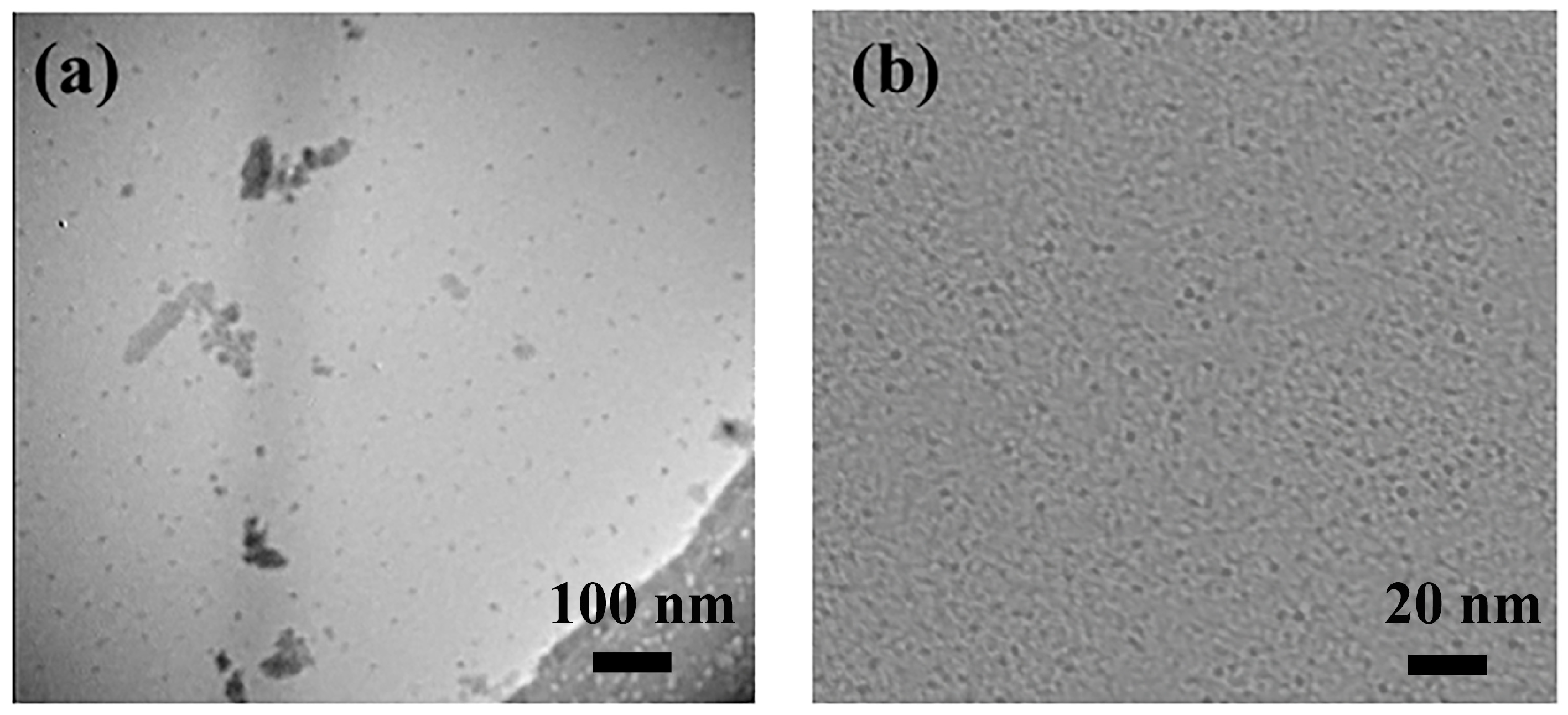
3.1.2. Morphologies of CDs-PNAT
3.2. Fluorescence Performances
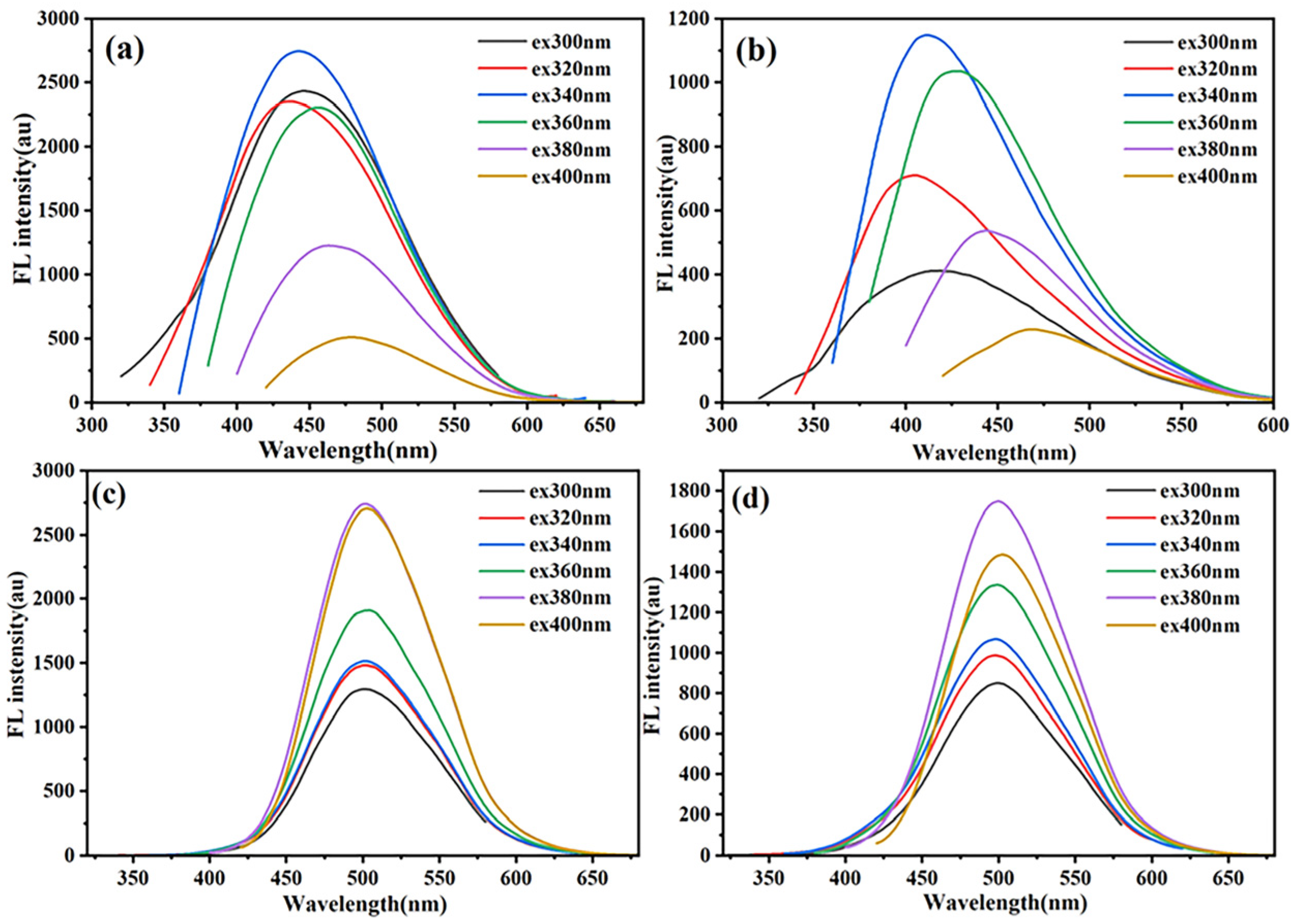
3.2.1. Characterization of Fluorescence Performances
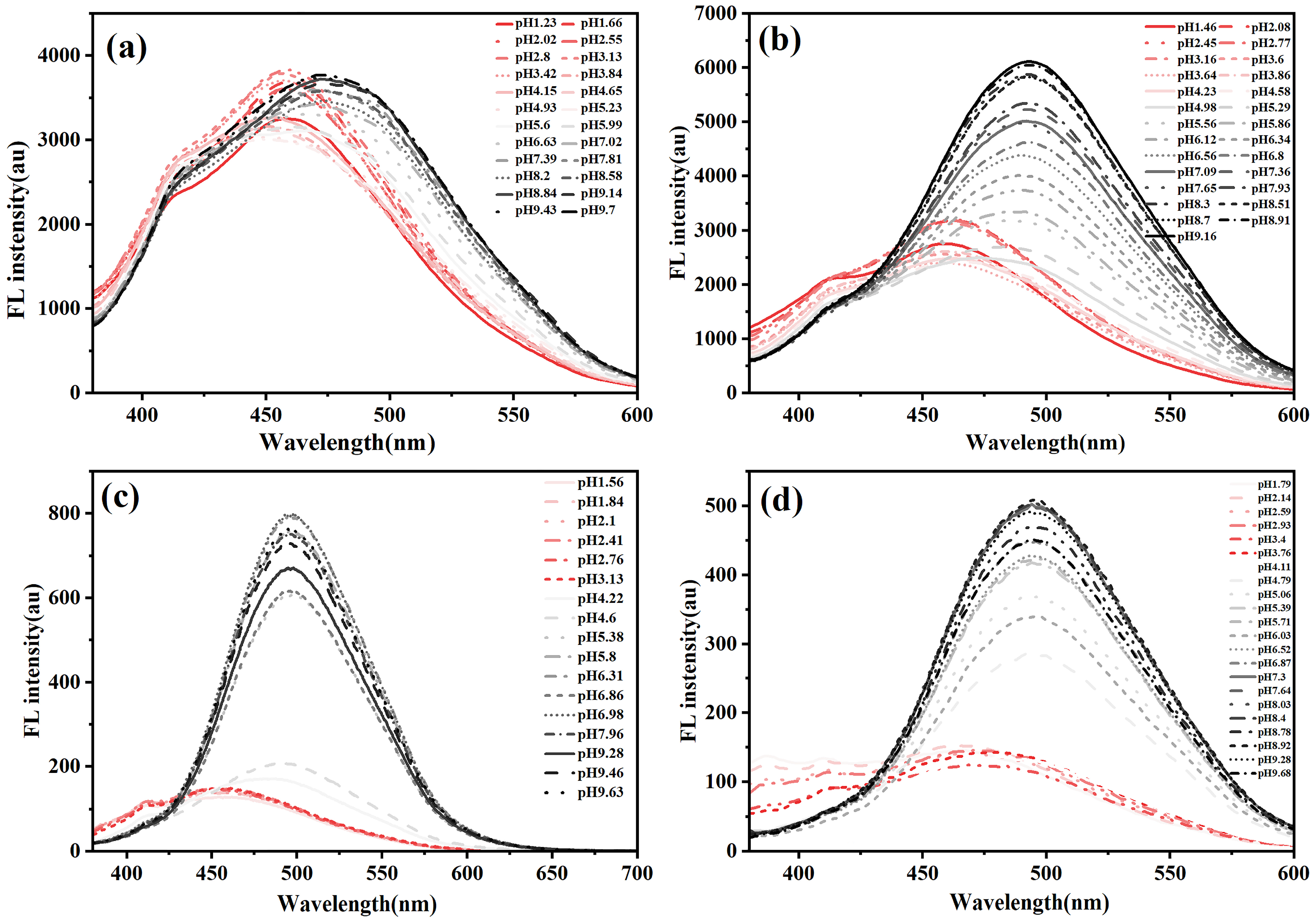
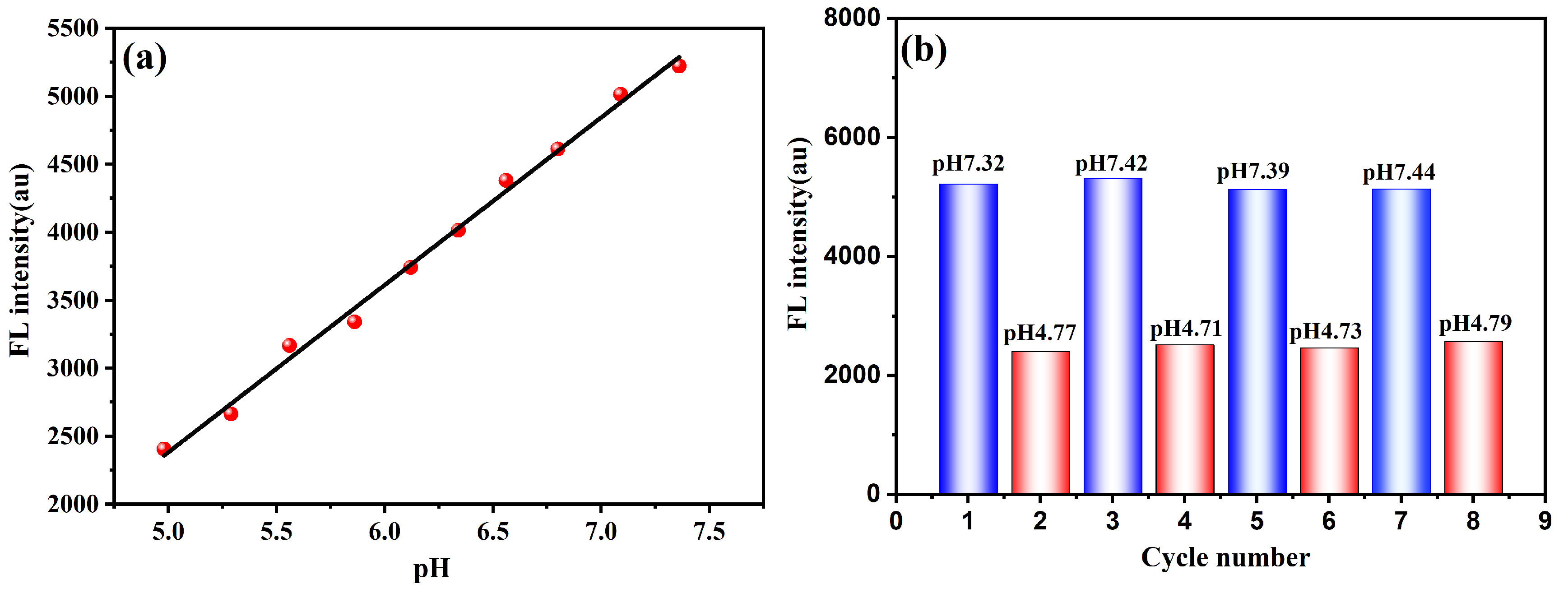
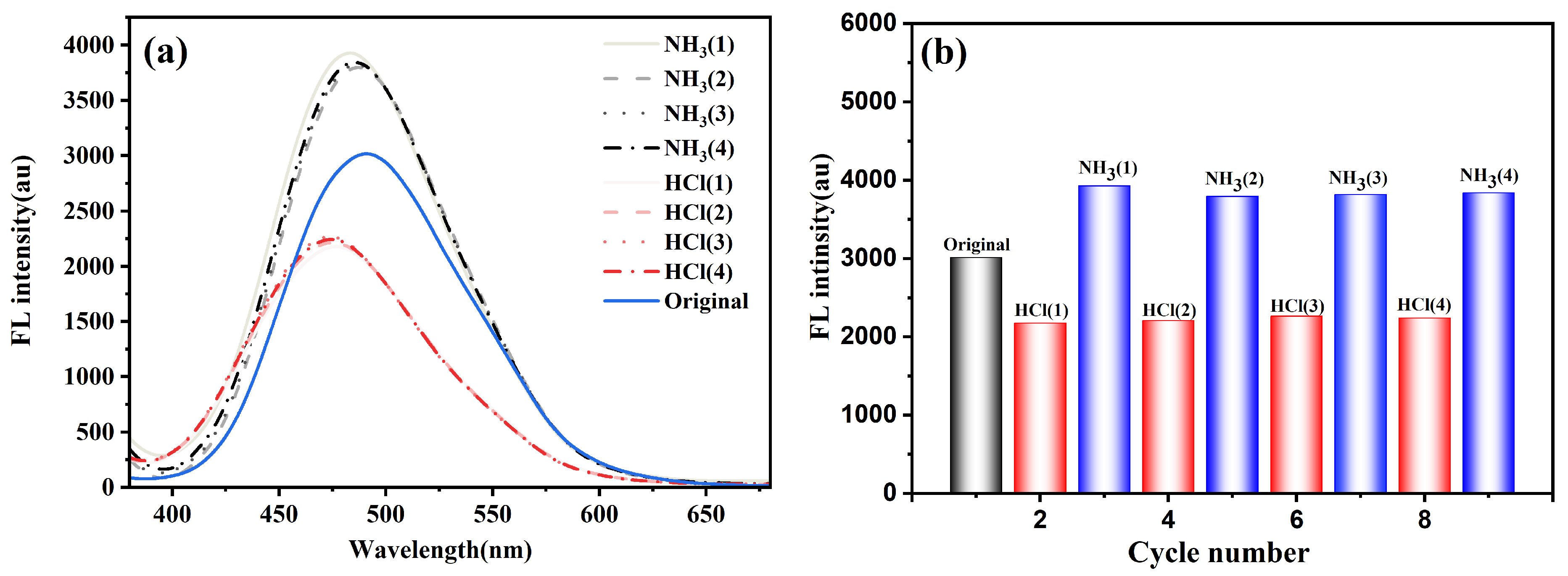
3.2.2. pH-Responsive Fluorescence Behavior
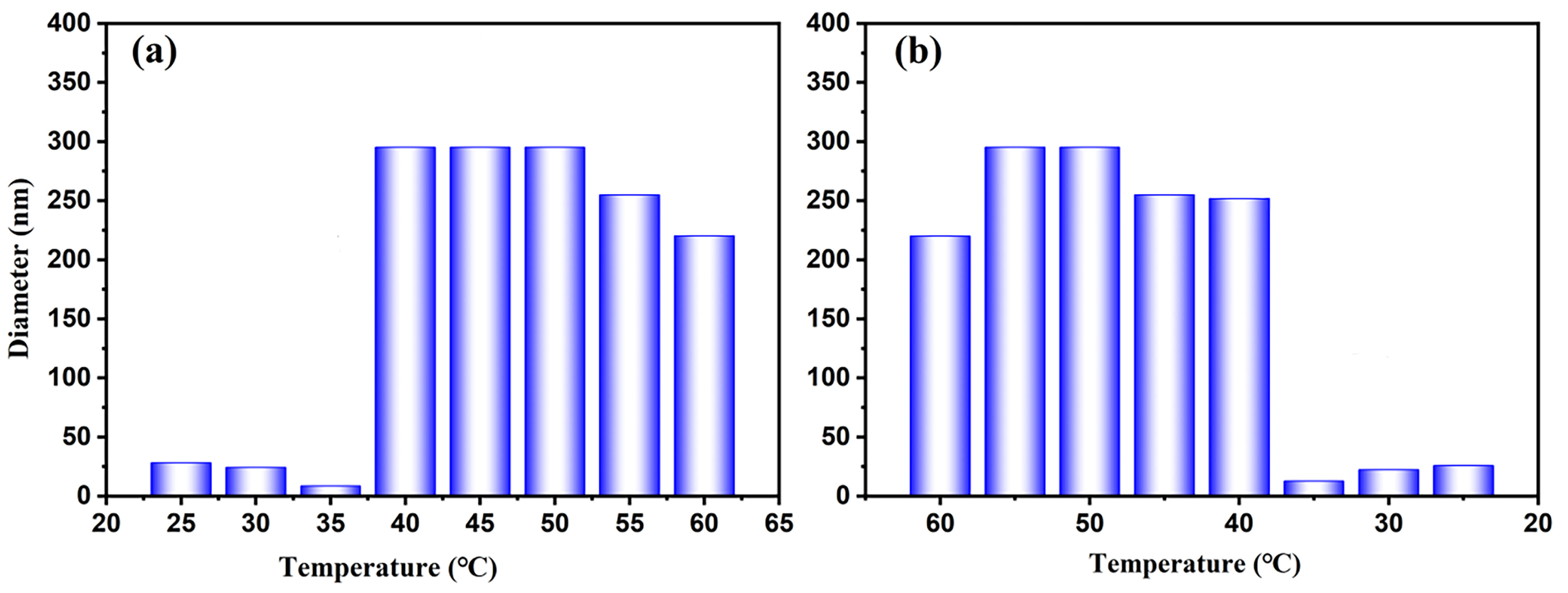
3.2.3. Fluorescence Responsiveness to Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Madhusudhan, A.; Suhagia, T.A.; Sharma, C.; Jaganathan, S.K.; Purohit, S.D. Carbon based polymeric nanocomposite hydrogel bioink: A review. Polymers 2024, 16, 3318. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Malfatti, L.; Innocenzi, P. Citric acid derived carbon dots, the challenge of understanding the synthesis-structure relationship. C 2021, 7, 2. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Pieber, B.; Delbianco, M. Modulating the surface and photophysical properties of carbon dots to access colloidal photocatalysts for cross-couplings. ACS Catal. 2022, 12, 13831. [Google Scholar] [CrossRef]
- Araújo, C.; Rodrigues, R.O.; Bañobre-López, M.; Silva, A.M.T.; Ribeiro, R.S. Carbon dots as a fluorescent nanosystem for crossing the blood-brain barrier with plausible application in neurological diseases. Pharmaceutics 2025, 17, 477. [Google Scholar] [CrossRef] [PubMed]
- Suner, S.S.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Rapid pathogen purge by photosensitive arginine-riboflavin carbon dots without toxicity. Materials 2023, 16, 6512. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, Z.; Li, Y.; Xi, F.; Liu, J. Magnetic nanozyme based on loading nitrogen-doped carbon dots on mesoporous Fe3O4 nanoparticles for the colorimetric detection of glucose. Molecules 2023, 28, 4573. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Z.; Ma, T.; Bai, J. Aptamer paper-based fluorescent sensor for determination of SARS-CoV-2 spike protein. Sensors 2025, 25, 1637. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G. Recent advancements in doped/co-doped carbon quantum dots for multi-potential applications. C 2019, 5, 24. [Google Scholar] [CrossRef]
- Liao, B.; Long, P.; He, B.Q. Reverible fluorescence modulation of spiropyran-functionalized carbon nanparticles. J. Mater. Chem. C 2013, 1, 3716. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, H.; Jiang, H.; Sun, L.; Sun, Y.; Liu, Q.; Ren, S.; Zhuang, Y.; Gong, X. Controllable functionalization of carbon dots as selective and sensitive fluorescent probes for sensing Cu(II) ions. Crystals 2025, 15, 205. [Google Scholar] [CrossRef]
- Egorova, M.; Tomskaya, A.; Smagulova, S.A. Optical properties of carbon dots synthesized by the hydrothermal method. Materials 2023, 16, 4018. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Tian, R.; Dong, Y.; Yang, J.; Liu, J.; Chang, Q. Modulation and effects of surface groups on photoluminescence and photocatalytic activity of carbon dots. Nanoscale 2013, 5, 11665. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.C.; Sun, J.D.; Ji, J.; Yang, X.X.; Wei, K.M.; Xu, H.W.; Gu, Q.Y.; Zhang, Y.Z.; Sun, X.L. Carbon dots-releasing hydrogels with antibacterial activity, high biocompatibility, and fluorescence performance as candidate materials for wound healing. J. Hazard. Mater. 2021, 3, 124330. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Wang, W.; Long, P.; Deng, X.; He, B.Q.; Liu, Q.Q.; Yi, S.J. The carbon nanoparticles grafted with copolymers of styrene and spiropyran with reversibly photoswitchable fluorescence. Carbon 2015, 91, 30. [Google Scholar] [CrossRef]
- Liao, B.; Wang, W.; Long, P.; Li, F.W.; Liu, Q.Q. Synthesis of fluorescent carbon nanoparticles grafted with polystyrene and their fluorescent fibers processed by electrospinning. RSC Adv. 2014, 4, 57683. [Google Scholar] [CrossRef]
- Yang, S.L.; Liao, B.; Yi, S.J.; Liang, E.X.; He, B. Tetraphenylethylene and N-isopropylacrylamide block polymergrafted carbon dots with their application in cellular imaging. Mater. Today Chem. 2022, 23, 100703. [Google Scholar] [CrossRef]
- Yang, S.L.; Liao, B.; Liang, E.X.; Yi, S.J.; Liao, Q. Reversible light-controlled fluorescence switch of block polymer-grafted carbon dots and cellular imaging. Soft Matter 2022, 18, 8017. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Liu, X.Q.; Liao, S.Y.; Liu, W.; Yi, S.J.; Liu, Q.; He, B. N-isopropylacrylamide and spiropyran copolymer-grafted fluorescent carbon nanoparticles with dual responses to light and temperature stimuli. Polym. J. 2020, 52, 1289. [Google Scholar] [CrossRef]
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Chen, H.Y.; Qiu, C.F.; Kwok, H.S.; Zhan, X.W.; Liu, Y.Q.; Zhu, D.B.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.M.; Hou, M.; Zhou, W.; Ning, Z.W.; Zhang, Y.; Xing, G.W. An AIE-active nir fluorescent probe with good water solubility for the detection of Aβ1–42 aggregates in Alzheimer’s disease. Molecules 2023, 28, 5110. [Google Scholar] [CrossRef] [PubMed]
- Picchi, A.; Wang, Q.; Ventura, F.; Micheletti, C.; Heijkoop, J.; Picchioni, F.; Ciofini, I.; Adamo, C.; Pucci, A. Effect of polymer composition on the optical properties of a new aggregation-induced emission fluorophore: A combined experimental and computational approach. Polymers 2023, 15, 3530. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Zhou, T.; Zhu, J.; Tang, Y.; Xiong, J.; Min, X.; Qin, Q.; Li, M.; Zhao, N.; Wan, C. Multiple light-activated photodynamic therapy of tetraphenylethylene derivative with AIE characteristics for hepatocellular carcinoma via dual-organelles targeting. Pharmaceutics 2022, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, H.; Yu, L.; Zhou, J. Aggregation-induced emission (AIE)-labeled cellulose nanocrystals for the detection of nitrophenolic explosives in aqueous solutions. Nanomaterials 2019, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Bender, M.; Seehafer, K.; Wacker, I.; Schröder, R.R.; Bunz, U.H.F. Novel functional TPE polymers: Aggregation-induced emission, pH response, and solvatochromic behavior. Macrom. Rapid Comm. 2019, 40, 1800774. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly (N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Edit. 2015, 54, 15342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.P.; Liao, B.; Yang, S.L.; Yi, S.J. Carbon dots and gold nanocluster-based hybrid microgels and their application in cells imaging. J. Nanopart. Res. 2022, 24, 140. [Google Scholar] [CrossRef]
- Colaco, R.; Appiah, C.; Staubitz, A. Controlling the LCST-phase transition in azobenzene-functionalized poly(N-isopropylacrlyamide) hydrogels by light. Gels 2023, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Stetsyshyn, Y.; Ohar, H.; Budkowski, A.; Lazzara, G. Molecular design and role of the dynamic hydrogen bonds and hydrophobic interactions in temperature-switchable polymers: From understanding to applications. Polymers 2025, 17, 1580. [Google Scholar] [CrossRef] [PubMed]
- Kostyurina, E.; De Mel, J.U.; Vasilyeva, A.; Kruteva, M.; Frielinghaus, H.; Dulle, M.; Allgaier, J. Controlled LCST behavior and structure formation of alternating amphiphilic copolymers in water. Macromolecules 2022, 55, 1552. [Google Scholar] [CrossRef]
- Manfredini, N.; Gardoni, G.; Sponchioni, M.; Moscatelli, D. Thermo-responsive polymers as surface active compounds: A review. Eur. Polym. J. 2023, 198, 112421. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Lam, J.W.; Tang, B.Z. Restriction of intramolecular motion (RIM): Investigating AIE mechanism from experimental and theoretical studies. Chem. Res. Chinese Univ. 2021, 37, 1. [Google Scholar] [CrossRef]
- Liao, Q.Y.; Li, Q.Q.; Li, Z. The key role of molecular packing in luminescence property: From adjacent molecules to molecular aggregates. Adv. Mater. 2023, 35, 2306617. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shan, G.; Qin, C.; Liu, S. Polymerization-enhanced photophysical performances of aiegens for chemo/bio-sensing and therapy. Molecules 2022, 28, 78. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ding, Y.; Zhou, L.; Xu, S.; Liao, B. Dual pH- and Temperature-Responsive Fluorescent Hybrid Materials Based on Carbon Dot-Grafted Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers. C 2025, 11, 53. https://doi.org/10.3390/c11030053
Liu H, Ding Y, Zhou L, Xu S, Liao B. Dual pH- and Temperature-Responsive Fluorescent Hybrid Materials Based on Carbon Dot-Grafted Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers. C. 2025; 11(3):53. https://doi.org/10.3390/c11030053
Chicago/Turabian StyleLiu, Huan, Yuxin Ding, Longping Zhou, Shirui Xu, and Bo Liao. 2025. "Dual pH- and Temperature-Responsive Fluorescent Hybrid Materials Based on Carbon Dot-Grafted Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers" C 11, no. 3: 53. https://doi.org/10.3390/c11030053
APA StyleLiu, H., Ding, Y., Zhou, L., Xu, S., & Liao, B. (2025). Dual pH- and Temperature-Responsive Fluorescent Hybrid Materials Based on Carbon Dot-Grafted Triamino-Tetraphenylethylene/N-Isopropylacrylamide Copolymers. C, 11(3), 53. https://doi.org/10.3390/c11030053







