CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Isolation, Culture, and Differentiation of Rat Oligodendrocyte Precursor Cells
2.3. Nucleotide Transfection for siRNA, gRNA, or Oligopeptide Expression Plasmid
2.4. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.5. Immunoblotting and Immunoprecipitation
2.6. Affinity-Precipitation Assay for Ras
2.7. Statistical Analyses
2.8. Ethics Statement
3. Results
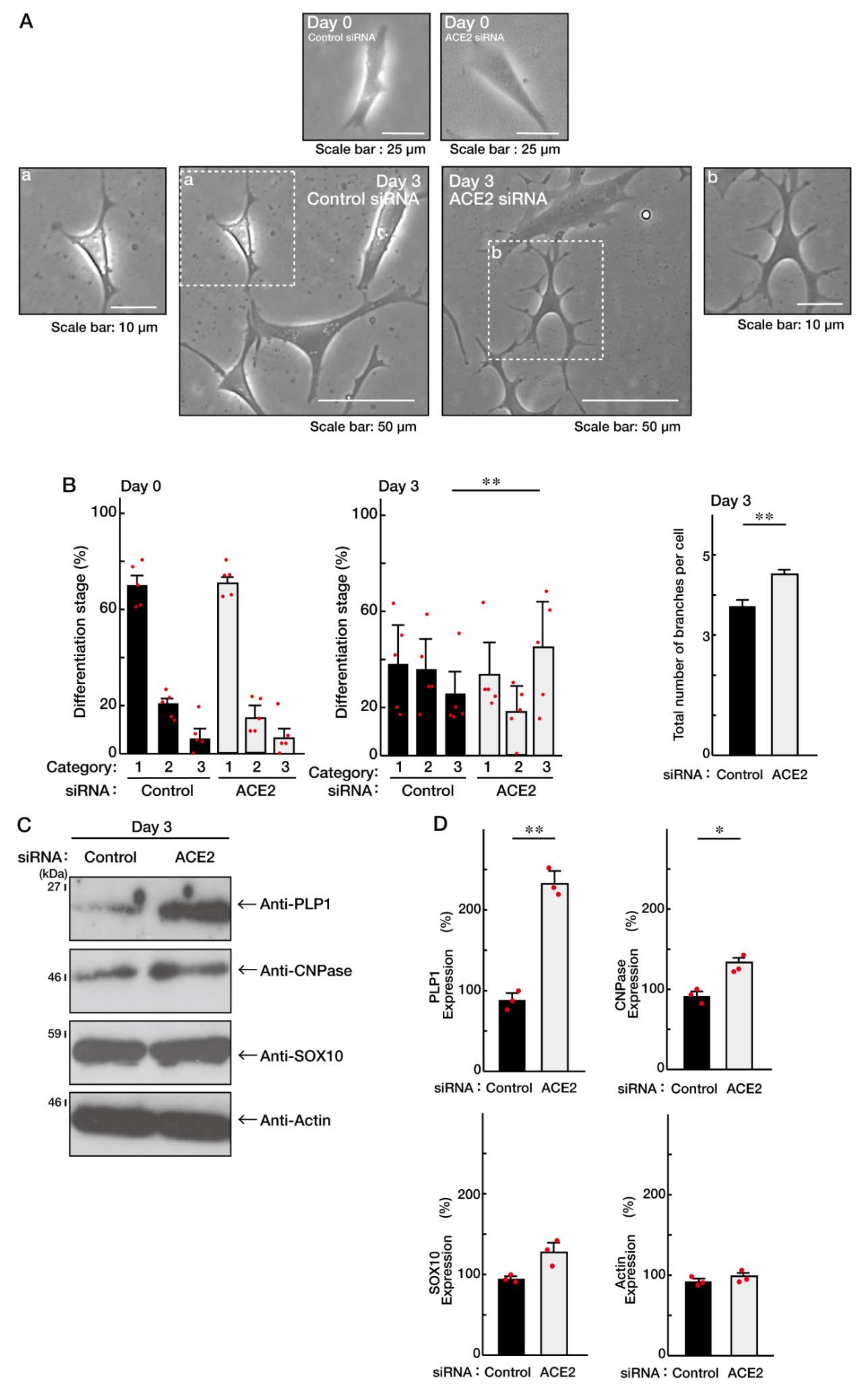
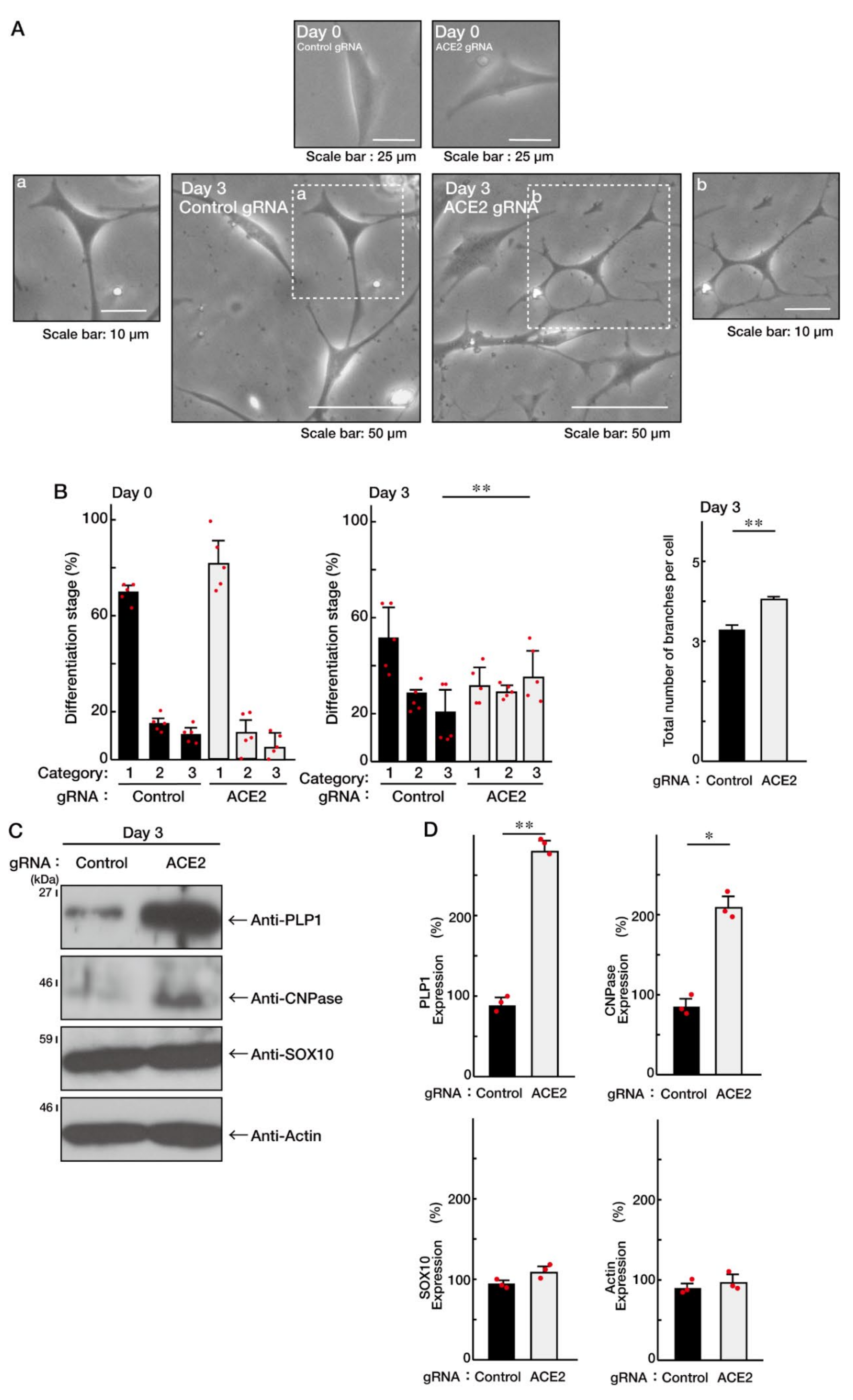
3.1. ACE2 Negatively Regulates Oligodendroglial Cell Morphological Differentiation
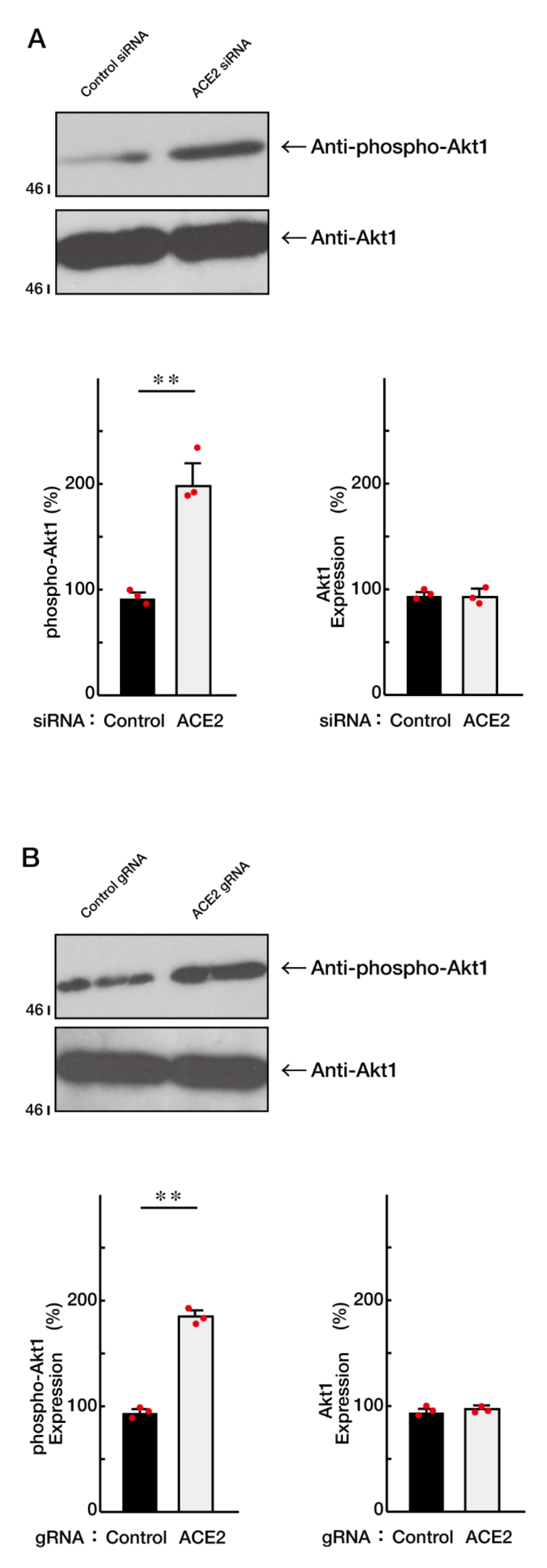
3.2. ACE2 Preferentially Interacts with an Active GTP-Bound Ras to Interact with Ras
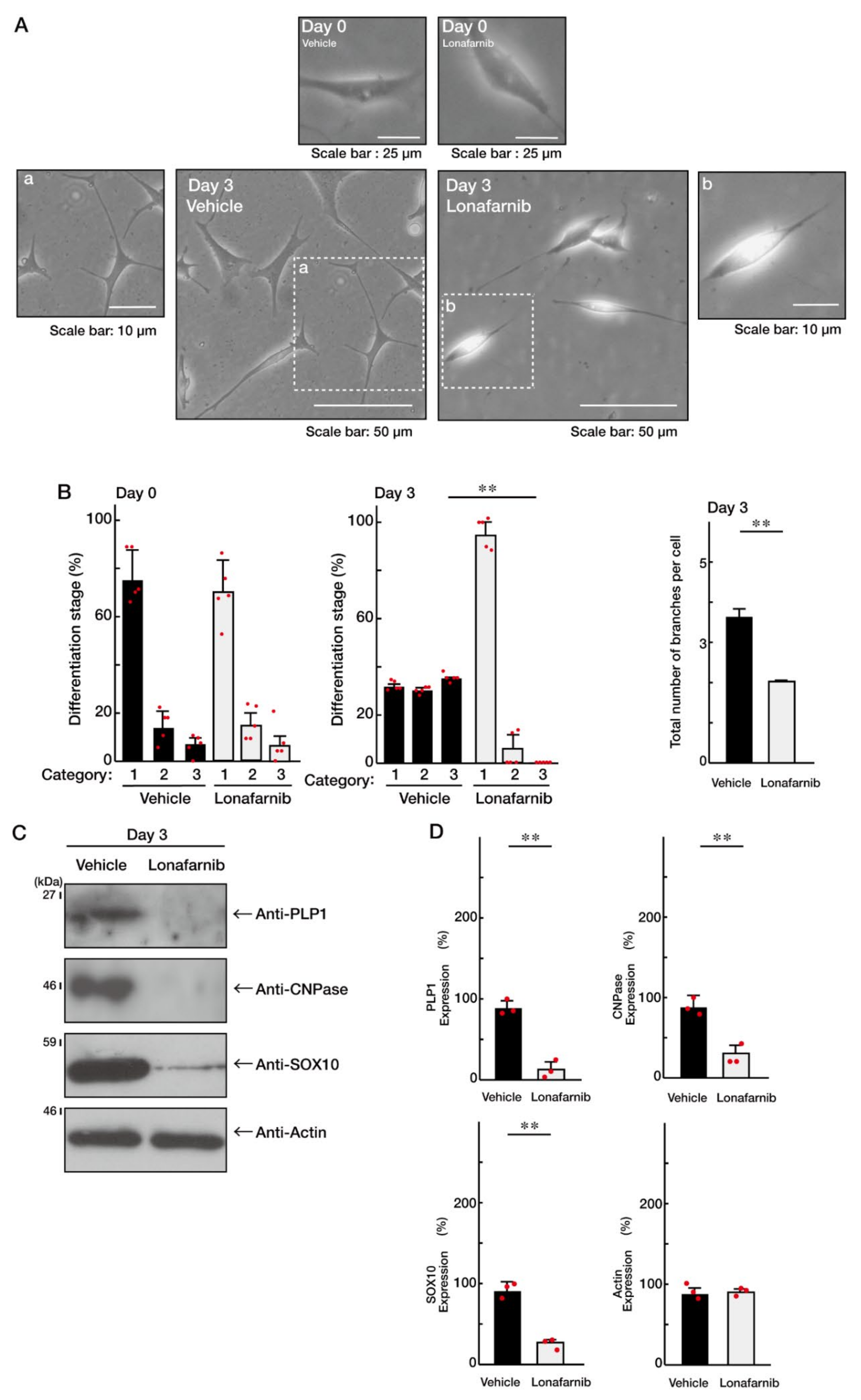
3.3. Ras Positively Regulates Morphological Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simons, M.; Lyons, D.A. Axonal selection and myelin sheath generation in the central nervous system. Curr. Opin. Cell Biol. 2013, 25, 512–519. [Google Scholar] [CrossRef]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A.; Gallo, V. Congenital cardiac anomalies and white matter injury. Trends Neurosci. 2015, 38, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Abu-Rub, M.; Miller, R.H. Emerging cellular and molecular strategies for enhancing central nervous system (CNS) remyelination. Brain Sci. 2018, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Nave, K.-A.; Werner, H.B. Ensheathment and Myelination of Axons: Evolution of Glial Functions. Annu. Rev. Neurosci. 2021, 44, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Danilczyk, U.; Penninger, J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006, 98, 463–471. [Google Scholar] [CrossRef]
- Mertens, B.; Vanderheyden, P.; Michotte, Y.; Sarre, S. The role of the central renin-angiotensin system in Parkinson’s disease. J. Renin. Angiotensin. Aldosterone Syst. 2009, 11, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Riet, L.T.; van Esch, J.H.M.; Roks, A.J.M.; van den Meiracker, A.H.; Jan Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/angiotensin-(1-7)/MAS axis of the renin-angiotensin system: Focus on angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.-E.; Madore, C.; Bordeleau, M.; Tian, L.; Verkhratsky, A. Neuropathobiology of COVID-19: The Role for Glia. Front. Cell. Neurosci. 2020, 14, 592214. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Yamauchi, J.; Chan, J.R.; Okada, A.; Tomooka, Y.; Hisanaga, S.-I.; Tanoue, A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J. Cell Sci. 2007, 120, 4355–4366. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Ochiai, A.; Seki, Y.; Morimoto, T.; Oizumi, H.; Ohbuchi, K.; Mizoguchi, K.; Yamamoto, M.; Sakagami, H.; Miyamoto, Y.; et al. Phospholipase D and phosphatidylinositol-4-phosphate 5-kinase 1 are involved in the regulation of oligodendrocyte morphological differentiation. Exp. Cell Res. 2021, 405, 112654. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 2018, 173, 665–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, W.X.; Chong, S.; Zhang, H.; Makarova, K.S.; Koonin, E.V.; Cheng, D.R.; Scott, D.A. Cas13d Is a Compact RNA-Targeting Type VI CRISPR Effector Positively Modulated by a WYL-Domain-Containing Accessory Protein. Mol. Cell 2018, 70, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Galabova-Kovacs, G.; Catalanotti, F.; Matzen, D.; Reyes, G.X.; Zezula, J.; Herbst, R.; Silva, A.; Walter, I.; Baccarini, M. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J. Cell Biol. 2008, 180, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Mayes, D.A.; Rizvi, T.A.; Titus-Mitchell, H.; Oberst, R.; Ciraolo, G.M.; Vorhees, C.V.; Robinson, A.P.; Miller, S.D.; Cancelas, J.A.; Stemmer-Rachamimov, A.O.; et al. Nf1 loss and Ras hyperactivation in oligodendrocytes induce NOS-driven defects in myelin and vasculature. Cell Rep. 2013, 4, 1197–1212. [Google Scholar] [CrossRef] [Green Version]
