Annealing Effects of ZnO Thin Film on Photocatalytic Performances of Graphene Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. ZnO Thin Film Preparation
2.3. Selection of Annealing Temperatures
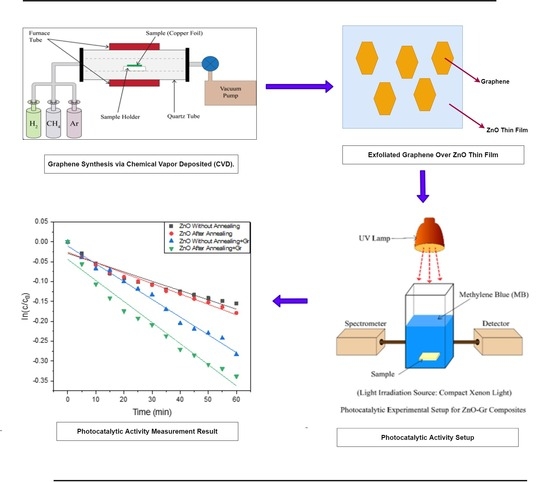
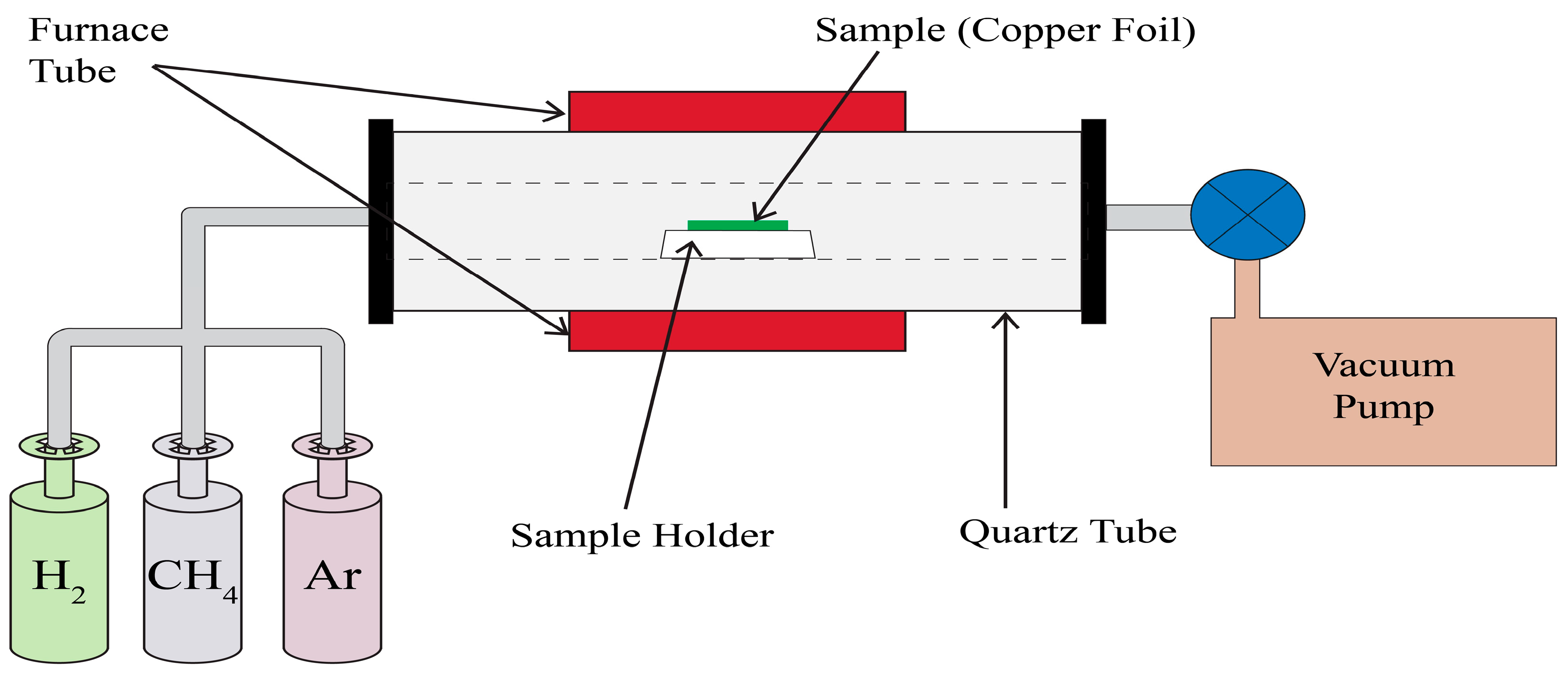
2.4. Graphene Synthesis via CVD Method
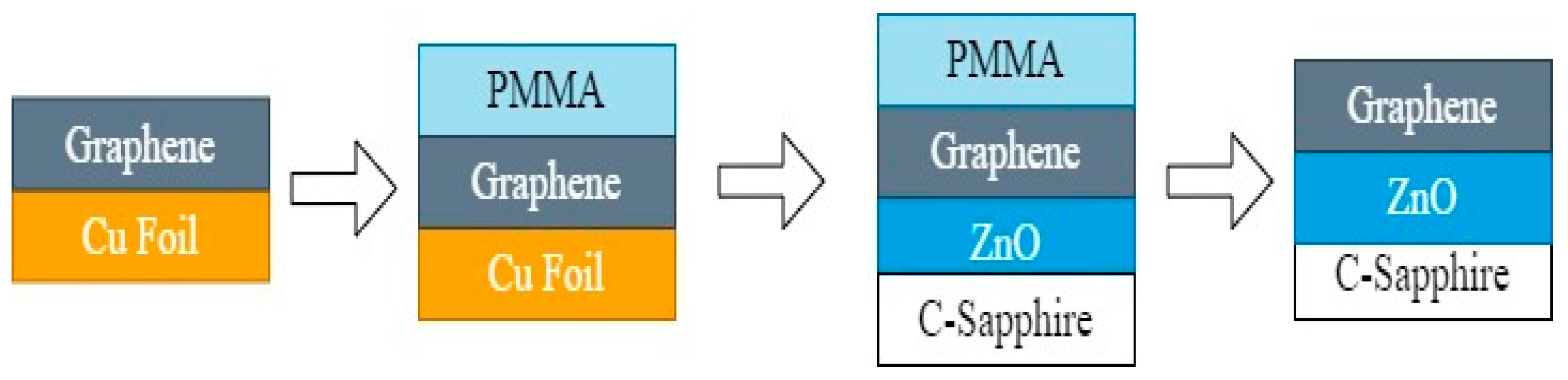
2.5. Exfoliation of Graphene Layer from Copper Foil
2.6. ZnO-Graphene Composite Preparations
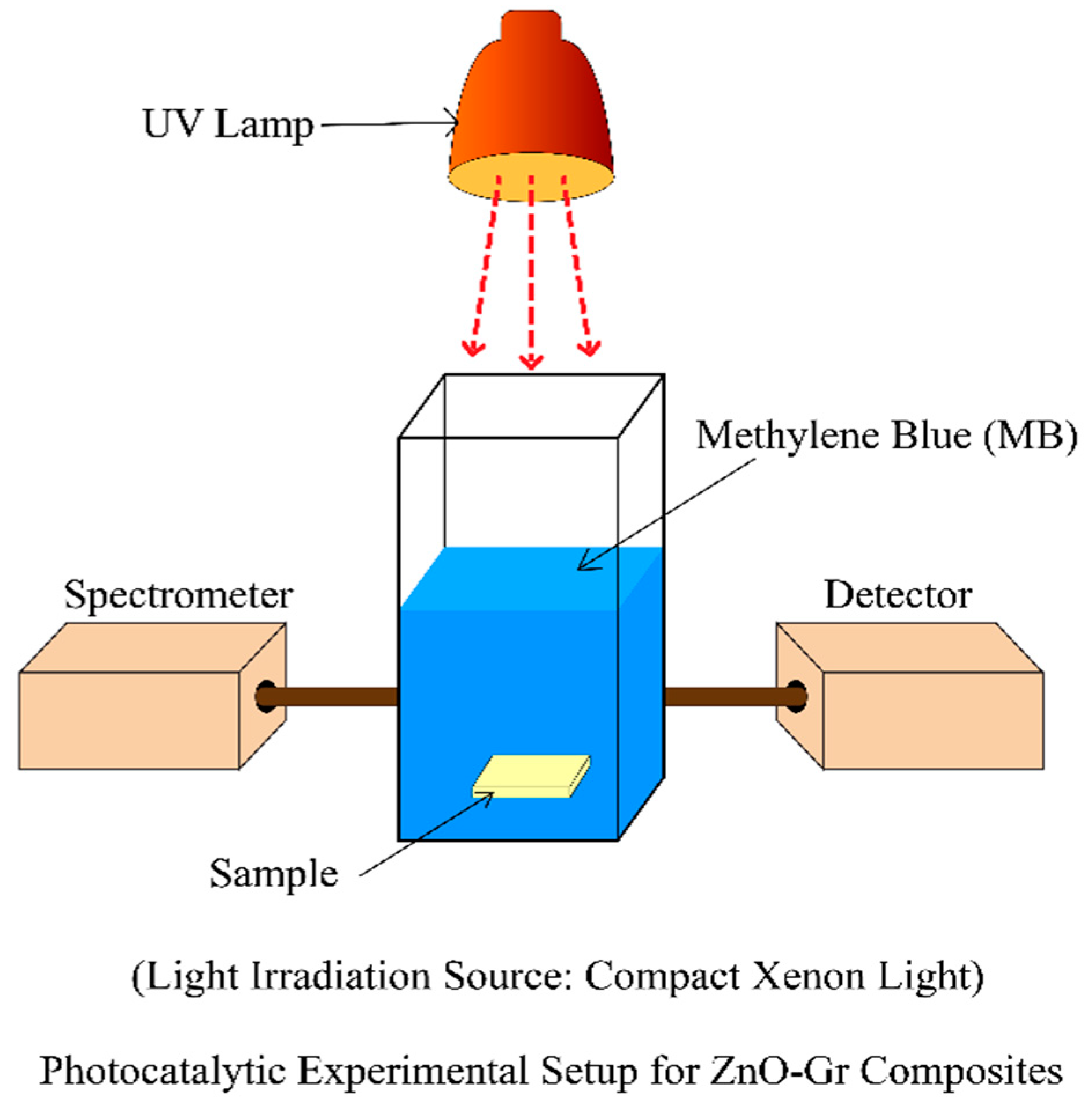
2.7. Experimental Setup for Photocatalytic Activity Measurement
2.8. Material Characterization Apparatus
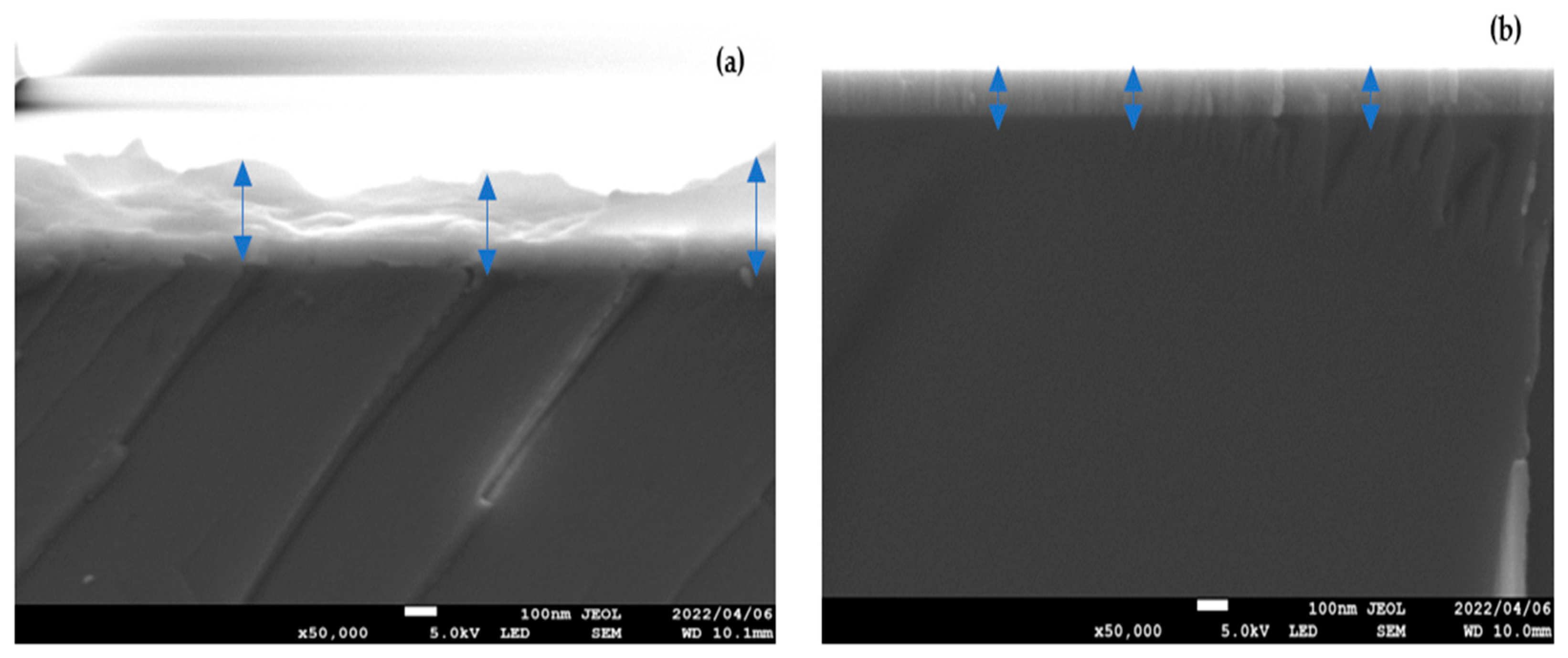
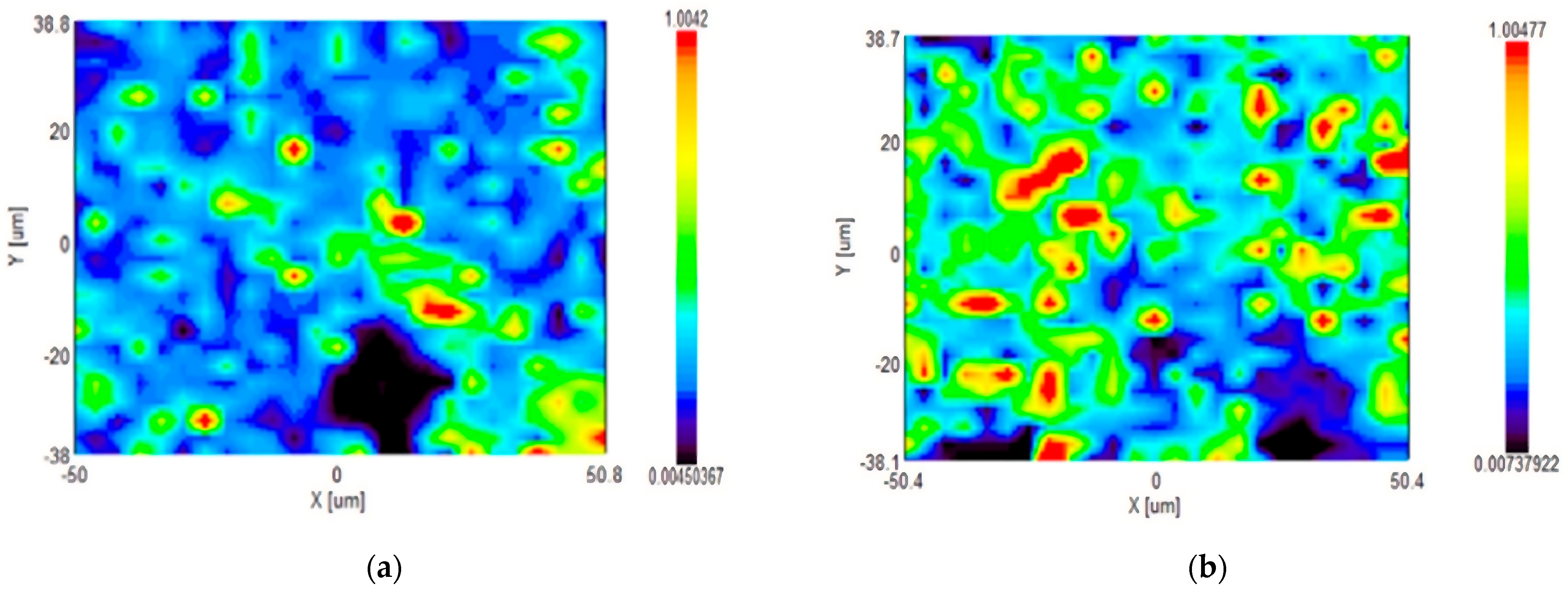
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.M.; Pyun, Y.B.; Yi, J.; Choung, J.W.; Park, W., II. ZnO Nanorod−Graphene Hybrid Architectures for Multifunctional Conductors. J. Phys. Chem. 2009, 113, 19134–19138. [Google Scholar] [CrossRef]
- Lin, J.; Penchev, M.; Wang, G.; Paul, R.K.; Zhong, J.; Jing, X.; Ozkan, M. Heterogeneous Graphene Nanostructures: ZnO Nanostructures Grown on Large-Area Graphene Layers. J. Nano Micro Small 2010, 6, 2448–2452. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Hadiyawarmam; Yoon, A.; Kim, M.; Yi, G.-C.; Liu, C. Hydrothermally grown ZnO nanostructures on few-layer graphene sheets. J. Nanotechnol. 2011, 22, 245603. [Google Scholar] [CrossRef]
