Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development
Abstract
1. Introduction
2. Results
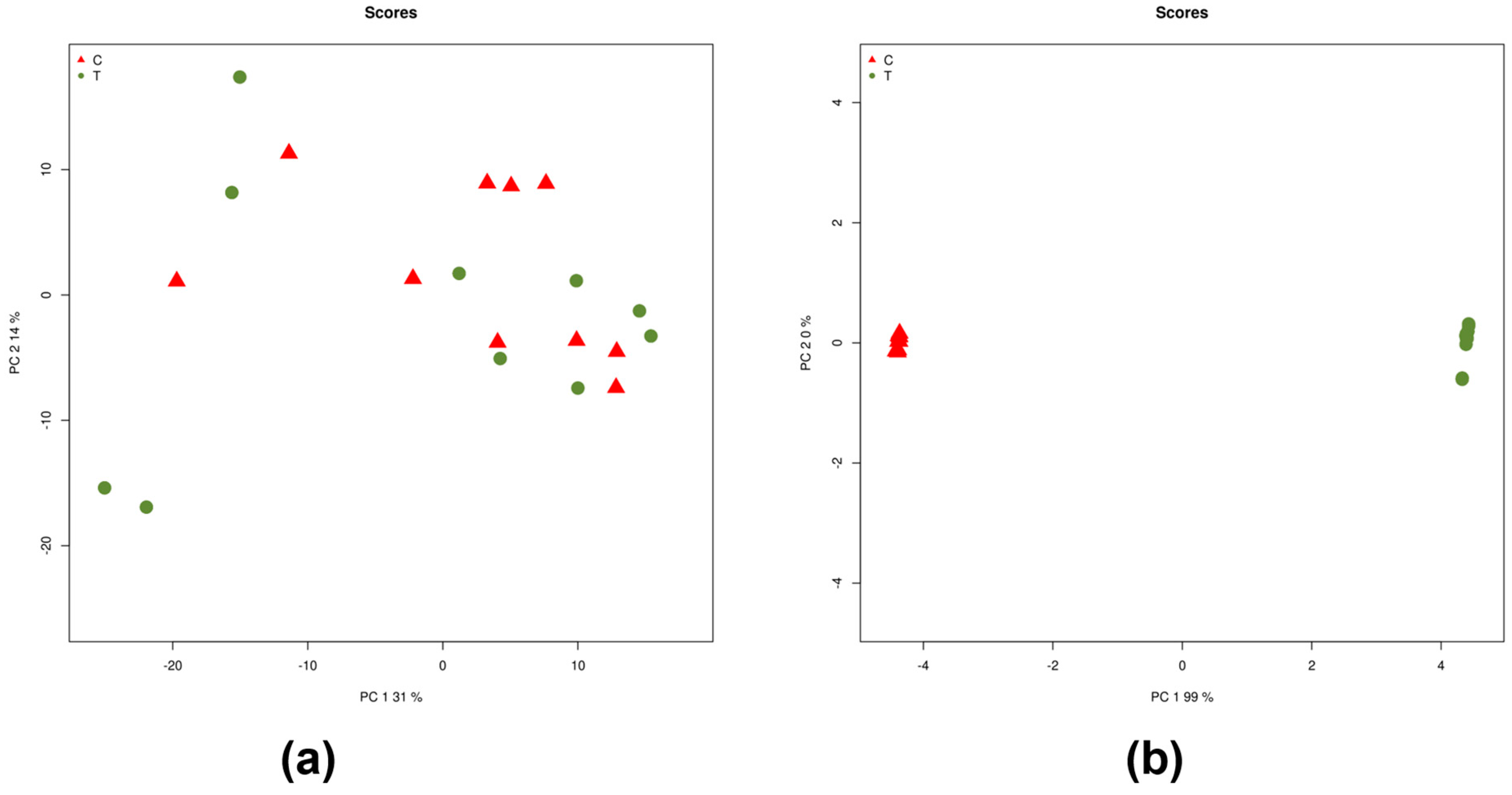
2.1. Differentially Expressed miRNAs
2.2. MicroRNA Gene Targets
