Abstract
In recent years, long non-coding RNAs (lncRNAs) have been shown to play important regulatory roles in cellular processes. Growth arrests specific transcript 5 (GAS5) is a lncRNA that is highly expressed during the cell cycle arrest phase but is downregulated in actively growing cells. Growth arrests specific transcript 5 was discovered to be downregulated in several cancers, primarily solid tumors, and it is known as a tumor suppressor gene that regulates cell proliferation, invasion, migration, and apoptosis via multiple molecular mechanisms. Furthermore, GAS5 polymorphism was found to affect GAS5 expression and functionality in a cell-specific manner. This review article focuses on GAS5’s tumor-suppressive effects in regulating oncogenic signaling pathways, cell cycle, apoptosis, tumor-associated genes, and treatment-resistant cells. We also discussed genetic polymorphisms of GAS5 and their association with cancer susceptibility.
1. Introduction
The Encyclopaedia of DNA Elements (ENCODE) project revealed that the non- coding section of DNA, which was long regarded to be “junk DNA,” is now recognized as functional elements in the human genome and produces functional non-coding RNAs that regulate cellular functions in a variety of ways [1,2]. LncRNAs are non-coding RNAs that contain more than 200 nucleotides and are the result of extensive genome transcription. Even though lncRNAs are not translated into functional proteins, they are referred to as regulatory RNAs because of their role in gene regulation via epigenetic, transcription, and post-transcriptional activities [3]. Recent studies have indicated that the dysregulation of lncRNA expression can result in tumor initiation and progression [4]. LncRNA GAS5 is located at chromosome 1q25 [5] and belongs to the 5’-terminal oligopyrimidine (5’TOP) gene family, which encodes 10 box C/D short nucleolar RNAs (snoRNAs) within the introns (Figure 1) [6]. GAS5 was initially isolated and discovered from a group of genes expressed only during the growth arrest phase of NIH 3T3 cells [7]. During the growth-arrested phase, spliced GAS5 mRNA accumulates into messenger ribonucleoprotein (mRNP) particles, sequestered away from active ribosomes and remains untranslated [6], resulting in higher GAS5 expression in growth-arrested cells than in actively growing cells. However, in growth-induced cells, transcriptional activity is unaffected, implying that post-transcriptional mechanisms regulate GAS5 mRNA levels [8,9]. As a member of 5′TOP mRNAs, GAS5 translation is regulated by the mammalian targets of rapamycin (mTOR) pathway [10], and its expression is influenced by the nonsense-mediated mRNA decay (NMD) pathway during active translation [11]. The NMD pathway can change GAS5 functionalities such as apoptotic-related gene transcriptional activity by regulating GAS5 expression levels and decay rates [12].
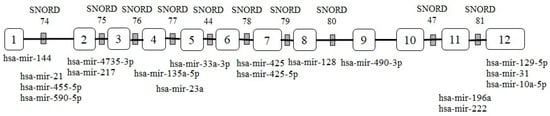
Figure 1.
Diagram representation of GAS5 transcript (GenBank ID: NR_002578.3) with the sequence length of 656 nt encodes 10 snoRNAs within introns. The sponging regions of miRNAs predicted by miRcode [13] are located at exon 1 (hsa-mir-144), intron 1 (hsa-mir-21, hsa-mir-455-5p and hsa-mir-590-5p), exon 2 (hsa-mir-4735-3p and hsa-mir-217), exon 4 (hsa-mir-135a-5p), exon 5 (hsa-mir-33a-3p), intron 4 (hsa-mir-23a), exon 7 (hsa-mir-425 and hsa-mir-425-5p, exon 8 (hsa-mir-128), exon 9 (hsa-mir-490-3p), intron 11 (hsa-mir-196a and hsa-mir-222) and exon 12 (hsa-mir-129-5p, hsa-mir-31 and hsa-mir-10a-5p).
Aberrant GAS5 expression was reported in actively proliferating cancer cells including breast cancer [14], cervical cancer [15], colorectal cancer [16], gastric cancer [17], hepatocellular carcinoma [18], renal cell carcinoma [19], and glioma [20]. The prognostic value of GAS5 in cancer was explored by evaluating the relationship between the GAS5 expression with clinicopathological features and patients’ survival. Cao and colleagues [15] demonstrated that decreased GAS5 expression was negatively correlated with the FIGO (International Federation of Gynecology and Obstetrics) stage, vascular invasion, and lymph node metastasis in cervical cancer. Furthermore, studies in clear cell renal carcinoma [21] and laryngeal squamous carcinoma [22] have linked lower GAS5 expression to the advanced tumor stage and tumor size, respectively. Another study by Sun et al. showed that downregulated GAS5 was significantly correlated with the shorter survival time in patients with gastric cancer [17]. Besides, GAS5 is also known as a tumor suppressor gene due to its role in inhibiting cell proliferation, migration, invasion, and promoting apoptosis in cancer cells through binding and downregulating oncomirs [16,21,22,23]. GAS5 genetic polymorphisms, on the other hand, have recently been linked to cancer susceptibility and progression [24,25,26,27]. This review summarises GAS5’s tumor-suppressive effects in cancer cells, including modulation of oncogenic signaling pathways, cell cycle, tumor-suppressor genes, apoptosis, and treatment-resistant cells. We also discussed the impact of GAS5 polymorphisms on cancer patients. Figure 2 depicts the GAS5 secondary structure and sponging regions of microRNAs (miRNAs).
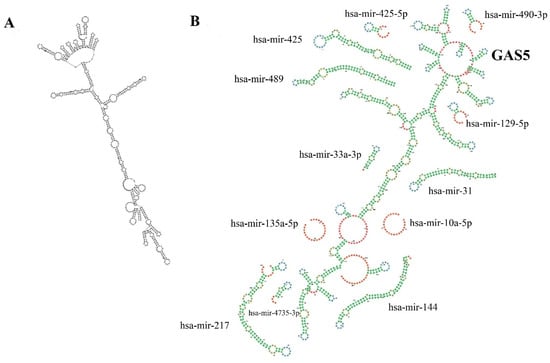
Figure 2.
Diagram representation of (A) The minimum free energy (MFE) secondary structure of GAS5 predicted using RNA-fold web server (http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi) (accessed on 24 March 2022). (B) The secondary structure of GAS5 regions sponging miRNAs in GAS5 variant 1 is shown using forna (force directed graph layout) [28].