- Chandandraiahgari, C.R.; De Bellis, G.; Balijepalli, S.K.; Kaciulis, S.; Ballirano, P.; Migliori, A.; Morandi, V.; Caneve, L.; Sarto, F.; Sarto, M.S. Control of the size and density of ZnO-nanorods grown onto graphene nanoplatelets in aqueous suspensions. J. RSC Adv. 2016, 6, 83217–83225. [Google Scholar] [CrossRef]
- Gon, K.J.; Byungrak, S.; Santanu, M.; Nicholas, S.; Alex, B.; Osung, K.; Jong, C.M.; Yeol, C.H.; Sam, P. A review of lithium and non-lithium based solid state batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium Batteries: Status, Prospects and Future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Raub, A.A.M.; Yunas, J.; Mohamed, M.A.; Kazmi, J.; Ridwan, J. Structural and Optical Properties Investigation of Graphene Oxide coated ZnO nanorods for Enhanced Photocatalytic Effect. In Proceedings of the 2021 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Kuala Lumpur, Malaysia, 2–4 August 2021; pp. 104–107. [Google Scholar]
- Machado, B.F.; Serp, P. Graphene-based mater ials for catalysis. J. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Islam, M.A.; Al Rafi, J.; Uddin, M.A. Modeling and formation of a single-walled carbon nanotube (SWCNT) based heterostructure for efficient solar energy: Performance and defect analysis by numerical simulation. AIP Adv. 2023, 13, 115201. [Google Scholar] [CrossRef]
- Uddin, M.A.; Rafi, J.A.; Islam, M.A.; Mominuzzaman, S.M.; Nath, I.D. Modeling and Numerical Analysis of Heterostructure Single-Walled Carbon Nanotube (SWCNT) Solar Cell. In Proceedings of the 2022 12th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 21–23 December 2022; pp. 388–391. [Google Scholar]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef]
- Wang, Z.G.; Chen, Y.F.; Li, P.J.; Hao, X.; Liu, J.B.; Huang, R.; Li, Y.R. Flexible graphene-based electroluminescent devices. ACS Nano 2011, 5, 7149–7154. [Google Scholar] [CrossRef]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Sun, S.; Salim, T.; Wu, S.; Huan, X.; He, Q.; Lam, Y.M.; Zhang, H. Organic photovoltaic devices using highly flexible reduced graphene oxide films as transparent electrodes. ACS Nano 2010, 4, 5263–5268. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Y.M.; Bol, A.A.; Jenkins, K.A.; Xia, F.; Farmer, D.B.; Zhu, Y.; Avouris, P. High-frequency, scaled graphene transistors on diamond-like carbon. Nature 2011, 472, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.H.C.; Guinea, F.; Peres, N.M.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2014, 306, 666–669. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Gao, L.; Guest, J.R.; Guisinger, N.P. Epitaxial graphene on Cu (111). Nano Lett. 2010, 10, 3512–3516. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef]
- Wang, X.; You, H.; Liu, F.; Li, M.; Wan, L.; Li, S.; Li, Q.; Xu, Y.; Tian, R.; Yu, Z.; et al. Large-Scale Synthesis of Few-Layered Graphene using CVD. Chem. Vap. Depos. 2009, 15, 53–56. [Google Scholar] [CrossRef]
- Al Rafi, J.; Islam, A.; Nuzhat, T.; Honda, M.; Ichikawa, Y. Effects of Argon (Ar) on Synthesis and Photocatalytic Activities of Graphene. IEEE Trans. Nanotechnol. 2023, 22, 321–327. [Google Scholar] [CrossRef]
- Ismach, A.; Druzgalski, C.; Penwell, S.; Schwartzberg, A.; Zheng, M.; Javey, A.; Bokor, J.; Zhang, Y. Direct chemical vapor deposition of graphene on dielectric surfaces. Nano Lett. 2010, 10, 1542–1548. [Google Scholar] [CrossRef]