- López-Juárez, A.; Titus, H.; Silbak, S.; Pressler, J.W.; Rizvi, T.A.; Bogard, M.; Bennett, M.R.; Ciraolo, G.; Williams, M.; Vorhees, C.; et al. Oligodendrocyte Nf1 Controls Aberrant Notch Activation and Regulates Myelin Structure and Behavior. Cell Rep. 2017, 19, 545–557. [Google Scholar] [CrossRef]
- Tago, K.; Kaziro, Y.; Satoh, T. Functional Involvement of mSos in Interleukin-3 and Thrombin Stimulation of the Ras, Mitogen-Activated Protein Kinase Pathway in BaF3 Murine Hematopoietic Cells. J. Biochem. 1998, 123, 659–667. [Google Scholar] [CrossRef]
- South, A.M.; Brady, T.M.; Flynn, J.T. ACE2 (Angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (angiotensin II) receptor blocker use during the pandemic: The pediatric perspective. Hypertension 2020, 76, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.I.; Narayanan, S.P.; Morse, E.N.; Shick, H.E.; Yin, X.; Kidd, G.; Avila, R.L.; Kirschner, D.A.; Macklin, W.B. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008, 28, 7174–7783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffries, M.A.; McLane, L.E.; Khandker, L.; Mather, M.L.; Evangelou, A.V.; Kantak, D.; Bourne, J.N.; Macklin, W.B.; Wood, T.L. mTOR signaling regulates metabolic function in oligodendrocyte precursor cells and promotes efficient brain remyelination in the cuprizone model. J. Neurosci. 2021, 41, 8321–8337. [Google Scholar] [CrossRef]
- Franke, T.F.; Kaplan, D.R.; Cantley, L.C. PI3K: Downstream AKTion Blocks Apoptosis. Cell 1997, 88, 435–437. [Google Scholar] [CrossRef] [Green Version]
- Golla, U.; Sesham, K.; Dallavalasa, S.; Manda, N.K.; Unnam, S.; Sanapala, A.K.; Nalla, S.; Kondam, S.; Kumar, R. ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies. Non-Coding RNA 2022, 8, 21. [Google Scholar] [CrossRef]
- Bachegowda, L.; Gligich, O.; Mantzaris, I.; Schinke, C.; Wyville, D.; Carrillo, T.; Braunschweig, I.; Steidl, U.; Verma, A. Signal transduction inhibitors in treatment of myelodysplastic syndromes. J. Hematol. Oncol. 2013, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Katanov, C.; Novak, N.; Vainshtein, A.; Golani, O.; DuPree, J.L.; Peles, E. N-Wasp Regulates Oligodendrocyte Myelination. J. Neurosci. 2020, 40, 6103–6111. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Torii, T.; Terao, M.; Takada, S.; Tanoue, A.; Katoh, H.; Yamauchi, J. Rnd2 differentially regulates oligodendrocyte myelination at different developmental periods. Mol. Biol. Cell 2021, 32, 769–787. [Google Scholar] [CrossRef]
- Yamashita, T.; Tucker, K.L.; Barde, Y.A. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Tohyama, M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat. Neurosci. 2003, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Oinuma, I.; Ishikawa, Y.; Katoh, H.; Negishi, M. The Semaphorin 4D Receptor Plexin-B1 Is a GTPase Activating Protein for R-Ras. Science 2004, 305, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ke, P.; Kuo, Y.-C.; Wang, Y.; Zhang, X.; Song, C.; Shan, Y. A putative structural mechanism underlying the antithetic effect of homologous RND1 and RhoD GTPases in mammalian plexin regulation. eLife 2021, 10, e64304. [Google Scholar] [CrossRef]
- Chandra, A.; Grecco, H.E.; Pisupati, V.; Perera, D.; Cassidy, L.; Skoulidis, F.; Ismail, S.A.; Hedberg, C.; Hanzal-Bayer, M.; Venkitaraman, A.R.; et al. The GDI-like solubilizing factor PDEδ sustains the spatial organization and signalling of Ras family proteins. Nat. Cell Biol. 2011, 14, 148–158. [Google Scholar] [CrossRef]
- Miller, R.H. Calcium control of myelin sheath growth. Nat. Neurosci. 2017, 21, 2–3. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wu, S.; Zhao, X. Ca2+ Signaling in Oligodendrocyte Development. Cell. Mol. Neurobiol. 2019, 39, 1071–1080. [Google Scholar] [CrossRef]
- Paez, P.M.; Lyons, D.A. Calcium signaling in the oligodendrocyte lineage: Regulators and consequences. Annu. Rev. Neurosci. 2020, 43, 163–186. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Torii, T.; Tanoue, A.; Yamauchi, J. VCAM1 acts in parallel with CD69 and is required for the initiation of oligodendrocyte myelination. Nat. Commun. 2016, 7, 13478. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Kawanami, T.; Eriguchi, M.; Hara, H. SARS-CoV-2-related Myelin oligodendrocyte glycoprotein antibody-associated disease: A case report and literature review. Intern. Med. 2022; in press. [Google Scholar] [CrossRef] [PubMed]



| Reagent or Source | Company or Source | Cat. No. | Lot. No. | Concentration Used |
|---|---|---|---|---|
| Antibodies | ||||
| Anti-proteolipid protein (PLP) 1 | Atlas Antibodies | HPA004128 | B115828 | IB, 1/500 |
| Anti-cyclic nucleotide 3′-phosphodiesterase (CNPase) | BioLegend | 836404 | B278794 | IB, 1/500 |
| Anti-SRY-related HMG-box protein (SOX) 10 | Santa Cruz Biotechnology | sc-365692 | F1621 | IB, 1/500 |
| Anti-Actin | MBL | M177-3 | 007 | IB, 1/80,000 |
| Anti-phospho-Akt1 (pSer473, which is essential for Akt1 activation) | Cell Signaling Technology | 9018S | 13 | IB, 1/500 |
| Anti-Akt1 | Cell Signaling Technology | 2938S | 2 | IB, 1/500 |
| Anti-angiotensin-converting enzyme (ACE) 2 | Santa Cruz Biotechnology | sc-390851 | F2520 | IB, 1/500 |
| Anti-pan-Ras | Santa Cruz Biotechnology | sc-166691 | H1220 | IB, 1/500 |
| Anti-green fluorescence protein (GFP) | Nacalai Tesque | 04404-84 | M7H8151 | immunoprecipitation, 1 mg for 1 mg proteins |
| Anti-GFP | MBL | M048-3 | 066 | IB, 1/1000 |
| Anti-DDDDK | MBL | M185-3L | 002 | IB, 1/10,000 |
| Anti-IgG (H+L chain) (Mouse) pAb-HRP | MBL | 330 | 365 | IB, 1/5000 |
| Anti-IgG (H+L chain) (Rabbit) pAb-HRP | MBL | 458 | 353 | IB, 1/5000 |
| Anti-PI 3-kinase p110α (PI3Ka) | Santa Cruz Biotechnology | sc-518070 | D2519 | IB, 1/250 |
| Anti-PI 3-kinase p110β (PI3Kb) | Santa Cruz Biotechnology | sc-376641 | H1320 | IB, 1/250 |
| Key chemicals | ||||
| guanosine triphosphate (GTP) | Merk | G8634-1MG | 056K1555 | 1 μM for immunoprecipitation |
| guanosine diphosphate (GDP) | Merk | 371545 | B62411 | 1 μM for immunoprecipitation |
| Glutathion-sepharose 4B | GE Healthcare | 17-0756-05 | 10058508 | 45 μL of 33% slurry (containing 30 μg of glutathion-S-transferase (GST)-Ras binding domain proteins) for 1 mg of total proteins in cell lysates |
| Protein G-sepharose 4FastFlow | GE Healthcare | 17-0618-01 | 10081061 | 45 μL of 33% slurry for 1 mg of total proteins in cell lysates |
| Lonafarnib as a Ras inhibitor | CAYMAN CHEMICAL | 11746 | 10081061 | 5 μM for cell treatment |
| MLN-4760 as an ACE2 inhibitor | MedChemExpress | HY-19414 | 305335-31-3 | 1 nM for cell treatment |
| Key reagents | ||||
| ScreenFect TM siRNA Transfection Reagent | FUJIFILM Wako Pure Chemical Corporation | 292-75013 | CAM0357 | |
| ScreenFect TM Dilution Buffer | FUJIFILM Wako Pure Chemical Corporation | 194-18181 | SKF5794 | |
| ImmunoStar Zeta | FUJIFILM Wako Pure Chemical Corporation | 295-72404 | LEP1844 | |
| Chemi-Lumi One Ultra | Nacalai Tesque | 11644-24 | L1G6389 | |
| Skim Milk Powder | FUJIFILM Wako Pure Chemical Corporation | 190-12865 | SKG4901 | |
| Western blotting (WB) Stripping Solution | Nacalai Tesque | 05364-55 | L5M5218 | |
| Gflex DNA Polymerase | TaKaRa Bio | R060A | AL80564A | |
| 2× Gflex PCR Buffer (Mg2+, dNTP plus) | TaKaRa Bio | R060A | AL80564A | |
| ISOGEN | Nippon Gene | 311-02501 | 75009K | |
| Sample Buffer Solution (2+Mercaptoethanol) (X4) | FUJIFILM Wako Pure Chemical Corporation | 191-13272 | WDP4995 | |
| Pre-stained Protein Markers (Broad Range) for SDS-PAGE | Nacalai Tesque | 02525 | L9M9989 | |
| 5×Prime Script Master Mix | TaKaRa Bio | RR036A | AIE0440A | |
| Recombinant protein | ||||
| Ras·GTP (active Ras)-binding domain of human c-Raf | Dr. Kenji Tago (Jichi Medical University, Tochigi, Japan) | N/A | N/A | |
| Cell line | ||||
| FBD-102b cells (mouse cells) | Dr. Yasuhiro Tomo-oka (Tokyo University of Science, Chiba, Japan) | N/A | N/A | |
| Plasmids and vectors | ||||
| pcDNA3.1-N-EGFP as the N-terminal GFP-tag expression vector | GenScript | not described | N/A | |
| pXR001: EF1a-CasRx-2A-EGFP | Addgene | 109049 | N/A | |
| pSINmU6 as the oligonucleotide transcription vector | TaKaRa Bio | not described | N/A | |
| ACE2 intracellular domain sequences (5′ to 3′) inserted into the plasmid (pcDNA3.1-N-EGFP) | ||||
| Sense-KpnI-oligonucleotide for human ACE2 intracellular domain-KpnI 5′-GGTACCGGGATCAGAGATCGGAAGAAGAAAAATAAAGCAAGAAGTGGAGAAAATCCTTATGCCTCCATCGATATTAGCAAAGGAGAAAATAATCCAGGATTCCAAAACACTGATGATGTTCAGACCTCCTTTTAGGGTACC-3′ Antisense-KpnI-oligonucleotide for human ACE2 intracellular domain-KpnI 5′-GGTACCCTAAAAGGAGGTCTGAACATCATCAGTGTTTTGGAATCCTGGATTATTTTCTCCTTTGCTAATATCGATGGAGGCATAAGGATTTTCTCCACTTCTTGCTTTATTTTTCTTCTTCCGATCTCTGATCCCGGTACC-3′ | This manuscript | N/A | N/A | |
| gRNA sequences (5′ to 3′) inserted into the plasmid (pSINmU6) | ||||
| Sense-BamHI-gLuciferase-ClaI gatccGCACCCGTGCAAAAATGCAGGGGTCTAAAACGGCGCCATTCTATCCTCTAGAGTTTTTTat Antisense-BamHI-gLuciferase-ClaI cgatAAAAAACTCTAGAGGATAGAATGGCGCCGTTTTAGACCCCTGCATTTTTGCACGGGTGCg | This manuscript | N/A | N/A | |
| Sense-BamHI-gACE2-93th-ClaI gatccGCACCCGTGCAAAAATGCAGGGGTCTAAAACGTCTTCAGCTTCCTGATTAAAGTTTTTTat Antisense-BamHI-gACE2-93th-ClaI cgatAAAAAACTTTAATCAGGAAGCTGAAGACGTTTTAGACCCCTGCATTTTTGCACGGGTGCg | This manuscript | N/A | N/A | |
| Sense-BamHI-gACE2-169th-ClaI gatccGCACCCGTGCAAAAATGCAGGGGTCTAAAACCACTCATCTTTTGGGCATTTTCTTTTTTat Antisense-BamHI-gACE2-169th-ClaI cgatAAAAAAGAAAATGCCCAAAAGATGAGTGGTTTTAGACCCCTGCATTTTTGCACGGGTGCg | This manuscript | N/A | N/A | |
| siRNA sequences (5′ to 3′) | ||||
| Sense chain for siLuciferase (siControl) GCCAUUCUAUCCUCUAGAG-dTdT Antisense chain for siLuciferase (siControl) CUCUAGAGGAUAGAAUGGC-dTdT | Yamauchi, J. et al. Exp. Cell Res. (2009) 315:2043-2052 | N/A | N/A | |
| Sense chain for siACE2-128th GUUCACUUGCUUCUUGGAA-dTdT Antisense chain for siACE2-128th UUCCAAGAAGCAAGUGAAC-dTdT | This manuscript | N/A | N/A | |
| Sense chain for siACE2-169th GAAAAUGCCCAAAAGAUGA-dTdT Antisense chain for siACE2-169th UCAUCUUUUGGGCAUUUUC-dTdT | This manuscript | N/A | N/A | |
| RT-PCR primers (5′ to 3′) | ||||
| Sense primer for Arf1 (internal control) ATGGGTGGCTTTTTCTCAAGTATTTTTTC Antisense primer for Arf1 (internal control) TCACTGTCTGCTTTTCAGGGTTTC | This manuscript | N/A | N/A | |
| Sense primer for ACE2 ATGTCCAGCTCCTCCTGGTCCTTC Antisense primer for ACE2 TCAGCATAGAGTTTGCCCAGAATCCTTGAGTCATATG | This manuscript | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y.; Tago, K.; Fukatsu, S.; Okabe, M.; Shirai, R.; Oizumi, H.; Ohbuchi, K.; Yamamoto, M.; Mizoguchi, K.; Miyamoto, Y.; et al. CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation. Non-Coding RNA 2022, 8, 42. https://doi.org/10.3390/ncrna8030042
Kato Y, Tago K, Fukatsu S, Okabe M, Shirai R, Oizumi H, Ohbuchi K, Yamamoto M, Mizoguchi K, Miyamoto Y, et al. CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation. Non-Coding RNA. 2022; 8(3):42. https://doi.org/10.3390/ncrna8030042
Chicago/Turabian StyleKato, Yukino, Kenji Tago, Shoya Fukatsu, Miyu Okabe, Remina Shirai, Hiroaki Oizumi, Katsuya Ohbuchi, Masahiro Yamamoto, Kazushige Mizoguchi, Yuki Miyamoto, and et al. 2022. "CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation" Non-Coding RNA 8, no. 3: 42. https://doi.org/10.3390/ncrna8030042
APA StyleKato, Y., Tago, K., Fukatsu, S., Okabe, M., Shirai, R., Oizumi, H., Ohbuchi, K., Yamamoto, M., Mizoguchi, K., Miyamoto, Y., & Yamauchi, J. (2022). CRISPR/CasRx-Mediated RNA Knockdown Reveals That ACE2 Is Involved in the Regulation of Oligodendroglial Cell Morphological Differentiation. Non-Coding RNA, 8(3), 42. https://doi.org/10.3390/ncrna8030042








