Potential miRNAs for miRNA-Based Therapeutics in Breast Cancer
Abstract
1. Introduction
2. miRNA Biogenesis and Mode of Action
3. Cell Lines as In Vitro Model
4. Roles of miRNAs in Cancer
- Sole function as onco-miR or ts-miR;
- miRNA signatures (miRNA profiles in tumor stages and subtypes);
- Validation of miRNA functions (loss/gain of function);
- Multiple reports supporting its functions (same cell types/subtypes);
- Pharmacological studies—non-toxic (in vitro and in vivo studies);
- Sensitizing cancer cells to standard therapy (optional).
4.1. Tumor-Suppressive miRNAs (ts-miRs)
4.2. Oncogenic miRNAs (onco-miRs)
5. miRNA-Based Therapeutics
5.1. miRNA Suppression (Synthetic miRNA-Induced Inhibition)
- Addition of methoxyethyl group at the RNA 2′-OH (2-MOE);
- Addition of fluorine 2′-hydroxyl group at C2 carbon of the sugar group (2′-F);
- Substitution of oxygen of the phosphate backbone to sulfur to form phosphonothioate linkage;
- Substitution of phosphate with the uncharged phosphonodiamidite group to form phosphorothioate linkage, known as phosphorodiamidate morpholino oligomers (PMOs);
- Substitution of phosphate backbone with a pseudo-peptide polymer (N-(2-aminoethyl) glycine) to form an uncharged synthetic DNA, known as peptide nucleic acid (PNA).
5.2. miRNA Replenishment (Delivery Systems)
6. Conclusions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hudis, C.A.; Gianni, L. Triple-Negative Breast Cancer: An Unmet Medical Need. Oncologist 2011, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Majid, S.; Hassan, T. MicroRNAs and Its Emerging Role as Breast Cancer Diagnostic Marker—A Review. Adv. Biomark. Sci. Technol. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W. Current view of microRNA processing. Signal Transduct. Insights 2016, 5, STI.S12317. [Google Scholar] [CrossRef]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Iorio, M.V.; Casalini, P.; Piovan, C.; Braccioli, L.; Tagliabue, E. Breast Cancer and MicroRNAs: Therapeutic Impact. Breast 2011, 20, S63–S70. [Google Scholar] [CrossRef]
- Abdelfattah, A.M.; Park, C.; Choi, M.Y. Update on Non-Canonical MicroRNAs. Biomol. Concepts 2014, 5, 275–287. [Google Scholar] [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-Induced Silencing Complex: A Versatile Gene-Silencing Machine. J. Boil. Chem. 2009, 284, 17897–17901. [Google Scholar] [CrossRef]
- Fukao, A.; Mishima, Y.; Takizawa, N.; Oka, S.; Imataka, H.; Pelletier, J.; Sonenberg, N.; Thoma, C.; Fujiwara, T. MicroRNAs Trigger Dissociation of EIF4AI and EIF4AII from Target MRNAs in Humans. Mol. Cell 2014, 56, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.E.; Huntzinger, E.; Fauser, M.; Izaurralde, E. GW182 Proteins Directly Recruit Cytoplasmic Deadenylase Complexes to MiRNA Targets. Mol. Cell 2011, 44, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and Molecular Characterization of the Claudin-Low Intrinsic Subtype of Breast Cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppé, J.-P.; Tong, F.; et al. A Collection of Breast Cancer Cell Lines for the Study of Functionally Distinct Cancer Subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef]
- Hughes, P.; Marshall, D.; Reid, Y.; Parkes, H.; Gelber, C. The Costs of Using Unauthenticated, over-Passaged Cell Lines: How Much More Data Do We Need? BioTechniques. 2007, 43, 575–586. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Gurski, L.A.; Petrelli, N.J.; Jia, X.; Farach-Carson, M.C. 3D Matrices for Anti-cancer Drug Testing and Development. Oncol. Issues 2010, 25, 20–25. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, C.H.; Lee, H. Mouse Models of Breast Cancer in Preclinical Research. Lab. Anim. Res. 2018, 34, 160–165. [Google Scholar] [CrossRef]
- Fantozzi, A.; Christofori, G. Mouse Models of Breast Cancer Metastasis. Breast Cancer Res. 2006, 8, 1–11. [Google Scholar]
- Whittle, J.R.; Lewis, M.T.; Lindeman, G.J.; Visvader, J.E. Patient-derived Xenograft Models of Breast Cancer and Their Predictive Power. Breast Cancer Res. 2015, 17, 1–13. [Google Scholar]
- Hiraga, T.; Ninomiya, T. Establishment and Characterization of a C57BL/6 Mouse Model of Bone Metastasis of Breast Cancer. J. Bone Miner. Metab. 2019, 37, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, A.; Castellví, J.; Artero-Castro, A.; Leal, J.A.; Romagosa, C.; Hernández-Losa, J.; Peg, V.; Fabra, A.; Vidal, F.; Kondoh, H.; et al. MiR-125b Acts as a Tumor Suppressor in Breast Tumorigenesis via Its Novel Direct Targets ENPEP, CK2-α, CCNJ, and MEGF9. PLoS ONE 2013, 8, e76247. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Y.; Fan, X.; Zhang, P.; Wang, P.; Cheng, S.; Zhang, J. MicroRNA-125b as a Tumor Suppressor by Targeting MMP11 in Breast Cancer. Thorac. Cancer 2020. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, H.; Zhang, Z.; Li, N. MiR-125b-5p Inhibits Breast Cancer Cell Proliferation, Migration and Invasion by Targeting KIAA1522. Biochem. Biophys. Res. Commun. 2018, 504, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Jiang, H.C.; Zhou, Y.C.; Jiang, B.; He, W.J.; Wang, Y.F.; Dong, J. MiR-125b Regulates the Proliferation and Metastasis of Triple Negative Breast Cancer Cells via the Wnt/β-Catenin Pathway and EMT. Biosci. Biotechnol. Biochem. 2019, 83, 1062–1071. [Google Scholar] [CrossRef]
- Imani, S.; Wu, R.C.; Fu, J. MicroRNA-34 Family in Breast Cancer: From Research to Therapeutic Potential. J. Cancer 2018, 9, 3765–3775. [Google Scholar] [CrossRef]
- Mattiske, S.; Suetani, R.J.; Neilsen, P.M.; Callen, D.F. The Oncogenic Role of MiR-155 in Breast Cancer. Cancer Epidemiology Biomarkers Prev. 2012, 21, 1236–1243. [Google Scholar] [CrossRef]
- Chen, W.X.; Hu, Q.; Qiu, M.T.; Zhong, S.L.; Xu, J.J.; Tang, J.H.; Zhao, J.H. MiR-221/222: Promising Biomarkers for Breast Cancer. Tumor Biol. 2013, 34, 1361–1370. [Google Scholar] [CrossRef]
- Mayr, C.; Hemann, M.T.; Bartel, D.P. Disrupting the Pairing between Let-7 and Hmga2 Enhances Oncogenic Transformation. Science 2007, 315, 1576–1579. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, J.Y.; Lee, K.D.; Bae, Y.K.; Sung, K.W.; Nam, S.J.; Chun, K.H. MicroRNA Let-7a Suppresses Breast Cancer Cell Migration and Invasion through Downregulation of C-C Chemokine Receptor Type 7. Breast Cancer Res. 2012, 14, R14. [Google Scholar] [CrossRef]
- Mi, Y.Z.; Liu, F.; Liang, X.L.; Liu, S.N.; Huang, X.C.; Sang, M.X.; Geng, C.Z. Tumor Suppressor Let-7a Inhibits Breast Cancer Cell Proliferation, Migration and Invasion by Targeting MAGE-A1. Neoplasma 2019, 66, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, G.; Wu, B.; Yuan, Y.; Pan, Y. Let-7d Inhibits Growth and Metastasis in Breast Cancer by Targeting Jab1/Cops5. Cell. Physiol. Biochem. 2018, 47, 2126–2135. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Lai, Y.; Liao, Y.; Liu, R.; Qiu, W. Hsa-MiR-1 Suppresses Breast Cancer Development by down-Regulating K-Ras and Long Non-Coding RNA MALAT1. Int. J. Biol. Macromol. 2015, 81, 491–497. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, F.; Chen, P. MiR-7 and MiR-218 Epigenetically Control Tumor Suppressor Genes RASSF1A and Claudin-6 by Targeting HoxB3 in Breast Cancer. Biochem. Biophys. Res. Commun. 2012, 424, 28–33. [Google Scholar] [CrossRef]
- Reddy, S.D.N.; Ohshiro, K.; Rayala, S.K.; Kumar, R. MicroRNA-7, a Homeobox D10 Target, Inhibits P21-Activated Kinase 1 and Regulates Its Functions. Cancer Res. 2008, 68, 8195–8200. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.J.; Giles, K.M.; Price, K.J.; Zhang, P.M.; Mattick, J.S.; Leedman, P.J. Regulation of Epidermal Growth Factor Receptor Signaling in Human Cancer Cells by MicroRNA-7. J. Biol. Chem. 2009, 284, 5731–5741. [Google Scholar] [CrossRef]
- Okuda, H.; Xing, F.; Pandey, P.R.; Sharma, S.; Watabe, M.; Pai, S.K.; Mo, Y.-Y.; Iiizumi-Gairani, M.; Hirota, S.; Liu, Y.; et al. MiR-7 Suppresses Brain Metastasis of Breast Cancer Stem-like Cells by Modulating KLF4. Cancer Res. 2013, 73, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Moazzeni, H.; Najafi, A.; Khani, M. Identification of Direct Target Genes of MiR-7, MiR-9, MiR-96, and MiR-182 in the Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231. Mol. Cell. Probes 2017, 34, 45–52. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, X.; Li, P.; Tan, J.; Wang, X.; Xiang, T.; Ren, G. MiR-7-5p Suppresses Cell Proliferation and Induces Apoptosis of Breast Cancer Cells Mainly by Targeting REGγ. Cancer Lett. 2015, 358, 27–36. [Google Scholar] [CrossRef]
- Cui, Y.X.; Bradbury, R.; Flamini, V.; Wu, B.; Jordan, N.; Jiang, W.G. MicroRNA-7 Suppresses the Homing and Migration Potential of Human Endothelial Cells to Highly Metastatic Human Breast Cancer Cells. Br. J. Cancer 2017, 117, 89–101. [Google Scholar] [CrossRef]
- Hong, T.; Ding, J.; Li, W. Mir-7 Reverses Breast Cancer Resistance to Chemotherapy by Targeting MRP1 and BCL2. OncoTargets Ther. 2019, 12, 11097–11105. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Garikapati, K.R.; Ramaiah, M.J.; Polavarapu, K.K.; Bhadra, U.; Bhadra, M.P. MiR-15a/MiR-16 Induces Mitochondrial Dependent Apoptosis in Breast Cancer Cells by Suppressing Oncogene BMI1. Life Sci. 2016, 164, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Janaki Ramaiah, M.; Lavanya, A.; Honarpisheh, M.; Zarea, M.; Bhadra, U.; Bhadra, M.P. MiR-15/16 Complex Targets P70S6 Kinase1 and Controls Cell Proliferation in MDA-MB-231 Breast Cancer Cells. Gene 2014, 552, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Haghi, M.; Taha, M.F.; Javeri, A. Suppressive Effect of Exogenous MiR-16 and MiR-34a on Tumorigenesis of Breast Cancer Cells. J. Cell. Biochem. 2019, 120, 13342–13353. [Google Scholar] [CrossRef]
- Ruan, L.; Qian, X. MIR-16-5p Inhibits Breast Cancer by Reducing AKT3 to Restrain NF-ΚB Pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Fan, M.; Sethuraman, A.; Brown, M.; Sun, W.; Pfeffer, L.M. Systematic Analysis of Metastasis-Associated Genes Identifies MiR-17-5p as a Metastatic Suppressor of Basal-like Breast Cancer. Breast Cancer Res. Treat. 2014, 146, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, C.; Wang, M.; Li, Z.; Casimiro, M.C.; Liu, M.; Wu, K.; Whittle, J.; Ju, X.; Hyslop, T.; et al. A Cyclin D1/MicroRNA 17/20 Regulatory Feedback Loop in Control of Breast Cancer Cell Proliferation. J. Cell Biol. 2008, 182, 509–517. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, P.; Li, X.; Wang, Q.; Deng, Z.-B.; Zhuang, X.; Mu, J.; Zhang, L.; Wang, B.; Yan, J.; et al. Restoration of MiR17/20a in Solid Tumor Cells Enhances the Natural Killer Cell Antitumor Activity by Targeting Mekk2. Cancer Immunol. Res. 2014, 2, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Krutilina, R.; Sun, W.; Sethuraman, A.; Brown, M.; Seagroves, T.N.; Pfeffer, L.M.; Ignatova, T.; Fan, M. MicroRNA-18a Inhibits Hypoxia-Inducible Factor 1α Activity and Lung Metastasis in Basal Breast Cancers. Breast Cancer Res. 2014, 16, R78. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Liu, Y.; Su, P.; Zhang, J.; Wang, X.; Sun, M.; Chen, B.; Zhao, W.; Wang, L.; et al. SREBP1, Targeted by MiR-18a-5p, Modulates Epithelial-Mesenchymal Transition in Breast Cancer via Forming a Co-Repressor Complex with Snail and HDAC1/2. Cell Death Differ. 2019, 26, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-X.; Liu, Y.-H.; Xiang, J.-W.; Wu, Q.-N.; Xu, L.-B.; Luo, X.-L.; Zhu, X.-L.; Liu, C.; Xu, F.-P.; Luo, D.-L.; et al. PIK3R1 Targeting by MiR-21 Suppresses Tumor Cell Migration and Invasion by Reducing PI3K/AKT Signaling and Reversing EMT, and Predicts Clinical Outcome of Breast Cancer. Int. J. Oncol. 2016, 48, 471–484. [Google Scholar] [CrossRef]
- Huang, Z.M.; Ge, H.F.; Yang, C.C.; Cai, Y.; Chen, Z.; Tian, W.Z.; Tao, J.L. MicroRNA-26a-5p inhibits breast cancer cell growth by suppressing RNF6 expression. Kaohsiung J. Med Sci. 2019, 35, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ding, K.; Li, R.; Zhang, W.; Li, G.; Kong, X.; Qian, P.; Lobie, P.E.; Zhu, T. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Res. 2014, 16, R40. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tang, H.; Chen, B.; He, Z.; Deng, M.; Wu, M.; Liu, X.; Yang, L.; Ye, F.; Xie, X. MiR-26a Suppresses Tumour Proliferation and Metastasis by Targeting Metadherin in Triple Negative Breast Cancer. Cancer Lett. 2015, 357, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, L.; Wu, M.; Liu, M.; Xie, X.; Guo, J.; Tang, H.; Xie, X. MiR-26a inhibits proliferation and migration of breast cancer through repression of MCL-1. PLoS ONE 2013, 8, 41309. [Google Scholar] [CrossRef]
- Tormo, E.; Adam-Artigues, A.; Ballester, S.; Pineda, B.; Zazo, S.; González-Alonso, P.; Albanell, J.; Rovira, A.; Rojo, F.; Lluch, A.; et al. The role of miR-26a and miR-30b in HER2+ breast cancer trastuzumab resistance and regulation of the CCNE2 gene. Scientific Rep. 2017, 7, 41309. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ding, K.; Chong, Q.Y.; Zhao, J.; Liu, Y.; Shao, Y.; Zhang, Y.; Yu, Q.; Xiong, Z.; Zhang, W.; et al. Decreased miR26a/b and increased HuR expression post-transcriptionally upregulates ERBB2 to mediate acquired tamoxifen resistance in ER+ breast cancer cells. J. Biol. Chem. 2017, 292, 13551–13564. [Google Scholar] [CrossRef]
- Li, J.; Kong, X.; Zhang, J.; Luo, Q.; Li, X.; Fang, L. MiRNA-26b Inhibits Proliferation by Targeting PTGS2 in Breast Cancer. Cancer Cell Int. 2013, 13, 7. [Google Scholar] [CrossRef]
- Zhang, L.; Du, Y.; Xu, S.; Jiang, Y.; Yuan, C.; Zhou, L.; Ma, X.; Bai, Y.; Lu, J.; Ma, J. DEPDC1, Negatively Regulated by MiR-26b, Facilitates Cell Proliferation via the up-Regulation of FOXM1 Expression in TNBC. Cancer Lett. 2019, 442, 242–251. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, B.; Ye, Q.; Liu, W. MiR-30a-5p/UBE3C Axis Regulates Breast Cancer Cell Proliferation and Migration. Biochem. Biophys. Res. Commun. 2019, 516, 1013–1018. [Google Scholar] [CrossRef]
- Li, L.; Kang, L.; Zhao, W.; Feng, Y.; Liu, W.; Wang, T.; Mai, H.; Huang, J.; Chen, S.; Liang, Y.; et al. MiR-30a-5p Suppresses Breast Tumor Growth and Metastasis through Inhibition of LDHA-Mediated Warburg Effect. Cancer Lett. 2017, 400, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.D.; Jiang, L.H.; Sun, D.W.; Li, J.; Tang, J.H. MIR.30a Inhibits the Biological Function of Breast Cancer Cells by Targeting Notch1. Int. J. Mol. Med. 2017, 40, 1235–1242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, J.; Xu, X.; Kang, L.; Zhou, L.; Wang, S.; Lu, J.; Cheng, L.; Fan, Z.; Yuan, B.; Tian, P.; et al. MiR-30a Suppresses Breast Cancer Cell Proliferation and Migration by Targeting Eya2. Biochem. Biophys. Res. Commun. 2014, 445, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Ouzounova, M.; Vuong, T.; Ancey, P.B.; Ferrand, M.; Durand, G.; Le-Calvez Kelm, F.; Croce, C.; Matar, C.; Herceg, Z.; Hernandez-Vargas, H. MicroRNA MiR-30 Family Regulates Non-Attachment Growth of Breast Cancer Cells. BMC Genom. 2013, 14, 139. [Google Scholar] [CrossRef]
- Xiao, B.; Shi, X.; Bai, J. MiR-30a Regulates the Proliferation and Invasion of Breast Cancer Cells by Targeting Snail. Oncol. Lett. 2019, 17, 406–413. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, H.; Tang, R.; Song, H.; Pan, H.; Feng, Z.; Chen, L. MiR-30a Inhibits Epithelial-Mesenchymal Transition and Metastasis in Triple-Negative Breast Cancer by Targeting ROR1. Oncol. Rep. 2018, 39, 2635–2643. [Google Scholar] [CrossRef]
- Croset, M.; Pantano, F.; Kan, C.W.S.; Bonnelye, E.; Descotes, F.; Alix-Panabieres, C.; Lecellier, C.H.; Bachelier, R.; Allioli, N.; Hong, S.S.; et al. MiRNA-30 Family Members Inhibit Breast Cancer Invasion, Osteomimicry, and Bone Destruction by Directly Targeting Multiple Bone Metastasis–Associated Genes. Cancer Res. 2018, 78, 5259–5273. [Google Scholar] [CrossRef]
- Bao, S.; Wang, X.; Wang, Z.; Yang, J.; Liu, F.; Yin, C. MicroRNA-30 Mediates Cell Invasion and Metastasis in Breast Cancer. Biochem. Cell Biol. 2018, 96, 825–831. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Downs-Kelly, E.; Das, M.; Izem, L.; Tubbs, R.; Plow, E.F. WAVE3, an Actin Remodeling Protein, Is Regulated by the Metastasis Suppressor MicroRNA, MiR-31, during the Invasion-Metastasis Cascade. Int. J. Cancer 2011, 129, 1331–1343. [Google Scholar] [CrossRef]
- Luo, L.J.; Yang, F.; Ding, J.J.; Yan, D.L.; Wang, D.D.; Yang, S.J.; Ding, L.; Li, J.; Chen, D.; Ma, R.; et al. MiR-31 Inhibits Migration and Invasion by Targeting SATB2 in Triple Negative Breast Cancer. Gene 2016, 594, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.A.K.; Teo, C.R.; Beillard, E.J.; Voorhoeve, P.M.; Zhou, W.; Ghosh, S.; Casey, P.J. MicroRNA-31 Controls G Protein Alpha-13 (GNA13) Expression and Cell Invasion in Breast Cancer Cells. Mol. Cancer 2015, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Weihua, Z.; Guorong, Z.; Xiaolong, C.; Weizhan, L. MiR-33a Functions as a Tumor Suppressor in Triple-Negative Breast Cancer by Targeting EZH2. Cancer Cell Int. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Gao, J.; Zhang, T.; Li, S.; Luo, A.; Chen, H.; Ding, F.; Wang, X.; Liu, Z. MicroRNA-34 Suppresses Breast Cancer Invasion and Metastasis by Directly Targeting Fra-1. Oncogene 2013, 32, 4294–4303. [Google Scholar] [CrossRef]
- Wu, J.; Li, W.-Z.; Huang, M.-L.; Wei, H.-L.; Wang, T.; Fan, J.; Li, N.-L.; Ling, R. Regulation of Cancerous Progression and Epithelial-Mesenchymal Transition by MiR-34c-3p via Modulation of MAP3K2 Signaling in Triple-Negative Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 483, 10–16. [Google Scholar] [CrossRef]
- Si, W.; Li, Y.; Shao, H.; Hu, R.; Wang, W.; Zhang, K.; Yang, Q. MiR-34a Inhibits Breast Cancer Proliferation and Progression by Targeting Wnt1 in Wnt/β-Catenin Signaling Pathway. Am. J. Med. Sci. 2016, 352, 191–199. [Google Scholar] [CrossRef]
- Wu, M.Y.; Fu, J.; Xiao, X.; Wu, J.; Wu, R.C. MiR-34a Regulates Therapy Resistance by Targeting HDAC1 and HDAC7 in Breast Cancer. Cancer Lett. 2014, 354, 311–319. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, H.S.; Li, X.Y.; Lee, I.; Choi, H.S.; Kang, S.E.; Cha, S.Y.; Ryu, J.K.; Yoon, D.; Fearon, E.R.; et al. A P53/MiRNA-34 Axis Regulates Snail1-Dependent Cancer Cell Epithelial-Mesenchymal Transition. J. Cell Biol. 2011, 195, 417–433. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, J.Y.; Ho, C.C.; Hong, Q.S.; Yu, S.L.; Tzeng, C.R.; Yang, P.C.; Chen, H.W. MiRNA-34b as a Tumor Suppressor in Estrogen-Dependent Growth of Breast Cancer Cells. Breast Cancer Res. 2011, 13, R116. [Google Scholar] [CrossRef]
- Achari, C.; Winslow, S.; Ceder, Y.; Larsson, C. Expression of MiR-34c Induces G2/M Cell Cycle Arrest in Breast Cancer Cells. BMC Cancer 2014, 14, 538. [Google Scholar] [CrossRef]
- Huang, X.; Xie, X.; Wang, H.; Xiao, X.; Yang, L.; Tian, Z.; Guo, X.; Zhang, L.; Tang, H.; Xie, X. PDL1 and LDHA Act as CeRNAs in Triple Negative Breast Cancer by Regulating MiR-34a. J. Exp. Clin. Cancer Res. 2017, 36, 129. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Zhao, H.; Xiao, X.; Wang, L.; Mo, L.; Yao, Y. Microrna-34a Suppresses Breast Cancer Cell Proliferation and Invasion by Targeting Notch1. Exp. Ther. Med. 2018, 16, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, L.; Dong, D.; Wang, Z.; Ji, W.; Yu, M.; Zhang, F.; Niu, R.; Zhou, Y. MiR-34b/c-5p and the Neurokinin-1 Receptor Regulate Breast Cancer Cell Proliferation and Apoptosis. Cell Prolif. 2019, 52, e12527. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.; Yang, C.; Ji, J.S. MicroRNA-34a Inhibition of the TLR Signaling Pathway Via CXCL10 Suppresses Breast Cancer Cell Invasion and Migration. Cell. Physiol. Biochem. 2018, 46, 1286–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, D.; Kovalchuk, I.; Apel, I.J.; Chinnaiyan, A.M.; Wóycicki, R.K.; Cantor, C.R.; Kovalchuk, O. MiR-34a Directly Targets TRNAiMet Precursors and Affects Cellular Proliferation, Cell Cycle, and Apoptosis. Proc. Natl. Acad. Sci. USA 2018, 115, 7392–7397. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Sang, M.; Meng, L.; Gu, L.; Liu, S.; Li, J.; Geng, C. MiR-92b Promotes Autophagy and Suppresses Viability and Invasion in Breast Cancer by Targeting EZH2. Int. J. Oncol. 2018, 53, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Wang, Q.Y.; Zhou, C.X.; Yin, Q.Q.; He, M.; Yu, X.T.; Cao, D.X.; Chen, G.Q.; He, J.R.; Zhao, Q. MiR-124 targets Slug to regulate epithelial–mesenchymal transition and metastasis of breast cancer. Carcinogenesis 2013, 34, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, J.; Wang, B.; Wang, D.; Xie, X.; Yuan, L.; Guo, J.; Xi, S.; Gao, J.; Lin, X.; et al. Microrna-124 Targets Flotillin-1 to Regulate Proliferation and Migration in Breast Cancer. Mol. Cancer 2013, 12, 163. [Google Scholar] [CrossRef]
- Cai, W.L.; Huang, W.D.; Li, B.; Chen, T.R.; Li, Z.X.; Zhao, C.L.; Li, H.Y.; Wu, Y.M.; Yan, W.J.; Xiao, J.R. MicroRNA-124 Inhibits Bone Metastasis of Breast Cancer by Repressing Interleukin-11. Mol. Cancer 2018, 17, 9. [Google Scholar] [CrossRef]
- Dong, L.L.; Chen, L.M.; Wang, W.M.; Zhang, L.M. Decreased expression of microRNA-124 is an independent unfavorable prognostic factor for patients with breast cancer. Diagnostic Pathol. 2015, 10, 45. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Chen, X.; Liu, B.; Han, J. MicroRNA-124-3p Directly Targets PDCD6 to Inhibit Metastasis in Breast Cancer. Oncol. Lett. 2018, 15, 984–990. [Google Scholar] [CrossRef]
- Ji, H.; Sang, M.; Liu, F.; Ai, N.; Geng, C. MiR-124 Regulates EMT Based on ZEB2 Target to Inhibit Invasion and Metastasis in Triple-Negative Breast Cancer. Pathol. Res. Pr. 2019, 215, 697–704. [Google Scholar] [CrossRef]
- Shi, P.; Chen, C.; Li, X.; Wei, Z.; Liu, Z.; Liu, Y. MicroRNA-124 Suppresses Cell Proliferation and Invasion of Triple Negative Breast Cancer Cells by Targeting STAT3. Mol. Med. Rep. 2019, 49, 3667–3675. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Wu, Z.; Wang, M.; Jin, F.; Wang, N.; Hu, X.; Liu, Z.; Zhang, C.-Y.; Zen, K.; et al. MiR-124-3p Functions as a Tumor Suppressor in Breast Cancer by Targeting CBL. BMC Cancer 2016, 16, 826. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Long, H.; Yu, J.; Li, F.; Hou, H.; Yang, Q. Decreased miR-124-3p Expression Prompted Breast Cancer Cell Progression Mainly by Targeting Beclin-1. Clinical Lab. 2016, 62, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xing, H.; Guo, C.; Yang, Z.; Wang, Y.; Wang, Y. MiR-124 Reversed the Doxorubicin Resistance of Breast Cancer Stem Cells through STAT3/HIF-1 Signaling Pathways. Cell Cycle 2019, 18, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, M.; Su, J.; Miao, K.; Qiu, X. Dual-Targeting of MiR-124-3p and ABCC4 Promotes Sensitivity to Adriamycin in Breast Cancer Cells. Genet. Test. Mol. Biomark. 2019, 23, 156–165. [Google Scholar] [CrossRef]
- Lv, X.B.; Jiao, Y.; Qing, Y.; Hu, H.; Cui, X.; Lin, T.; Song, E.; Yu, F. MiR-124 Suppresses Multiple Steps of Breast Cancer Metastasis by Targeting A Cohort of Pro-metastatic Genes In Vitro. Chin. J. Cancer 2011, 30, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Hsu, C.Y.; Tsai, C.F.; Long, C.Y.; Chai, C.Y.; Hou, M.F.; Lee, J.N.; Wu, D.C.; Wang, S.C.; Tsai, E.M. MiR-125a-5p is a Prognostic Biomarker That Targets HDAC4 to Suppress Breast Tumorigenesis. Oncotarget 2014, 6, 494–509. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Hartley, R.S. MicroRNA-125a Represses Cell Growth by Targeting HuR in Breast Cancer. RNA Biol. 2009, 6, 575–583. [Google Scholar] [CrossRef]
- Yadav, V.; Denning, M.F. Fyn is Induced by Ras/PI3K/Akt Signaling and is Required for Enhanced Invasion/migration. Mol. Carcinog. 2011, 50, 346–352. [Google Scholar] [CrossRef]
- Ninio-Many, L.; Grossman, H.; Shomron, N.; Chuderland, D.; Shalgi, R. MicroRNA-125a-3p Reduces Cell Proliferation and Migration by Targeting Fyn. J. Cell Sci. 2013, 126, 2867–2876. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yu, M.C.; Gao, G.L.; Liang, H.W.; Zhou, X.Y.; Zhu, Z.T.; Zhang, C.Y.; Wang, Y.B.; Chen, X. MiR-125a-5p Functions as a Tumour Suppressor in Breast Cancer by Downregulating BAP1. J. Cell. Biochem. 2018, 119, 8773–8783. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Pan, Q.; Zhang, Z.; Huang, C.; Yan, Z.; Zhang, Y.; Li, J. MicroRNA-125a-5p Controls the Proliferation, Apoptosis, Migration and PTEN/MEK1/2/ERK1/2 Signaling Pathway in MCF-7 Breast Cancer Cells. Mol. Med. Rep. 2019, 20, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Meng, X.; Li, X.; Zhang, Y.; Li, C.; Xiang, C.; Xing, Y.; Xia, Y.; Xi, T. MIR-125a-3p Inhibits ERα Transactivation and Overrides Tamoxifen Resistance by Targeting CDK3 in Estrogen Receptor-Positive Breast Cancer. Faseb J. 2018, 32, 588–600. [Google Scholar] [CrossRef]
- Ninio-Many, L.; Hikri, E.; Burg Golani, T.; Stemmer, S.M.; Shalgi, R.; Ben-Aharon, I. miR-125a induces HER2 expression and sensitivity to trastuzumab in triple negative breast cancer lines. Front. Oncol. 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Hsu, C.Y.; Tsai, C.F.; Long, C.Y.; Wu, C.H.; Wu, D.C.; Lee, J.N.; Chang, W.C.; Tsai, E.M. HDAC Inhibitors Target HDAC5, Upregulate MicroRNA-125a-5p, and Induce Apoptosis in Breast Cancer Cells. Mol. Ther. 2015, 23, 656–666. [Google Scholar] [CrossRef]
- Png, K.J.; Halberg, N.; Yoshida, M.; Tavazoie, S.F. A MicroRNA Regulon That Mediates Endothelial Recruitment and Metastasis by Cancer Cells. Nature 2012, 481, 190–196. [Google Scholar] [CrossRef]
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous Human MicroRNAs That Suppress Breast Cancer Metastasis. Nature 2008, 451, 147–152. [Google Scholar] [CrossRef]
- Alhasan, L. MiR-126 modulates angiogenesis in breast cancer by targeting VEGF-A -mRNA Asian Pacific. J. Cancer Prev. 2019, 20, 193–197. [Google Scholar]
- Hong, Z.; Hong, C.; Ma, B.; Wang, Q.; Zhang, X.; Li, L.; Wang, C.; Chen, D. MicroRNA-126-3p Inhibits the Proliferation, Migration, Invasion, and Angiogenesis of Triple-Negative Breast Cancer Cells by Targeting RGS3. Oncol. Rep. 2019, 42, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, F.; Jiao, Y.; Feng, J.; Tang, W.; Yao, H.; Gong, C.; Chen, J.; Su, F.; Zhang, Y.; et al. Reduced MiR-128 in Breast Tumor-Initiating Cells Induces Chemotherapeutic Resistance via Bmi-1 and ABCC5. Clin. Cancer Res. 2011, 17, 7105–7115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, D.; Fang, L. MiR-128-3p Suppresses Breast Cancer Cellular Progression via Targeting LIMK1. Biomed. Pharmacother. 2019, 115, 108947. [Google Scholar] [CrossRef]
- Xiao, M.; Lou, C.; Xiao, H.; Yang, Y.; Cai, X.; Li, C.; Jia, S.; Huang, Y. MiR-128 Regulation of Glucose Metabolism and Cell Proliferation in Triple-Negative Breast Cancer. Br. J. Surg. 2018, 105, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, L.; Wang, J.; Chen, T.; Li, H.; Zhang, K.; Chen, J.; Zhen, S.; Tuluhong, D.; Li, J.; et al. MicroRNA-129-5p Suppresses Adriamycin Resistance in Breast Cancer by Targeting SOX2. Arch. Biochem. Biophys. 2018, 651, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, Y.; Sun, X.H.; Ge, J.; Zhang, B.; Wang, X.; Cao, X.C. Down-Regulation of MiR-129-5p via the Twist1-Snail Feedback Loop Stimulates the Epithelial-Mesenchymal Transition and Is Associated with Poor Prognosis in Breast Cancer. Oncotarget 2015, 6, 34423–34436. [Google Scholar] [CrossRef] [PubMed]
- Shui, Y.; Yu, X.; Duan, R.; Bao, Q.; Wu, J.; Yuan, H.; Ma, C. MiR-130b-3p Inhibits Cell Invasion and Migration by Targeting the Notch Ligand Delta-like 1 in Breast Carcinoma. Gene 2017, 609, 80–87. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, M.; Hu, H.; Wang, J.; Ang, L.; Zheng, L. MicroRNA-130a Reduces Drug Resistance in Breast Cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 2699–2705. [Google Scholar]
- Chen, X.; Zhao, M.; Huang, J.; Li, Y.; Wang, S.; Harrington, C.A.; Qian, D.Z.; Sun, X.X.; Dai, M.S. MicroRNA-130a Suppresses Breast Cancer Cell Migration and Invasion by Targeting FOSL1 and Upregulating ZO-1. J. Cell. Biochem. 2018, 119, 4945–4956. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Li, J.; Shao, J.; Fang, L. MiR-130a-3p Inhibits Migration and Invasion by Regulating RAB5B in Human Breast Cancer Stem Cell-like Cells. Biochem. Biophys. Res. Commun. 2018, 501, 486–493. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Sahlberg, K.K.; Mäkelä, R.; Due, E.U.; Kallioniemi, O.; Børresen-Dale, A.L.; Perälä, M. High-Throughput Screens Identify MicroRNAs Essential for HER2 Positive Breast Cancer Cell Growth. Mol. Oncol. 2014, 8, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, N.; Hu, Y.; Li, X.; Zhang, K. MiR-137 Suppresses Triple-Negative Breast Cancer Stemness and Tumorigenesis by Perturbing BCL11A-DNMT1 Interaction. Cell. Physiol. Biochem. 2018, 47, 2147–2158. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Lou, G.; Zhao, L.; Xu, Z.; Zhang, Y.; He, F. MiR-137 Targets Estrogen-related Receptor Alpha and Impairs the Proliferative and Migratory Capacity of Breast Cancer Cells. PLoS ONE 2012, 7, e39102. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Yu, L.; Wu, Y.; Wang, S.; Yao, J.; Zheng, X.; Xie, S.; Zhang, S.; Lu, X.; Liu, Y.; et al. MiR-137 Alleviates Doxorubicin Resistance in Breast Cancer through Inhibition of Epithelial-Mesenchymal Transition by Targeting DUSP4. Cell Death Dis. 2019, 10, 922. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.H.; Lee, M.N.; Oh, G.T.; Choi, K.C.; Choi, E.Y. P53 Regulates the Transcription of the Anti-inflammatory Molecule Developmental Endothelial Locus-1 (Del-1). Oncotarget 2013, 4, 1976. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.M.; Cho, K.W.; Kim, E.J.; Tang, Q.; Kim, K.S.; Tickle, C.; Jung, H.S. A Contrasting Function for MiR-137 in Embryonic Mammogenesis and Adult Breast Carcinogenesis. Oncotarget 2015, 6, 22048. [Google Scholar] [CrossRef][Green Version]
- Denis, H.; Van Grembergen, O.; Delatte, B.; Dedeurwaerder, S.; Putmans, P.; Calonne, E.; Rothé, F.; Sotiriou, C.; Fuks, F.; Deplus, R. MicroRNAs Regulate KDM5 Histone Demethylases in Breast Cancer Cells. Mol. BioSyst. 2016, 12, 404–413. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Y.; Lou, C.; He, Y.; Zhang, Y.; Zhang, Q. FSTL1 Enhances Chemoresistance and Maintains Stemness in Breast Cancer Cells via Integrin β3/Wnt Signaling Under MiR-137 Regulation. Cancer Biol. Ther. 2019, 20, 328–337. [Google Scholar] [CrossRef]
- Ying, X.; Sun, Y.; He, P. MicroRNA-137 Inhibits BMP7 to Enhance the Epithelialmesenchymal Transition of Breast Cancer Cells. Oncotarget 2017, 8, 18348–18358. [Google Scholar] [CrossRef]
- Liang, Z.; Feng, Q.; Xu, L.; Li, S.; Zhou, L. CREPT Regulated by MiR-138 Promotes Breast Cancer Progression. Biochem. Biophys. Res. Commun. 2017, 493, 263–269. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Feng, Z.; Mao, J.; Zhang, C.; Lu, Y.; Li, J.; Zhang, Q.; Li, Q.; Li, L. MicroRNA-138 Modulates Metastasis and EMT in Breast Cancer Cells by Targeting Vimentin. Biomed. Pharmacother. 2016, 77, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ling, X.; Li, X.; Hou, X.; Zhao, D. MicroRNA-138-5p Inhibits Cell Migration, Invasion and EMT in Breast Cancer by Directly Targeting RHBDD1. Breast Cancer 2019, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Steptoe, A.L.; Martin, H.C.; Pattabiraman, D.R.; Nones, K.; Waddell, N.; Mariasegaram, M.; Simpson, P.T.; Lakhani, S.R.; Vlassov, A.; et al. MiR-139-5p Is A Regulator of Metastatic Pathways in Breast Cancer. RNA 2013, 19, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Gallagher, D.; Schmitt, S.; Pessetto, Z.Y.; Fan, F.; Godwin, A.K.; Tawfik, O. Role of MiR-139 as a Surrogate Marker for Tumor Aggression in Breast Cancer. Hum. Pathol. 2017, 61, 68–77. [Google Scholar] [CrossRef]
- Hua, W.; Sa, K.D.; Zhang, X.; Jia, L.T.; Zhao, J.; Yang, A.G.; Zhang, R.; Fan, J.; Bian, K. MicroRNA-139 Suppresses Proliferation in Luminal Type Breast Cancer Cells by Targeting Topoisomerase II Alpha. Biochem. Biophys. Res. Commun. 2015, 463, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.; Wang, K.; Tang, X.; He, J. MIR-139-3p Suppresses the Invasion and Migration Properties of Breast Cancer Cells by Targeting RAB1A. Oncol. Rep. 2019, 42, 1699–1708. [Google Scholar] [CrossRef]
- Li, H.C.; Chen, Y.F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.M.; Chen, T.; Xu, K.; Feng, D.X. Loss of the Opa Interacting Protein 5 Inhibits Breast Cancer Proliferation through MiR-139-5p/NOTCH1 Pathway. Gene 2017, 603, 1–8. [Google Scholar] [CrossRef]
- Pajic, M.; Froio, D.; Daly, S.; Doculara, L.; Millar, E.; Graham, P.H.; Drury, A.; Steinmann, A.; de Bock, C.E.; Boulghourjian, A.; et al. miR-139-5p Modulates Radiotherapy Resistance in Breast Cancer by Repressing Multiple Gene Networks of DNA Repair and ROS Defense. Cancer Res. 2018, 78, 501–515. [Google Scholar] [CrossRef]
- Zhang, H.D.; Sun, D.W.; Mao, L.; Zhang, J.; Jiang, L.H.; Li, J.; Wu, Y.; Ji, H.; Chen, W.; Wang, J.; et al. MiR-139-5p Inhibits the Biological Function of Breast Cancer Cells by Targeting Notch1 and Mediates Chemosensitivity to Docetaxel. Biochem. Biophys. Res. Commun. 2015, 465, 702–713. [Google Scholar] [CrossRef]
- Salem, O.; Erdem, N.; Jung, J.; Münstermann, E.; Wörner, A.; Wilhelm, H.; Wiemann, S.; Körner, C. The Highly Expressed 5′isomiR of Hsa-MiR-140-3p Contributes to the Tumor-Suppressive Effects of MiR-140 by Reducing Breast Cancer Proliferation and Migration. BMC Genom. 2016, 17, 566. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Lu, Y.; Bo, S.; Li, L.; Wang, L.; Zhang, Q.; Mao, J. MiR-140-5p Inhibits the Proliferation and Enhances the Efficacy of Doxorubicin to Breast Cancer Stem Cells by Targeting Wnt1. Cancer Gene Ther. 2019, 26, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Ghasabi, M.; Shirjang, S.; Dehghan, R.; Montazeri, V.; Holmskov, U.; Kazemi, T.; Duijf, P.; Gjerstorff, M.; et al. MiR-142-3p as Tumor Suppressor MiRNA in the Regulation of Tumorigenicity, Invasion and Migration of Human Breast Cancer by Targeting Bach-1 Expression. J. Cell. Physiol. 2019, 234, 9816–9825. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, W. MicroRNA-142-5p Modulates Breast Cancer Cell Proliferation and Apoptosis by Targeting Phosphatase and Tensin Homolog. Mol. Med. Rep. 2018, 17, 7529–7536. [Google Scholar] [CrossRef]
- Tavanafar, F.; Safaralizadeh, R.; Hosseinpour-Feizi, M.A.; Mansoori, B.; Shanehbandi, D.; Mohammadi, A.; Baradaran, B. Restoration of MiR-143 Expression Could Inhibit Migration and Growth of MDA-MB-468 Cells through down-Regulating the Expression of Invasion-Related Factors. Biomed. Pharmacother. 2017, 91, 920–924. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X.; Liang, H.; Deng, T.; Chen, W.; Zhang, S.; Liu, M.; Gao, X.; Liu, Y.; Zhao, C.; et al. MiR-143 and MiR-145 Synergistically Regulate ERBB3 to Suppress Cell Proliferation and Invasion in Breast Cancer. Mol. Cancer 2014, 13, 220. [Google Scholar] [CrossRef]
- Yin, Y.; Cai, J.; Meng, F.; Sui, C.; Jiang, Y. MiR-144 Suppresses Proliferation, Invasion, and Migration of Breast Cancer Cells through Inhibiting CEP55. Cancer Biol. Ther. 2018, 19, 306–315. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, X.Y.; Chen, W.Q.; Hu, H.; Luo, B.; Shi, Y.X.; Wu, T.W.; Li, Y.; Kong, Q.Z.; Lu, H.D.; et al. Demethylation of the MIR145 Promoter Suppresses Migration and Invasion in Breast Cancer. Oncotarget. 2017, 8, 61731. [Google Scholar] [CrossRef]
- Manvati, S.; Mangalhara, K.C.; Kalaiarasan, P.; Chopra, R.; Agarwal, G.; Kumar, R.; Saini, S.K.; Kaushik, M.; Arora, A.; Kumari, U.; et al. MiR-145 Supports Cancer Cell Survival and Shows Association with DDR Genes, methylation pattern, and epithelial to mesenchymal transition. Cancer Cell Int. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. P53 Represses C-Myc Through Induction of the Tumor Suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Mo, Y.Y. MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Res. 2010, 70, 378–387. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X.; Tan, W.; Gao, J.; Pan, L.; Ye, X.; Chen, L.; Zheng, W. MiR-145-5p Suppresses Breast Cancer Progression by Inhibiting SOX2. J. Surg. Res. 2019, 236, 278–287. [Google Scholar] [CrossRef] [PubMed]
- García-García, F.; Salinas-Vera, Y.M.; García-Vázquez, R.; Marchat, L.A.; Rodríguez-Cuevas, S.; López-González, J.S.; Carlos-Reyes, Á.; Ramos-Payán, R.; Aguilar-Medina, M.; Pérez-Plasencia, C.; et al. MiR-145-5p Is Associated with Pathological Complete Response to Neoadjuvant Chemotherapy and Impairs Cell Proliferation by Targeting TGFβR2 in Breast Cancer. Oncol. Rep. 2019, 41, 3527–3534. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, C.; Zhang, J.; Zhang, N.; Li, T.; Fang, J.; Zhang, Y.; Zuo, F.; Tao, Z.; Tang, S.; et al. miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression. Int. J. Oncol. 2017, 50, 1701–1710. [Google Scholar] [CrossRef]
- Götte, M.; Mohr, C.; Koo, C.Y.; Stock, C.; Vaske, A.K.; Viola, M.; Ibrahim, S.A.; Peddibhotla, S.; Teng, Y.H.F.; Low, J.Y.; et al. MiR-145-Dependent Targeting of Junctional Adhesion Molecule A and Modulation of Fascin Expression Are Associated with Reduced Breast Cancer Cell Motility and Invasiveness. Oncogene 2010, 29, 6569–6580. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Ren, H.; Shi, Y.; Gao, Y. MiR-145-Targeted HBXIP Modulates Human Breast Cancer Cell Proliferation. Thorac. Cancer 2019, 10, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bian, C.; Yang, Z.; Bo, Y.; Li, J.; Zeng, L.; Zhou, H.; Zhao, R.C. MiR-145 Inhibits Breast Cancer Cell Growth through RTKN. Int. J. Oncol. 2009, 34, 1461–1466. [Google Scholar] [CrossRef]
- Zou, C.; Xu, Q.; Mao, F.; Li, D.; Bian, C.; Liu, L.Z.; Jiang, Y.; Chen, X.; Qi, Y.; Zhang, X.; et al. MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle 2012, 11, 2137–2145. [Google Scholar] [CrossRef]
- Bellissimo, T.; Tito, C.; Ganci, F.; Sacconi, A.; Masciarelli, S.; Di Martino, G.; Porta, N.; Cirenza, M.; Sorci, M.; De Angelis, L.; et al. Argonaute 2 drives miR-145-5p-dependent gene expression program in breast cancer cells. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Hu, J.; Guo, H.; Li, H.; Liu, Y.; Liu, J.; Chen, L.; Zhang, J.; Zhang, N. MiR-145 Regulates Epithelial to Mesenchymal Transition of Breast Cancer Cells by Targeting Oct4. PLoS ONE 2012, 7, e45965. [Google Scholar] [CrossRef]
- Dong, Y.; Chang, C.; Liu, J.; Qiang, J. Targeting of GIT1 by MiR-149* in Breast Cancer Suppresses Cell Proliferation and Metastasis in Vitro and Tumor Growth in Vivo. Onco. Targets. Ther. 2017, 10, 5873–5882. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhang, L.; Song, C.; Yu, H. Tumor-Suppressor MicroRNA-151-5p Regulates the Growth, Migration and Invasion of Human Breast Cancer Cells by Inhibiting SCOS5. Am. J. Transl. Res. 2019, 11, 7376–7384. [Google Scholar]
- Sengupta, D.; Deb, M.; Rath, S.K.; Kar, S.; Parbin, S.; Pradhan, N.; Patra, S.K. DNA Methylation and Not H3K4 Trimethylation Dictates the Expression Status of MiR-152 Gene Which Inhibits Migration of Breast Cancer Cells via DNMT1/CDH1 Loop. Exp. Cell Res. 2016, 346, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Ye, F.; Liu, Z.; Huang, J.; Gong, Y. MicroRNA-153 Inhibits Cell Proliferation, Migration, Invasion and Epithelial-mesenchymal Transition in Breast Cancer via Direct Targeting of RUNX2. Exp. Ther. Med. 2019, 17, 4693. [Google Scholar] [CrossRef]
- Shi, D.; Li, Y.; Fan, L.; Zhao, Q.; Tan, B.; Cui, G. Upregulation of Mir-153 Inhibits Triple-Negative Breast Cancer Progression by Targeting Zeb2-Mediated Emt and Contributes to Better Prognosis. Onco. Targets Ther. 2019, 12, 9611–9625. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ge, F.; Xu, Y.; Xiao, J.; Zhou, Z.; Liu, R.; Chen, C. MiR-153 Inhibits the Migration and the Tube Formation of Endothelial Cells by Blocking the Paracrine of Angiopoietin 1 in Breast Cancer Cells. Angiogenesis 2018, 21, 849–860. [Google Scholar] [CrossRef]
- Xu, H.; Fei, D.; Zong, S.; Fan, Z. MicroRNA-154 Inhibits Growth and Invasion of Breast Cancer Cells through Targeting E2F5. Am. J. Transl. Res. 2016, 8, 2620. [Google Scholar] [PubMed]
- Zhu, X.; Qiu, J.; Zhang, T.; Yang, Y.; Guo, S.; Li, T.; Jiang, K.; Zahoor, A.; Deng, G.; Qiu, C. MicroRNA-188-5p Promotes Apoptosis and Inhibits Cell Proliferation of Breast Cancer Cells via the MAPK Signaling Pathway by Targeting Rap2c. J. Cell. Physiol. 2020, 235, 2389–2402. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, W.; Yu, Z.H.; Zhou, Y.J.; Chi, J.R.; Ge, J.; Cao, X.C. MiR-190 Enhances Endocrine Therapy Sensitivity by Regulating SOX9 Expression in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Luo, W.; Yang, Z.J.; Chi, J.R.; Li, Y.R.; Ding, Y.; Ge, J.; Wang, X.; Cao, X.C. MiR-190 Suppresses Breast Cancer Metastasis by Regulation of TGF-β-Induced Epithelial-Mesenchymal Transition. Mol. Cancer 2018, 17. [Google Scholar] [CrossRef]
- Hu, F.; Meng, X.; Tong, Q.; Liang, L.; Xiang, R.; Zhu, T.; Yang, S. BMP-6 Inhibits Cell Proliferation by Targeting MicroRNA-192 in Breast Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2379–2390. [Google Scholar] [CrossRef]
- Chen, P.; Feng, Y.; Zhang, H.; Shi, X.; Li, B.; Ju, W.; Yu, X.; Zhang, N.; Luo, X. MicroRNA-192 Inhibits Cell Proliferation and Induces Apoptosis in Human Breast Cancer by Targeting Caveolin 1. Oncol. Rep. 2019, 42, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Hulin, J.A.; Tommasi, S.; Elliot, D.; Hu, D.G.; Lewis, B.C.; Mangoni, A.A. MiR-193b regulates breast cancer cell migration and vasculogenic mimicry by targeting dimethylarginine dimethylaminohydrolase 1. Sci. Rep. 2017, 7, 13996. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, Z.; Liu, Y.; Zhou, X.; Sun, F.; Liu, Y.; Li, L.; Hua, S.; Zhao, Y.; Gao, H.; et al. PTP1B Markedly Promotes Breast Cancer Progression and Is Regulated by MiR-193a-3p. FEBS J. 2019, 286, 1136–1153. [Google Scholar] [CrossRef]
- Yang, Z.; Zhuang, Q.; Hu, G.; Geng, S. MORC4 Is a Novel Breast Cancer Oncogene Regulated by MiR-193b-3p. J. Cell. Biochem. 2019, 120, 4634–4643. [Google Scholar] [CrossRef]
- Xie, F.Y.; Hosany, S.; Zhong, S.; Jiang, Y.; Zhang, F.; Lin, L.L.; Wang, X.B.; Gao, S.M.; Hu, X.Q. MicroRNA-193a Inhibits Breast Cancer Proliferation and Metastasis by Downregulating WT1. PLoS ONE 2017, 12, e0185565. [Google Scholar] [CrossRef]
- Sun, L.; He, M.; Xu, N.; Xu, D.H.; Ben-David, Y.; Yang, Z.Y.; Li, Y.J. Regulation of RAB22A by MiR-193b Inhibits Breast Cancer Growth and Metastasis Mediated by Exosomes. Int. J. Oncol. 2018, 53, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ji, Z.; Jiang, K.; Wang, Z.; Meng, G. miR-193b Modulates Resistance to Doxorubicin in Human Breast Cancer Cells by Downregulating MCL-1. BioMed Res. Int. 2015, 2015, 373574. [Google Scholar] [CrossRef]
- Li, X.F.; Yan, P.J.; Shao, Z.M. Downregulation of miR-193b Contributes to Enhance Urokinase-Type Plasminogen Activator (uPA) Expression and Tumor Progression and Invasion in Human Breast Cancer. Oncogene. 2009, 28, 3937–3948. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zou, C.; Kung, H.F.; Lin, M.C.; Dress, A.; Wardle, F.; Jiang, B.H.; Lai, L. MiR-195 Inhibits Tumor Growth and Angiogenesis through Modulating IRS1 in Breast Cancer. Biomed. Pharmacother. 2016, 80, 95–101. [Google Scholar] [CrossRef]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A Review of Its Role in Cancers. OncoTargets Ther. 2018, 11, 7109–7123. [Google Scholar] [CrossRef]
- Zhu, X.; Rao, X.; Yao, W.; Zou, X. Downregulation of MiR-196b-5p Impedes Cell Proliferation and Metastasis in Breast Cancer through Regulating COL1A1. Am. J. Transl. Res. 2018, 10, 3122–3132. [Google Scholar] [PubMed]
- Lin, X.; Qiu, W.; Xiao, Y.; Ma, J.; Xu, F.; Zhang, K.; Gao, Y.; Chen, Q.; Li, Y.; Li, H.; et al. MiR-199b-5p Suppresses Tumor Angiogenesis Mediated by Vascular Endothelial Cells in Breast Cancer by Targeting ALK1. Front. Genet. 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Chen, Y.; Liu, Y.; Lai, Y.; Liu, D. MiR-199b-5p Inhibits Triple Negative Breast Cancer Cell Proliferation, Migration and Invasion by Targeting DDR1. Oncol. Lett. 2018, 16, 4889–4896. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Xu, L.-Y.; Li, E.-M. A Family of Pleiotropically Acting MicroRNAs in Cancer Progression, MiR-200: Potential Cancer Therapeutic Targets. Curr. Pharm. Des. 2014, 20, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Castilla, M.Á.; Díaz-Martín, J.; Sarrió, D.; Romero-Pérez, L.; López-García, M.Á.; Vieites, B.; Biscuola, M.; Ramiro-Fuentes, S.; Isacke, C.M.; Palacios, J. MicroRNA-200 Family Modulation in Distinct Breast Cancer Phenotypes. PLoS ONE 2012, 7, e47709. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The MiR-200 Family and MiR-205 Regulate Epithelial to Mesenchymal Transition by Targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Duhachek-Muggy, S.; Zolkiewska, A. ADAM12-L Is a Direct Target of the MiR-29 and MiR-200 Families in Breast Cancer. BMC Cancer 2015, 15, 93. [Google Scholar] [CrossRef]
- Lu, Z.; Jiao, D.; Qiao, J.; Yang, S.; Yan, M.; Cui, S.; Liu, Z. Restin Suppressed Epithelial-Mesenchymal Transition and Tumor Metastasis in Breast Cancer Cells through Upregulating Mir-200a/b Expression via Association with P73. Mol. Cancer 2015, 14, 102. [Google Scholar] [CrossRef][Green Version]
- Sossey-Alaoui, K.; Pluskota, E.; Szpak, D.; Schiemann, W.P.; Plow, E.F. The Kindlin-2 Regulation of Epithelial-to-Mesenchymal Transition in Breast Cancer Metastasis Is Mediated through MiR-200b. Sci. Rep. 2018, 8, 7360. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, X.; Shen, C.; Shi, Y. MicroRNA-203 Suppresses Cell Proliferation and Migration by Targeting BIRC5 and LASP1 in Human Triple-Negative Breast Cancer Cells. J. Exp. Clin. Cancer Res. 2012, 31, 58. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y. Trichostatin A and Tamoxifen Inhibit Breast Cancer Cell Growth by MIR-204 and ERα Reducing AKT/MTOR Pathway. Biochem. Biophys. Res. Commun. 2015, 467, 242–247. [Google Scholar] [CrossRef]
- Hong, B.S.; Ryu, H.S.; Kim, N.; Kim, J.; Lee, E.; Moon, H.; Kim, K.H.; Jin, M.S.; Kwon, N.H.; Kim, S.; et al. Tumor Suppressor MiRNA-204-5p Regulates Growth, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Cancer Res. 2019, 79, 1520–1534. [Google Scholar] [CrossRef]
- Gao, J.B.; Zhu, M.N.; Zhu, X.L. MiRNA-215-5p Suppresses the Aggressiveness of Breast Cancer Cells by Targeting Sox9. FEBS Open Bio 2019, 9, 1957–1967. [Google Scholar] [CrossRef]
- Roscigno, G.; Cirella, A.; Affinito, A.; Quintavalle, C.; Scognamiglio, I.; Palma, F.; Ingenito, F.; Nuzzo, S.; De Micco, F.; Cuccuru, A.; et al. miR-216a Acts as a Negative Regulator of Breast Cancer by Modulating Stemness Properties and Tumor Microenvironment. Int. J. Mol. Sci. 2020, 21, 2313. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, J.; Liu, S.; Qu, D.; Jin, H.; Zhu, L.; Yang, J.; Zhang, J.; Li, Q.; Zhang, Y.; et al. MiR-216a Promotes Breast Cancer Cell Apoptosis by Targeting PKCα. Fundam. Clin. Pharmacol. 2019, 33, 397–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, P.; Zou, J.Y.; Zou, G.; Wang, W.Z.; Liu, Y.L.; Zhao, H.W.; Fang, A.P. MiR-216a-5p act as a tumor suppressor, regulating the cell proliferation and metastasis by targeting PAK2 in breast cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2469–2475. [Google Scholar]
- Jana, S.; Sengupta, S.; Biswas, S.; Chatterjee, A.; Roy, H.; Bhattacharyya, A. MiR-216b Suppresses Breast Cancer Growth and Metastasis by Targeting SDCBP. Biochem. Biophys. Res. Commun. 2017, 482, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, X.; Yang, F.; Zhu, J.; Zhou, P.; Yu, F.; Hou, L.; Xiao, L.; He, Q.; Wang, B. Regulation of the P2 × 7R by MicroRNA-216b in Human Breast Cancer. Biochem. Biophys. Res. Commun. 2014, 452, 197–204. [Google Scholar] [CrossRef]
- Menbari, M.N.; Rahimi, K.; Ahmadi, A.; Elyasi, A.; Darvishi, N.; Hosseini, V.; Mohammadi-Yeganeh, S.; Abdi, M. MiR-216b-5p Inhibits Cell Proliferation in Human Breast Cancer by down-Regulating HDAC8 Expression. Life Sci. 2019, 237, 116945. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, S.; Zhao, Y.; Zhang, Z.; Qin, C.; Yang, X. MicroRNA-216a Suppresses the Proliferation and Migration of Human Breast Cancer Cells via the Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2019, 41, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Setijono, S.R.; Park, M.; Kim, G.; Kim, Y.; Cho, K.W.; Song, S.J. MiR-218 and MiR-129 Regulate Breast Cancer Progression by Targeting Lamins. Biochem. Biophys. Res. Commun. 2018, 496, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Q.; Wang, Q.; Jiang, Z.; Zhang, L. Silencing of MiRNA-218 Promotes Migration and Invasion of Breast Cancer via Slit2-Robo1 Pathway. Biomed. Pharmacother. 2012, 66, 535–540. [Google Scholar] [CrossRef]
- Gong, B.; Hu, H.; Chen, J.; Cao, S.; Yu, J.; Xue, J.; Chen, F.; Cai, Y.; He, H.; Zhang, L. Caprin-1 Is a Novel MicroRNA-223 Target for Regulating the Proliferation and Invasion of Human Breast Cancer Cells. Biomed. Pharmacother. 2013, 67, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, Z.; Ma, N.; Wang, B.; Liu, J.; Zhang, L.; Gu, L. MicroRNA-223 Targeting STIM1 Inhibits the Biological Behavior of Breast Cancer. Cell. Physiol. Biochem. 2018, 45, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Zheng, M.; Zuo, W.; Zheng, W. MicroRNA-223 Increases the Sensitivity of Triple-negative Breast Cancer Stem Cells to TRAIL-induced Apoptosis by Targeting HAX-1. PLoS ONE 2016, 11, e0162754. [Google Scholar] [CrossRef] [PubMed]
- Pinatel, E.M.; Orso, F.; Penna, E.; Cimino, D.; Elia, A.R.; Circosta, P.; Dentelli, P.; Brizzi, M.F.; Provero, P.; Taverna, D. MiR-223 Is A Coordinator of Breast Cancer Progression As Revealed by Bioinformatics Predictions. PLoS ONE 2014, 9, e84859. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Berton, S.; Citron, F.; D’Andrea, S.; Segatto, I.; Nicoloso, M.S.; Massarut, S.; Armenia, J.; Zafarana, G.; Rossi, S.; et al. Radiotherapy-induced MiR-223 Prevents Relapse of Breast Cancer by Targeting The EGF Pathway. Oncogene 2016, 35, 4914–4926. [Google Scholar] [CrossRef]
- Li, C.; Wang, A.; Chen, Y.; Liu, Y.; Zhang, H.; Zhou, J. MicroRNA-299-5p Inhibits Cell Metastasis in Breast Cancer by Directly Targeting Serine/Threonine Kinase 39. Oncol. Rep. 2020, 43, 1221–1233. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, Y.; Mao, F.; Lin, Y.; Shen, S.; Zhang, Y.; Sun, Q. MicroRNA-320a Suppresses Tumor Cell Growth and Invasion of Human Breast Cancer by Targeting Insulin-like Growth Factor 1 Receptor. Oncol. Rep. 2018, 40, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, R.; Zhao, S.; Chen, Y.; Hong, S.; Wang, K.; Wang, T.; Cheng, J.; Zhang, T.; Chen, D. Decreased MiR-320 Expression Is Associated with Breast Cancer Progression, Cell Migration, and Invasiveness via Targeting Aquaporin 1. Acta Biochim. Biophys. Sin. 2018, 50, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, Z.; Soltani, B.M.; Mowla, S.J. MicroRNA-326 Functions as a Tumor Suppressor in Breast Cancer by Targeting ErbB/PI3K Signaling Pathway. Front. Oncol. 2019, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, M.; Tao, Z.; Cao, J.; Wang, L.; Hu, X. MiR-331-3p Suppresses Cell Proliferation in TNBC Cells by Downregulating NRP2. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kim, K.H.; Jin, U.H.; Pfent, C.; Cao, H.; Amendt, B.; Liu, X.; Wilson-Robles, H.; Safe, S. Aryl Hydrocarbon Receptor Agonists Induce MicroRNA-335 Expression and Inhibit Lung Metastasis of Estrogen Receptor Negative Breast Cancer Cells. Mol. Cancer Ther. 2012, 11, 108–118. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wu, Q.; Wang, C.Q.; Wang, X.N.; Wang, Y.; Zhao, J.J.; Mao, S.S.; Zhang, G.H.; Zhang, N.; Xu, X.C. MiR-339-5p Inhibits Breast Cancer Cell Migration and Invasion in Vitro and May Be a Potential Biomarker for Breast Cancer Prognosis. BMC Cancer 2010, 10, 542. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Chen, X.; Liu, H.; Yu, K.; Bao, Y.; Chai, J.; Gao, H.; Zou, L. LGR5 Acts as a Target of MiR-340-5p in the Suppression of Cell Progression and Drug Resistance in Breast Cancer via Wnt/β-Catenin Pathway. Gene 2019, 683, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Assiri, A.A.; Mourad, N.; Shao, M.; Kiel, P.; Liu, W.; Skaar, T.C.; Overholser, B.R. MicroRNA 362-3p Reduces HERG-Related Current and Inhibits Breast Cancer Cells Proliferation. Cancer Genom. Proteom. 2019, 16, 433–442. [Google Scholar] [CrossRef]
- Hao, S.; Tian, W.; Chen, Y.; Wang, L.; Jiang, Y.; Gao, B.; Luo, D. MicroRNA-374c-5p Inhibits the Development of Breast Cancer through TATA-Box Binding Protein Associated Factor 7-Mediated Transcriptional Regulation of DEP Domain Containing 1. J. Cell. Biochem. 2019, 120, 15360–15368. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, J. MicroRNA-384 Inhibits the Progression of Breast Cancer by Targeting ACVR1. Oncol. Rep. 2018, 39, 2563–2574. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Xu, H.; Yang, Q. MIR-409-3p Suppresses Breast Cancer Cell Growth and Invasion by Targeting Akt1. Biochem. Biophys. Res. Commun. 2016, 469, 189–195. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Guo, E.; Luo, S.; Wang, G. MiR-410 Acts as a Tumor Suppressor in Estrogen Receptor-Positive Breast Cancer Cells by Directly Targeting ERLIN2 via the ERS Pathway. Cell. Physiol. Biochem. 2018, 48, 461–474. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, D.; Fang, K.; Guo, Z.; Li, L. Med19 Is Targeted by MiR-101–3p/MiR-422a and Promotes Breast Cancer Progression by Regulating the EGFR/MEK/ERK Signaling Pathway. Cancer Lett. 2019, 444, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, Y.; Yao, S.; Deng, G.; Liu, D.; Yuan, X.; Liu, S.; Rao, J.; Xiong, H.; Yuan, X.; et al. MiR-422a Weakened Breast Cancer Stem Cells Properties by Targeting PLP2. Cancer Biol. Ther. 2018, 19, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; He, W.; Zhao, P.; Ye, C. MicroRNA-433 Targets AKT3 and Inhibits Cell Proliferation and Viability in Breast Cancer. Oncol. Lett. 2018, 15, 3998–4004. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, K.; Zhu, X.; Zhao, G.; Wu, H.; Deng, G.; Qiu, C. MiR-433 Inhibits Breast Cancer Cell Growth via the MAPK Signaling Pathway by Targeting Rap1a. Int. J. Biol. Sci. 2018, 14, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Song, H.; Wu, T.; Li, D.; Hua, K.; Xu, H.; Zhao, B.; Wu, C.; Hu, J.; Ji, C.; et al. MicroRNA-424 Serves an Anti-Oncogenic Role by Targeting Cyclin-Dependent Kinase 1 in Breast Cancer Cells. Oncol. Rep. 2018, 40, 3416–3426. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Zhou, J.; Qian, Q. MiR-424-5p Regulates Cell Proliferation, Migration and Invasion by Targeting Doublecortin-like Kinase 1 in Basal-like Breast Cancer. Biomed. Pharmacother. 2018, 102, 147–152. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Chong, Q.-Y.; Zhang, W.; Qian, P.; Yan, H.; Qian, W.; Zhang, M.; Lobie, P.E.; Zhu, T. Amplification of Hsa-MiR-191/425 Locus Promotes Breast Cancer Proliferation and Metastasis by Targeting DICER1. Carcinogenesis 2018, 39, 1506–1516. [Google Scholar] [CrossRef]
- Ma, P.; Ni, K.; Ke, J.; Zhang, W.; Feng, Y.; Mao, Q. MiR-448 Inhibits the Epithelial-Mesenchymal Transition in Breast Cancer Cells by Directly Targeting the E-Cadherin Repressor ZEB1/2. Exp. Biol. Med. 2018, 243, 473–480. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Sun, A.J.; Xue, J.L. NEAT1 Contributes to Breast Cancer Progression through Modulating MiR-448 and ZEB1. J. Cell. Physiol. 2018, 233, 8558–8566. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Wang, S.; Shi, B. MiR-449a Suppresses Cell Migration and Invasion by Targeting PLAGL2 in Breast Cancer. Pathol. Res. Pr. 2018, 214, 790–795. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, X.; He, X.; Ma, W.; Wang, J.; Zhou, Q.; Li, M.; Yu, S. MicroRNA-449b-5p Suppresses the Growth and Invasion of Breast Cancer Cells via Inhibiting CREPT-Mediated Wnt/β-Catenin Signaling. Chem. Biol. Interact. 2019, 302, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lyu, J. Tumor Suppressor Function of MiR-483-3p on Breast Cancer via Targeting of the Cyclin E1 Gene. Exp. Ther. Med. 2018, 16, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Menbari, M.N.; Rahimi, K.; Ahmadi, A.; Mohammadi-Yeganeh, S.; Elyasi, A.; Darvishi, N.; Hosseini, V.; Abdi, M. MiR-483-3p Suppresses the Proliferation and Progression of Human Triple Negative Breast Cancer Cells by Targeting the HDAC8>oncogene. J. Cell. Physiol. 2020, 235, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; He, D.; Yang, D.; Chen, Z.; Pan, Q.; Mao, A.; Cai, Y.; Li, X.; Xing, H.; Shi, M.; et al. MiR-489 Regulates Chemoresistance in Breast Cancer via Epithelial Mesenchymal Transition Pathway. Febs Lett. 2014, 588. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.; Patel, Y.; Markoutsa, E.; Jie, C.; Liu, S.; Xu, P.; Chen, H. Autophagy, Cell Viability, and Chemoresistance Are Regulated by MiR-489 in Breast Cancer. Mol. Cancer Res. 2018, 16, 1348–1360. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Gao, Q.; Han, Y.; Zhang, G.; Xu, S.; Cheng, K.; Zou, W. MiR-490-3p Inhibits the Growth and Invasiveness in Triple-Negative Breast Cancer by Repressing the Expression of TNKS2. Gene 2016, 593, 41–47. [Google Scholar] [CrossRef]
- Hui, Z.; Yiling, C.; Wenting, Y.; Xuqun, H.; Chuanyi, Z.; Hui, L. MiR-491-5p Functions as a Tumor Suppressor by Targeting JMJD2B in ERα-Positive Breast Cancer. Febs Lett. 2015, 589, 812–821. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, L.; Zhao, D.; Liu, Y.; Ma, X.; Fan, Y.; Wan, L.; Huang, T.; Cheng, Z.; Shen, B. Overexpression of MiRNA-497 Inhibits Tumor Angiogenesis by Targeting VEGFR2. Sci. Rep. 2015, 5, 13827. [Google Scholar] [CrossRef]
- Luo, Q.; Li, X.; Gao, Y.; Long, Y.; Chen, L.; Huang, Y.; Fang, L. MiRNA-497 Regulates Cell Growth and Invasion by Targeting Cyclin E1 in Breast Cancer. Cancer Cell Int. 2013, 13, 95. [Google Scholar] [CrossRef]
- Chai, C.; Wu, H.; Wang, B.; Eisenstat, D.D.; Leng, R.P. MicroRNA-498 Promotes Proliferation and Migration by Targeting the Tumor Suppressor PTEN in Breast Cancer Cells. Carcinogenesis 2018, 39, 1185–1196. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Li, M. Downregulation of MiR-505 Promotes Cell Proliferation, Migration and Invasion, and Predicts Poor Prognosis in Breast Cancer. Oncol. Lett. 2019, 18, 247–254. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, W.; Yang, N.; Hao, L.; Wang, L. MicroRNA-511 Inhibits Malignant Behaviors of Breast Cancer by Directly Targeting SOX9 and Regulating the PI3K/Akt Pathway. Int. J. Oncol. 2018, 53, 2715–2726. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Wu, T.; Xie, D.; Hu, J.; Zhao, J.; Shen, Q.; Fang, L. MiR-519d-3p Suppresses Breast Cancer Cell Growth and Motility via Targeting LIM Domain Kinase 1. Mol. Cell. Biochem. 2018, 444, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, X.; Bai, X.; Zhao, T.; Wang, M.; Xu, R.; Li, M.; Hu, Y.; Li, W.; Yang, L.; et al. MiR-519d Suppresses Breast Cancer Tumorigenesis and Metastasis via Targeting MMP3. Int. J. Biol. Sci. 2018, 14, 228–236. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, T.; Ding, J.; Liu, W.; Meng, X.; Zhang, P.; Liu, K.; Wang, P. MiR-520d-3p Antitumor Activity in Human Breast Cancer via Post-Transcriptional Regulation of Spindle and Kinetochore Associated 2 Expression. Am. J. Transl. Res. 2018, 10, 1097–1108.108. [Google Scholar] [PubMed]
- Guo, J.; Gong, G.; Zhang, B. MiR-539 Acts as a Tumor Suppressor by Targeting Epidermal Growth Factor Receptor in Breast Cancer. Sci. Rep. 2018, 8, 2073. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Wang, S.; Huang, J.; Wang, B.; He, Z.; Liu, B. Survivin-Targeting MiR-542-3p Overcomes HER3 Signaling-Induced Chemoresistance and Enhances the Antitumor Activity of Paclitaxel against HER2-Overexpressing Breast Cancer. Cancer Lett. 2018, 420, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Mou, Q.; Pan, X.; Zhang, G.; Li, H.; Sun, Y. MiR-577 Suppresses Epithelial-Mesenchymal Transition and Metastasis of Breast Cancer by Targeting Rab25. Thorac. Cancer 2018, 9, 472–479. [Google Scholar] [CrossRef]
- Chu, J. MicroRNA-589 Serves as a Tumor Suppressor MicroRNA through Directly Targeting Metastasis-Associated Protein 2 in Breast Cancer. Oncol. Lett. 2019, 18, 2232–2239. [Google Scholar] [CrossRef]
- Abdolvahabi, Z.; Nourbakhsh, M.; Hosseinkhani, S.; Hesari, Z.; Alipour, M.; Jafarzadeh, M.; Ghorbanhosseini, S.S.; Seiri, P.; Yousefi, Z.; yarahmadi, S.; et al. MicroRNA-590-3P Suppresses Cell Survival and Triggers Breast Cancer Cell Apoptosis via Targeting Sirtuin-1 and Deacetylation of P53. J. Cell. Biochem. 2019, 120, 9356–9368. [Google Scholar] [CrossRef]
- Rohini, M.; Gokulnath, M.; Miranda, P.J.; Selvamurugan, N. MiR-590–3p Inhibits Proliferation and Promotes Apoptosis by Targeting Activating Transcription Factor 3 in Human Breast Cancer Cells. Biochimie 2018, 154, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, F.; Weng, Z.; Zhou, M.; Zhang, Q. MiR-591 Functions as Tumor Suppressor in Breast Cancer by Targeting TCF4 and Inhibits Hippo-YAP/TAZ Signaling Pathway. Cancer Cell Int. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- El Helou, R.; Pinna, G.; Cabaud, O.; Wicinski, J.; Bhajun, R.; Guyon, L.; Rioualen, C.; Finetti, P.; Gros, A.; Mari, B.; et al. MiR-600 Acts as a Bimodal Switch That Regulates Breast Cancer Stem Cell Fate through WNT Signaling. Cell Rep. 2017, 18, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Yi, W.; Wei, X.; Zhang, M.Y.; Xu, R.; Zeng, L.S.; Huang, Z.J.; Chen, J.S. MiR-601 Is a Prognostic Marker and Suppresses Cell Growth and Invasion by Targeting PTP4A1 in Breast Cancer. Biomed. Pharmacother. 2016, 79, 247–253. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, P.; Mao, Q.; Lu, P.; Xie, T.; Yang, H.; Wang, C. MiR-613 Inhibits Proliferation and Invasion of Breast Cancer Cell via VEGFA. Biochem. Biophys. Res. Commun. 2016, 478, 274–278. [Google Scholar] [CrossRef]
- Xiong, H.; Yan, T.; Zhang, W.; Shi, F.; Jiang, X.; Wang, X.; Li, S.; Chen, Y.; Chen, C.; Zhu, Y. MiR-613 Inhibits Cell Migration and Invasion by Downregulating Daam1 in Triple-Negative Breast Cancer. Cell. Signal. 2018, 44, 33–42. [Google Scholar] [CrossRef]
- Zhou, W.B.; Zhong, C.N.; Luo, X.P.; Zhang, Y.Y.; Zhang, G.Y.; Zhou, D.X.; Liu, L.P. MIR-625 Suppresses Cell Proliferation and Migration by Targeting HMGA1 in Breast Cancer. Biochem. Biophys. Res. Commun. 2016, 470, 838–844. [Google Scholar] [CrossRef]
- Lin, C.; Gao, B.; Yan, X.; Lei, Z.; Chen, K.; Li, Y.; Zeng, Q.; Chen, Z.; Li, H. MicroRNA 628 Suppresses Migration and Invasion of Breast Cancer Stem Cells through Targeting SOS1. Onco. Targets. Ther. 2018, 11, 5419–5428. [Google Scholar] [CrossRef]
- Gong, X.F.; Yu, A.L.; Tang, J.; Wang, C.L.; He, J.R.; Chen, G.Q.; Zhao, Q.; He, M.; Zhou, C.X. MicroRNA-630 Inhibits Breast Cancer Progression by Directly Targeting BMI1. Exp. Cell Res. 2018, 362, 378–385. [Google Scholar] [CrossRef]
- Meng, D.; Lei, M.; Han, Y.; Zhao, D.; Zhang, X.; Yang, Y.; Liu, R. MicroRNA-645 Targets Urokinase Plasminogen Activator and Decreases the Invasive Growth of MDA-MB-231 Triple-Negative Breast Cancer Cells. Onco. Targets. Ther. 2018, 11, 7733–7743. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Fu, Y.; Peng, J.; Mo, M.H.; Stamatakos, M.; Teal, C.B.; Brem, R.F.; Stojadinovic, A.; Grinkemeyer, M.; et al. Role of Deregulated MicroRNAs in Breast Cancer Progression Using FFPE Tissue. PLoS ONE. 2013, 8, e54213. [Google Scholar] [CrossRef]
- Halvorsen, A.R.; Helland, Å.; Gromov, P.; Wielenga, V.T.; Talman, M.L.M.; Brunner, N.; Sandhu, V.; Børresen-Dale, A.L.; Gromova, I.; Haakensen, V.D. Profiling of Micro RNA s in Tumor Interstitial Fluid of Breast Tumors–A Novel Resource to Identify Biomarkers for Prognostic Classification and Detection of Cancer. Mol. Oncol. 2017, 11, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Z.; Ren, S.; Rezaei, K.; Pan, Q.; Goldstein, A.T.; Macri, C.J.; Cao, D.; Brem, R.F.; Fu, S.W. Dynamically Decreased MiR-671-5p Expression Is Associated with Oncogenic Transformation and Radiochemoresistance in Breast Cancer. Breast Cancer Res. 2019, 21, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Fu, Y.; Chen, L.; Lee, W.; Lai, Y.; Rezaei, K.; Tabbara, S.; Latham, P.; Teal, C.B.; Man, Y.G.; et al. MiR-671-5p Inhibits Epithelial-to-mesenchymal Transition by Downregulating FOXM1 Expression in Breast Cancer. Oncotarget 2016, 7, 293. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.D.; Chen, H.; He, R.Q.; Lan, A.H.; Zhong, J.C.; Chen, G.; Feng, Z.B.; Wei, K.L. MicroRNA-671-3p Inhibits the Development of Breast Cancer: A Study Based on In Vitro Experiments, In-house Quantitative Polymerase Chain Reaction and Bioinformatics Analysis. Int. J. Oncol. 2018, 52, 1801–1814. [Google Scholar] [CrossRef]
- Chen, X.; Lu, P.; Wang, D.D.; Yang, S.J.; Wu, Y.; Shen, H.Y.; Zhong, S.L.; Zhao, J.H.; Tang, J.H. The Role of MiRNAs in Drug Resistance and Prognosis of Breast Cancer Formalin-fixed paraffin-embedded Tissues. Gene 2016, 595, 221–226. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, X.; Wang, D.; Zhang, X.; Shen, H.; Yang, S.; Lv, M.; Tang, J.; Zhao, J. MicroRNA Expression Profiles of Drug-resistance Breast Cancer Cells and Their Exosomes. Oncotarget 2016, 7, 19601. [Google Scholar] [CrossRef]
- Ryu, S.; McDonnell, K.; Choi, H.; Gao, D.; Hahn, M.; Joshi, N.; Park, S.M.; Catena, R.; Do, Y.; Brazin, J.; et al. Suppression of MiRNA-708 by Polycomb Group Promotes Metastases by Calcium-Induced Cell Migration. Cancer Cell 2013, 23, 63–76. [Google Scholar] [CrossRef]
- Lee, J.W.; Guan, W.; Han, S.; Hong, D.K.; Kim, L.S.; Kim, H. MicroRNA-708-3p Mediates Metastasis and Chemoresistance through Inhibition of Epithelial-to-Mesenchymal Transition in Breast Cancer. Cancer Sci. 2018, 109, 1404–1413. [Google Scholar] [CrossRef]
- Han, M.L.; Wang, F.; Gu, Y.T.; Pei, X.H.; Ge, X.; Guo, G.C.; Li, L.; Duan, X.; Zhu, M.Z.; Wang, Y.M. MicroR-760 Suppresses Cancer Stem Cell Subpopulation and Breast Cancer Cell Proliferation and Metastasis: By down-Regulating NANOG. Biomed. Pharmacother. 2016, 80, 304–310. [Google Scholar] [CrossRef]
- Li, Y.; Liang, Y.; Sang, Y.; Song, X.; Zhang, H.; Liu, Y.; Jiang, L.; Yang, Q. MIR-770 Suppresses the Chemo-Resistance and Metastasis of Triple Negative Breast Cancer via Direct Targeting of STMN1 Article. Cell Death Dis. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dong, Y.; Liu, H.; Ji, N.; Cao, J.; Liu, A.; Tang, X.; Ren, Y. Loss of MiR-873 Contributes to Gemcitabine Resistance in Triple-Negative Breast Cancer via Targeting ZEB1. Oncol. Lett. 2019, 18, 3837–3844. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, W.; Hu, F.; Xu, Z.; Wang, F. MicroRNA-874 Inhibits Cell Proliferation and Induces Apoptosis in Human Breast Cancer by Targeting CDK9. FEBS Lett. 2014, 588, 4527–4535. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, J.; Wang, J.; Shao, J. MiR-876-5p Suppresses Breast Cancer Progression through Targeting TFAP2A. Exp. Ther. Med. 2019, 18, 1458–1464. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L. MiR-888 Regulates Side Population Properties and Cancer Metastasis in Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2014, 450, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, C.; Niu, R.; Hu, G.; Gu, Z.; Zhuang, Z. MiR-890 Inhibits Proliferation and Invasion and Induces Apoptosis in Triple-Negative Breast Cancer Cells by Targeting CD147. BMC Cancer 2019, 19, 577. [Google Scholar] [CrossRef]
- Li, W.J.; Xie, X.X.; Bai, J.; Wang, C.; Zhao, L.; Jiang, D.Q. Increased Expression of MiR-1179 Inhibits Breast Cancer Cell Metastasis by Modulating Notch Signaling Pathway and Correlates with Favorable Prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8374–8382. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, Q.; Zhao, S.; Wang, J.; LU, K.; Song, Y.; Zhao, L.; Kang, X.; Wang, J.; Xu, S.; et al. The Expression and Clinical Significance of MicroRNA-1258 and Heparanase in Human Breast Cancer. Clin. Biochem. 2013, 46, 926–932. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, F.; Luo, Q.; Yan, D.; Sun, S. MiR-1284 Inhibits the Growth and Invasion of Breast Cancer Cells by Targeting ZIC2. Oncol. Res. 2019, 27, 253–260. [Google Scholar] [CrossRef]
- Hironaka-Mitsuhashi, A.; Otsuka, K.; Gailhouste, L.; Sanchez Calle, A.; Kumazaki, M.; Yamamoto, Y.; Fujiwara, Y.; Ochiya, T. MiR-1285-5p/TMEM194A Axis Affects Cell Proliferation in Breast Cancer. Cancer Sci. 2020, 111, 395–405. [Google Scholar] [CrossRef]
- Schwarzenbacher, D.; Klec, C.; Pasculli, B.; Cerk, S.; Rinner, B.; Karbiener, M.; Ivan, C.; Barbano, R.; Ling, H.; Wulf-Goldenberg, A.; et al. MiR-1287-5p Inhibits Triple Negative Breast Cancer Growth by Interaction with Phosphoinositide 3-Kinase CB, Thereby Sensitizing Cells for PI3Kinase Inhibitors. Breast Cancer Res. 2019, 21, 20. [Google Scholar] [CrossRef]
- Wang, B.; Wu, H.; Chai, C.; Lewis, J.; Pichiorri, F.; Eisenstat, D.D.; Pomeroy, S.L.; Leng, R.P. MicroRNA-1301 Suppresses Tumor Cell Migration and Invasion by Targeting the P53/UBE4B Pathway in Multiple Human Cancer Cells. Cancer Lett. 2017, 401, 20–32. [Google Scholar] [CrossRef]
- Peng, X.; Yan, B.; Shen, Y. MiR-1301-3p Inhibits Human Breast Cancer Cell Proliferation by Regulating Cell Cycle Progression and Apoptosis through Directly Targeting ICT1. Breast Cancer 2018, 25, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, M. MicroRNA-1323 Downregulation Promotes Migration and Invasion of Breast Cancer Cells by Targeting Tumor Protein D52. J. Biochem. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Zhao, H.; Yu, Y.; Cao, X.; Zhang, B. Downregulation of MicroRNA-1469 Promotes the Development of Breast Cancer via Targeting HOXA1 and Activating PTEN/PI3K/AKT and Wnt/β-Catenin Pathways. J. Cell. Biochem. 2019, 120, 5097–5107. [Google Scholar] [CrossRef]
- Kong, P.; Chen, L.; Yu, M.; Tao, J.; Liu, J.; Wang, Y.; Pan, H.; Zhou, W.; Wang, S. MiR-3178 Inhibits Cell Proliferation and Metastasis by Targeting Notch1 in Triple-Negative Breast Cancer. Cell Death Dis. 2018, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.C.; Han, S.H.; Xing, Y.F. Overexpression of MiR-3196 Suppresses Cell Proliferation and Induces Cell Apoptosis through Targeting ERBB3 in Breast Cancer. Eur. Rev. Med. Pharm. Pharmacol. Sci. 2018, 22, 8383–8390. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, D.; Han, C.; Zhao, Z.; Wang, X.; Jiang, T.; Li, Q.; Liu, S.; Chen, L.; Chen, Y.; et al. Blockade of MiR-3614 Maturation by IGF2BP3 Increases TRIM25 Expression and Promotes Breast Cancer Cell Proliferation. EBioMedicine 2019, 41, 357–369. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, G.Q. MiR-4282 Inhibits Proliferation, Invasion and Metastasis of Human Breast Cancer by Targeting Myc. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8763–8771. [Google Scholar] [CrossRef]
- Gholipour, N.; Ohradanova-Repic, A.; Ahangari, G. A Novel Report of MiR-4301 Induces Cell Apoptosis by Negatively Regulating DRD2 Expression in Human Breast Cancer Cells. J. Cell. Biochem. 2018, 119, 6408–6417. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Hu, R.; Zhang, Y.; Luo, W. MicroRNA-4317 Predicts the Prognosis of Breast Cancer and Inhibits Tumor Cell Proliferation, Migration, and Invasion. Clin. Exp. Med. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Li, Y.; Fan, X.; Ma, J.; Li, J.; Lu, G.; Zhang, Y.; Huang, Y.; Li, W.; Huang, X.; et al. MiR-4319 Suppress the Malignancy of Triple-Negative Breast Cancer by Regulating Self-Renewal and Tumorigenesis of Stem Cells. Cell. Physiol. Biochem. 2018, 48, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Zhang, G. MiR-4458 Regulates Cell Proliferation and Apoptosis through Targeting SOCS1 in Triple-Negative Breast Cancer. J. Cell. Biochem. 2019, 120, 12943–12948. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, P.; Zhang, H. MiR-6838-5p Suppresses Cell Metastasis and the EMT Process in Triple-Negative Breast Cancer by Targeting WNT3A to Inhibit the Wnt Pathway. J. Gene Med. 2019, 21, e3129. [Google Scholar] [CrossRef]
- Selcuklu, S.D.; Donoghue, M.T.A.; Rehmet, K.; De Gomes, M.S.; Fort, A.; Kovvuru, P.; Muniyappa, M.K.; Kerin, M.J.; Enright, A.J.; Spillane, C. MicroRNA-9 Inhibition of Cell Proliferation and Identification of Novel MiR-9 Targets by Transcriptome Profiling in Breast Cancer Cells. J. Biol. Chem. 2012, 287, 29516–29528. [Google Scholar] [CrossRef]
- Ma, L.; Young, J.; Prabhala, H.; Pan, E.; Mestdagh, P.; Muth, D.; Teruya-Feldstein, J.; Reinhardt, F.; Onder, T.T.; Valastyan, S.; et al. MiR-9, a MYC/MYCN-Activated MicroRNA, Regulates E-Cadherin and Cancer Metastasis. Nat. Cell Biol. 2010, 12, 247–256. [Google Scholar] [CrossRef]
- Yang, J.; Li, T.; Gao, C.; Lv, X.; Liu, K.; Song, H.; Xing, Y.; Xi, T. FOXO1 3′UTR Functions as a CeRNA in Repressing the Metastases of Breast Cancer Cells via Regulating MiRNA Activity. FEBS Lett. 2014, 588, 3218–3224. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; He, Y.; Mao, Y. MiR-19b Promotes Breast Cancer Metastasis through Targeting MYLIP and Its Related Cell Adhesion Molecules. Oncotarget 2017, 8, 64330–64343. [Google Scholar] [CrossRef]
- Jin, J.; Sun, Z.; Yang, F.; Tang, L.; Chen, W.; Guan, X. MiR-19b-3p Inhibits Breast Cancer Cell Proliferation and Reverses Saracatinib-Resistance by Regulating PI3K/Akt Pathway. Arch. Biochem. Biophys. 2018, 645, 54–60. [Google Scholar] [CrossRef]
- Yin, R.; Guo, L.; Gu, J.; Li, C.; Zhang, W. Over Expressing MiR-19b-1 Suppress Breast Cancer Growth by Inhibiting Tumor Microenvironment Induced Angiogenesis. Int. J. Biochem. Cell Biol. 2018, 97, 43–51. [Google Scholar] [CrossRef]
- Bai, X.; Han, G.; Liu, Y.; Jiang, H.; He, Q. MiRNA-20a-5p Promotes the Growth of Triple-Negative Breast Cancer Cells through Targeting RUNX3. Biomed. Pharmacother. 2018, 103, 1482–1489. [Google Scholar] [CrossRef]
- Li, S.; Qiang, Q.; Shan, H.; Shi, M.; Gan, G.; Ma, F.; Chen, B. MiR-20a and MiR-20b Negatively Regulate Autophagy by Targeting RB1CC1/FIP200 in Breast Cancer Cells. Life Sci. 2016, 147, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shi, G.; Zhang, Q.; Wu, Q.; Li, B.; Zhang, Z. MicroRNA-20b Promotes Cell Growth of Breast Cancer Cells Partly via Targeting Phosphatase and Tensin Homologue (PTEN). Cell Biosci. 2014, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Das, S.; Mukherjee, S.; Das, S.; Sengupta (Bandyopadhyay), S. Establishment of Twist-1 and TGFBR2 as Direct Targets of MicroRNA-20a in Mesenchymal to Epithelial Transition of Breast Cancer Cell-Line MDA-MB-231. Exp. Cell Res. 2017, 361, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Tang, Q.; Pan, Y.; Wang, X.; Dong, X.; Liang, Z.; Huang, D. MicroRNA-22 Inhibits Cell Growth and Metastasis in Breast Cancer via Targeting of SIRT1. Exp. Ther. Med. 2017, 14, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tang, H.; Liu, X.; Liu, P.; Yang, L.; Xie, X.; Ye, F.; Song, C.; Xie, X.; Wei, W. MiR-22 as a Prognostic Factor Targets Glucose Transporter Protein Type 1 in Breast Cancer. Cancer Lett. 2015, 356, 410–417. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Wang, D.; Wei, X. MiR-22 Suppresses Tumorigenesis and Improves Radiosensitivity of Breast Cancer Cells by Targeting Sirt1. Biol. Res. 2017, 50, 27. [Google Scholar] [CrossRef]
- Song, Y.K.; Wang, Y.; Wen, Y.Y.; Zhao, P.; Bian, Z.J. MicroRNA-22 Suppresses Breast Cancer Cell Growth and Increases Paclitaxel Sensitivity by Targeting NRAS. Technol. Cancer Res. Treat. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Tong, Y.; Yu, M.; Wang, M.; Dong, D.; Shao, J.; Zhang, F.; Niu, R.; Zhou, Y. MicroRNA-22 Inhibits Proliferation, Invasion and Metastasis of Breast Cancer Cells through Targeting Truncated Neurokinin-1 Receptor and ERα. Life Sci. 2019, 217, 57–69. [Google Scholar] [CrossRef]
- Song, S.J.; Poliseno, L.; Song, M.S.; Ala, U.; Webster, K.; Ng, C.; Beringer, G.; Brikbak, N.J.; Yuan, X.; Cantley, L.C.; et al. XMicroRNA-Antagonism Regulates Breast Cancer Stemness and Metastasis via TET-Family-Dependent Chromatin Remodeling. Cell 2013, 154, 311. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, H.; Cao, Y.; Wu, Q.; Li, W.; Zhang, J. Suppression of OGT by MicroRNA24 Reduces FOXA1 Stability and Prevents Breast Cancer Cells Invasion. Biochem. Biophys. Res. Commun. 2017, 487, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Liao, X.; Ye, C.; Yin, X.; Liu, M.; Hong, Y.; Yu, M.; Liu, Y.; Liang, H.; Zhang, C.Y.; et al. ING5 Suppresses Breast Cancer Progression and Is Regulated by MiR-24. Mol. Cancer 2017, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Dong, S.; Ivan, C.; Alahari, S.K. Suppression of PDHX by MicroRNA-27b Deregulates Cell Metabolism and Promotes Growth in Breast Cancer. Mol. Cancer 2018, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Hannafon, B.N.; Cai, A.; Calloway, C.L.; Xu, Y.F.; Zhang, R.; Fung, K.M.; Ding, W.Q. MiR-23b and MiR-27b Are Oncogenic MicroRNAs in Breast Cancer: Evidence from a CRISPR/Cas9 Deletion Study. BMC Cancer 2019, 19, 642. [Google Scholar] [CrossRef]
- Takahashi, R.U.; Miyazaki, H.; Takeshita, F.; Yamamoto, Y.; Minoura, K.; Ono, M.; Kodaira, M.; Tamura, K.; Mori, M.; Ochiya, T. Loss of MicroRNA-27b Contributes to Breast Cancer Stem Cell Generation by Activating ENPP1. Nat. Commun. 2015, 6, 7318. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakajima, M.; Takagi, S.; Taniya, T.; Yokoi, T. MicroRNA Regulates the Expression of Human Cytochrome P450 1B1. Cancer Res. 2006, 66, 9090–9098. [Google Scholar] [CrossRef]
- Chen, D.; Si, W.; Shen, J.; Du, C.; Lou, W.; Bao, C.; Zheng, H.; Pan, J.; Zhong, G.; Xu, L.; et al. MiR-27b-3p Inhibits Proliferation and Potentially Reverses Multi-Chemoresistance by Targeting CBLB/GRB2 in Breast Cancer Cells. Cell Death Dis. 2018, 9, 188. [Google Scholar] [CrossRef]
- Tang, W.; Yu, F.; Yao, H.; Cui, X.; Jiao, Y.; Lin, L.; Chen, J.; Yin, D.; Song, E.; Liu, Q. MiR-27a Regulates Endothelial Differentiation of Breast Cancer Stem like Cells. Oncogene 2014, 33, 2629–2638. [Google Scholar] [CrossRef]
- Zhang, B.; Shetti, D.; Fan, C.; Wei, K. MiR-29b-3p Promotes Progression of MDA-MB-231 Triple-Negative Breast Cancer Cells through Downregulating TRAF3. Biol. Res. 2019, 52, 38. [Google Scholar] [CrossRef]
- Li, Y.; Cai, B.; Shen, L.; Dong, Y.; Lu, Q.; Sun, S.; Liu, S.; Ma, S.; Ma, P.X.; Chen, J. MiRNA-29b Suppresses Tumor Growth through Simultaneously Inhibiting Angiogenesis and Tumorigenesis by Targeting Akt3. Cancer Lett. 2017, 397, 111–119. [Google Scholar] [CrossRef]
- Rostas, J.W.; Pruitt, H.C.; Metge, B.J.; Mitra, A.; Bailey, S.K.; Bae, S.; Singh, K.P.; Devine, D.J.; Dyess, D.L.; Richards, W.O.; et al. MicroRNA-29 Negatively Regulates EMT Regulator N-Myc Interactor in Breast Cancer. Mol. Cancer 2014, 13, 200. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.R.; Taipaleenmäki, H.; Hu, Y.J.; Hong, D.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B.; Pratap, J. Hsa-Mir-30c Promotes the Invasive Phenotype of Metastatic Breast Cancer Cells by Targeting NOV/CCN3. Cancer Cell Int. 2014, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.; Sharma, A.K.; Ward, A.; Will, R.; Hielscher, T.; Balwierz, A.; Breunig, C.; Münstermann, E.; König, R.; Keklikoglou, I.; et al. MicroRNA-30c-2-3p Negatively Regulates NF-ΚB Signaling and Cell Cycle Progression through Downregulation of TRADD and CCNE1 in Breast Cancer. Mol. Oncol. 2015, 9, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Chen, J.; Wang, X.P.; Wu, Y.Q.; Peng, H.T.; Lin, X.H.; Wang, W.J. Collagen Triple Helix Repeat Containing-1 Negatively Regulated by MicroRNA-30c Promotes Cell Proliferation and Metastasis and Indicates Poor Prognosis in Breast Cancer. J. Exp. Clin. Cancer Res. 2017, 36, 92. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Zhou, W.; Wang, Z.; Qiong, G.; Chen, Z.; Deng, S. MicroRNAs-107 Inhibited Autophagy, Proliferation, and Migration of Breast Cancer Cells by Targeting HMGB1. J. Cell. Biochem. 2019, 120, 8696–8705. [Google Scholar] [CrossRef]
- Wang, G.; Ma, C.; Shi, X.; Guo, W.; Niu, J. MiR-107 Enhances the Sensitivity of Breast Cancer Cells to Paclitaxel. Open Med. 2019, 14, 456–466. [Google Scholar] [CrossRef]
- Martello, G.; Rosato, A.; Ferrari, F.; Manfrin, A.; Cordenonsi, M.; Dupont, S.; Enzo, E.; Guzzardo, V.; Rondina, M.; Spruce, T.; et al. A MicroRNA Targeting Dicer for Metastasis Control. Cell 2010, 141, 1195–1207. [Google Scholar] [CrossRef]
- Ferracin, M.; Bassi, C.; Pedriali, M.; Pagotto, S.; D’Abundo, L.; Zagatti, B.; Corrà, F.; Musa, G.; Callegari, E.; Lupini, L.; et al. MiR-125b Targets Erythropoietin and Its Receptor and Their Expression Correlates with Metastatic Potential and ERBB2/HER2 Expression. Mol. Cancer 2013, 12, 130. [Google Scholar] [CrossRef]
- Wang, S.; Huang, J.; Lyu, H.; Lee, C.K.; Tan, J.; Wang, J.; Liu, B. Functional Cooperation of MiR-125a, MiR-125b, and MiR-205 in Entinostat-Induced Downregulation of ErbB2/ErbB3 and Apoptosis in Breast Cancer Cells. Cell Death Dis. 2013, 4, e556. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ding, J.; Chao, Z.; Dress, A.; Lai, L. MKNK2 Is a Valid Target of MiR-125b in Breast Cancer. Gene Rep. 2016, 5, 92–97. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Wang, H.; Zhao, J.; Xu, C.; Du, Y.; Luo, X.; Zheng, F.; Liu, R.; Zhang, H.; et al. MiRNA-135a Promotes Breast Cancer Cell Migration and Invasion by Targeting HOXA10. BMC Cancer 2012, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Zhang, W.; Wu, M.; Tan, S.; Zhu, T. Tumor-Suppressive MiRNA-135a Inhibits Breast Cancer Cell Proliferation by Targeting ELK1 and ELK3 Oncogenes. Genes Genom. 2018, 40, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Du, R.; Zhou, L.; Xu, J.; Chen, S.; Chen, J.; Yang, X.; Liu, D.X.; Shao, Z.M.; Zhang, L.; et al. MiR-200c/141 Regulates Breast Cancer Stem Cell Heterogeneity via Targeting HIPK1/β-Catenin Axis. Theranostics 2018, 8, 5801–5813. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, W.; Yang, X.; Zhao, D.; Li, F.; Wang, H. MicroRNA-146a Inhibits Cell Migration and Invasion by Targeting RhoA in Breast Cancer. Oncol. Rep. 2016, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.; Jiang, X.-Q.; Li, D.-Q.; Jiang, X.-C.; Wu, X.-B.; Cao, Y.-L. MiR-146a Promoted Breast Cancer Proliferation and Invasion by Regulating NM23-H1. J. Biochem. 2020, 167, 41–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, S.; Yang, J.; Chen, X.; Huang, W. Identification of MiR-146a Is Associated with the Aggressiveness and Suppresses Proliferation via Targeting CDKN2A in Breast Cancer. Pathol. Oncol. Res. 2018, 26, 1–7. [Google Scholar] [CrossRef]
- Liang, R.; Li, Y.; Wang, M.; Tang, S.C.; Xiao, G.; Sun, X.; Li, G.; Du, N.; Liu, D.; Ren, H. MiR-146a Promotes the Asymmetric Division and Inhibits the Self-Renewal Ability of Breast Cancer Stem-like Cells via Indirect Upregulation of Let-7. Cell Cycle 2018, 17, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wang, L.; Yang, C.; Li, X.; Jia, C.; Croteau, S.; Ruan, X.; Hardy, P. Micro-RNA-181a Suppresses Progestin-Promoted Breast Cancer Cell Growth. Maturitas 2018, 114, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Fu, X.; Li, Q.; Wang, Y.; Fan, D.; Zhou, Q.; Kuang, W.; Shen, L. MicroRNA-181 Serves an Oncogenic Role in Breast Cancer via the Inhibition of SPRY4. Mol. Med. Rep. 2018, 18, 5603–5613. [Google Scholar] [CrossRef]
- Yang, C.; Tabatabaei, S.N.; Ruan, X.; Hardy, P. The dual regulatory role of MiR-181a in breast cancer. Cell. Physiol. Biochem. 2017, 44, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xie, F.; Gao, A.; Zhang, R.; Zhang, L.; Xiao, Z.; Hu, Q.; Huang, W.; Huang, Q.; Lin, B.; et al. SOX2 Regulates Multiple Malignant Processes of Breast Cancer Development through the SOX2/MiR-181a-5p, MiR-30e-5p/TUSC3 Axis. Mol. Cancer 2017, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xue, A.; Chi, Y.; Xue, J.; Wang, W.; Zhao, Z.; Fan, M.; Yang, C.H.; Shao, Z.M.; Pfeffer, L.M.; et al. Induction of MiRNA-181a by Genotoxic Treatments Promotes Chemotherapeutic Resistance and Metastasis in Breast Cancer. Oncogene 2016, 35, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. MiR181b Is Induced by the Chemopreventive Polyphenol Curcumin and Inhibits Breast Cancer Metastasis via Down-Regulation of the Inflammatory Cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Yoo, J.O.; Kwak, S.Y.; An, H.J.; Bae, I.H.; Park, M.J.; Han, Y.H. MiR-181b-3p Promotes Epithelial-Mesenchymal Transition in Breast Cancer Cells through Snail Stabilization by Directly Targeting YWHAG. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Sossey-Alaoui, K.; Thompson, C.L.; Danielpour, D.; Schiemann, W.P. TGF-β Upregulates MiR-181a Expression to Promote Breast Cancer Metastasis. J. Clin. Invest. 2013, 123, 150–163. [Google Scholar] [CrossRef]
- Langer, E.M.; Kendsersky, N.D.; Daniel, C.J.; Kuziel, G.M.; Pelz, C.; Murphy, K.M.; Capecchi, M.R.; Sears, R.C. ZEB1-repressed microRNAs inhibit autocrine signaling that promotes vascular mimicry of breast cancer cells. Oncogene 2018, 37, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Sheng, C.; Huang, L.; Zhang, H.; Huang, L.; Cheng, Z.; Zhu, Q. MiR-183/-96/-182 Cluster Is up-Regulated in Most Breast Cancers and Increases Cell Proliferation and Migration. Breast Cancer Res. 2014, 16, 473. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Dwyer, R.M.; Kerin, M.J. Dysregulated MiR-183 Inhibits Migration in Breast Cancer Cells. Bmc Cancer 2010, 10, 502. [Google Scholar] [CrossRef]
- Cuiffo, B.G.; Campagne, A.; Bell, G.W.; Lembo, A.; Orso, F.; Lien, E.C.; Bhasin, M.K.; Raimo, M.; Hanson, S.E.; Marusyk, A.; et al. MSC-Regulated MicroRNAs Converge on the Transcription Factor FOXP2 and Promote Breast Cancer Metastasis. Cell Stem Cell 2014, 15, 762–774. [Google Scholar] [CrossRef]
- Chen, J.; Shin, V.Y.; Siu, M.T.; Ho, J.C.W.; Cheuk, I.; Kwong, A. MiR-199a-5p Confers Tumor-Suppressive Role in Triple-Negative Breast Cancer. BMC Cancer 2016, 16, 887. [Google Scholar] [CrossRef]
- Huang, R.; Li, J.; Pan, F.; Zhang, B.; Yao, Y. The Activation of GPER Inhibits Cells Proliferation, Invasion and EMT of Triple-Negative Breast Cancer via CD151/MiR-199a-3p Bio-Axis. Am. J. Transl. Res. 2020, 12, 32–44. [Google Scholar] [PubMed]
- Yuan, X.; Liu, J.; Ye, X. Effect of MiR-200c on the Proliferation, Migration and Invasion of Breast Cancer Cells and Relevant Mechanisms. J. BUON. 2019, 24, 61–67. [Google Scholar] [PubMed]
- Bian, X.; Liang, Z.; Feng, A.; Salgado, E.; Shim, H. HDAC Inhibitor Suppresses Proliferation and Invasion of Breast Cancer Cells through Regulation of MiR-200c Targeting CRKL. Biochem. Pharmacol.. 2018, 147, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Li, Y.; Xu, Y.; Kim, J.; Huang, S. Phosphodiesterase 7B/MicroRNA-200c Relationship Regulates Triple-Negative Breast Cancer Cell Growth. Oncogene 2019, 38, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.M.; Qiu, J.; Chen, X.W.; Liao, R.X.; Liao, X.Y.; Zhang, L.P.; Chen, X.; Li, Y.; Chen, Z.T.; Sun, J.G. Essential Role of MiR-200c in Regulating Self-Renewal of Breast Cancer Stem Cells and Their Counterparts of Mammary Epithelium. BMC Cancer 2015, 15, 1–12. [Google Scholar] [CrossRef][Green Version]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of MiRNA-200c Links Breast Cancer Stem Cells with Normal Stem Cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Chen, J.; Tian, W.; He, H.; Chen, F.; Huang, J.; Wang, X.; Chen, Z. Downregulation of MiR-200c-3p Contributes to the Resistance of Breast Cancer Cells to Paclitaxel by Targeting SOX2. Oncol. Rep. 2018, 40, 3821–3829. [Google Scholar] [CrossRef]
- Elgamal, O.A.; Park, J.K.; Gusev, Y.; Azevedo-Pouly, A.C.P.; Jiang, J.; Roopra, A.; Schmittgen, T.D. Tumor Suppressive Function of Mir-205 in Breast Cancer Is Linked to HMGB3 Regulation. PLoS ONE 2013, 8, e76402. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, H.; Deng, Z.; Yang, P.; Du, N.; Zhanng, Y.; Ren, H. MiRNA-205 Affects Infiltration and Metastasis of Breast Cancer. Biochem. Biophys. Res. Commun. 2013, 441, 139–143. [Google Scholar] [CrossRef]
- Piovan, C.; Palmieri, D.; Di Leva, G.; Braccioli, L.; Casalini, P.; Nuovo, G.; Tortoreto, M.; Sasso, M.; Plantamura, I.; Triulzi, T.; et al. Oncosuppressive Role of P53-Induced MiR-205 in Triple Negative Breast Cancer. Mol. Oncol. 2012, 6, 458–472. [Google Scholar] [CrossRef]
- Adachi, R.; Horiuchi, S.; Sakurazawa, Y.; Hasegawa, T.; Sato, K.; Sakamaki, T. ErbB2 Down-Regulates MicroRNA-205 in Breast Cancer. Biochem. Biophys. Res. Commun. 2011, 411, 804–808. [Google Scholar] [CrossRef]
- Ma, C.; Shi, X.; Guo, W.; Feng, F.; Wang, G. MiR-205-5p Downregulation Decreases Gemcitabine Sensitivity of Breast Cancer Cells via ERp29 Upregulation. Exp. Ther. Med. 2019, 18, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Huang, F.; Zhang, Q.; Chen, W.; Zhang, H. MiR-205-3p Promotes Proliferation and Reduces Apoptosis of Breast Cancer MCF-7 Cells and Is Associated with Poor Prognosis of Breast Cancer Patients. J. Clin. Lab. Anal. 2019, 33, e22966. [Google Scholar] [CrossRef]
- Fu, Y.; Shao, Z.M.; He, Q.Z.; Jiang, B.Q.; Wu, Y.; Zhuang, Z.G. Hsa-MiR-206 Represses the Proliferation and Invasion of Breast Cancer Cells by Targeting Cx43. Eur. Rev. Med. Pharm. Sci. 2015, 19, 2091–2104. [Google Scholar]
- Liang, Z.; Bian, X.; Shim, H. Downregulation of MicroRNA-206 Promotes Invasion and Angiogenesis of Triple Negative Breast Cancer. Biochem. Biophys. Res. Commun. 2016, 477, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Lyu, P.; Cao, Z.; Li, J.; Guo, G.; Xia, W.; Gu, Y. Overexpression of MiR-206 Suppresses Glycolysis, Proliferation and Migration in Breast Cancer Cells via PFKFB3 Targeting. Biochem. Biophys. Res. Commun. 2015, 463, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Amir, S.; Simion, C.; Umeh-Garcia, M.; Krig, S.; Moss, T.; Carraway, K.L.; Sweeney, C. Regulation of the T-Box Transcription Factor Tbx3 by the Tumour Suppressor MicroRNA-206 in Breast Cancer. Br. J. Cancer 2016, 114, 1125–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Tsouko, E.; Jonsson, P.; Bergh, J.; Hartman, J.; Aydogdu, E.; Williams, C. MiR-206 Inhibits Cell Migration through Direct Targeting of the Actin-Binding Protein Coronin 1C in Triple-Negative Breast Cancer. Mol. Oncol. 2014, 8, 1690–1702. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, Y.; Li, J.; Lu, B.; Sun, M.; Zou, Y.; Kong, R.; Luo, Y.; Shi, Y.; Wang, K.; et al. MiR-206 Is down-Regulated in Breast Cancer and Inhibits Cell Proliferation through the up-Regulation of CyclinD2. Biochem. Biophys. Res. Commun. 2013, 433, 207–212. [Google Scholar] [CrossRef]
- Huang, L.; Dai, T.; Lin, X.; Zhao, X.; Chen, X.; Wang, C.; Li, X.; Shen, H.; Wang, X. MicroRNA-224 Targets RKIP to Control Cell Invasion and Expression of Metastasis Genes in Human Breast Cancer Cells. Biochem. Biophys. Res. Commun. 2012, 425, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Z.; Xie, J.; Wang, P.; Zhu, J.; Li, Y.; Wang, Y. MiRNA-224-5p Inhibits Autophagy in Breast Cancer Cells via Targeting Smad4. Biochem. Biophys. Res. Commun. 2018, 506, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Wang, X.; He, M.; Qiao, S. MicroRNA-224 Promotes Tumorigenesis through Downregulation of Caspase-9 in Triple-Negative Breast Cancer. Dis. Markers 2019, 7378967. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Shen, J.; Zhang, G.; Han, J. MicroRNA-224 Inhibits Proliferation and Migration of Breast Cancer Cells by down-Regulating Fizzled 5 Expression. Oncotarget 2016, 7, 49130–49142. [Google Scholar] [CrossRef] [PubMed]
- Lettlova, S.; Brynychova, V.; Blecha, J.; Vrana, D.; Vondrusova, M.; Soucek, P.; Truksa, J. MiR-301a-3p Suppresses Estrogen Signaling by Directly Inhibiting ESR1 in ERα Positive Breast Cancer. Cell. Physiol. Biochem. 2018, 46, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, J.; Zhong, L.; Wang, L.; Liu, Y.; Wang, Y.; Peng, L.; Guo, B. Upregulated MicroRNA-301a in Breast Cancer Promotes Tumor Metastasis by Targeting PTEN and Activating Wnt/β-Catenin Signaling. Gene 2014, 535, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Yan, Y.; He, Q.; Ma, X.; Wang, W. MiR-328-5p Inhibits MDA-MB-231 Breast Cancer Cell Proliferation by Targeting RAGE. Oncol. Rep. 2018, 39, 2906–2914. [Google Scholar] [CrossRef]
- Xiao, B.; Chen, D.; Zhou, Q.; Hang, J.; Zhang, W.; Kuang, Z.; Sun, Z.; Li, L. Glutamate Metabotropic Receptor 4 (GRM4) Inhibits Cell Proliferation, Migration and Invasion in Breast Cancer and Is Regulated by MiR-328-3p and MiR-370-3p. BMC Cancer 2019, 19. [Google Scholar] [CrossRef]
- Hua, B.; Li, Y.; Yang, X.; Niu, X.; Zhao, Y.; Zhu, X. MicroRNA-361-3p Promotes Human Breast Cancer Cell Viability by Inhibiting the E2F1/P73 Signalling Pathway. Biomed. Pharm.. 2020, 125, 109994. [Google Scholar] [CrossRef]
- Han, J.; Yu, J.; Dai, Y.; Li, J.; Guo, M.; Song, J.; Zhou, X. Overexpression of MiR-361-5p in Triple-Negative Breast Cancer (TNBC) Inhibits Migration and Invasion by Targeting RQCD1 and Inhibiting the EGFR/PI3K/Akt Pathway. Bosn. J. Basic Med. Sci. 2019, 19, 52–59. [Google Scholar] [CrossRef]
- Huang, Q.; Gumireddy, K.; Schrier, M.; le Sage, C.; Nagel, R.; Nair, S.; Egan, D.A.; Li, A.; Huang, G.; Klein-Szanto, A.J.; et al. The MicroRNAs MiR-373 and MiR-520c Promote Tumour Invasion and Metastasis. Nat. Cell Biol. 2008, 10, 202–210. [Google Scholar] [CrossRef]
- Keklikoglou, I.; Koerner, C.; Schmidt, C.; Zhang, J.D.; Heckmann, D.; Shavinskaya, A.; Allgayer, H.; Gückel, B.; Fehm, T.; Schneeweiss, A.; et al. MicroRNA-520/373 Family Functions as a Tumor Suppressor in Estrogen Receptor Negative Breast Cancer by Targeting NF-B and TGF-Β Signaling Pathways. Oncogene 2012, 31, 4150–4163. [Google Scholar] [CrossRef] [PubMed]
- Rocha Simonini, P.D.S.; Breiling, A.; Gupta, N.; Malekpour, M.; Youns, M.; Omranipour, R.; Malekpour, F.; Volinia, S.; Croce, C.M.; Najmabadi, H.; et al. Epigenetically Deregulated MicroRNA-375 Is Involved in a Positive Feedback Loop with Estrogen Receptor α in Breast Cancer Cells. Cancer Res. 2010, 70, 9175–9184. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Noh, H.; Teng, Y.; Shao, J.; Rehmani, H.; Ding, H.F.; Dong, Z.; Su, S.B.; Shi, H.; Kim, J.; et al. SHOX2 Is a Direct MiR-375 Target and a Novel Epithelial-to-Mesenchymal Transition Inducer in Breast Cancer Cells. Neoplasia 2014, 16, 279–290.e5. [Google Scholar] [CrossRef]
- Pan, Y.; Jiao, G.; Wang, C.; Yang, J.; Yang, W. MicroRNA-421 Inhibits Breast Cancer Metastasis by Targeting Metastasis Associated 1. Biomed. Pharmacother. 2016, 83, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Shen, J. MicroRNA-421-Targeted PDCD4 Regulates Breast Cancer Cell Proliferation. Int. J. Mol. Med. 2019, 43, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Aili, T.; Paizula, X.; Ayoufu, A. MiR-455-5p Promotes Cell Invasion and Migration in Breast Cancer. Mol. Med. Rep. 2018, 17, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zou, A.; Ma, L.; Chen, X.; Wang, L.; Zeng, X.; Tan, T. MiR-455 Inhibits Breast Cancer Cell Proliferation through Targeting CDK14. Eur. J. Pharmacol. 2017, 807, 138–143. [Google Scholar] [CrossRef]
- Ma, L.; Teruya-Feldstein, J.; Weinberg, R.A. Tumour Invasion and Metastasis Initiated by MicroRNA-10b in Breast Cancer. Nature 2007, 449, 682–688. [Google Scholar] [CrossRef]
- Wu, B.; Liu, G.; Jin, Y.; Yang, T.; Zhang, D.; Ding, L.; Zhou, F.; Pan, Y.; Wei, Y. MiR-15b-5p Promotes Growth and Metastasis in Breast Cancer by Targeting HPSE2. Front. Oncol. 2020, 10, 108. [Google Scholar] [CrossRef]
- Han, M.; Liu, M.; Wang, Y.; Chen, X.; Xu, J.; Sun, Y.; Zhao, L.; Qu, H.; Fan, Y.; Wu, C. Antagonism of MiR-21 Reverses Epithelial-Mesenchymal Transition and Cancer Stem Cell Phenotype through AKT/ERK1/2 Inactivation by Targeting PTEN. PLoS ONE 2012, 7, e39520. [Google Scholar] [CrossRef]
- Xiao-mei, W.; Jing, X.; Zhi-qiang, C.; Quan-zhou, P.; Jin-tao, H.; Li-kun, G.; Shi-fen, Z.; Jin-zhong, C. Programmed Cell Death 4 (PDCD4) Is an Important Functional Target of MicroRNA-21 in Cervical Cancer Cell. Chin. J. Diagn. Pathology 2011, 18, 199–202. [Google Scholar]
- Li, J.; Zhang, Y.; Zhang, W.; Jia, S.; Tian, R.; Kang, Y.; Ma, Y.; Li, D. Genetic Heterogeneity of Breast Cancer Metastasis May Be Related to MiR-21 Regulation of TIMP-3 in Translation. Int. J. Surg. Oncol. 2013, 2013, 875078. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, T.A.; Schwartz, G.N.; Calderone, H.M.; Graveel, C.R.; Winn, M.E.; Hostetter, G.; Wells, W.A.; Sempere, L.F. Stromal Expression of MiR-21 Identifies High-Risk Group in Triple-Negative Breast Cancer. Am. J. Pathol. 2014, 184, 3217–3225. [Google Scholar] [CrossRef]
- Song, B.; Wang, C.; Liu, J.; Wang, X.; Lv, L.; Wei, L.; Xie, L.; Zheng, Y.; Song, X. MicroRNA-21 Regulates Breast Cancer Invasion Partly by Targeting Tissue Inhibitor of Metalloproteinase 3 Expression. J. Exp. Clin. Cancer Res. 2010, 29, 29. [Google Scholar] [CrossRef]
- Yan, L.X.; Wu, Q.N.; Zhang, Y.; Li, Y.Y.; Liao, D.Z.; Hou, J.H.; Fu, J.; Zeng, M.S.; Yun, J.P.; Wu, Q.L.; et al. Knockdown of MiR-21 in Human Breast Cancer Cell Lines Inhibits Proliferation, in Vitro Migration and in Vivo Tumor Growth. Breast Cancer Res. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Y.; Fan, X.; Zhang, L.; Li, Q.; Li, S.; Wang, H.; Xiao, Y. MicroRNA-21 Promotes Progression of Breast Cancer via Inhibition of Mitogen-Activated Protein Kinase10 (MAPK10). Biosci. Rep. 2020. [Google Scholar] [CrossRef]
- Wang, H.; Tan, Z.; Hu, H.; Liu, H.; Wu, T.; Zheng, C.; Wang, X.; Luo, Z.; Wang, J.; Liu, S.; et al. MicroRNA-21 Promotes Breast Cancer Proliferation and Metastasis by Targeting LZTFL1. BMC Cancer 2019, 19, 738. [Google Scholar] [CrossRef]
- Ma, F.; Li, W.; Liu, C.; Li, W.; Yu, H.; Lei, B.; Ren, Y.; Li, Z.; Pang, D.; Qian, C. MiR-23a Promotes TGF-Β1-Induced EMT and Tumor Metastasis in Breast Cancer Cells by Directly Targeting CDH1 and Activating Wnt/β-Catenin Signaling. Oncotarget 2017, 8, 69538–69550. [Google Scholar] [CrossRef]
- Hesari, A.; Azizian, M.; Darabi, H.; Nesaei, A.; Hosseini, S.A.; Salarinia, R.; Motaghi, A.A.; Ghasemi, F. Expression of circulating miR-17, miR-25, and miR-133 in breast cancer patients. J. Cell. Biochem. 2019, 120, 7109–7114. [Google Scholar] [CrossRef]
- Chen, H.; Pan, H.; Qian, Y.; Zhou, W.; Liu, X. MiR-25-3p Promotes the Proliferation of Triple Negative Breast Cancer by Targeting BTG2. Mol. Cancer 2018, 17, 4. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Z.; Sun, H.; Li, Y. MicroRNA-27a Promotes Tumorigenesis via Targeting AKT in Triple Negative Breast Cancer. Mol. Med. Rep. 2018, 17, 562–570. [Google Scholar] [CrossRef]
- Guttilla, I.K.; White, B.A. Coordinate Regulation of FOXO1 by MiR-27a, MiR-96, and MiR-182 in Breast Cancer Cells. J. Biol. Chem. 2009, 284, 23204–23216. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lei, Y.; Liu, X. MiR-29a Promotes Cell Proliferation and EMT in Breast Cancer by Targeting Ten Eleven Translocation 1. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 2177–2185. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, W.; Tang, T.; Wang, Y.; Yin, X.; Chen, Y.; Zhang, Y.; Xing, Y.; Shen, Y.; Xia, T.; et al. MiR-29a Contributes to Breast Cancer Cells Epithelial–Mesenchymal Transition, Migration, and Invasion via down-Regulating Histone H4K20 Trimethylation through Directly Targeting SUV420H2. Cell Death Dis. 2019, 10, 176. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; Guo, G.; Li, L.; Dou, D.; Ge, X.; Lv, P.; Wang, F.; Gu, Y. MicroRNA-30d Mediated Breast Cancer Invasion, Migration, and EMT by Targeting KLF11 and Activating STAT3 Pathway. J. Cell. Biochem. 2018, 119, 8138–8145. [Google Scholar] [CrossRef]
- Xia, W.; Zhou, J.Y.; Luo, H.B.; Liu, Y.Z.; Peng, C.C.; Zheng, W.L.; Ma, W.L. MicroRNA-32 Promotes Cell Proliferation, Migration and Suppresses Apoptosis in Breast Cancer Cells by Targeting FBXW7. Cancer Cell Int. 2017, 17, 14. [Google Scholar] [CrossRef]
- Xia, H.; Long, J.; Zhang, R.; Yang, X.; Ma, Z. MiR-32 Contributed to Cell Proliferation of Human Breast Cancer Cells by Suppressing of PHLPP2 Expression. Biomed. Pharmacother. 2015, 75, 105–110. [Google Scholar] [CrossRef]
- Lin, H.; Dai, T.; Xiong, H.; Zhao, X.; Chen, X.; Yu, C.; Li, J.; Wang, X.; Song, L. Unregulated miR-96 Induces Cell Proliferation in Human Breast Cancer by Downregulating Transcriptional Factor FOXO3a. PLoS ONE. 2010, 5, e15797. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Liang, H.; Wang, Y.; Zhang, W.; Zhou, Y.; Yu, M.; Cui, S.; Liu, M.; Wang, N.; Ye, C.; et al. MiR-96 Promotes Cell Proliferation, Migration and Invasion by Targeting PTPN9 in Breast Cancer. Sci. Rep. 2016, 6, 37421. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, Y.; Shao, N.; Ye, R.; Lin, Y.; Zhang, N.; Li, W.; Zhang, Y.; Wang, S. Overexpression of MicroRNA-96-5p Inhibits Autophagy and Apoptosis and Enhances the Proliferation, Migration and Invasiveness of Human Breast Cancer Cells. Oncology Lett. 2017, 13, 4402–4412. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.A.; Pierce, M.L.; Soukup, G.A. Sensational MicroRNAs: Neurosensory Roles of the MicroRNA-183 Family. Mol. Neurobiol. 2019, 57, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Sun, F.; Chen, L.; Cao, X. MiR-96 Promotes Breast Cancer Metastasis by Suppressing MTSS1. Oncology Lett. 2018, 15, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, P.; Zhang, X.; Zhang, M.; Wang, H.; Wu, M.; Kong, X.; Tan, S.; Ding, K.; Perry, J.K.; et al. Autocrine/Paracrine Human Growth Hormone-Stimulated MicroRNA 96-182-183 Cluster Promotes Epithelial-Mesenchymal Transition and Invasion in Breast Cancer. J. Biol. Chem. 2015, 290, 13812–13829. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.; Guttilla Reed, I.K. Regulation of cell growth and migration by miR-96 and miR-183 in a breast cancer model of epithelial-mesenchymal transition. PLoS ONE. 2020, 15, e0233187. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Liang, J.L.; Kuo, Y.L.; Lee, H.H.; Calkins, M.J.; Chang, H.T.; Lin, F.C.; Chen, Y.C.; Hsu, T.I.; Hsiao, M.; et al. MiR-105/93-3p Promotes Chemoresistance and Circulating MiR-105/93-3p Acts as a Diagnostic Biomarker for Triple Negative Breast Cancer. Breast Cancer Res. 2017, 19, 133. [Google Scholar] [CrossRef]
- Wang, Z.; Li, T.E.; Chen, M.; Pan, J.J.; Shen, K.W. MiR-106b-5p Contributes to the Lung Metastasis of Breast Cancer via Targeting CNN1 and Regulating Rho/ROCK1 Pathway. Aging 2020, 12, 1867–1887. [Google Scholar] [CrossRef]
- You, F.; Luan, H.; Sun, D.; Cui, T.; Ding, P.; Tang, H.; Sun, D. MiRNA-106a Promotes Breast Cancer Cell Proliferation, Clonogenicity, Migration, and Invasion Through Inhibiting Apoptosis and Chemosensitivity. DNA Cell Biol. 2019, 38, 198–207. [Google Scholar] [CrossRef]
- Manne, R.K.; Agrawal, Y.; Bargale, A.; Patel, A.; Paul, D.; Gupta, N.A.; Rapole, S.; Seshadri, V.; Subramanyam, D.; Shetty, P.; et al. A MicroRNA/Ubiquitin Ligase Feedback Loop Regulates Slug-Mediated Invasion in Breast Cancer. Neoplasia 2017, 19, 483–495. [Google Scholar] [CrossRef]
- Ivanovska, I.; Ball, A.S.; Diaz, R.L.; Magnus, J.F.; Kibukawa, M.; Schelter, J.M.; Kobayashi, S.V.; Lim, L.; Burchard, J.; Jackson, A.L.; et al. MicroRNAs in the MiR-106b Family Regulate P21/CDKN1A and Promote Cell Cycle Progression. Mol. Cell. Biol. 2008, 28, 2167–2174. [Google Scholar] [CrossRef]
- Zhang, C.M.; Zhao, J.; Deng, H.Y. MiR-155 Promotes Proliferation of Human Breast Cancer MCF-7 Cells through Targeting Tumor Protein 53-Induced Nuclear Protein 1. J. Biomed. Sci. 2013, 20, 79. [Google Scholar] [CrossRef]
- Kong, W.; He, L.; Coppola, M.; Guo, J.; Esposito, N.N.; Coppola, D.; Cheng, J.Q. MicroRNA-155 Regulates Cell Survival, Growth, and Chemosensitivity by Targeting FOXO3a in Breast Cancer. J. Biol. Chem. 2010, 285, 17869–17879. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.W.; Lu, M.H.; He, X.H.; Li, Y.; Gu, H.; Liu, M.F.; Wang, E.D. MicroRNA-155 Functions as an OncomiR in Breast Cancer by Targeting the Suppressor of Cytokine Signaling 1 Gene. Cancer Res. 2010, 70, 3119–3127. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.-J.; Guo, G.-L. MiR-155 Promotes the Proliferation and Migration of Breast Cancer Cells via Targeting SOCS1 and MMP16. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7323–7332. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, T.; Yan, L.; Xu, H.; Wang, Y.; Li, Y.; Wang, H.; Chen, S.; Wang, W.; Chen, C.; et al. MiR-155-3p Acts as a Tumor Suppressor and Reverses Paclitaxel Resistance via Negative Regulation of MYD88 in Human Breast Cancer. Gene 2019, 700, 85–95. [Google Scholar] [CrossRef]
- Liu, J.-H.; Yang, Y.; Song, Q.; Li, J.B. MicroRNA-155 regulates the Proliferation and Metastasis of Human Breast Cancers by Targeting MAPK7. J. Buon. 2019, 24, 1075–1080. [Google Scholar] [PubMed]
- Chen, Y.; Wei, H.; Liu, Y.; Zheng, S. Promotional Effect of MicroRNA-194 on Breast Cancer Cells via Targeting F-Box/WD Repeat-Containing Protein 7. Oncol. Lett. 2018, 15, 4439–4444. [Google Scholar] [CrossRef]
- Yang, F.; Xiao, Z.; Zhang, S. Knockdown of MiR-194-5p Inhibits Cell Proliferation, Migration and Invasion in Breast Cancer by Regulating the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Med. 2018, 42, 3355–3363. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, S.; Dong, L.; Li, Y.; Mao, Y.; Liang, Y.; Tao, Y.; Ma, J. Inhibition of MiR-214 Attenuates the Migration and Invasion of Triple-negative Breast Cancer Cells. Mol. Med. Rep. 2019, 49, 4035–4042. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liu, J.; Guo, H.; Xu, H.; Zhang, G. PGC-1 Alpha Interacts with MicroRNA-217 to Functionally Regulate Breast Cancer Cell Proliferation. Biomed. Pharmacother. 2017, 85, 541–548. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cui, J.; Xiao, T.; Jiang, D. MiR-217 Promotes Tumor Proliferation in Breast Cancer via Targeting DACH1. J. Cancer 2015, 6, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, Y.; Sun, X.; Xu, T.; Cheng, X.; Qin, Y. MiR-221/222 Promote Tumor Growth and Suppress Apoptosis by Targeting LncRNA GAS5 in Breast Cancer. Biosci. Rep. 2019, 39, 39. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, Y.; Wang, H.; Han, X.; Mao, J.; Li, J.; Yu, L.; Wang, B.; Fan, S.; Yu, X.; et al. MiR-221/222 Enhance the Tumorigenicity of Human Breast Cancer Stem Cells via Modulation of PTEN/Akt Pathway. Biomed. Pharmacother. 2016, 79, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, Y.; Yu, L.; Han, X.; Wang, H.; Mao, J.; Shen, J.; Wang, B.; Tang, J.; Li, C.; et al. MiR-221/222 Promote Cancer Stem-like Cell Properties and Tumor Growth of Breast Cancer via Targeting PTEN and Sustained Akt/NF-ΚB/COX-2 Activation. Chem. Biol. Interact. 2017, 277, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, D.; Wu, T.; Xie, D.; Hua, K.; Hu, J.; Deng, X.; Ji, C.; Deng, Y.; Fang, L. MicroRNA-301b Promotes Cell Proliferation and Apoptosis Resistance in Triple-Negative Breast Cancer by Targeting CYLD. BMB Rep. 2018, 51, 602–607. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Kuo, W.H.; Hung, J.H.; Lee, C.Y.; Lee, Y.H.; Chang, Y.C.; Lin, W.C.; Shen, C.Y.; Huang, C.S.; Hsieh, F.J.; et al. Deregulated microRNAs in triple-negative breast cancer revealed by deep sequencing. Mol. Cancer 2015, 14, 36. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X. MicroRNA-370 Promotes Cell Growth by Targeting WNK2 in Breast Cancer. DNA Cell Biol. 2019, 38, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Xia, K.; Xu, P.; Sun, E.; Ma, J.; Gao, S.; Zhou, Q.; Zhang, M.; Wang, F.; Chen, F.; et al. miRNA expression patterns in chemoresistant breast cancer tissues. Biomed. Pharmacother. 2014, 68, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, J.; Huang, Z. MiR-372 Promotes Breast Cancer Cell Proliferation by Directly Targeting LATS2. Exp. Ther. Med. 2018, 15, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Huang, X.; Li, Z.; Ma, X. MicroRNA-372-3p Promotes the Epithelial-Mesenchymal Transition in Breast Carcinoma by Activating the Wnt Pathway. J. Buon. 2018, 23, 1309–1315. [Google Scholar]
- Son, D.; Kim, Y.; Lim, S.; Kang, H.G.; Kim, D.H.; Park, J.W.; Cheong, W.; Kong, H.K.; Han, W.; Park, W.Y.; et al. MiR-374a-5p Promotes Tumor Progression by Targeting ARRB1 in Triple Negative Breast Cancer. Cancer Lett. 2019, 454, 224–233. [Google Scholar] [CrossRef]
- Cai, J.; Guan, H.; Fang, L.; Yang, Y.; Zhu, X.; Yuan, J.; Wu, J.; Li, M. MicroRNA-374a Activates Wnt/β-Catenin Signaling to Promote Breast Cancer Metastasis. J. Clin. Invest. 2013, 123, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Wang, Y.; Ding, Y.; Chen, T.; Wang, Y.; Wang, H.; Li, Y.; Duan, K.; Chen, S.; et al. Involvement of MiR-451 in Resistance to Paclitaxel by Regulating YWHAZ in Breast Cancer. Cell Death Dis. 2017, 8, e3071. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Meng, X.; Pang, R.; Li, J. MicroRNA-454 May Function as an Oncogene via Targeting AKT in Triple Negative Breast Cancer. J. Biol. Res. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, H.; Song, J.; Chen, X.; Lin, C.; Zhang, X.; Hou, N.; Pan, J.; Zhou, Z.; Wang, L.; et al. MiR-454-3p-Mediated Wnt/β-Catenin Signaling Antagonists Suppression Promotes Breast Cancer Metastasis. Theranostics 2019, 9, 449–465. [Google Scholar] [CrossRef]
- Guo, Q.J.; Mills, J.N.; Bandurraga, S.G.; Nogueira, L.M.; Mason, N.J.; Camp, E.R.; Larue, A.C.; Turner, D.P.; Findlay, V.J. MicroRNA-510 Promotes Cell and Tumor Growth by Targeting Peroxiredoxin1 in Breast Cancer. Breast Cancer Res. 2013, 15, R70. [Google Scholar] [CrossRef]
- Navarro, P.; Ramkissoon, S.H.; Shah, S.; Park, J.M.; Murthy, R.G.; Patel, S.A.; Greco, S.J.; Rameshwar, P. An Indirect Role for the Oncomir-519b in the Expression of Truncated Neurokinin-1 in Breast Cancer Cells. Exp. Cell Res. 2012, 318, 2604–2615. [Google Scholar] [CrossRef]
- Hunter, S.; Nault, B.; Ugwuagbo, K.C.; Maiti, S.; Majumder, M. Mir526b and Mir655 Promote Tumour Associated Angiogenesis and Lymphangiogenesis in Breast Cancer. Cancers 2019, 11, 938. [Google Scholar] [CrossRef]
- Huang, L.; Tang, X.; Shi, X.; Su, L. MiR-532-5p Promotes Breast Cancer Proliferation and Migration by Targeting RERG. Exp. Ther. Med. 2019, 19, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Liang, X.; Li, L.; Wang, B.; Ding, F.; Li, Y.; Wang, X.; Zhan, Q.; Liu, Z. MicroRNA-548j Functions as a Metastasis Promoter in Human Breast Cancer by Targeting Tensin1. Mol. Oncol. 2016, 10, 838–849. [Google Scholar] [CrossRef]
- Mitra, A.; Rostas, J.W.; Dyess, D.L.; Shevde, L.A.; Samant, R.S. Micro-RNA-632 Downregulates DNAJB6 in Breast Cancer. Lab. Investig. 2012, 92, 1310–1317. [Google Scholar] [CrossRef]
- Li, L.; Qiu, X.G.; Lv, P.W.; Wang, F. MiR-639 Promotes the Proliferation and Invasion of Breast Cancer Cell in Vitro. Cancer Cell Int. 2014, 14, 39. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.G.; Hu, J.Y.; Tang, J.; Yi, W.; Zhang, M.Y.; Deng, R.; Mai, S.J.; Weng, N.Q.; Wang, R.Q.; Liu, J.; et al. MiR-665 Expression Predicts Poor Survival and Promotes Tumor Metastasis by Targeting NR4A3 in Breast Cancer. Cell Death Dis. 2019, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast Cancer-Secreted MiR-939 Downregulates VE-Cadherin and Destroys the Barrier Function of Endothelial Monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef]
- Liu, X.; Bi, L.; Wang, Q.; Wen, M.; Li, C.; Ren, Y.; Jiao, Q.; Mao, J.H.; Wang, C.; Wei, G.; et al. MiR-1204 Targets VDR to Promotes Epithelial-Mesenchymal Transition and Metastasis in Breast Cancer. Oncogene 2018, 37, 3426–3439. [Google Scholar] [CrossRef]
- Li, X.J.; Ren, Z.J.; Tang, J.H.; Yu, Q. Exosomal MicroRNA MiR-1246 Promotes Cell Proliferation, Invasion and Drug Resistance by Targeting CCNG2 in Breast Cancer. Cell. Physiol. Biochem. 2018, 44, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, P.; Wang, J.; Wang, L.; Ren, M.; Zhang, R.; He, J. MicroRNA-1254 Exerts Oncogenic Effects by Directly Targeting RASSF9 in Human Breast Cancer. Int. J. Oncol. 2018, 53, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zou, H.; Lee, J.W.; Han, J.; Kim, H.C.; Cheol, J.J.; Kim, L.S.; Kim, H. MiR-1307-3p Stimulates Breast Cancer Development and Progression by Targeting SMYD4. J. Cancer. 2019, 10, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.N.; Deng, X.Y.; Gan, X.X.; Zhang, J.H.; Ren, G.H.; Shen, F.; Feng, J.H.; Cai, W.S.; Xu, B. Targeting of TLE3 by MiR-3677 in Human Breast Cancer Promotes Cell Proliferation, Migration and Invasion. Oncol. Lett. 2020, 19, 1409–1417. [Google Scholar]
- Li, Y.; Wang, Y.W.; Chen, X.; Ma, R.R.; Guo, X.Y.; Liu, H.T.; Jiang, S.J.; Wei, J.M.; Gao, P. MicroRNA-4472 Promotes Tumor Proliferation and Aggressiveness in Breast Cancer by Targeting RGMA and Inducing EMT. Clin. Breast Cancer 2020, 20, e113–e126. [Google Scholar] [CrossRef]
- Liang, F.; Fu, X.; Wang, L. MiR-5590-3p-YY1 Feedback Loop Promotes the Proliferation and Migration of Triple-Negative Breast Cancer Cells. J. Cell. Biochem. 2019, 120, 18415–18424. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, H.; Gao, C.; Lv, Y.; Chen, X.H.; Xu, R.; Sun, M.; Liu, X.; Lu, X.; Pei, X.; et al. Tumor-Promoting Properties of MiR-8084 in Breast Cancer through Enhancing Proliferation, Suppressing Apoptosis and Inducing Epithelial-Mesenchymal Transition. J. Transl. Med. 2018, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baumann, V.; Winkler, J. MiRNA-based Therapies: Strategies and Delivery Platforms for Oligonucleotide and Non-oligonucleotide Agents. Futur. Med. Chem. 2014, 6, 1967–1984. [Google Scholar] [CrossRef]
- Lima, J.F.; Cerqueira, L.; Figueiredo, C.; Oliveira, C.; Azevedo, N.F. Anti-miRNA Oligonucleotides: A Comprehensive Guide for Design. RNA Biol. 2018, 15, 338–352. [Google Scholar] [CrossRef]
- Gao, S.; Tian, H.; Guo, Y.; Li, Y.; Guo, Z.; Zhu, X.; Chen, X. MiRNA Oligonucleotide and Sponge For MiRNA-21 Inhibition Mediated by PEI-PLL in Breast Cancer Therapy. Acta Biomater. 2015, 25, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Escobar, G.; Moi, D.; Ranghetti, A.; Ozkal-Baydin, P.; Squadrito, M.L.; Kajaste-Rudnitski, A.; Bondanza, A.; Gentner, B.; De Palma, M.; Mazzieri, R.; et al. Genetic Engineering of Hematopoiesis for Targeted IFN-α Delivery Inhibits Breast Cancer Progression. Sci. Transl. Med. 2014, 6, 217ra3. [Google Scholar] [CrossRef] [PubMed]
- Trepel, M.; Körbelin, J.; Spies, E.; Heckmann, M.B.; Hunger, A.; Fehse, B.; Katus, H.A.; Kleinschmidt, J.A.; Müller, O.J.; Michelfelder, S. Treatment of Multifocal Breast Cancer by Systemic Delivery of Dual-targeted Adeno-associated Viral Vectors. Gene Ther. 2015, 22, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wu, K.; Zeng, Z.; Huang, S.; Ji, X.; Yuan, C.; Zhang, L.; Liu, W.; Huang, B.; Feng, Y.; et al. A Simplified System to Express Circularized Inhibitors of MiRNA for Stable and Potent Suppression of MiRNA Functions. Mol. Ther. Nucleic Acids 2018, 13, 556–567. [Google Scholar] [CrossRef]
- Ramchandani, D.; Lee, S.K.; Yomtoubian, S.; Han, M.S.; Tung, C.H.; Mittal, V. Nanoparticle Delivery of MiR-708 Mimetic Impairs Breast Cancer Metastasis. Mol. Cancer Ther. 2019, 18, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Lujan, H.; Griffin, W.C.; Taube, J.H.; Sayes, C.M. Synthesis and Characterization of Nanometer-sized Liposomes for Encapsulation and MicroRNA Transfer to Breast Cancer Cells. Int. J. Nanomed. 2019, 14, 5159. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.Q.; Duan, J.L.; Bao, C.J.; Cui, Y.N.; Su, Z.B.; Xu, J.R.; Luo, Q.; Chen, M.; Xie, Y.; et al. Nanosized Functional MiRNA Liposomes and Application in The Treatment of TNBC by Silencing Slug Gene. Int. J. Nanomed. 2019, 14, 3645. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, J.; Ho, P.Y.; Luo, Z.; Ma, W.; Zhao, W.; Rathod, S.B.; Fernandez, C.A.; Venkataramanan, R.; Xie, W.; et al. Creatine Based Polymer for Codelivery of Bioengineered MicroRNA and Chemodrugs Against Breast Cancer Lung Metastasis. Biomaterials 2019, 210, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Genepharma. miRNA Agomir & Antagomir. 2017. Available online: http://www.genepharma.com/en/productsview.php?id=594&content=1 (accessed on 29 April 2020).
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Surapaneni, S.K.; Bashir, S.; Tikoo, K. Gold Nanoparticles-induced Cytotoxicity in Triple Negative Breast Cancer Involves Different Epigenetic Alterations Depending Upon the Surface Charge. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
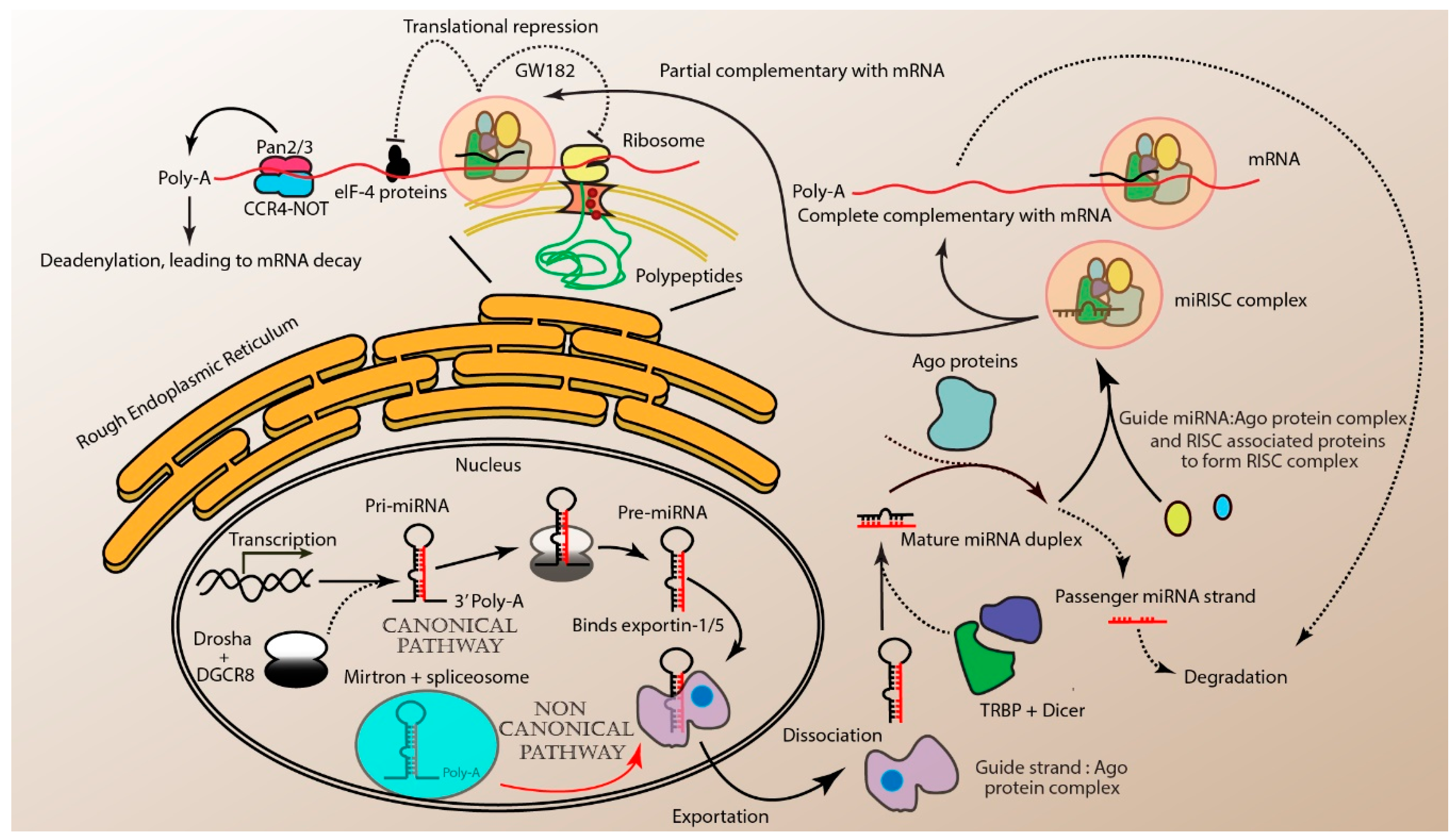

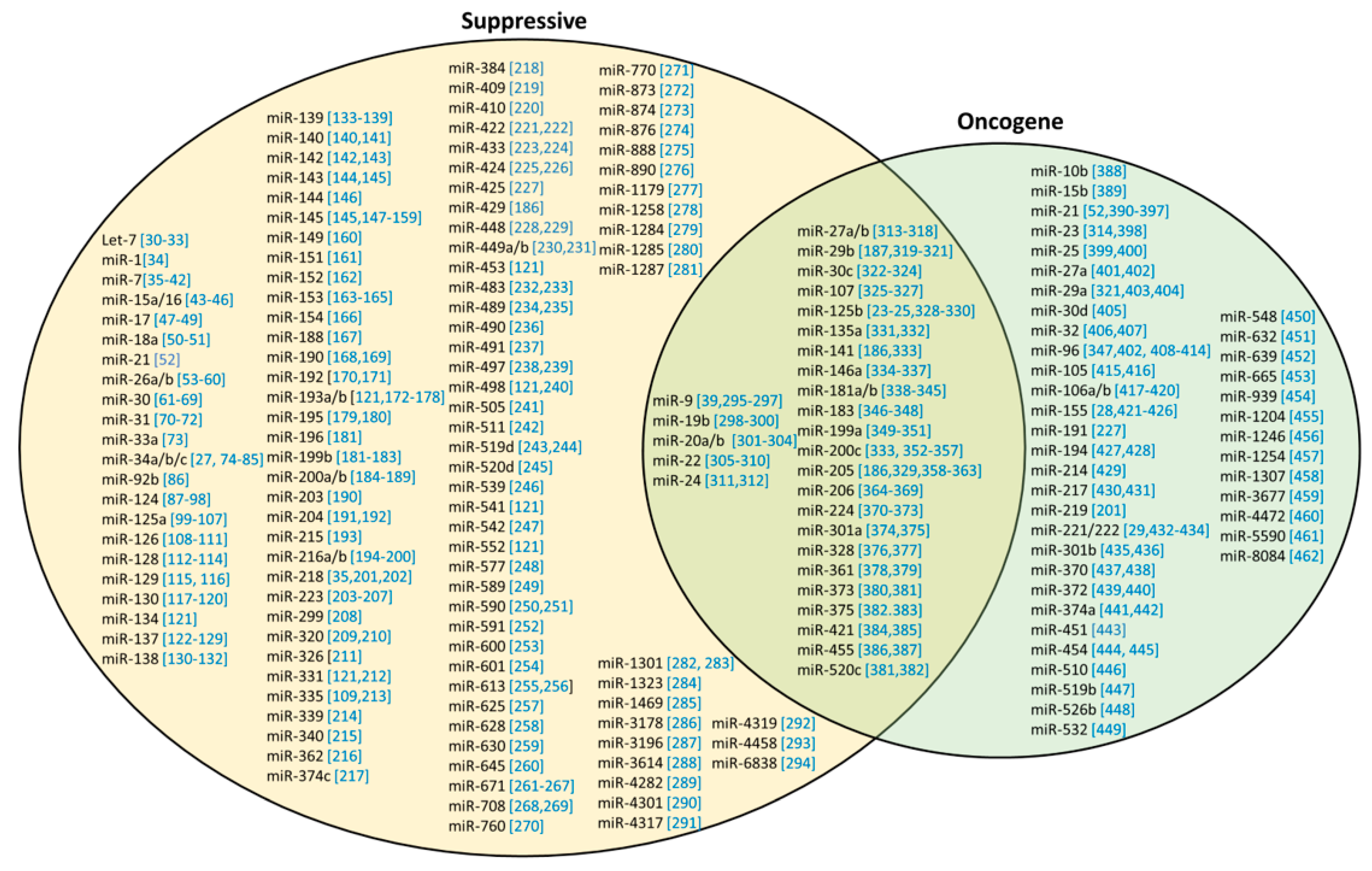
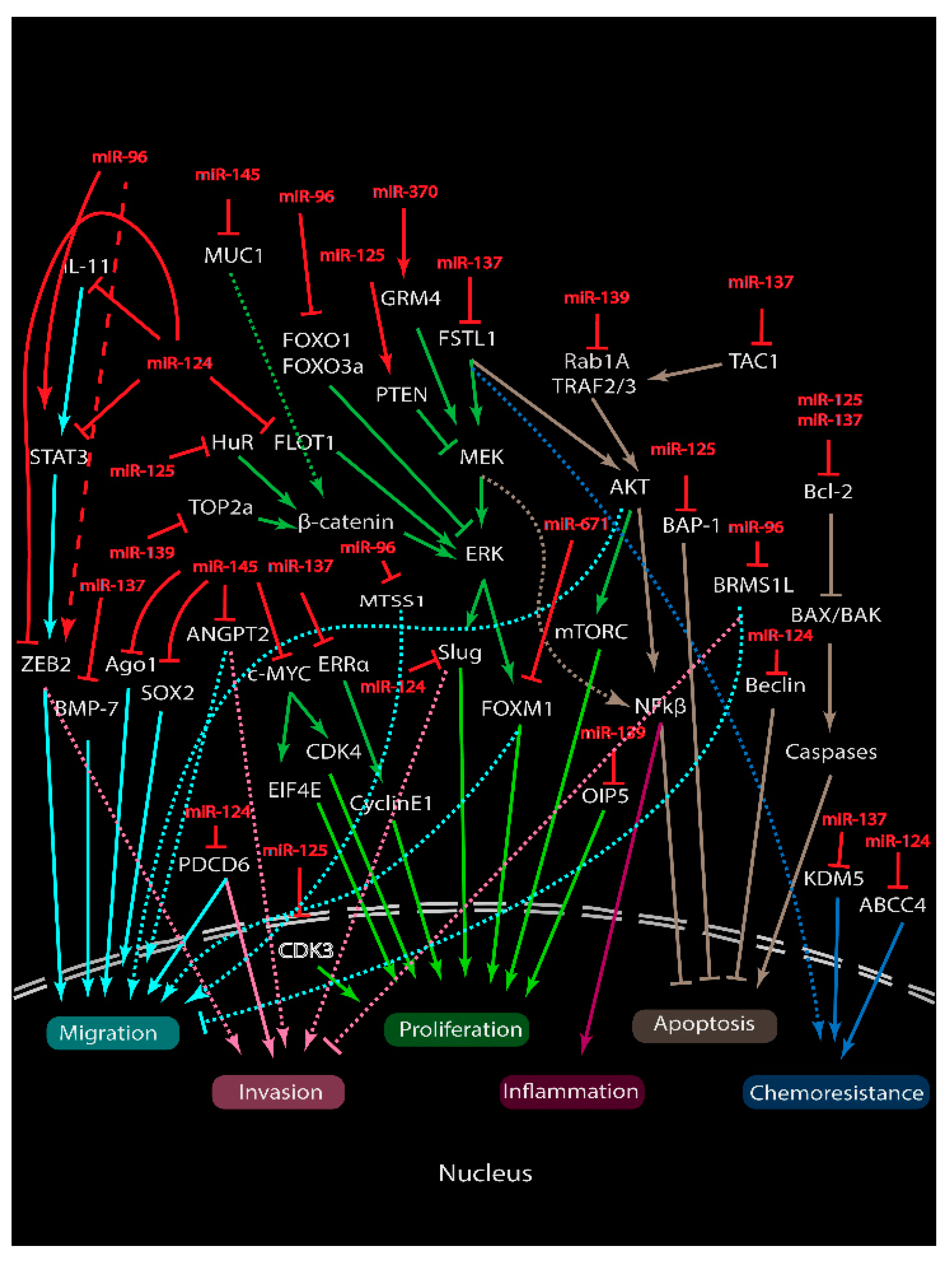
| Function | miRNA | Target | Effects | Ref. |
|---|---|---|---|---|
| Tumor suppressive (inhibition) | miR-26a | RNF6 | Proliferation | [53] |
| CHD1, GREB1 and KPNA2 | [54] | |||
| Metadherin | Proliferation and metastasis | [55] | ||
| MCL-1 | [56] | |||
| CCNE2 | Sensitize to trastuzumab in Her2+ subtype | [57] | ||
| ERBB2 | Sensitize to tamoxifen in ER+ subtype | [58] | ||
| miR-193a/b | DDAH1 | Angiogenesis | [172] | |
| PTP1B | Proliferation and survival | [173] | ||
| MORC4 | [174] | |||
| WT1 | Proliferation and metastasis | [175] | ||
| RAB22A | [176] | |||
| MCL-1 | Sensitize to doxorubicin | [177] | ||
| uPA | Proliferation, cell invasion and metastasis | [178] | ||
| miR-216a/b | TLR4 | Suppresses stemness and the release of soluble factors associated with cancer-associated fibroblast activation | [194] | |
| PKCα | Survival and migration | [195] | ||
| PAK2 | Proliferation and metastasis | [196] | ||
| SDCBP | [197] | |||
| P2X7R | Proliferation and survival | [198] | ||
| HDAC8 | Proliferation and colony formation | [199] | ||
| miR-223 | Caprin-1 | Proliferation and invasion | [203] | |
| STIM1 | [204] | |||
| HAX-1 | Survival and sensitize to doxorubicin and cisplatin | [205] | ||
| STAT5A | Survival and sensitize to paclitaxel | [206] | ||
| EGF | Proliferation and sensitize radiation-treated breast cancer cells to lapatinib | [207] | ||
| Oncogenic (promotion) | miR-1246 | CCNG2 | Proliferation, migration, chemoresistance to docetaxel, epirubicin and gemcitabine | [456] |
| Treatment | Modes | Methods | miRNA | Types of Studies In Vivo/In Vitro | Ref |
|---|---|---|---|---|---|
| miRNA inhibition therapy | AMOs | 2-OMe | miR-451 | In vitro (MCF-7, SKBR3) In vivo (BALB/c) | [443] |
| miR-21 | In vitro (MCF-7 and HeLa) | [465] | |||
| Gene editing | Crispr-cas9 | miR-23b, miR-27b | In vitro (MCF7) In vivo (nude) | [314] | |
| miRNA replacement therapy | Virus | Lentivirus | miR-126, miR-130a | In vitro (MDA-MB-231) In vivo (PyMT, C57Bl/6) | [466] |
| Adeno-associated virus | miR-1d | In vitro (HEK293T and HeLa) in vivo (PyMT) | [467] | ||
| Retroviral | miR-21 | In vitro (SKBR3, MCF7, Jurkat, HEK-293T) In vivo (nude) | [468] | ||
| Nanoparticle | Gold (PLL) | mir-708 | In vitro (MDA-MB-231, HEK-293, 4T1) In vivo (CB-17 SCID and CB17.Cg-PrkdcscidHrhr/IcrCrl) | [469] | |
| Lipid | miR-203 | In vitro (MDA-MB-231 and Hs578t cells) | [470] | ||
| miR-203 | In vitro (MDA-MB 231) In vivo (BALB/c) | [471] | |||
| Polymer | Creatine | mir-34a | In vitro (4T1.2 and MDA-MB-231) In vivo (BALB/c) | [472] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, J.S.; Cheah, Y.K. Potential miRNAs for miRNA-Based Therapeutics in Breast Cancer. Non-Coding RNA 2020, 6, 29. https://doi.org/10.3390/ncrna6030029
Wong JS, Cheah YK. Potential miRNAs for miRNA-Based Therapeutics in Breast Cancer. Non-Coding RNA. 2020; 6(3):29. https://doi.org/10.3390/ncrna6030029
Chicago/Turabian StyleWong, Jun Sheng, and Yoke Kqueen Cheah. 2020. "Potential miRNAs for miRNA-Based Therapeutics in Breast Cancer" Non-Coding RNA 6, no. 3: 29. https://doi.org/10.3390/ncrna6030029
APA StyleWong, J. S., & Cheah, Y. K. (2020). Potential miRNAs for miRNA-Based Therapeutics in Breast Cancer. Non-Coding RNA, 6(3), 29. https://doi.org/10.3390/ncrna6030029