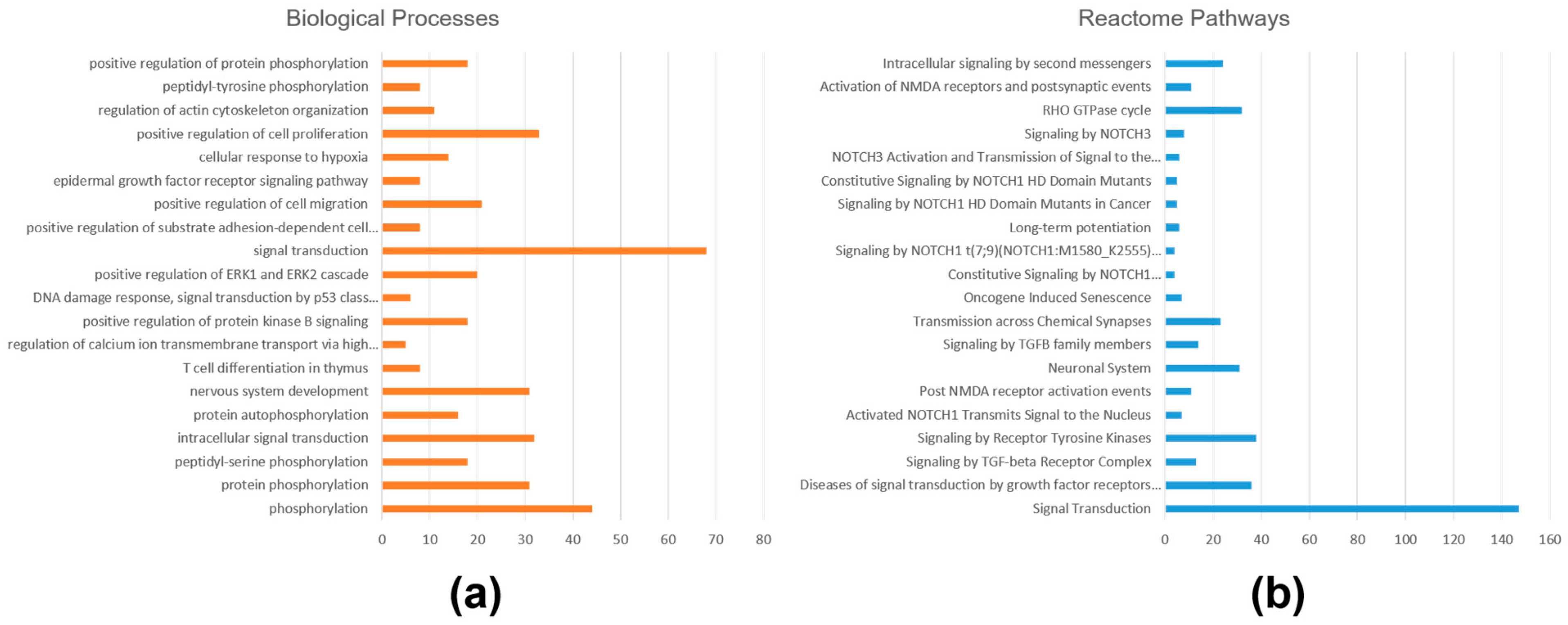
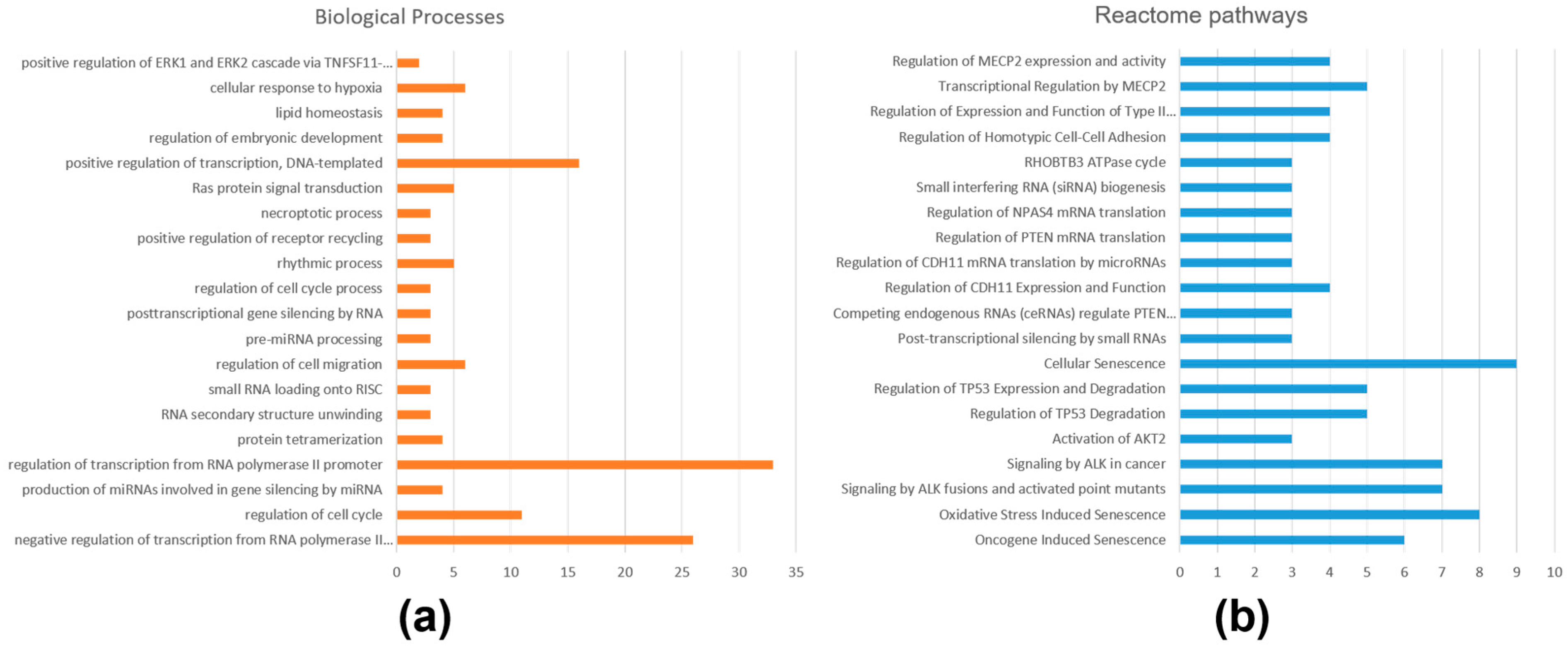
2.3. Functional Annotations and Pathway Analysis
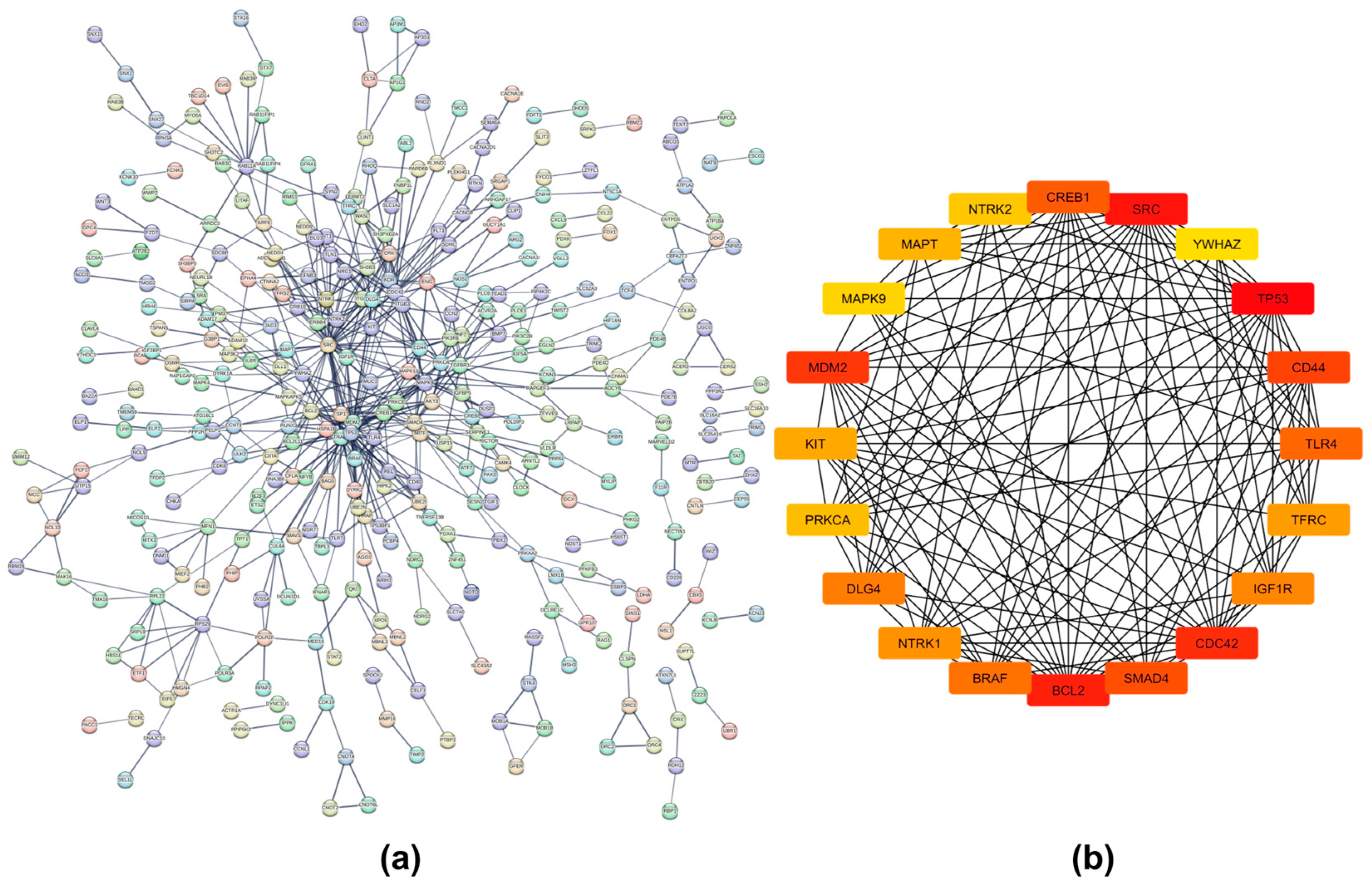
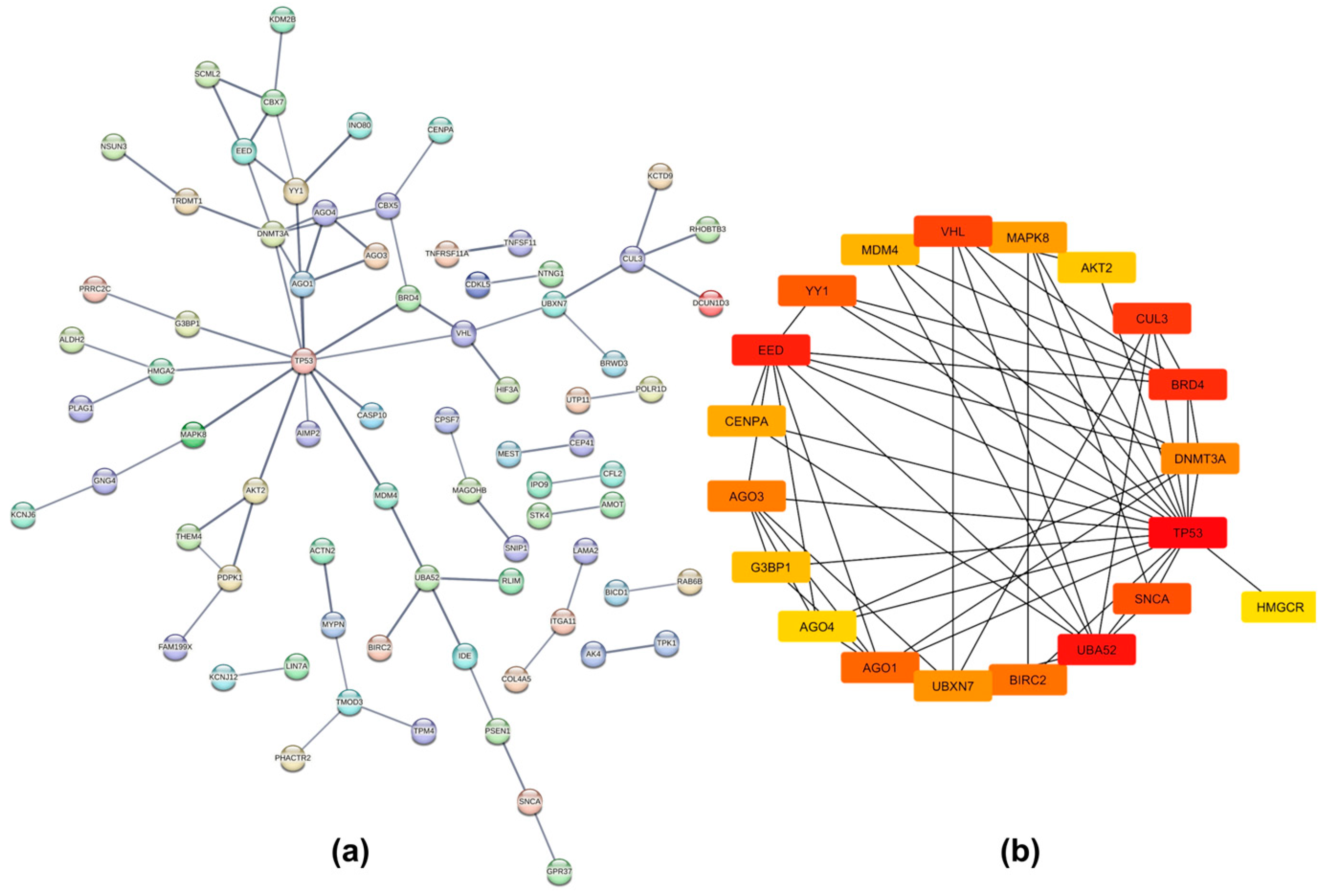
2.4. Protein–Protein Interaction Networks
2.5. MicroRNA Expression Confirmation by Quantitative RT-PCR
3. Discussion
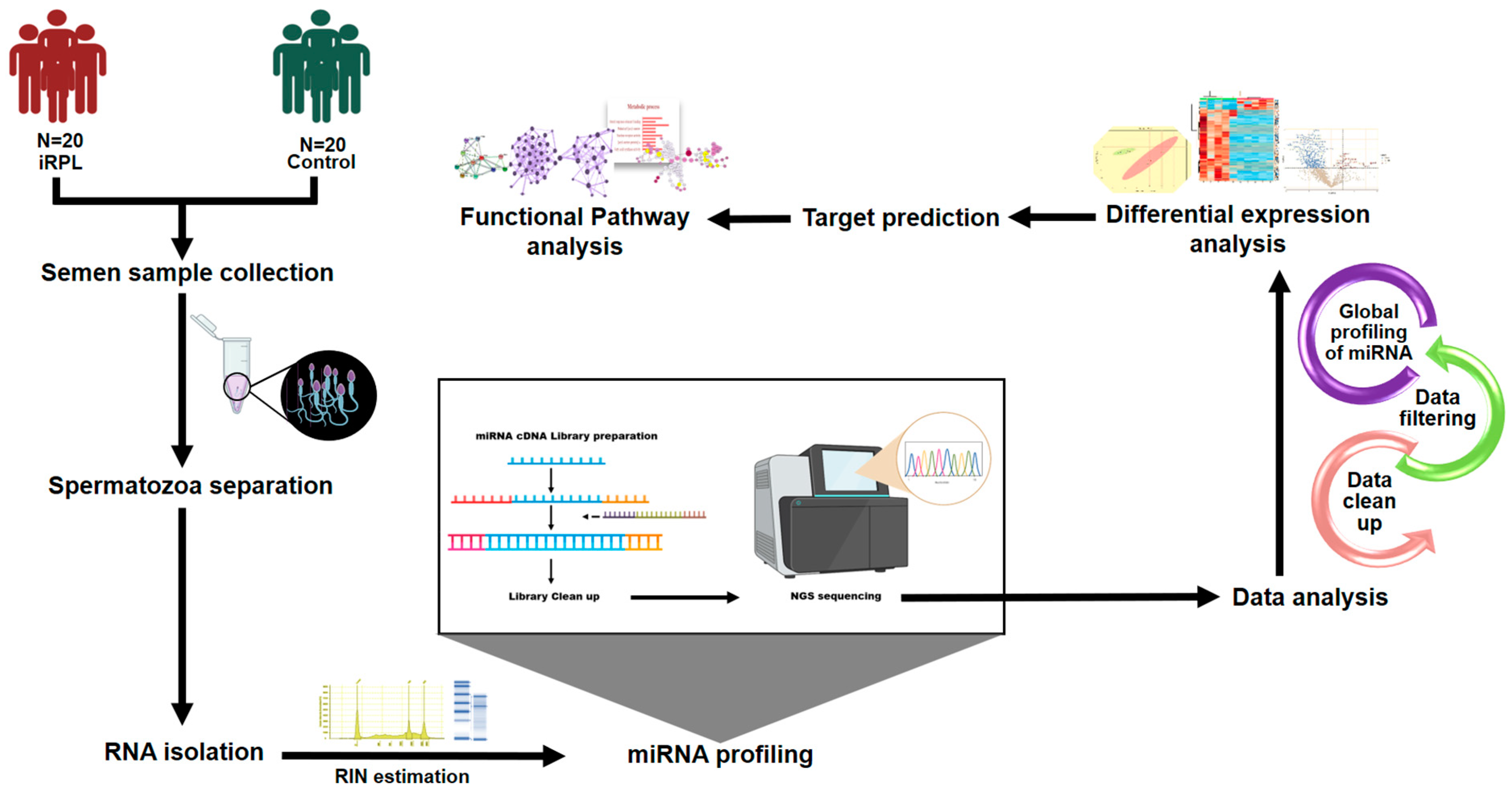
4. Materials and Methods
4.1. Recruitment of Study Participants
- Male partners of confirmed iRPL women with a history of 2 or more spontaneous abortions in the clinical first trimester
- Age < 45 years
- Normal semen profile
- No history of autoimmune or endocrine disorder
- Clinical workup should have ruled out maternal RPL etiologies, including genetic, anatomic, immunological factors, inherited thrombophilia, infections, environmental and lifestyle factors, birth defects in families, heart disease, diabetes, etc.
- Age-matched volunteers who have fathered a healthy child within the last 2 years.
- Normal semen profile
- No history of RPL in female partner
- Participants who did not meet the inclusion criteria
- Male partners of iRPL patients who had abnormal karyotypes, endocrine problems, immunological disorders, or any other significant illnesses.
- Semen samples that exhibited abnormal semen parameters or a substantial presence of round cells
4.2. Semen Collection and Analysis
4.3. Separation of Spermatozoa
4.4. RNA Extraction
4.5. MicroRNA Library Preparation and Sequencing
4.6. Expression Estimation of Sperm miRNAs
4.7. MicroRNA Target Prediction
4.8. Bioinformatics Analysis
4.9. MicroRNA Expression Analysis by Quantitative RT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeve, Y.B.; Davies, W. Evidence-based management of recurrent miscarriages. J. Hum. Reprod. Sci. 2014, 7, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Regan, L. Recurrent miscarriage. Lancet 2006, 368, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.W.; Brambati, B.; Eskes, T.K.; Exalto, N.; Fox, H.; Geraedts, J.P.; Gerhard, I.; Gonzales Gomes, F.; Grudzinskas, J.G.; Hustin, J. The Euro-Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Hum. Reprod. 1995, 10, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Ford, H.B.; Schust, D.J. Recurrent pregnancy loss: Etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2009, 2, 76–83. [Google Scholar] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Giorlandino, C.; Calugi, G.; Iaconianni, L.; Santoro, M.L.; Lippa, A. Spermatozoa with chromosomal abnormalities may result in a higher rate of recurrent abortion. Fertil. Steril. 1998, 70, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Zidi-Jrah, I.; Hajlaoui, A.; Mougou-Zerelli, S.; Kammoun, M.; Meniaoui, I.; Sallem, A.; Brahem, S.; Fekih, M.; Bibi, M.; Saad, A.; et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil. Steril. 2016, 105, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Kiani-Esfahani, A.; Bahrami, S.; Tavalaee, M.; Deemeh, M.R.; Mahjour, A.A.; Nasr-Esfahani, M.H. Cytosolic and mitochondrial ROS: Which one is associated with poor chromatin remodeling? Syst. Biol. Reprod. Med. 2013, 59, 352–359. [Google Scholar] [CrossRef]
- Kumar, K.; Deka, D.; Singh, A.; Mitra, D.K.; Vanitha, B.R.; Dada, R. Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J. Assist. Reprod. Genet. 2012, 29, 861–867. [Google Scholar] [CrossRef]
- Venkatesh, S.; Kumar, R.; Deka, D.; Deecaraman, M.; Dada, R. Analysis of sperm nuclear protein gene polymorphisms and DNA integrity in infertile men. Syst. Biol. Reprod. Med. 2011, 57, 124–132. [Google Scholar] [CrossRef]
- Bronet, F.; Martinez, E.; Gaytan, M.; Linan, A.; Cernuda, D.; Ariza, M.; Nogales, M.; Pacheco, A.; San Celestino, M.; Garcia-Velasco, J.A. Sperm DNA fragmentation index does not correlate with the sperm or embryo aneuploidy rate in recurrent miscarriage or implantation failure patients. Hum. Reprod. 2012, 27, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Bellver, J.; Meseguer, M.; Muriel, L.; Garcia-Herrero, S.; Barreto, M.A.; Garda, A.L.; Remohi, J.; Pellicer, A.; Garrido, N. Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Hum. Reprod. 2010, 25, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, G.; Jena, S.R.; Nayak, J.; Kar, S.; Samanta, L. Proteomic Signatures in Spermatozoa Reveal the Role of Paternal Factors in Recurrent Pregnancy Loss. World J. Men’s Health 2020, 38, 103–114. [Google Scholar] [CrossRef]
- Naglot, S.; Tomar, A.K.; Singh, N.; Yadav, S. Label-free proteomics of spermatozoa identifies candidate protein markers of idiopathic recurrent pregnancy loss. Reprod. Biol. 2021, 21, 100539. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, A.; Tomar, A.K.; Chandra, K.B.; Naglot, S.; Dhiman, S.; Singh, N.; Sharma, J.B.; Yadav, S. Differential Sperm Proteomics Reveals the Significance of Fatty Acid Synthase and Clusterin in Idiopathic Recurrent Pregnancy Loss. Reprod. Sci. 2023, 30, 3456–3468. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, G.C.; Miller, D.; Huntriss, J.D.; Diamond, M.P.; Krawetz, S.A. Reproductive biology: Delivering spermatozoan RNA to the oocyte. Nature 2004, 429, 154. [Google Scholar] [CrossRef] [PubMed]
- Champroux, A.; Cocquet, J.; Henry-Berger, J.; Drevet, J.R.; Kocer, A. A Decade of Exploring the Mammalian Sperm Epigenome: Paternal Epigenetic and Transgenerational Inheritance. Front. Cell Dev. Biol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Patronia, M.-M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Vrachnis, D.; Moustakli, E.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 3361. [Google Scholar] [CrossRef]
- Alipour, M.; Abtin, M.; Hosseinzadeh, A.; Maleki, M. Association between miR-146a C>G, miR-149 T>C, miR-196a2 T>C, and miR-499 A>G polymorphisms and susceptibility to idiopathic recurrent pregnancy loss. J. Assist. Reprod. Genet. 2019, 36, 2237–2244. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Li, M.Q.; Xu, J.; Zhang, J.P.; Jin, L.P. MicroRNA-184 promotes apoptosis of trophoblast cells via targeting WIG1 and induces early spontaneous abortion. Cell Death Dis. 2019, 10, 223. [Google Scholar] [CrossRef]
- Dong, X.; Yang, L.; Wang, H. miR-520 promotes DNA-damage-induced trophoblast cell apoptosis by targeting PARP1 in recurrent spontaneous abortion (RSA). Gynecol. Endocrinol. 2017, 33, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gu, W.W.; Gu, Y.; Yan, N.N.; Mao, Y.Y.; Zhen, X.X.; Wang, J.M.; Yang, J.; Shi, H.J.; Zhang, X.; et al. Association of the peripheral blood levels of circulating microRNAs with both recurrent miscarriage and the outcomes of embryo transfer in an in vitro fertilization process. J. Transl. Med. 2018, 16, 186. [Google Scholar] [CrossRef]
- Wang, J.M.; Gu, Y.; Zhang, Y.; Yang, Q.; Zhang, X.; Yin, L.; Wang, J. Deep-sequencing identification of differentially expressed miRNAs in decidua and villus of recurrent miscarriage patients. Arch. Gynecol. Obstet. 2016, 293, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.; Amati, F.; Ticconi, C.; Riccio, S.; Vancheri, C.; Rizzacasa, B.; Splendiani, E.; Ferretti, E.; Ernerudh, J.; Piccione, E.; et al. Low molecular weight heparin -induced miRNA changes in peripheral blood mononuclear cells in pregnancies with unexplained recurrent pregnancy loss. J. Reprod. Immunol. 2022, 151, 103502. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Shaman, J.A.; Ward, W.S. Non-genetic contributions of the sperm nucleus to embryonic development. Asian J. Androl. 2011, 13, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Paradowska, A.; Steger, K. Analysing the sperm epigenome: Roles in early embryogenesis and assisted reproduction. Nat. Rev. Urol. 2012, 9, 609–619. [Google Scholar] [CrossRef]
- Davies, R.; Jayasena, C.N.; Rai, R.; Minhas, S. The Role of Seminal Oxidative Stress in Recurrent Pregnancy Loss. Antioxidants 2023, 12, 723. [Google Scholar] [CrossRef]
- Jena, S.R.; Nayak, J.; Kumar, S.; Kar, S.; Dixit, A.; Samanta, L. Paternal contributors in recurrent pregnancy loss: Cues from comparative proteome profiling of seminal extracellular vesicles. Mol. Reprod. Dev. 2021, 88, 96–112. [Google Scholar] [CrossRef]