2. GAS5 Expression Is Regulated by the mTOR Signaling Pathway
The 70-kDa ribosomal protein S6 kinase (p70S6K) and eukaryotic initiation factor 4E/4E-binding protein 1 (eIF4E/4E-BP1) are two independent mTOR downstream effectors that are involved in cell cycle control [29]. In response to mitogenic stimulation or nutrient availability, mTOR downstream signaling effectors regulate cell growth and cell cycle progression through controlling cellular translation [29]. The p70S6 kinase plays an important role in regulating translational of 5′TOP mRNAs through phosphorylation of ribosomal S6 protein [30]. The mTOR signaling pathway selectively regulates GAS5 translation [10] via mitogen-induced translation by p70S6K [31]. However, due to a short reading frame [32], GAS5 is subjected to rapid degradation by the NMD pathway, thus lowering GAS5 expression during the normal cell growth process [11,12]. Inhibition of GAS5 translation by the immunosuppressant rapamycin, on the other hand, can cause cell cycle arrest by increasing GAS5 levels [10].
3. Role of GAS5 in Oncogenic Signaling Pathways
3.1. PI3K/AKT/mTOR Pathway
In the activated phosphatidylinositol-3-kinase (PI3K)/AKT pathway, 4E-BP1 is phosphorylated [33] and dissociated from the eIF4E/4E-BP1 complex [34] resulting in increased cyclin D1 protein levels in response to increased eIF4E expression [35] and thus promoting the cell cycle progression [36]. GAS5 plays an important regulatory role in modulating the PI3K/AKT/mTOR pathway via sponging oncomirs. Xue et al. revealed that GAS5 overexpression in prostate cancer cells can significantly reduce the phosphorylation of AKT and its downstream proteins mTOR and S6K1 through targeting miR-103 [23]. Dong et al. have further demonstrated that the ectopic expression of GAS5 could affect gastric cancer progression through the inactivation of the AKT/mTOR pathway by negatively regulating miR-106a-5p expression [37]. Similarly, J. Liu et al. have also confirmed that GAS5 can also inactivate the PI3K/AKT pathway and inhibit cell growth in osteosarcoma cells by upregulating phosphatase and tensin homologue (PTEN) via sponging miR-23a-3p [38]. Figure 3 depicts the downregulation of GAS5 in actively growing cells.
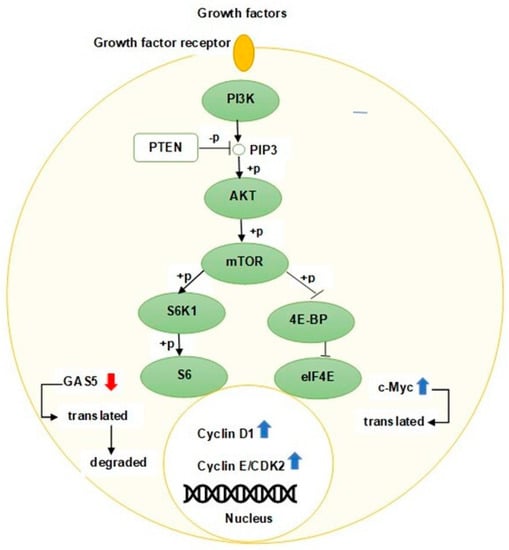
Figure 3.
Schematic representation of proliferating cell with activated PI3K/AKT/mTOR pathway. The ribosomal S6 protein is phosphorylated (+p) when the PI3K/AKT/mTOR pathway is activated, resulting in translation and degradation of GAS5 by the NMD pathway. Downregulation of GAS5 causes an increase in c-Myc protein, thereby facilitating cell cycle progression via inducing activation of cyclin-CDK complexes. PTEN, a tumor suppressor protein, blocks the PI3K/AKT/mTOR signaling pathway by dephosphorylating (−p) PIP3.
3.2. PTEN/AKT Pathway
PTEN gene plays a critical role in controlling cell proliferation and cell growth by regulating the AKT signaling pathway [39]. The ability of PTEN to induce cell-cycle inhibition depends on the negative regulation of the PI3K/AKT signaling pathway [40]. Immunohistochemical analysis revealed that the loss of PTEN triggers AKT phosphorylation in endometrial carcinoma [41]. Meanwhile, a study in non-small cell lung cancer cells found that overexpression of PTEN inhibits cell growth by inducing G0/G1 arrest [42]. According to recent research, GAS5 may regulate PTEN expression by competitively binding to miRNAs and thus reducing PTEN suppression. Moreover, GAS5 can also sensitize the lung cancer cells to chemotherapeutic [43] and radiosensitivity treatments [44] through miR-21/PTEN/AKT axis by sponging miR-21, thereby upregulating PTEN expression and consequently suppressing AKT phosphorylation. Likewise, downregulated GAS5 in hepatocellular cancer (HCC) cells resulted in decreased PTEN levels and increased doxorubicin resistance in HCC [45]. As shown in Table 1, GAS5 inhibits tumors via sponging oncomirs and upregulating PTEN expression.

Table 1.
GAS5 regulates PTEN expression via acting as a molecular sponge.
4. GAS5 as Part of Cell Cycle Regulatory Mechanism
4.1. c-Myc Expression
c-Myc is required for the activation of Cyclin-Dependent Kinase (CDK) complexes during cell growth and is selectively translated via eIF4E activation [50]. By increasing cyclin E/CDK2 function and decreasing cyclin E-associated p27 levels, c-Myc could suppress p27-induced growth arrest [51]. GAS5 was found to regulate c-Myc protein levels by interacting with eIF4E during the translation initiation phase and suppressing c-Myc translation by preventing c-Myc mRNA from entering the polysome [52]. By blocking c-Myc translation, GAS5 could potentially induce cell growth arrest.
4.2. CDK Inhibitors
Inhibitors such as p27kip1, p21, and cyclin-dependent kinase inhibitor 1C (CDKN1C) (known as p57kip2) are of cyclin-dependent kinases (CDK) and are negative regulators of cell cycle progression [53,54]. GAS5 induces cell growth arrest by regulating the expression of CDK inhibitors. For instance, GAS5 promotes cell growth arrest by increasing the promoter activity of p27kip1, through enhancing the binding of E2F1 to p27kip1 promoter and subsequently upregulating p27 kip1 expression [55]. Additionally, GAS5 can upregulate p27 expression by acting as a molecular sponge to miR-222-3p and abrogates its ability to inhibit p27 protein expression [56]. According to a study on stomach cancer, GAS5 modulates p21 expression and hence maintains G1 phase cell cycle arrest. Depletion of GAS5 may increase the turnover of the transcriptional activator Y-box-binding protein 1 (YBX1), reducing p21 expression and so preventing G1 phase arrest [57].
The enhancer of zeste homolog 2 (EZH2) epigenetic modifier was found to be overexpressed in melanoma, resulting in tumor growth and spread by inhibiting tumor-suppressive genes [58]. Xu et al. showed that GAS5 could reverse the effect of EZH2 in melanoma cells by recruiting transcription factor E2F4, which acts as transcriptional repressor to the EZH2 promoter region, therefore affecting the EZH2 expression from the transcriptional level and eventually leads to upregulation of tumor suppressor gene CDKN1C [59]. Likewise, a study in bladder cancer has further verified the GAS5 role in promoting apoptosis through inhibiting EZH2 transcription similarly by recruiting transcription factor E2F4 to EZH2 promoter [60]. Figure 4 illustrates the role of GAS5 as a guide.
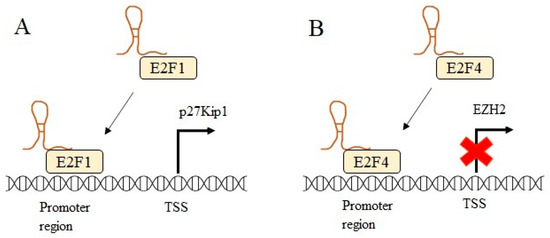
Figure 4.
GAS5 acts as a guide by directing transcription factors to the promoter region of the gene. (A) GAS5 guides transcriptional activator E2F1 to the promoter region of p27Kip1 and promotes transcription. (B) GAS5 enhances transcriptional repression by guiding the transcriptional repressor E2F4 to the promoter region of EZH2. Transcription start site (TSS).
5. GAS5 Regulates Cellular Apoptosis
Glucocorticoid receptor (GR) is a member of the nuclear receptor family and activation of GR by glucocorticoids (GCs) results in translocation of GR from the cytoplasm to the nucleus, which then interacts with glucocorticoid response elements (GREs), thereby regulating the expression of target genes [61]. In lymphoid cancer, GCs are used as anticancer drugs due to their inhibitory effects in lymphoid tissue [62]. Despite GCs’ antitumor effects in hematopoietic malignancies, a recent meta-analysis study on the significance of GR expression in cancer found that high GR expression was linked to cancer progression and a worse prognosis in certain solid tumors, including endometrial, ovarian, and early stages of untreated triple-negative breast cancers [63]. Glucocorticoids have cell-specific pro-apoptotic and anti-apoptotic effects, with lymphoid tissue showing pro-apoptotic and anti-proliferative effects and solid cancer cells showing anti-apoptotic and proliferation-promoting effects [64]. GAS5 serves as a riborepressor of the GR, suppressing GR-induced transcriptional activity by binding to the DNA binding domain of GR via its double-stranded GRE mimic sequence [65]. A stem-loop structure encoded in GAS5 exon 12 which serves as GRE-mimic was found to induce apoptosis in breast cancer cells [66]. The role of GAS5 as an apoptosis regulator was delineated by overexpressing GAS5 in breast epithelial cells which resulted in increased apoptosis in GAS5-overexpressed cells [14]. Similar findings in prostate cancer cells showed that high GAS5 levels increase both basal and drug-induced apoptosis, whereas downregulation of GAS5 attenuates apoptosis [67]. The anti-apoptotic activity of GCs was previously found to be attributable to the overexpression of the cellular inhibitor apoptosis 2 (cIAP2) [68]. Growth arrests specific transcript 5 can reduce the transcriptional activity of anti-apoptotic genes like cIAP2 and serum- and glucocorticoid-regulated kinase 1 (SGK1), as well as increase cellular death [12]. Figure 5 shows how GAS5 acts as a riborepressor for GR.
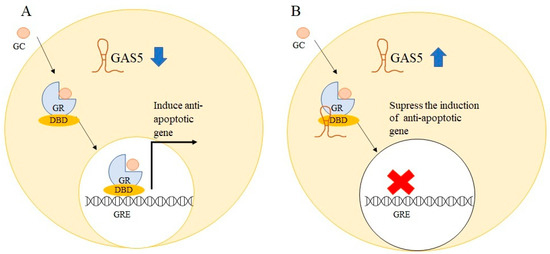
Figure 5.
Schematic representation of glucocorticoid receptor (GR) activation by glucocorticoid (GC). (A) The glucocorticoid-activated GR bind to the glucocorticoid responsive element (GRE) via the DNA binding domain (DBD) in downregulated GAS5 solid cancer cells to induce anti-apoptotic genes such as cIAP2, resulting in cell survival. (B) GAS5 acts as a GRE decoy in GAS5 elevated cells, repressing GR-mediated activation of anti-apoptotic genes and therefore sensitising cells to apoptosis.
6. Genetic Variants Affect GAS5 Expression and Cancer Susceptibility
In leukemia, GAS5 behaves differently than in solid tumors. According to Yan and colleagues, patients with GAS5 rs55829688 CC genotype exhibited higher GAS5 expression and were associated with poor prognosis in acute myeloid leukemia. Thus, overexpression of GAS5 was predicted to decrease GC function and aggravate chemotherapy-induced hematological damage [69]. On the other hand, Wang et al. demonstrated that rs55829688 CT/TT genotypes were linked to higher GAS5 expression and a greater risk of colorectal cancer when compared to the CC genotype. They also found that the rs55829688 T>C polymorphism downregulates GAS5 expression and ultimately slows CRC growth [24].
GAS5 expression was shown to be inconsistent in hepatocellular carcinoma. In previous studies, downregulation of GAS5 was found to promote HCC cell proliferation and growth [18,45]. On the other hand, Tao et al. reported that the GAS5 rs145204276 deletion allele was linked to higher GAS5 expression and an increased risk of HCC, implying that GAS5 may operate as a proto-oncogene in HCC [70]. Similarly, in oral cancer, GAS5 single nucleotide polymorphism (SNP) rs145204276 variants (Ins/Del or Del/Del) were associated with poor differentiation cell status in male patients. According to data from the GTEX database, individuals with these variants had significantly higher GAS5 expression in esophageal mucosa tissues [25]. Weng et al. revealed that cervical patients with allele deletion in the GAS5 rs145204276 are likely to have a poorer hazard ratio of 5 years survival [71]. In contrast, GAS5 rs145204276 allele deletion in cervical squamous cell carcinoma [26], urothelial cell carcinoma [24], and glioma [72] was associated with lower GAS5 expression and increased tumor risk. Likewise, GAS5 rs145204276 deletion allele was associated with a lower risk of breast cancer [73], and a lower risk of lymph node metastasis in prostate cancer [74].
In HCC [70], oral cancer [25], and uterine cervical cancer [71], the promoter variant rs145204276 may impact GAS5’s tumor suppressive effect; nevertheless, its tumor suppressive activity has been described in osteosarcoma, where allele deletion was associated with enhanced GAS5 expression and smaller tumors [27]. Apart from rs145204276, two other SNPs located at the promoter region, rs2067079 with a T allele and rs6790 with a G allele have been associated with an increased risk of bladder cancer [75]. Rakhshan et al. hypothesized that minor alleles of these SNPs could work together to disrupt GAS5 function [75]. Both SNPs have been identified as possible biomarkers for chemoradiotherapy-induced adverse responses in nasopharyngeal cancer [76]. Guo et al. have demonstrated that both SNPs may influence the transcription activity of GAS5, due to a strong expression of Quantitative Trait Locus (e-QTL) property in a variety of tissues. Furthermore, their structural analysis of GAS5 rs2067079 showed that GAS5 polymorphism has a clear effect on GAS5 secondary structure and could potentially disrupt the GAS5-miRNA sponge role through changes in the miRNA binding site [76]. We summarize that GAS5 variations may affect GAS5 function as a tumor suppressor by retaining or disrupting its function, regulating GAS5 expression, or modifying GAS5 secondary structure. The genetic variations of GAS5 in various malignancies are summarised in Table 2.

Table 2.
GAS5 polymorphisms in various cancers. ‘-’ indicates that Gas5 expression is not available.
7. GAS5 Regulates Target Genes via Competing Endogenous RNA (ceRNA) Network
MiRNAs are the small non-coding RNAs involved in various gene regulations through repressing the translation of the mRNAs. The interplay between coding and non-coding RNAs is known as ceRNA and the regulatory crosstalk between transcriptomes is mediated by miRNAs via miRNA response elements [78]. Perturbations in ceRNA regulatory components such as lncRNAs, miRNAs, and mRNAs are associated with the pathophysiology of cancer and several other diseases [79]. Growth arrests specific transcript 5 acts as a molecular sponge in the ceRNA network, absorbing oncogenic miRNAs and preventing the repression of tumor suppressor genes involved in inhibiting cell proliferation, migration, invasion, and promoting cell apoptosis in cancers such as colorectal cancer [80], glioma stem cells [81], triple-negative breast cancer [82], and clear cell renal cell carcinoma [21]. Figure 6 depicts GAS5’s function as a molecular sponge.
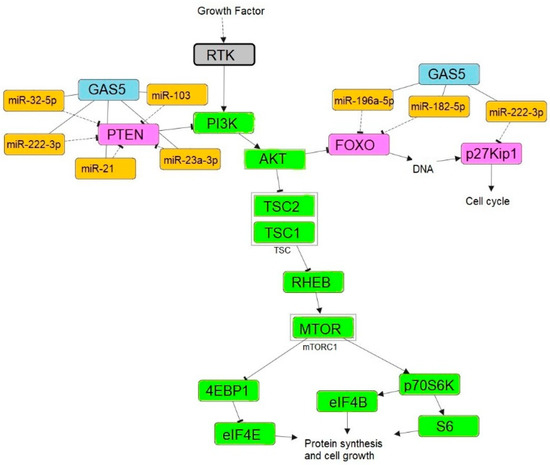
Figure 6.
Schematic representation of PI3K/AKT/mTOR pathway. GAS5 inhibits the repression of tumor suppressor gene expression such as PTEN, FOXO and p27KIP1 via sponging oncomirs. Pathway was constructed using PathwayMapper [83]. Receptor tyrosine kinase (RTK), Ras homolog enriched in brain (RHEB), Tuberous sclerosis complex 1 and 2 (TSC1 and TSC2).
7.1. FOXO
The forkhead box O (FOXO) is a subgroup of the forkhead-box (FOX) transcription factors superfamily [84]. FOXOs are considered tumor suppressors due to their inhibitory role in cancer. Evidence suggested that downregulation of FOXOs expression by miRNAs has been shown to facilitate cell proliferation, invasion, and metastasis in cancer cells [85]. Recent studies suggested that GAS5 could potentially upregulate FOXOs expression by abrogating the repressive function of miRNAs. A study in colorectal cancer showed that GAS5 can inhibit cell proliferation and promotes apoptosis by upregulating FOXO3a expression via sponging miR-182-5p [80]. Another study in glioma stem cells showed that GAS5 can also upregulate the other member of the FOXO family, namely FOXO1 via downregulating miR-196a-5p expression resulting in inhibition of cell proliferation, migration, invasion, and promotes apoptosis in glioma stem cells [81]. A similar study has further verified the tumor-suppressive effect of the GAS5/miR-196a-5p/FOXO1 signaling axis in triple-negative breast cancer cells [82].
7.2. hZIP1
Human ZIP1 (hZIP1) is known as a zinc transporter in human cells and is responsible for zinc uptake in cells [86]. The zinc status was evaluated in several cancers using in situ staining method and zinc levels were found markedly decreased in malignant cells namely hepatocellular cancer, pancreatic ductal adenocarcinoma, and breast invasive ductal adenocarcinoma as compared to their respective normal cells [87]. A study in prostate cancer indicated that the downregulation of hZIP1 transporter protein is accompanied by cellular zinc depletion in malignant prostate tissue [88]. Dong et al. reported that the hZIP1 expression was downregulated in clear cell renal cell carcinoma as compared to normal kidney samples [89]. In a separate study, Dong et al. revealed that GAS5 overexpression can upregulate hZIP1 protein expression and reduce tumorigenicity of clear cell renal cell carcinoma cells by sponging miR-223 [21].
8. GAS5 Modulates Chemosensitivity and Radiosensitivity in Cancer Cells
Chemotherapy and radiotherapy are used as standard treatments in cancer. However, the efficacy of these treatments is reduced in cancer resistance cells. Recently, non-coding RNAs have come to light as potential therapeutic targets for targeted and drug-resistance therapy in cancers [90,91]. This is typically due to the dysregulated non-coding RNAs that were found to have an essential role in cellular resistance towards chemotherapeutic drugs [92]. Growth arrests specific transcript 5 expression was verified to be significantly downregulated in resistant cancer cells. Its ectopic expression has been shown to improve treatment efficacy in radio- and chemo-resistant cancer cells by sponging oncomirs, thereby regulating tumor-suppressive effects by upregulating genes like reversion inducing cysteine rich protein with kazal motifs (RECK), dickkopf WNT signaling pathway inhibitor 2 (DKK2), immediate early response 3 (IER3) and phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP). Moreover, GAS5 can also sensitize cells to overcome treatment resistance allowing cells to undergo apoptosis [93,94,95,96]. Furthermore, via sponging miR-221 and elevating suppressor of cytokine signaling 3 (SOCS3) gene expression, overexpression of GAS5 was reported to inhibit gemcitabine resistance-mediated stem cell-like features, tumor metastasis, and epithelial-mesenchymal transition (EMT) of pancreatic cancer cells [97]. The roles of GAS5 in chemosensitivity and radiosensitivity regulation in cancer cells are summarised in Table 3.

Table 3.
GAS5 regulates chemosensitivity and radiosensitivity in cancer cells.
9. Conclusions
GAS5 is downregulated in a variety of solid tumors. Recent studies showed that ectopic expression of GAS5 inhibits cell growth and enhances cell death through altering oncomirs, tumor suppressor genes, and oncogenic signaling pathways. Although GAS5 has a tumor-suppressive role in a range of solid tumors, the precise molecular mechanism by which GAS5 acts in human cancers remains unknown, especially in hematological and solid cancers with GAS5 polymorphisms. GAS5 has been discovered to perform an adversarial role in leukemia carcinogenesis control. Besides, GAS5 polymorphism has been shown to have an opposing effect in cancers such as hepatocellular carcinoma, colorectal cancer, and oral cancer, where higher GAS5 expression has been linked to an increased risk of cancer. However, the underlying mechanisms of this opposing event remain unknown, necessitating further investigation. Research in hematopoietic malignancies is still lacking and further investigation is warranted to verify GAS5 functions in hematopoietic malignancies. Based on current research, GAS5 appears to be a potential therapeutic target, particularly in terms of restoring its tumor suppressor activity to restore dysregulated signaling pathways found in many cancers.
Author Contributions
Conceptualization of the manuscript, K.I., N.S., A.N. and J.K.; writing-original draft preparation, J.K.; writing-review and editing, K.I, J.K, A.N., N.S., A.A.R., N.M.A.N.A.R. and N.A.A.M.; supervision, K.I., N.S., A.N.; funding acquisition, K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundamental Research Grant Scheme (FRGS), Ministry of Higher Education, Malaysia, grant number FRGS/1/2019/STG03/UM/02/4.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diamantopoulos, M.A.; Tsiakanikas, P.; Scorilas, A. Non-coding RNAs: The riddle of the transcriptome and their perspectives in cancer. Ann. Transl. Med. 2018, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLOS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Okyay, T.M.; Yalcinkaya, B.; Karacaoglu, S.; Gocmen, M.; Akcakuyu, M.H. Tumor suppressor and oncogenic role of long non-coding RNAs in cancer. North Clin. Istanb. 2020, 7, 81–86. [Google Scholar] [CrossRef]
- Goustin, A.S.; Thepsuwan, P.; Kosir, M.A.; Lipovich, L. The Growth-Arrest-Specific (GAS)-5 Long Non-Coding RNA: A Fascinating lncRNA Widely Expressed in Cancers. Noncoding RNA 2019, 5, 46. [Google Scholar] [CrossRef]
- Smith, C.M.; Steitz, J.A. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol. Cell Biol. 1998, 18, 6897–6909. [Google Scholar] [CrossRef]
- Schneider, C.; King, R.M.; Philipson, L. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef]
- Coccia, E.M.; Cicala, C.; Charlesworth, A.; Ciccarelli, C.; Rossi, G.B.; Philipson, L.; Sorrentino, V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell Biol. 1992, 12, 3514–3521. [Google Scholar] [CrossRef]
- Ciccarelli, C.; Philipson, L.; Sorrentino, V. Regulation of expression of growth arrest-specific genes in mouse fibroblasts. Mol. Cell Biol. 1990, 10, 1525–1529. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hasan, A.M.; Farzaneh, F.; Williams, G.T. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Mol. Pharmacol. 2010, 78, 19–28. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Williams, G.T. Growth arrest on inhibition of nonsense-mediated decay is mediated by noncoding RNA GAS5. BioMed Res. Int. 2013, 2013, 358015. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Torimura, M.; Akimitsu, N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS ONE 2013, 8, e55684. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at UCSC. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef]
- Cao, S.; Liu, W.; Li, F.; Zhao, W.; Qin, C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 6776–6783. [Google Scholar] [PubMed]
- Li, J.; Wang, Y.; Zhang, C.-G.; Xiao, H.-J.; Xiao, H.-J.; Hu, J.-M.; Hou, J.-M.; He, J.-D. Effect of long non-coding RNA Gas5 on proliferation, migration, invasion and apoptosis of colorectal cancer HT-29 cell line. Cancer Cell Int. 2018, 18, 4. [Google Scholar] [CrossRef]
- Sun, M.; Jin, F.-y.; Xia, R.; Kong, R.; Li, J.-h.; Xu, T.-p.; Liu, Y.-w.; Zhang, E.-b.; Liu, X.-h.; De, W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer 2014, 14, 319. [Google Scholar] [CrossRef]
- Chang, L.; Li, C.; Lan, T.; Wu, L.; Yuan, Y.; Liu, Q.; Liu, Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol. Med. Rep. 2016, 13, 1541–1550. [Google Scholar] [CrossRef]
- Qiao, H.P.; Gao, W.S.; Huo, J.X.; Yang, Z.S. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1077–1082. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, S.; Zhang, K.; Shi, R.; Bao, X. Low GAS5 Levels as a Predictor of Poor Survival in Patients with Lower-Grade Gliomas. J. Oncol. 2019, 2019, 1785042. [Google Scholar] [CrossRef]
- Dong, X.; Kong, C.; Liu, X.; Bi, J.; Li, Z.; Li, Z.; Zhu, Y.; Zhang, Z. GAS5 functions as a ceRNA to regulate hZIP1 expression by sponging miR-223 in clear cell renal cell carcinoma. Am. J. Cancer Res. 2018, 8, 1414–1426. [Google Scholar] [PubMed]
- Lyu, K.; Xu, Y.; Yue, H.; Li, Y.; Zhao, J.; Chen, L.; Wu, J.; Zhu, X.; Chai, L.; Li, C.; et al. Long Noncoding RNA GAS5 Acts As A Tumor Suppressor In Laryngeal Squamous Cell Carcinoma Via miR-21. Cancer Manag. Res. 2019, 11, 8487–8498. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Zhou, C.; Lu, H.; Xu, R.; Xu, X.; He, X. LncRNA GAS5 inhibits proliferation and progression of prostate cancer by targeting miR-103 through AKT/mTOR signaling pathway. Tumor Biol. 2016, 37, 16187–16197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, S.; Yang, X.; Li, X.; Chen, R. Association between polymorphism in the promoter region of lncRNA GAS5 and the risk of colorectal cancer. Biosci. Rep. 2019, 39, BSR20190091. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-H.; Lu, H.-J.; Lin, C.-W.; Lee, C.-Y.; Yang, S.-J.; Wu, P.-H.; Chen, M.-K.; Yang, S.-F. Genetic Variants of lncRNA GAS5 Are Associated with the Clinicopathologic Development of Oral Cancer. J. Pers. Med. 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Feng, L.; Li, F.; Xue, Y.; Li, C.; Wang, H. A novel functional indel polymorphism within long non-coding RNAs growth arrest specific 5 conferred risk for cervical squamous cell carcinoma in Chinese Han populations. Transl. Cancer Res. 2017, 6, 424–431. [Google Scholar] [CrossRef]
- Xu, L.; Xia, C.; Xue, B.; Sheng, F.; Xiong, J.; Wang, S. A promoter variant of lncRNA GAS5 is functionally associated with the development of osteosarcoma. J. Bone Oncol. 2018, 12, 23–26. [Google Scholar] [CrossRef]
- Gendron, P.; Lemieux, S.; Major, F. Quantitative analysis of nucleic acid three-dimensional structures. J. Mol. Biol. 2001, 308, 919–936. [Google Scholar] [CrossRef]
- Fingar, D.C.; Salama, S.; Tsou, C.; Harlow, E.; Blenis, J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002, 16, 1472–1487. [Google Scholar] [CrossRef]
- Kawasome, H.; Papst, P.; Webb, S.; Keller, G.M.; Johnson, G.L.; Gelfand, E.W.; Terada, N. Targeted disruption of p70(s6k) defines its role in protein synthesis and rapamycin sensitivity. Proc. Natl. Acad. Sci. USA 1998, 95, 5033–5038. [Google Scholar] [CrossRef]
- Jefferies, H.B.; Fumagalli, S.; Dennis, P.B.; Reinhard, C.; Pearson, R.B.; Thomas, G. Rapamycin suppresses 5’TOP mRNA translation through inhibition of p70s6k. EMBO J. 1997, 16, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Raho, G.; Barone, V.; Rossi, D.; Philipson, L.; Sorrentino, V. The gas 5 gene shows four alternative splicing patterns without coding for a protein. Gene 2000, 256, 13–17. [Google Scholar] [CrossRef]
- Gingras, A.C.; Kennedy, S.G.; O’Leary, M.A.; Sonenberg, N.; Hay, N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998, 12, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Heesom, K.J.; Denton, R.M. Dissociation of the eukaryotic initiation factor-4E/4E-BP1 complex involves phosphorylation of 4E-BP1 by an mTOR-associated kinase. FEBS Lett. 1999, 457, 489–493. [Google Scholar] [CrossRef]
- Rosenwald, I.B.; Lazaris-Karatzas, A.; Sonenberg, N.; Schmidt, E.V. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol. Cell Biol. 1993, 13, 7358–7363. [Google Scholar] [CrossRef]
- Quelle, D.E.; Ashmun, R.A.; Shurtleff, S.A.; Kato, J.Y.; Bar-Sagi, D.; Roussel, M.F.; Sherr, C.J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993, 7, 1559–1571. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.; Liu, D. Overexpression of long noncoding RNA GAS5 suppresses tumorigenesis and development of gastric cancer by sponging miR-106a-5p through the Akt/mTOR pathway. Biol. Open 2019, 8, bio041343. [Google Scholar] [CrossRef]
- Liu, J.; Chen, M.; Ma, L.; Dang, X.; Du, G. LncRNA GAS5 Suppresses the Proliferation and Invasion of Osteosarcoma Cells via the miR-23a-3p/PTEN/PI3K/AKT Pathway. Cell Transplant. 2020, 29, 0963689720953093. [Google Scholar] [CrossRef]
- Gao, X.; Neufeld, T.P.; Pan, D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and -independent pathways. Dev. Biol. 2000, 221, 404–418. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Nakamura, N.; Vazquez, F.; Batt, D.B.; Perera, S.; Roberts, T.M.; Sellers, W.R. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 2110–2115. [Google Scholar] [CrossRef]
- Kanamori, Y.; Kigawa, J.; Itamochi, H.; Shimada, M.; Takahashi, M.; Kamazawa, S.; Sato, S.; Akeshima, R.; Terakawa, N. Correlation between Loss of PTEN Expression and Akt Phosphorylation in Endometrial Carcinoma. Clin. Cancer Res. 2001, 7, 892–895. [Google Scholar] [PubMed]
- Liu, L.; Huang, L.; He, J.; Cai, S.; Weng, Y.; Huang, S.; Ma, S. PTEN inhibits non-small cell lung cancer cell growth by promoting G(0)/G(1) arrest and cell apoptosis. Oncol. Lett. 2019, 17, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, J.; Ou, B.; Liu, C.; Zou, Y.; Chen, Q. GAS5 knockdown reduces the chemo-sensitivity of non-small cell lung cancer (NSCLC) cell to cisplatin (DDP) through regulating miR-21/PTEN axis. Biomed. Pharmacother. 2017, 93, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, P.; Zhang, Y.; Gong, B.; Yu, D.; Sun, X. Long non-coding RNA GAS5 increases the radiosensitivity of A549 cells through interaction with the miR-21/PTEN/Akt axis. Oncol. Rep. 2020, 43, 897–907. [Google Scholar] [CrossRef]
- Wang, C.; Ke, S.; Li, M.; Lin, C.; Liu, X.; Pan, Q. Downregulation of LncRNA GAS5 promotes liver cancer proliferation and drug resistance by decreasing PTEN expression. Mol. Genet. Genom. 2020, 295, 251–260. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.J.; Meng, T.; Lei, C.; Yang, X.H.; Wang, Q.S.; Jin, B.; Zhu, J.F. lncRNA GAS5 Inhibits Cell Migration and Invasion and Promotes Autophagy by Targeting miR-222-3p via the GAS5/PTEN-Signaling Pathway in CRC. Mol. Ther. Nucleic Acids 2019, 17, 644–656. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Ye, Y.; Zhao, S.-J. LncRNA Gas5 acts as a ceRNA to regulate PTEN expression by sponging miR-222-3p in papillary thyroid carcinoma. Oncotarget 2017, 9, 3519–3530. [Google Scholar] [CrossRef]
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in endometrial cancer cells. J. Biomed. Sci. 2015, 22, 100. [Google Scholar] [CrossRef]
- Gao, Z.-Q.; Wang, J.-f.; Chen, D.-H.; Ma, X.-S.; Wu, Y.; Tang, Z.; Dang, X.-W. Long non-coding RNA GAS5 suppresses pancreatic cancer metastasis through modulating miR-32-5p/PTEN axis. Cell Biosci. 2017, 7, 66. [Google Scholar] [CrossRef]
- West, M.J.; Stoneley, M.; Willis, A.E. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene 1998, 17, 769–780. [Google Scholar] [CrossRef]
- Vlach, J.; Hennecke, S.; Alevizopoulos, K.; Conti, D.; Amati, B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996, 15, 6595–6604. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Lou, Z.; Gupta, M. The long non-coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c-Myc translation. PLoS ONE 2014, 9, e107016. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, S.; Edwards, M.C.; Bai, C.; Parker, S.; Zhang, P.; Baldini, A.; Harper, J.; Elledge, S. p57KIP2, a structurally distinct member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor gene. Genes Dev. 1995, 9 6, 650–662. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995, 9, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, D.; Huang, C.; Wang, M.; Xiao, X.; Zeng, F.; Wang, L.; Jiang, G. LncRNA GAS5 Inhibits Cellular Proliferation by Targeting P27(Kip1). Mol. Cancer Res. 2017, 15, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Z.; Meng, X.; Zhou, S.; Xiao, S.; Li, X.; Liu, S.; Yu, P. Long noncoding RNA GAS5 impairs the proliferation and invasion of endometrial carcinoma induced by high glucose via targeting miR-222-3p/p27. Am. J. Transl. Res. 2019, 11, 2413–2421. [Google Scholar]
- Liu, Y.; Zhao, J.; Zhang, W.; Gan, J.; Hu, C.; Huang, G.; Zhang, Y. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci. Rep. 2015, 5, 10159. [Google Scholar] [CrossRef]
- Zingg, D.; Debbache, J.; Schaefer, S.M.; Tuncer, E.; Frommel, S.C.; Cheng, P.; Arenas-Ramirez, N.; Haeusel, J.; Zhang, Y.; Bonalli, M.; et al. The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors. Nat. Commun. 2015, 6, 6051. [Google Scholar] [CrossRef]
- Xu, W.; Yan, Z.; Hu, F.; Wei, W.; Yang, C.; Sun, Z. Long non-coding RNA GAS5 accelerates oxidative stress in melanoma cells by rescuing EZH2-mediated CDKN1C downregulation. Cancer Cell Int. 2020, 20, 116. [Google Scholar] [CrossRef]
- Wang, M.; Guo, C.; Wang, L.; Luo, G.; Huang, C.; Li, Y.; Liu, D.; Zeng, F.; Jiang, G.; Xiao, X. Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis. 2018, 9, 238. [Google Scholar] [CrossRef]
- Volden, P.A.; Conzen, S.D. The influence of glucocorticoid signaling on tumor progression. Brain Behav. Immun. 2013, 30, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Pufall, M.A. Glucocorticoids and Cancer. Adv. Exp. Med. Biol. 2015, 872, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Bakour, N.; Moriarty, F.; Moore, G.; Robson, T.; Annett, S.L. Prognostic Significance of Glucocorticoid Receptor Expression in Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Rutz, H.P.; Herr, I. Interference of glucocorticoids with apoptosis signaling and host-tumor interactions. Cancer Biol. Ther. 2004, 3, 715–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010, 3, ra8. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Williams, G.T. The hormone response element mimic sequence of GAS5 lncRNA is sufficient to induce apoptosis in breast cancer cells. Oncotarget 2016, 7, 10104–10116. [Google Scholar] [CrossRef]
- Pickard, M.R.; Mourtada-Maarabouni, M.; Williams, G.T. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta 2013, 1832, 1613–1623. [Google Scholar] [CrossRef]
- Runnebaum, I.B.; Brüning, A. Glucocorticoids Inhibit Cell Death in Ovarian Cancer and Up-regulate Caspase Inhibitor cIAP2. Clin. Cancer Res. 2005, 11, 6325–6332. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.-Y.; Li, X.; Yuan, X.-Q.; Yang, Y.-L.; Zhu, K.-W.; Zeng, H.; Li, X.-L.; Cao, S.; Zhou, H.-H.; et al. Long non-coding RNA GAS5 polymorphism predicts a poor prognosis of acute myeloid leukemia in Chinese patients via affecting hematopoietic reconstitution. Leuk. Lymphoma 2016, 58, 1948–1957. [Google Scholar] [CrossRef]
- Tao, R.; Hu, S.; Wang, S.; Zhou, X.; Zhang, Q.; Wang, C.; Zhao, X.; Zhou, W.; Zhang, S.; Li, C.; et al. Association between indel polymorphism in the promoter region of lncRNA GAS5 and the risk of hepatocellular carcinoma. Carcinogenesis 2015, 36, 1136–1143. [Google Scholar] [CrossRef]
- Weng, S.-L.; Ng, S.-C.; Lee, Y.-C.; Hsiao, Y.-H.; Hsu, C.-F.; Yang, S.-F.; Wang, P.-H. The relationships of genetic polymorphisms of the long noncoding RNA growth arrest-specific transcript 5 with uterine cervical cancer. Int. J. Med. Sci. 2020, 17, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, N.; Zheng, Y.; Chen, Y.-D.; Liu, J.; Yang, M. LncRNA GAS5 Indel Genetic Polymorphism Contributes to Glioma Risk Through Interfering Binding of Transcriptional Factor TFAP2A. DNA Cell Biol. 2018, 37, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Shahangian, S.S.; Salehi, Z.; Mashayekhi, F.; Talesh Sasani, S.; Mirzanezhad, L. Influence of a 5-bp Indel Polymorphism at Promoter of the GAS5 lncRNA and Risk of Breast Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 3705–3710. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, S.-S.; Yang, C.-K.; Li, J.-R.; Chen, C.-S.; Hung, S.-C.; Chiu, K.-Y.; Cheng, C.-L.; Ou, Y.-C.; Yang, S.-F. Impact of GAS5 genetic polymorphism on prostate cancer susceptibility and clinicopathologic characteristics. Int. J. Med. Sci. 2019, 16, 1424–1429. [Google Scholar] [CrossRef]
- Rakhshan, A.; Esmaeili, M.H.; Kahaei, M.S.; Taheri, M.; Omrani, M.D.; Noroozi, R.; Ghafouri-Fard, S. A Single Nucleotide Polymorphism in GAS5 lncRNA is Associated with Risk of Bladder Cancer in Iranian Population. Pathol. Oncol. Res. 2020, 26, 1251–1254. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Zhao, Y.; Jin, Y.; An, L.; Wu, B.; Liu, Z.; Chen, X.; Zhou, H.; Wang, H.; et al. Genetic polymorphisms of long non-coding RNA GAS5 predict platinum-based concurrent chemoradiotherapy response in nasopharyngeal carcinoma patients. Oncotarget 2017, 8, 62286–62297. [Google Scholar] [CrossRef]
- Weng, W.-C.; Chen, C.-J.; Chen, P.-N.; Wang, S.-S.; Hsieh, M.-J.; Yang, S.-F. Impact of Gene Polymorphisms in GAS5 on Urothelial Cell Carcinoma Development and Clinical Characteristics. Diagnostics 2020, 10, 260. [Google Scholar] [CrossRef]
- Ala, U. Competing Endogenous RNAs, Non-Coding RNAs and Diseases: An Intertwined Story. Cells 2020, 9, 1574. [Google Scholar] [CrossRef]
- Lekka, E.; Hall, J. Noncoding RNAs in disease. FEBS Lett. 2018, 592, 2884–2900. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, Z.; Wang, G.; Wang, J.; Zhu, W. lncRNA GAS5 inhibits colorectal cancer cell proliferation via the miR-182-5p/FOXO3a axis. Oncol. Rep. 2018, 40, 2371–2380. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Zheng, J.; Liu, X.; Chen, J.; Liu, L.; Wang, P.; Xue, Y. GAS5 suppresses malignancy of human glioma stem cells via a miR-196a-5p/FOXO1 feedback loop. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, J.; Wang, Z.; Wang, P.; Gao, X.; Wang, Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed. Pharmacother. 2018, 104, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Bahceci, I.; Dogrusoz, U.; La, K.C.; Babur, Ö.; Gao, J.; Schultz, N. PathwayMapper: A collaborative visual web editor for cancer pathways and genomic data. Bioinformatics 2017, 33, 2238–2240. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Ney, M. The FOXO’s Advantages of Being a Family: Considerations on Function and Evolution. Cells 2020, 9, 787. [Google Scholar] [CrossRef]
- Jiramongkol, Y.; Lam, E.W.F. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Gaither, L.A.; Eide, D.J. The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J. Biol. Chem. 2001, 276, 22258–22264. [Google Scholar] [CrossRef]
- Costello, L.C.; Franklin, R.B. Decreased zinc in the development and progression of malignancy: An important common relationship and potential for prevention and treatment of carcinomas. Expert Opin. Ther. Targets 2017, 21, 51–66. [Google Scholar] [CrossRef]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32. [Google Scholar] [CrossRef]
- Dong, X.; Kong, C.; Zhang, Z.; Liu, X.; Zhan, B.; Chen, Z.; Shi, D. hZIP1 that is down-regulated in clear cell renal cell carcinoma is negatively associated with the malignant potential of the tumor. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 885–892. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Guo, B.; Liu, D.; Shi, J.; Wu, C.; Chen, J.; Zhang, X.; Wu, J. Mechanisms of resistance to chemotherapy and radiotherapy in hepatocellular carcinoma. Transl. Cancer Res. 2018, 7, 765–781. [Google Scholar] [CrossRef]
- Wang, W.-T.; Han, C.; Sun, Y.-M.; Chen, T.-Q.; Chen, Y.-Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J. Hematol. Oncol. 2019, 12, 55. [Google Scholar] [CrossRef]
- Hahne, J.C.; Valeri, N. Non-Coding RNAs and Resistance to Anticancer Drugs in Gastrointestinal Tumors. Front. Oncol. 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pan, T.; Jiang, D.; Jin, L.; Geng, Y.; Feng, X.; Shen, A.; Zhang, L. The lncRNA-GAS5/miR-221-3p/DKK2 Axis Modulates ABCB1-Mediated Adriamycin Resistance of Breast Cancer via the Wnt/ß-Catenin Signaling Pathway. Mol. Ther.-Nucleic Acids 2020, 19, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, L.; Li, G.; Cai, M.; Tan, C.; Han, X.; Han, L. LncRNA GAS5 confers the radio sensitivity of cervical cancer cells via regulating miR-106b/IER3 axis. Int. J. Biol. Macromol. 2019, 126, 994–1001. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Z.; Liao, S.; Li, E.; Wu, X.; Zeng, W. Elevation of long non-coding RNA GAS5 and knockdown of microRNA-21 up-regulate RECK expression to enhance esophageal squamous cell carcinoma cell radio-sensitivity after radiotherapy. Genomics 2020, 112, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meng, L.; Zhong, Y.; Hu, F.; Wang, L.; Wang, M. The long intergenic noncoding RNA GAS5 reduces cisplatin-resistance in non-small cell lung cancer through the miR-217/LHPP axis. Aging 2021, 13, 2864–2884. [Google Scholar] [CrossRef]
- Liu, B.; Wu, S.; Ma, J.; Yan, S.; Xiao, Z.; Wan, L.; Zhang, F.; Shang, M.; Mao, A. lncRNA GAS5 Reverses EMT and Tumor Stem Cell-Mediated Gemcitabine Resistance and Metastasis by Targeting miR-221/SOCS3 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2018, 13, 472–482. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).