- Sattler, K.D. Carbon Nanomaterials. In Sourcebook: Graphene, Fullerenes, Nanotubes, and Nanodiamonds, 1st ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2016; Volume I, p. 630. [Google Scholar]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide. 1: Highyield synthesis and flexibility of the particles. Carbon 2014, 42, 2929–2937. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Sato, H.; Kagamitani, Y.; Sato, H.; Yoshikawa, A.; Fukuda, T. Solvothermal growth of ZnO. Prog. Cryst. Growth Charact. Mater. 2006, 52, 280–335. [Google Scholar] [CrossRef]
- Wang, L.; Tang, K.; Zhang, M.; Xu, J. Facile synthesis of Mn-doped ZnO porous nanosheets as anode materials for lithium-ion batteries with a better cycle durability. Nanoscale Res. Lett. 2015, 3, 3–7. [Google Scholar] [CrossRef]
- Sagar, R.U.R.; Mahmood, N.; Stadler, F.J.; Anwar, T.; Navale, S.T.; Shehzad, K.; Du, B. High-capacity retention anode material for lithium-ion battery. Electrochim. Acta 2016, 21, 156–163. [Google Scholar] [CrossRef]
- Coleman, V.A.; Jagadish, C. Basic properties and applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures: Processing, Properties, and Applications; Jagadish, S.P.E.C., Ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; Chapter 1; pp. 1–20. [Google Scholar]
- Oprea, O.; Andronescu, E.; Ficai, D.; Ficai, A.; Oktar, F.N.; Yetmez, M. ZnO applications and challenges. Curr. Org. Chem. 2014, 18, 192–203. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, K.; Yang, Z.; Ye, C.; Li, X.; Yan, K. Facile Synthesis of Porous ZnO Nanoparticles Efficient for Photocatalytic Degradation of Biomass-Derived Bisphenol A Under Simulated Sunlight Irradiation. Front. Bioeng. Biotechnol. 2021, 8, 616780. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 29. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Candal, R.; Martínez-de la Cruz, A. New Visible-Light Active Semiconductors. In Photocatalytic Semiconductors; Hernández-Ramírez, A., Medina-Ramírez, I., Eds.; Springer: Cham, Switzerland, 2015; pp. 41–67. [Google Scholar]
- Zhang, Q.; Honda, M.; Ichikawa, Y. Seed layer morphology influencing ZnO nanorod growth by hydrothermal synthesis. In Transactions of the Materials Research Society of Japan; The Materials Research Society of Japan: Yokohama, Japan, 2018; Volume 43, pp. 349–353. [Google Scholar]
- Vashista, M.; Paul, S. Correlation between full width at half maximum (FWHM) of XRD peak with residual stress on ground surfaces. Philos. Mag. 2012, 92, 4194–4204. [Google Scholar] [CrossRef]
- Honda, M.; Zhang, Q.; Tateyama, H.; Ichikawa, Y. Densely aligned ZnO nanoneedle arrays prepared via room temperature growth. J. Ceram. Soc. Jpn. 2018, 126, 367–371. [Google Scholar] [CrossRef]
- Thein, M.T.; Chim, J.E.; Pung, S.-Y.; Pung, Y.-F. Highly UV light driven WOx@ZnO nanocomposites synthesized by liquid impregnation method. J. Ind. Eng. Chem. 2017, 46, 119–129. [Google Scholar] [CrossRef]
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Lai, C.W.; Khee, C.-S.; Voona, C.H.; Hashim, U.; NHidayah, M.S. Synthesis of Single-layer Graphene: A Review of Recent Development. Procedia Chem. 2016, 19, 916–921. [Google Scholar] [CrossRef]


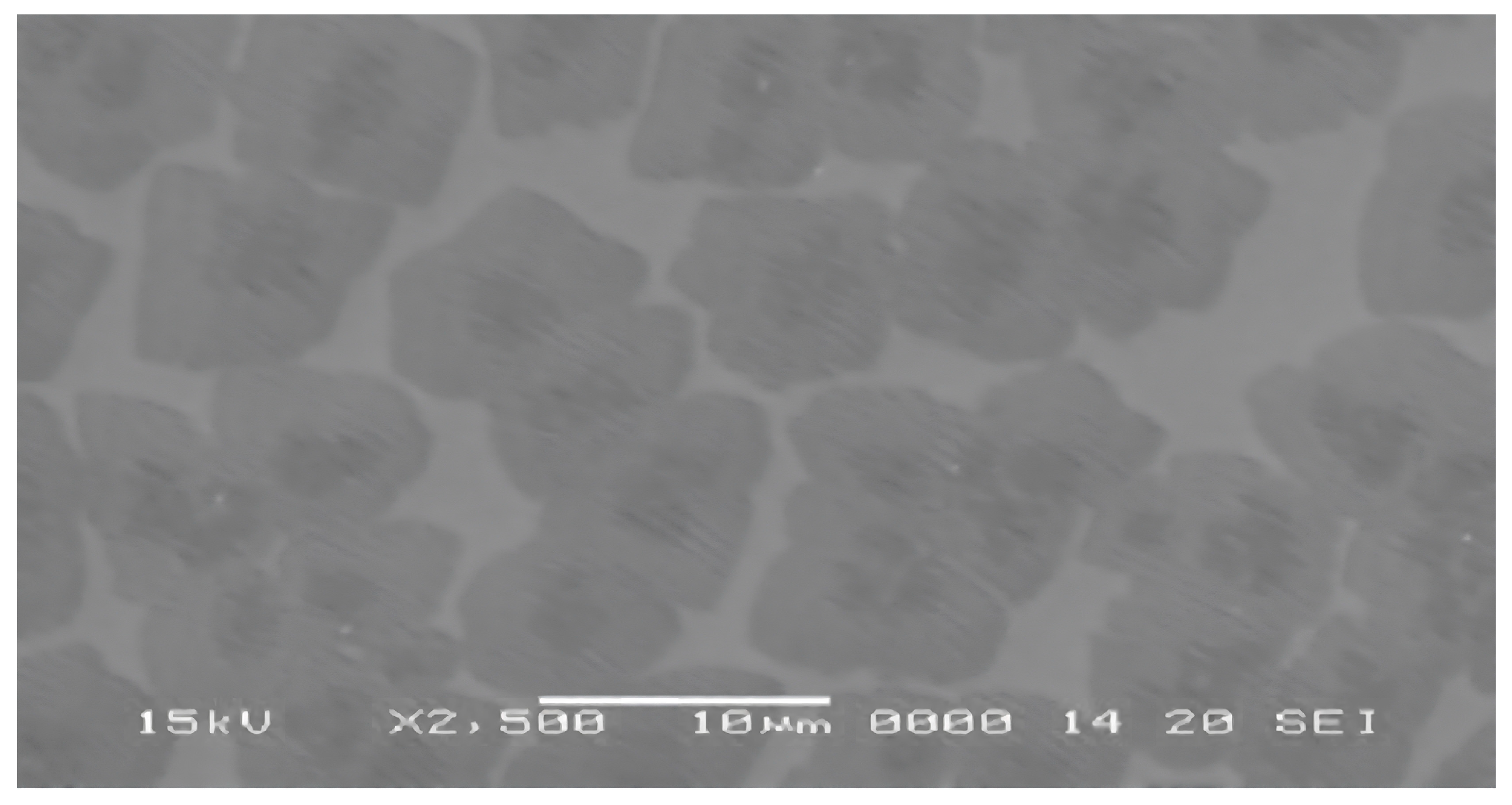
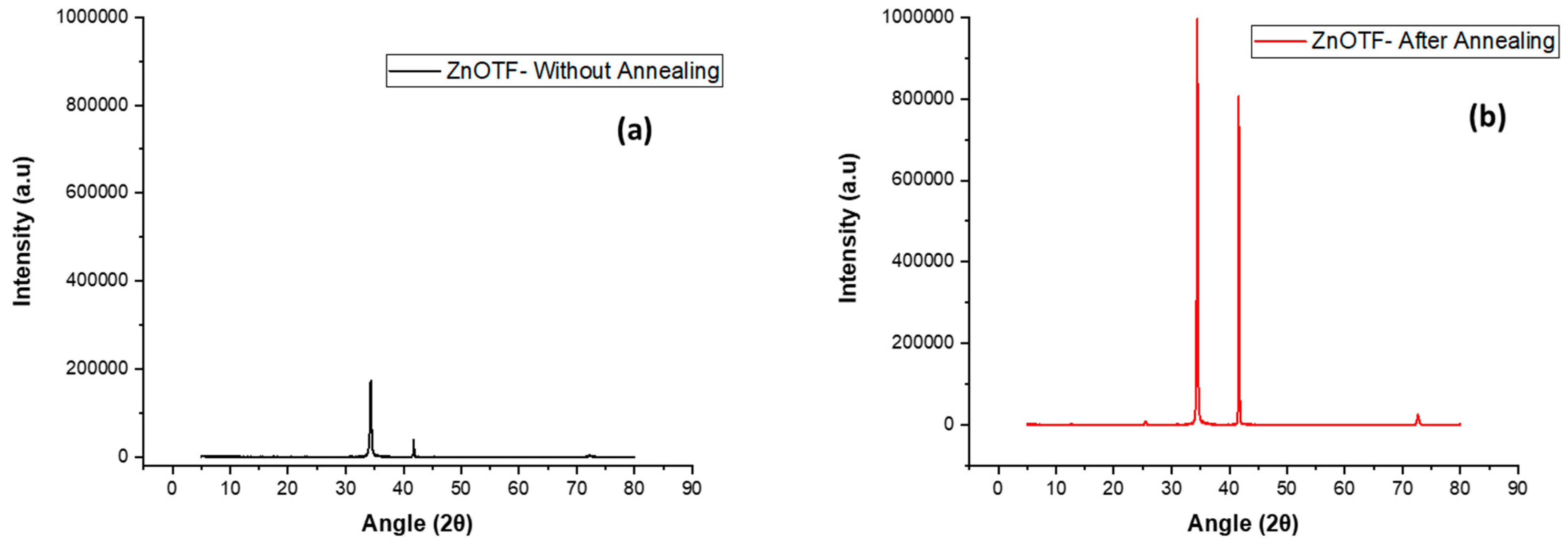
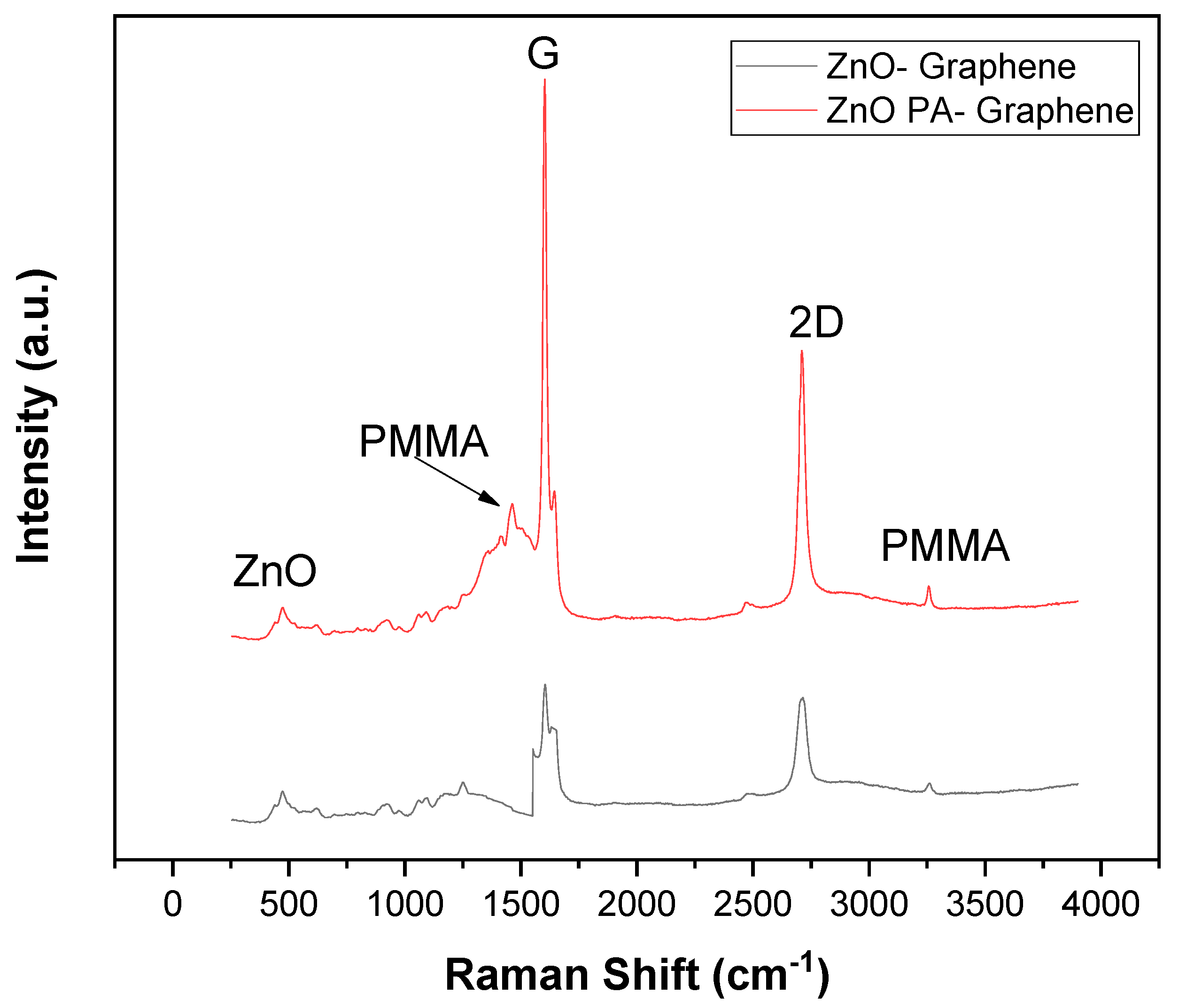
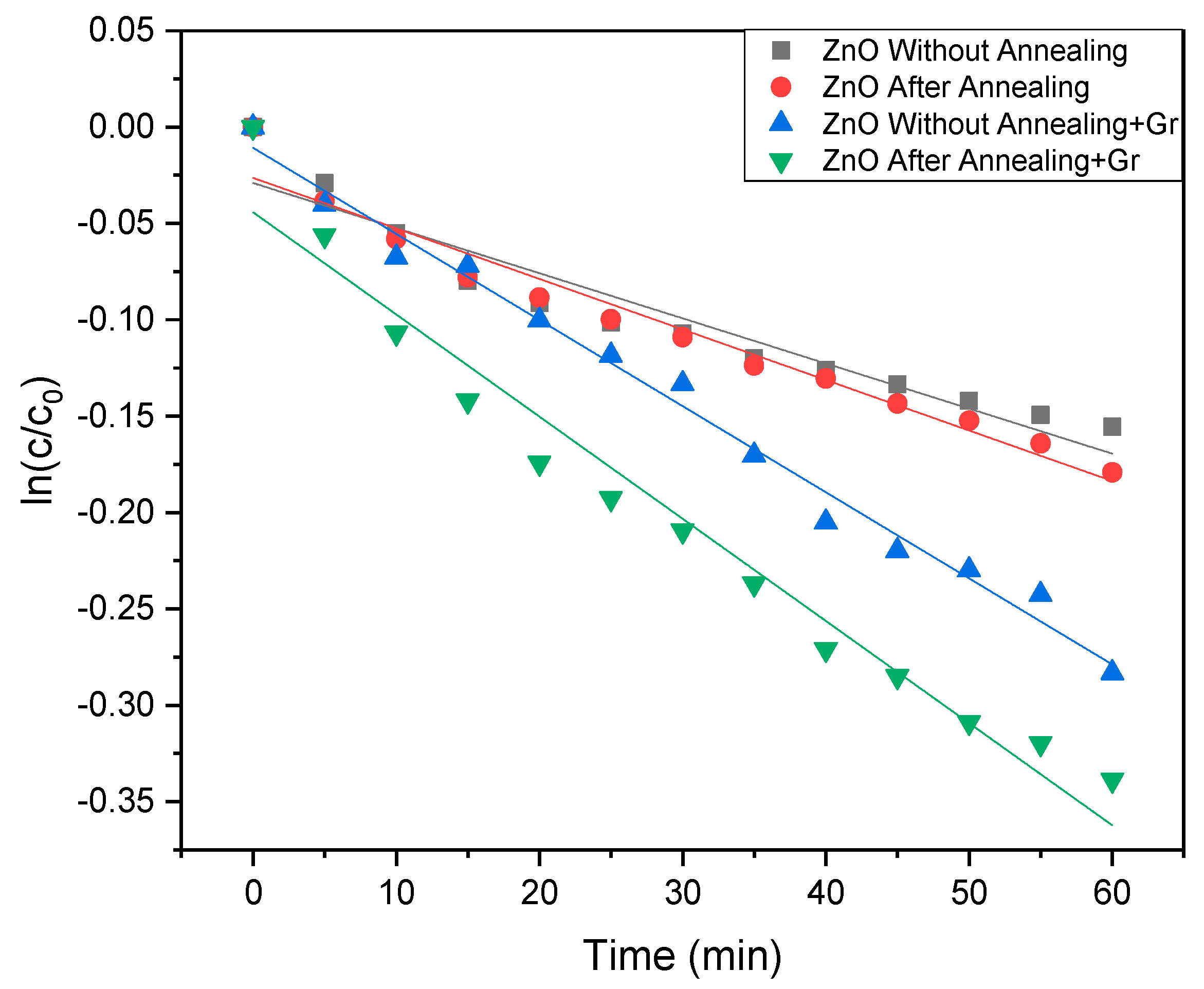

| Sample | FWHM | Crystalline Size (nm) |
|---|---|---|
| ZnO Thin Film | 0.31458 | 27.62 |
| Annealed ZnO Thin Film | 0.22133 | 39.27 |
| Sample | Constant Rate (C.R.) | C.R. Enhancement Compared to ZnO | %Photodegradation |
|---|---|---|---|
| ZnO Thin Film | 3.91 × 10−5/min | 1 | 14% |
| Annealed ZnO Thin Film | 4.36 × 10−5/min | 1.12 | 18% |
| ZnO Thin Film-Graphene | 7.45 × 10−5/min | 1.91 | 25% |
| Annealed ZnO Thin Film-Graphene | 8.83 × 10−5/min | 2.25 | 28% |
| Sample | Single Layer | Double Layer | Multilayer |
|---|---|---|---|
| ZnO Thin Film-Graphene | 11% | 28% | 61% |
| Annealed ZnO Thin Film-Graphene | 26% | 42% | 32% |
| Sample | Reaction Rate “k” (×10−5 min−1) | Perimeter (μm) |
|---|---|---|
| ZnO Thin Film-Graphene | 7.45 | 29.59 |
| Annealed ZnO Thin Film-Graphene | 8.83 | 45.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafi, J.A.; Kanda, Y.; Honda, M.; Ichikawa, Y. Annealing Effects of ZnO Thin Film on Photocatalytic Performances of Graphene Composites. C 2024, 10, 4. https://doi.org/10.3390/c10010004
Rafi JA, Kanda Y, Honda M, Ichikawa Y. Annealing Effects of ZnO Thin Film on Photocatalytic Performances of Graphene Composites. C. 2024; 10(1):4. https://doi.org/10.3390/c10010004
Chicago/Turabian StyleRafi, Jobair Al, Yu Kanda, Mitsuhiro Honda, and Yo Ichikawa. 2024. "Annealing Effects of ZnO Thin Film on Photocatalytic Performances of Graphene Composites" C 10, no. 1: 4. https://doi.org/10.3390/c10010004
APA StyleRafi, J. A., Kanda, Y., Honda, M., & Ichikawa, Y. (2024). Annealing Effects of ZnO Thin Film on Photocatalytic Performances of Graphene Composites. C, 10(1), 4. https://doi.org/10.3390/c10010004