- Imam, S.N.; Shamsi, M.B.; Kumar, K.; Deka, D.; Dada, R. Idiopathic recurrent pregnancy loss: Role of paternal factors; a pilot study. J. Reprod. Infertil. 2011, 12, 267–276. [Google Scholar]
- du Fosse, N.; van der Hoorn, M.L.; Eikmans, M.; Heidt, S.; le Cessie, S.; Mulders, A.; van Lith, J.; Lashley, E. Evaluating the role of paternal factors in aetiology and prognosis of recurrent pregnancy loss: Study protocol for a hospital-based multicentre case-control study and cohort study (REMI III project). BMJ Open 2019, 9, e033095. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, Y.; Wang, Y.; Wang, J.; An, F.; Sun, X.; Yu, Z. Quantitative proteomic analysis of sperm in unexplained recurrent pregnancy loss. Reprod. Biol. Endocrinol. RBE 2019, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Ostermeier, G.C. Towards a better understanding of RNA carriage by ejaculate spermatozoa. Hum. Reprod. Update 2006, 12, 757–767. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.Z.; Liu, Y.; Wang, H.J.; Pang, W.W.; Zhang, J.J. Downregulated MALAT1 relates to recurrent pregnancy loss via sponging miRNAs. Kaohsiung J. Med. Sci. 2018, 34, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Schmitt, J.; Leidinger, P.; Keller, A.; Meese, E.; Backes, C. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil. Steril. 2013, 99, 1249–1255.e16. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Backes, C.; Fischer, U.; Leidinger, P.; Lubbad, A.M.; Keller, A.; Meese, E. Panel of five microRNAs as potential biomarkers for the diagnosis and assessment of male infertility. Fertil. Steril. 2014, 102, 989–997.e1. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, C.; Chen, X.; Yao, B.; Yang, C.; Zhu, C.; Li, L.; Wang, J.; Li, X.; Shao, Y.; et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin. Chem. 2011, 57, 1722–1731. [Google Scholar] [CrossRef]
- Wu, C.; Blondin, P.; Vigneault, C.; Labrecque, R.; Sirard, M.A. Sperm miRNAs-potential mediators of bull age and early embryo development. BMC Genom. 2020, 21, 798. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; He, Y.; Lan, J.; Luo, S.; Sun, B.; Xiao, C.; Yu, W.; Zeng, Z.; Lei, S. FOXP3 promote the progression of glioblastoma via inhibiting ferroptosis mediated by linc00857/miR-1290/GPX4 axis. Cell Death Dis. 2024, 15, 239. [Google Scholar] [CrossRef]
- Hekim, N.; Gunes, S.; Ergun, S.; Barhan, E.N.; Asci, R. Investigation of sperm hsa-mir-145-5p and MLH1 expressions, seminal oxidative stress and sperm DNA fragmentation in varicocele. Mol. Biol. Rep. 2024, 51, 588. [Google Scholar] [CrossRef]
- Zhao, M.J.; Zhang, Y.N.; Zhao, Y.P.; Chen, X.B.; Han, B.S.; Ding, N.; Gu, Y.Q.; Wang, S.S.; Ma, J.; Liu, M.L. Altered microRNA expression profiles of human spermatozoa in normal fertile men of different ages. Asian J. Androl. 2023, 25, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bao, J.; Kim, M.; Yuan, S.; Tang, C.; Zheng, H.; Mastick, G.S.; Xu, C.; Yan, W. Two miRNA clusters, miR-34b/c and miR-449, are essential for normal brain development, motile ciliogenesis, and spermatogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2851–E2857. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, H.; Fang, S.; Xu, C. MiR-449c inhibits gastric carcinoma growth. Life Sci. 2015, 137, 14–19. [Google Scholar] [CrossRef]
- Sandbothe, M.; Buurman, R.; Reich, N.; Greiwe, L.; Vajen, B.; Gurlevik, E.; Schaffer, V.; Eilers, M.; Kuhnel, F.; Vaquero, A.; et al. The microRNA-449 family inhibits TGF-beta-mediated liver cancer cell migration by targeting SOX4. J. Hepatol. 2017, 66, 1012–1021. [Google Scholar] [CrossRef]
- Wang, X.; Guo, S.; Zhou, X.; Wang, Y.; Zhang, T.; Chen, R. