Industrial Off-Gas Fermentation for Acetic Acid Production: A Carbon Footprint Assessment in the Context of Energy Transition
Abstract
1. Introduction
- ∎
- Methanol synthesis
- ∎
- Methanol carbonylation
2. Materials and Methods
2.1. Foreground Inventory for Methanol Carbonylation Thermochemical Process
2.2. Foreground Inventory for Acetogenic Syngas Fermentation
2.3. Foreground Inventory for Other Biochemical Processes
2.3.1. PYROCO2
2.3.2. Microbial Electrosynthesis
2.4. Background Data
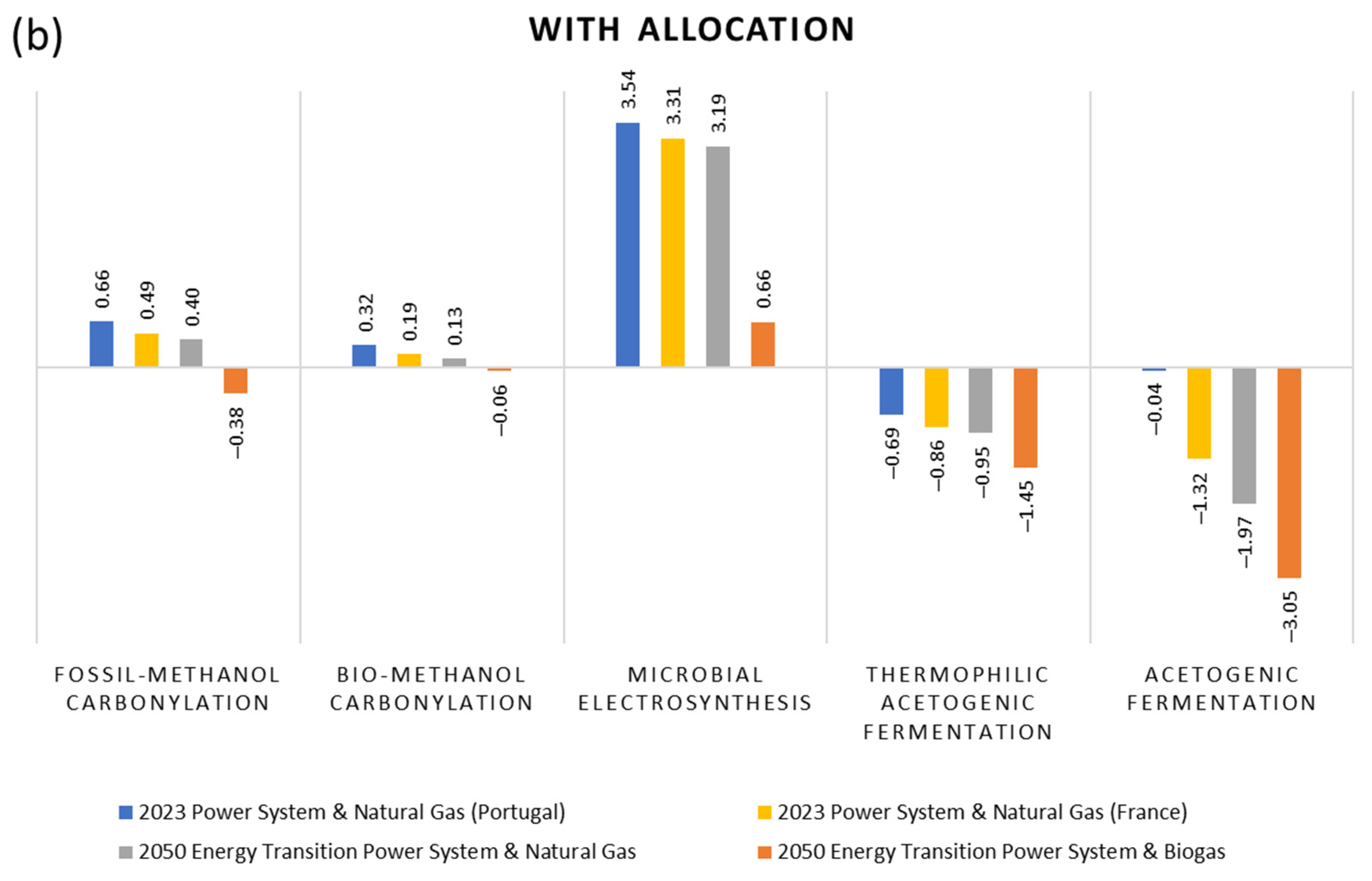
2.5. Allocation
2.6. Assuring the Same Level of Acetic Acid Purity
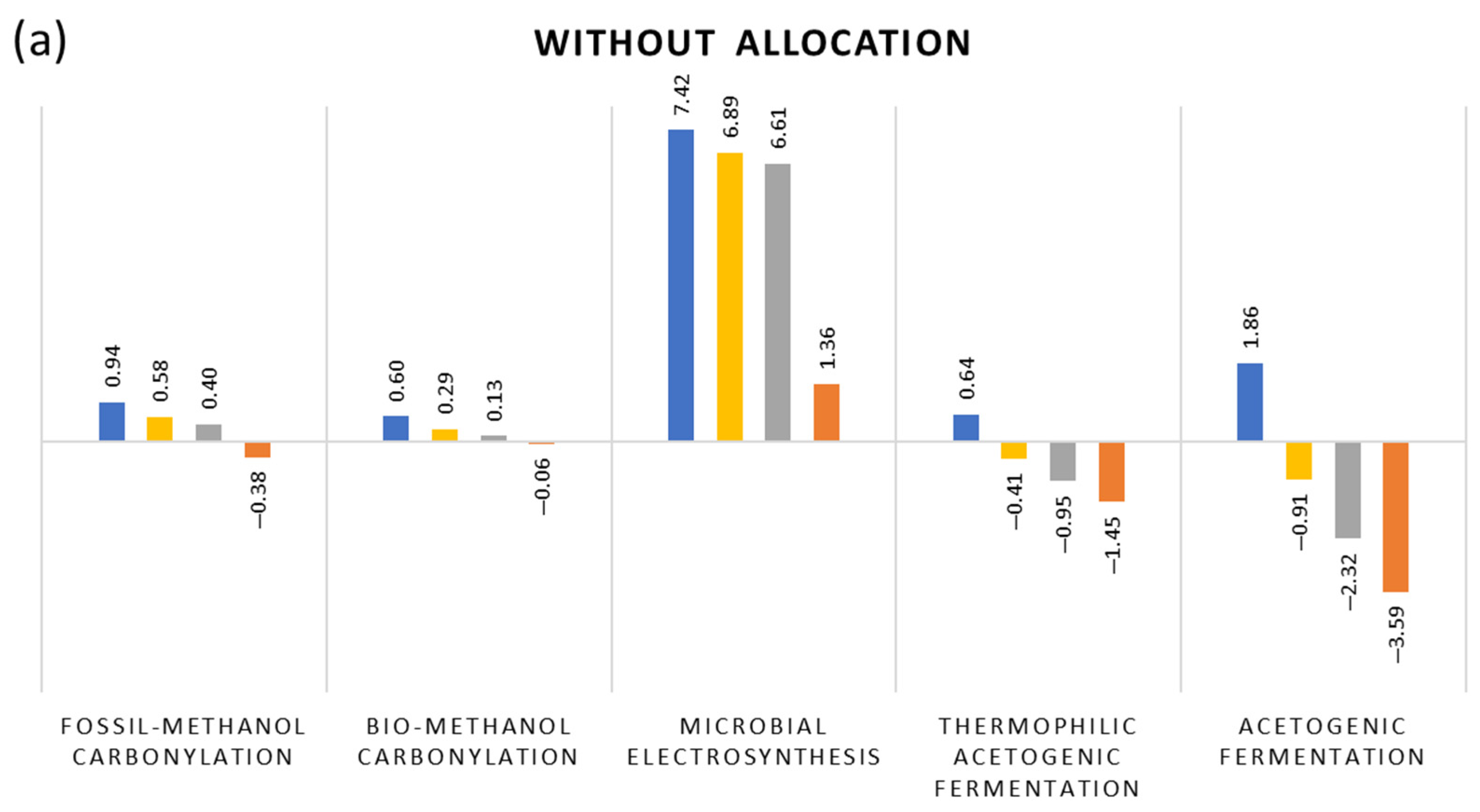
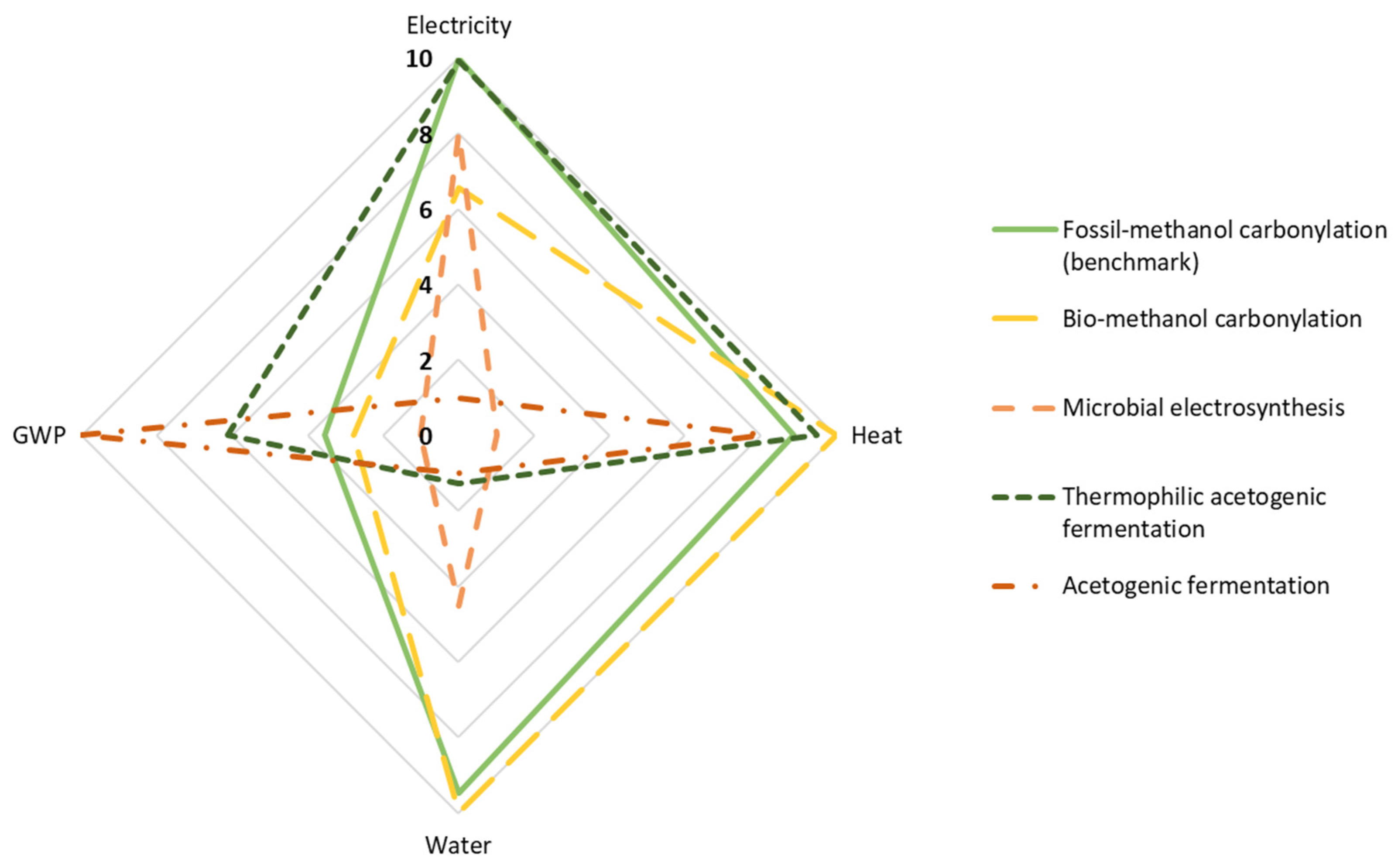
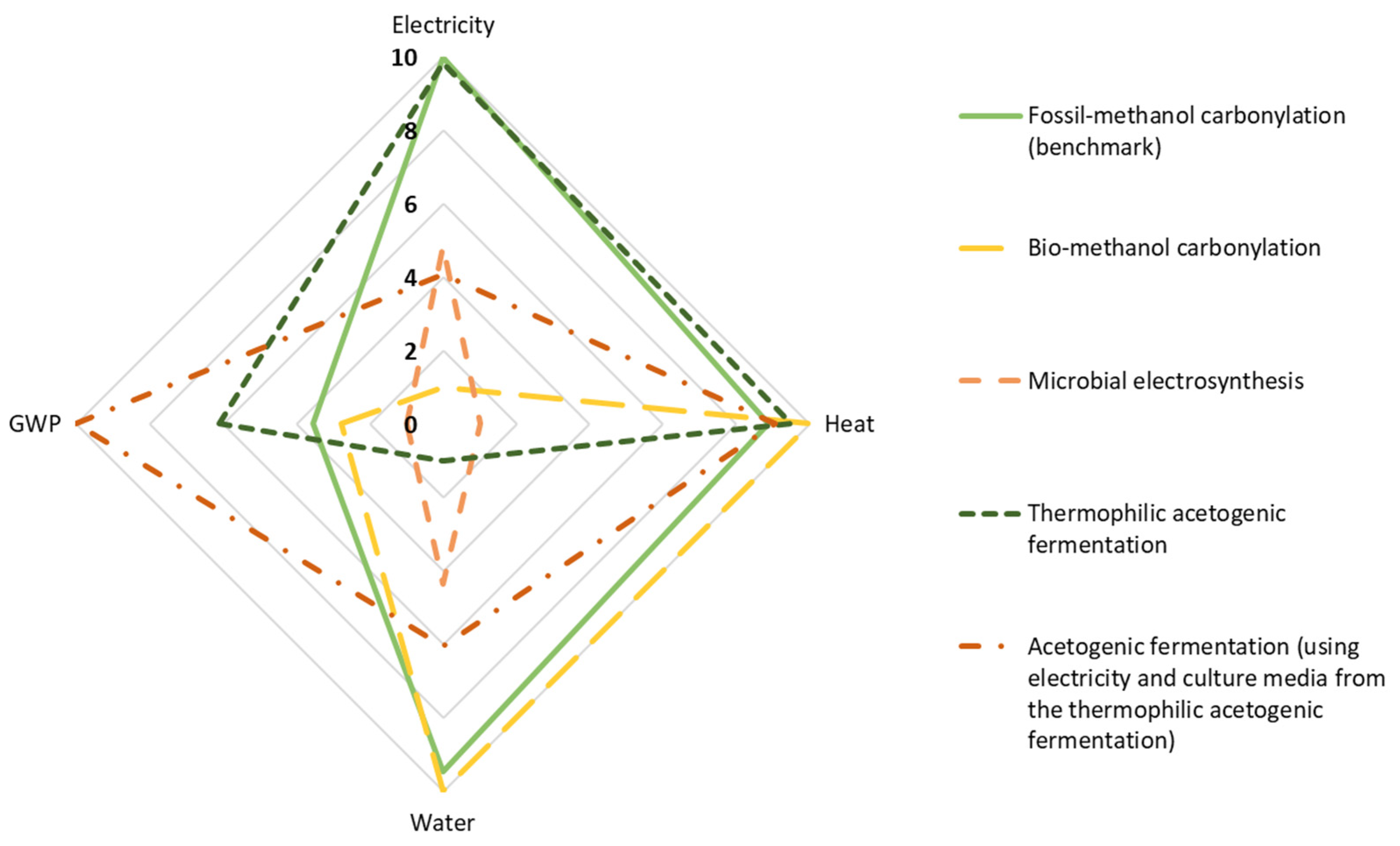
3. Results and Interpretation
- Thermochemical route, using natural gas reforming and methanol carbonylation (benchmark);
- Thermochemical route, using refuse-derived waste gasification and methanol carbonylation;
- Bio-electrochemical route, using MES;
- Biochemical route, using CO2 capture and hydrogen from water electrolysis and thermophilic acetogenic fermentation;
- Biochemical route, using CO2 capture and hydrogen from water electrolysis and acetogenic syngas fermentation under mesophilic conditions from lab-scale own experiments.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| CF | Carbon footprint |
| CP | Cementitious products |
| DAC | Direct air capture |
| DU | Declared unit |
| EF | Emission factor |
| e-LCA | Environmental life cycle assessment |
| FU | Functional unit |
| FW | Food waste |
| GHG | Greenhouse gas |
| GW | Garden waste |
| GWP100 | Average global warming potential over 100 years |
| KPI | Key performance indicator |
| MES | Microbial electrosynthesis |
| RWGS | Reverse water–gas shift reaction |
| USA | United States of America |
References
- Moussa, S.; Ibrahim, A.; Okba, A.; Hamza, H.; Opwis, K.; Schollmeyer, E. Antibacterial Action of Acetic Acid Soluble Material Isolated from Mucor rouxii and Its Application onto Textile. Int. J. Biol. Macromol. 2011, 48, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Acetic Acid Market: Trends & Challenges|Merchant Research & Consulting, Ltd. Available online: https://mcgroup.co.uk/news/20230407/acetic-acid-market-trends-challenges.html (accessed on 25 May 2025).
- Phan, H.N.; Nguyen, T.T.; Okubayashi, S. Textile Finishing for Bacterial Cellulose Modification: Organic Acid-Catalyzed Cellulosic Acetylation. J. Text. Inst. 2025, 116, 713–721. [Google Scholar] [CrossRef]
- Ren, Z.; Guan, X. Evaluating the Environmental and Easy-Care Benefits of Diacetate Fiber Blended Textiles: Implications for Sustainability in the Textile Industry. Res. J. Text. Appar. 2025; ahead-of-print. [Google Scholar] [CrossRef]
- Béligon, V.; Poughon, L.; Christophe, G.; Lebert, A.; Larroche, C.; Fontanille, P. Validation of a Predictive Model for Fed-Batch and Continuous Lipids Production Processes from Acetic Acid Using the Oleaginous Yeast Cryptococcus Curvatus. Biochem. Eng. J. 2016, 111, 117–128. [Google Scholar] [CrossRef]
- Pacheco, M.; Moura, P.; Silva, C. A Systematic Review of Syngas Bioconversion to Value-Added Products from 2012 to 2022. Energies. 2023, 16, 3241. [Google Scholar] [CrossRef]
- de Vicente, M.; Gonzalez-Fernández, C.; Nicaud, J.M.; Tomás-Pejó, E. Turning Residues into Valuable Compounds: Organic Waste Conversion into Odd-Chain Fatty Acids via the Carboxylate Platform by Recombinant Oleaginous Yeast. Microb. Cell Fact. 2025, 24, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tsui, T.-H.; Tong, Y.W.; Liu, R.; Smoliński, A. Microbial Lipid Technology Based on Oleaginous Yeasts. In Microbial Lipids and Biodiesel Technologies; Zhang, L., Tsui, T.H., Tong, Y.W., Liu, R., Eds.; Springer Nature Singapore: Singapore, 2025; pp. 17–50. [Google Scholar]
- Porsche’s Synthetic Fuel|Porsche Lauzon. Available online: https://dealer.porsche.com/ca/lauzon/en-CA/News-and-Events/Carburant-efuel (accessed on 25 May 2025).
- Lindstad, E.; Lagemann, B.; Rialland, A.; Gamlem, G.M.; Valland, A. Reduction of Maritime GHG Emissions and the Potential Role of E-Fuels. Transp. Res. Part D Transp. Environ. 2021, 101, 103075. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, Q.; Sun, N.; Wei, W. Life Cycle Assessment of Typical Methanol Production Routes: The Environmental Impacts Analysis and Power Optimization. J. Clean. Prod. 2019, 220, 408–416. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, Y.; Tang, Z.; Sun, Y. Comparative Environmental and Economic Performance of Solar Energy Integrated Methanol Production Systems in China. Energy Convers. Manag. 2019, 187, 63–75. [Google Scholar] [CrossRef]
- Jiang, P.; Li, L.; Zhao, G.; Zhang, H.; Ji, T.; Mu, L.; Lu, X.; Zhu, J. Seeking the Low-Carbon Route of Methanol Production with Sustainable Resources by Tracking Energy and Environment Indicators. Ind. Eng. Chem. Res. 2024, 63, 8261–8272. [Google Scholar] [CrossRef]
- Barbieri, M.; Manenti, F. Industrial Production of Acetic Acid: A Patent Landscape. SSRN Electron. J. 2021, 40. [Google Scholar] [CrossRef]
- Jones, B.J.H. The CativaTM Process for the Manufacture of Acetic Acid: Iridium Catalyst Improves Productivity in an Established Industrial Process. Platin. Met. Rev. 2000, 44, 94–105. [Google Scholar] [CrossRef]
- Global Acetic Acid Demand Share by Region. Available online: https://www.statista.com/statistics/1323645/distribution-of-acetic-acid-demand-worldwide-by-region/ (accessed on 25 May 2025).
- Martín-Espejo, J.L.; Gandara-Loe, J.; Odriozola, J.A.; Reina, T.R.; Pastor-Pérez, L. Sustainable Routes for Acetic Acid Production: Traditional Processes vs a Low-Carbon, Biogas-Based Strategy. Sci. Total Environ. 2022, 840, 156663. [Google Scholar] [CrossRef] [PubMed]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 25 May 2025).
- ISO/TR 14047:2012; Environmental Management—Life Cycle Assessment—Illustrative Examples on How to Apply ISO 14044 to Impact Assessment Situations. ISO: Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/57109.html (accessed on 25 May 2025).
- ISO 14067:2018; Greenhouse Gases—Carbon Footprint of Products. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/71206.html (accessed on 25 May 2025).
- Budsberg, E.; Morales-Vera, R.; Crawford, J.T.; Bura, R.; Gustafson, R. Production Routes to Bio-Acetic Acid: Life Cycle Assessment. Biotechnol. Biofuels 2020, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.R.; Rorrer, N.A.; Uekert, T.; Avery, G.; Carpenter, A.C.; Beckham, G.T. Manufacturing Energy and Greenhouse Gas Emissions Associated with United States Consumption of Organic Petrochemicals. ACS Sustain. Chem. Eng. 2023, 11, 2198–2208. [Google Scholar] [CrossRef]
- Flannery, B.; Mares, J. The Greenhouse Gas Index for Products in 39 Industrial Sectors. Resour. Future. 2022. Available online: https://www.rff.org/publications/working-papers/the-greenhouse-gas-index-for-products-in-39-industrial-sectors/ (accessed on 25 May 2025).
- Medrano-García, J.D.; Ruiz-Femenia, R.; Caballero, J.A. Revisiting Classic Acetic Acid Synthesis: Optimal Hydrogen Consumption and Carbon Dioxide Utilization. Comput. Aided Chem. Eng. 2019, 46, 145–150. [Google Scholar] [CrossRef]
- Smith, R.L.; Ruiz-Mercado, G.J.; Meyer, D.E.; Gonzalez, M.A.; Abraham, J.P.; Barrett, W.M.; Randall, P.M. Coupling Computer-Aided Process Simulation and Estimations of Emissions and Land Use for Rapid Life Cycle Inventory Modeling. ACS Sustain. Chem. Eng. 2017, 5, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Hischier, R.; Hellweg, S.; Capello, C.; Primas, A. Establishing Life Cycle Inventories of Chemicals Based on Differing Data Availability. Int. J. Life Cycle Assess. 2005, 10, 59–67. [Google Scholar] [CrossRef]
- Huber, E.; Bach, V.; Holzapfel, P.; Blizniukova, D.; Finkbeiner, M. An Approach to Determine Missing Life Cycle Inventory Data for Chemicals (RREM). Sustainability 2022, 14, 3161. [Google Scholar] [CrossRef]
- Pal, P.; Nayak, J. Acetic acid production and purification: Critical review towards process intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- BIO-TIC FP7 Project Acetic Acid; European Commission Environmental Factsheet, Brussels, Belgium. Available online: https://edepot.wur.nl/327986 (accessed on 25 May 2025).
- Gadkari, S.; Mirza Beigi, B.H.; Aryal, N.; Sadhukhan, J. Microbial Electrosynthesis: Is It Sustainable for Bioproduction of Acetic Acid? RSC Adv. 2021, 11, 9921–9932. [Google Scholar] [CrossRef] [PubMed]
- Lenzing Celebrates the 40th Anniversary of Excellence in LENZINGTM Acetic Acid Biobased. Available online: https://www.lenzing.com/newsroom/news-events/lenzing-celebrates-the-40th-anniversary-of-excellence-in-lenzingtm-acetic-acid-biobased/ (accessed on 25 May 2025).
- Flaiz, M.; Poehlein, A.; Wilhelm, W.; Mook, A.; Daniel, R.; Dürre, P.; Bengelsdorf, F.R. Refining and Illuminating Acetogenic Eubacterium Strains for Reclassification and Metabolic Engineering. Microb. Cell Fact. 2024, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Pinto, F.; Ortigueira, J.; Silva, C.; Gírio, F.; Moura, P. Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery. Energies 2021, 14, 7124. [Google Scholar] [CrossRef]
- European Commission International Reference Life Cycle Data System (ILCD) Handbook-General Guide for Life Cycle Assessment-Provisions and Action Steps; Publications Office of the European Union: Luxembourg, 2010; p. 150. [CrossRef]
- AR5 Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Available online: https://www.ipcc.ch/report/ar5/wg3/ (accessed on 25 May 2025).
- Borgogna, A.; Salladini, A.; Spadacini, L.; Pitrelli, A.; Annesini, M.C.; Iaquaniello, G. Methanol Production from Refuse Derived Fuel: Influence of Feedstock Composition on Process Yield through Gasification Analysis. J. Clean. Prod. 2019, 235, 1080–1089. [Google Scholar] [CrossRef]
- Pacheco, M.; Pinto, F.; Brunsvik, A.; André, R.; Marques, P.; Mata, R.; Ortigueira, J.; Gírio, F.; Moura, P. Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies 2023, 16, 1722. [Google Scholar] [CrossRef]
- Sternberg, A.D. System-Wide Perspective for Life Cycle Assessment of CO2-Based C1-Chemicals; Aachen University: Aachen, Germany, 2017. [Google Scholar]
- Tharak, A.; Katakojwala, R.; Kajla, S.; Venkata Mohan, S. Chemolithoautotrophic Reduction of CO2 to Acetic Acid in Gas and Gas-Electro Fermentation Systems: Enrichment, Microbial Dynamics, and Sustainability Assessment. Chem. Eng. J. 2023, 454, 140200. [Google Scholar] [CrossRef]
- “Data Page: Carbon Intensity of Electricity Generation”, Part of the Following Publication: Hannah Ritchie, Pablo Rosado, and Max Roser (2023)-“Energy”. Data Adapted from Ember, Energy Institute. Available online: https://ourworldindata.org/grapher/carbon-intensity-electricity (accessed on 25 May 2025).
- Bento, C.; Lopes, T.F.; Rodrigues, P.; Gírio, F.; Silva, C. Biogas Reforming as a Sustainable Solution for Hydrogen Production: Comparative Environmental Metrics with Steam-Methane Reforming and Water Electrolysis in the Portuguese Context. Int. J. Hydrogen Energy 2024, 66, 661–675. [Google Scholar] [CrossRef]
- Prussi, M.; Yugo, M.; De Padella, L.; Edwards, R.; Lonza, L. JEC Well-to-Tank Report V5; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Favoino, E.; Giavini, M.; Rupp, M. Bio-Waste Generation in the EU: Current Capture Levels and Future Potential; BIC/Zero Waste Europe: Brussels, Belgium, 2020. [Google Scholar]
- Papageorgiou, Z.; Founti, M.; Nikolaidis, G.N.; González, R.; García-Cascallana, J.; Gutiérrez-Bravo, J.; Gómez, X. Decentralized Biogas Production in Urban Areas: Studying the Feasibility of Using High-Efficiency Engines. Eng 2023, 4, 2204–2225. [Google Scholar] [CrossRef]
- Wang, J.; Okopi, S.I.; Ma, H.; Wang, M.; Chen, R.; Tian, W.; Xu, F. Life Cycle Assessment of the Integration of Anaerobic Digestion and Pyrolysis for Treatment of Municipal Solid Waste. Bioresour. Technol. 2021, 338, 125486. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Jia, W.; Chen, T.; Ling, Y. Life Cycle Assessment of Food Waste Anaerobic Digestion with Hydrothermal and Ionizing Radiation Pretreatment. J. Clean. Prod. 2022, 338, 130611. [Google Scholar] [CrossRef]
- Starr, K.; Gabarrell, X.; Villalba, G.; Talens, L.; Lombardi, L. Life Cycle Assessment of Biogas Upgrading Technologies. Waste Manag. 2012, 32, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, S.; Khadem Masjedi, S.; Kazemi, A.; Khaki, E.; Moeinaddini, M.; Irving Olsen, S. Life Cycle Assessment of Reverse Osmosis for High-Salinity Seawater Desalination Process: Potable and Industrial Water Production. J. Clean. Prod. 2023, 382, 135299. [Google Scholar] [CrossRef]
- Sutar, D.D.; Jadhav, S.V. Life Cycle Assessment of Methanol Production by Natural Gas Route. Mater. Today Proc. 2022, 57, 1559–1566. [Google Scholar] [CrossRef]
- Kumar, A.; Du, F.; Lienhard, J.H. Caustic Soda Production, Energy Efficiency, and Electrolyzers. ACS Energy Lett. 2021, 6, 3563–3566. [Google Scholar] [CrossRef]
- Kumar, A.; Phillips, K.R.; Thiel, G.P.; Schröder, U.; Lienhard, J.H. Direct Electrosynthesis of Sodium Hydroxide and Hydrochloric Acid from Brine Streams. Nat. Catal. 2019, 2, 106–113. [Google Scholar] [CrossRef]
- Singh, V.; Dincer, I.; Rosen, M.A. Chapter 4.2 -Life Cycle Assessment of Ammonia Production Methods. In Exergetic Energetic Environ. Dimens.; Dincer, I., Colpan, C.O., Kizilkan, O., Eds.; Academic Press: Cambridge, Massachusetts, 2018; pp. 935–959. [Google Scholar] [CrossRef]
- Chen, G. CEP Magazine. 2020. Available online: https://www.aiche-cep.com/cepmagazine/june_2020/MobilePagedArticle.action?articleId=1591898#articleId1591898 (accessed on 25 May 2025).
- Jiresten, E.; Larsson, O. Life Cycle Assessment of Acetone Production from Captured Carbon Dioxide: Using Bio-Fermentation at the PYROCO2-Pilot Plant. Master’s Thesis, Chalmers University of Technology, Chalmers, Sweeden, 2022. [Google Scholar]
- Von Der Assen, N.; Müller, L.J.; Steingrube, A.; Voll, P.; Bardow, A. Selecting CO2 Sources for CO2 Utilization by Environmental-Merit-Order Curves. Environ. Sci. Technol. 2016, 50, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Ecoinvent Ecoinvent Database V3.1 2014. Available online: https://ecoinvent.org/ (accessed on 25 May 2025).
- Naukkarinen, M. Life Cycle Assessment Study of a Sulfuric Acid Mmanufacturing Process in the Chemical Pulping Industry. Master’s Thesis, Lappeenranta–Lahti University of Technology, Lappeenranta, Finland, 2023. [Google Scholar]
- Young, B.; Hottle, T.; Hawkins, T.; Jamieson, M.; Cooney, G.; Motazedi, K.; Bergerson, J. Expansion of the Petroleum Refinery Life Cycle Inventory Model to Support Characterization of a Full Suite of Commonly Tracked Impact Potentials. Environ. Sci. Technol. 2019, 53, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Doran, P.M. Bioprocess Engineering Principles, 2nd ed.; Academic Press: New York, NY, USA, 2012; ISBN 9780080917702. [Google Scholar]
- Fábrica Cibra-Pataias|EMAS. Available online: https://emas.apambiente.pt/content/fabrica-cibra-pataias (accessed on 25 May 2025).
- Centro de Documentação. Available online: https://www.secil.pt/pt/centro-de-documentacao (accessed on 25 May 2025).

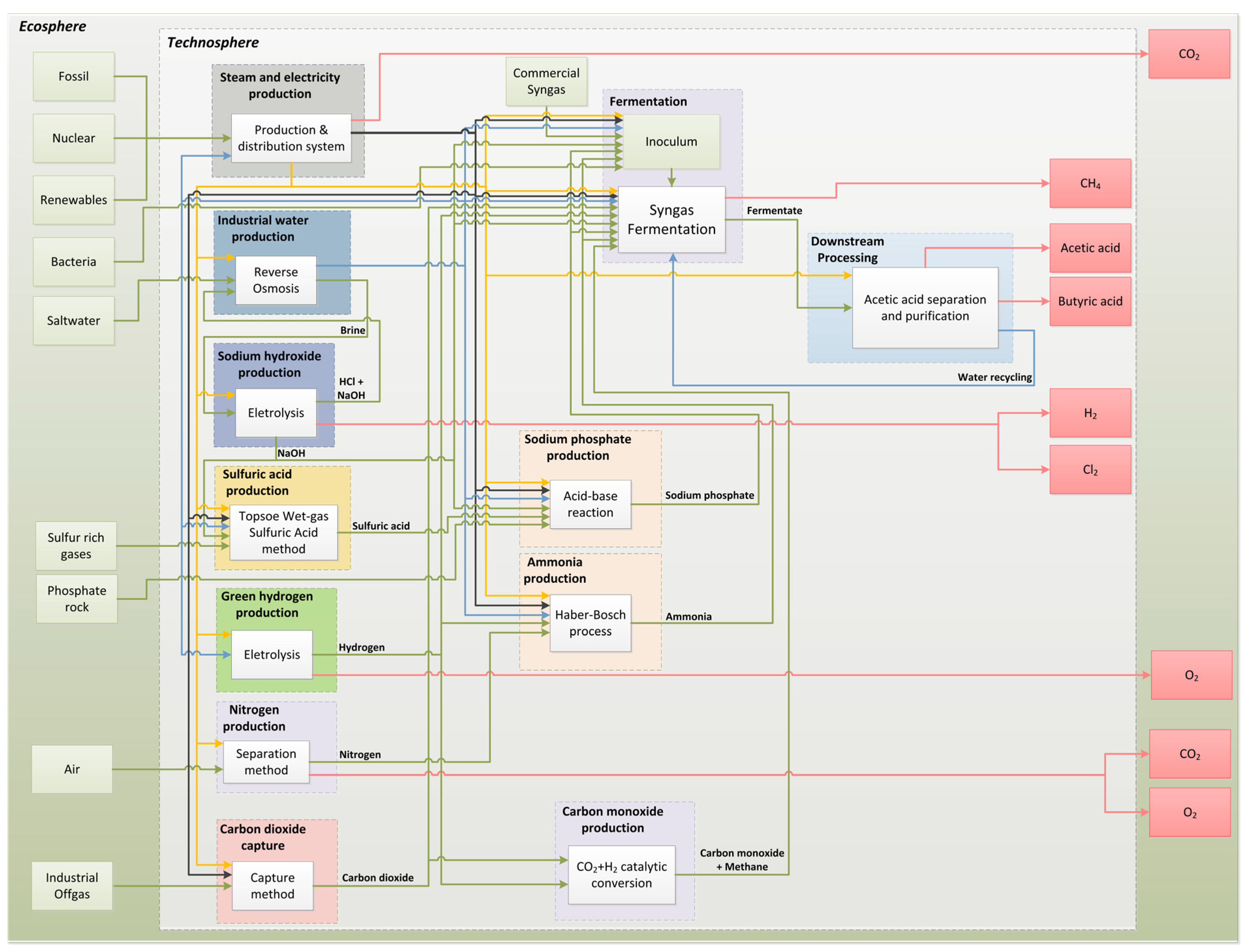

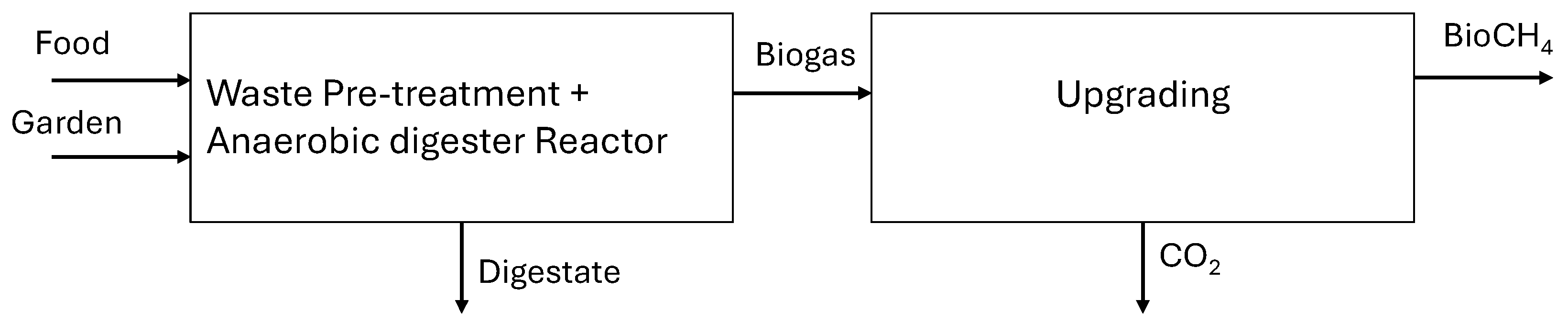

| Material Flows | Quantity | Unit |
|---|---|---|
| Inputs | ||
| Methanol | 0.539 | kg |
| CO | 0.509 | kg |
| Heat | 1.752 | MJ |
| Electricity | 0.002 | kWh |
| Outputs | ||
| CO | 0.022 | kg |
| CO2 | 0.002 | kg |
| CH4 | 0.001 | kg |
| Acetic acid * | 1 | kg |
| Material Flows | Quantity | Unit |
|---|---|---|
| Inputs | ||
| Water | 10.19 | kg |
| Yeast extract | 0.13 | kg |
| NaCl | 0.13 | kg |
| NH4Cl | 0.16 | kg |
| KCl | 0.02 | kg |
| KH2PO4 | 0.02 | kg |
| MgSO4.7H2O | 0.03 | kg |
| CaCl2.2H2O | 0.01 | kg |
| NaH2PO4 | 0.23 | kg |
| Na2HPO4 | 0.75 | kg |
| Inoculum | 0.03 | kg |
| Syngas | 4.01 | kg |
| CO | 1.51 | kg |
| CO2 | 1.90 | kg |
| H2 | 0.12 | kg |
| CH4 | 0.48 | kg |
| Energy | 8.30 | kWh |
| Reactor + support equipment | 1.10 | kWh |
| Centrifuge | 7.20 | kWh |
| Outputs | ||
| Liquid products | 1.18 | kg |
| Acetic acid * | 1.00 | kg |
| Butyric acid | 0.18 | kg |
| Off-gas | 3.56 | kg |
| CO | 0.53 | kg |
| CO2 | 2.43 | kg |
| H2 | 0.06 | kg |
| CH4 | 0.49 | kg |
| Dry Biomass | 0.0994 | kg |
| Material Flows | Quantity | Unit |
|---|---|---|
| Inputs | ||
| H2 | 0.158 | kg |
| CO2 | 1.710 | kg |
| NH3 | 0.019 | kg |
| Electricity | 0.003 | kWh |
| Water | 23.527 | kg |
| Outputs | ||
| Acetic acid * | 1 | kg |
| Material Flows | Quantity | Unit |
|---|---|---|
| Inputs | ||
| Energy for CO2 capture | 0.390 | kWh |
| MES electricity | 2.800 | kWh |
| Anolyte water | 0.680 | kg |
| CO2 capture | 1.470 | kg |
| Sodium phosphate | 1.000 | kg |
| Outputs | ||
| Acetic acid * | 1 | kg |
| O2 | 1.070 | kg |
| Electricity Mix/Literature Source | GWP100, IPCC AR5 (kg CO2-eq/kg AA) | ||||
|---|---|---|---|---|---|
| No Allocation | Mass Allocation | ||||
| Portugal | France | Portugal | France | ||
| Own calculations | 2023 | 0.94 | 0.58 | 0.66 | 0.49 |
| 5-year average (2019–2023) | 1.15 | 0.61 | 0.76 | 0.50 | |
| 2050—Natural gas | 0.40 | 0.40 | 0.40 | 0.40 | |
| 2050—Biomethane | −0.38 | –0.38 | −0.38 | −0.38 | |
| Literature | UK—2016 [30] | 1.3 | |||
| USA several years [17,21,22,23,24,25,26] | 1–2.84 | ||||
| Process | Electricity | Heat | Demineralized Water |
|---|---|---|---|
| Fossil–methanol carbonylation (benchmark) | CO production | Methanol synthesis | Methanol synthesis |
| Bio-methanol carbonylation | Methanol synthesis | Acetic acid production | Methanol synthesis |
| Microbial electrosynthesis | Acetic acid production | Acetic acid production | Sulfuric acid (for sodium phosphate production) |
| Thermophilic acetogenic fermentation | H2 production | Acetic acid production | Acetic acid production |
| Acetogenic fermentation | Acetic acid production CO production | Sodium phosphate production | Sulfuric acid (for sodium phosphate production) Acetic acid production |
| Year | CO2 Flow fromCement Plant (kton) | CO and H2 Adjusted for CO2 from Cement Plant | Acetic Acid Production (kton) | KPI Acetogenic Fermentation vs. Benchmark | ||||
|---|---|---|---|---|---|---|---|---|
| CO (kton) | H2 (kton) | Electricity (kWh) | Heat (MJ) | Water (L) | CO2-eq (kton) | |||
| 2019 | 1367 | 1087 | 88 | 721 | 2201 | −1883 | 6401 | −1911 |
| 2020 | 1962 | 1560 | 126 | 1035 | 3159 | −2703 | 9187 | −2743 |
| 2021 | 353 | 281 | 23 | 186 | 568 | −486 | 1653 | −493 |
| 2022 | 97 | 77 | 6 | 51 | 156 | −134 | 454 | −136 |
| 2023 | 63 | 50 | 4 | 33 | 101 | −87 | 295 | −88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.; Brac de la Perrière, A.; Moura, P.; Silva, C. Industrial Off-Gas Fermentation for Acetic Acid Production: A Carbon Footprint Assessment in the Context of Energy Transition. C 2025, 11, 54. https://doi.org/10.3390/c11030054
Pacheco M, Brac de la Perrière A, Moura P, Silva C. Industrial Off-Gas Fermentation for Acetic Acid Production: A Carbon Footprint Assessment in the Context of Energy Transition. C. 2025; 11(3):54. https://doi.org/10.3390/c11030054
Chicago/Turabian StylePacheco, Marta, Adrien Brac de la Perrière, Patrícia Moura, and Carla Silva. 2025. "Industrial Off-Gas Fermentation for Acetic Acid Production: A Carbon Footprint Assessment in the Context of Energy Transition" C 11, no. 3: 54. https://doi.org/10.3390/c11030054
APA StylePacheco, M., Brac de la Perrière, A., Moura, P., & Silva, C. (2025). Industrial Off-Gas Fermentation for Acetic Acid Production: A Carbon Footprint Assessment in the Context of Energy Transition. C, 11(3), 54. https://doi.org/10.3390/c11030054








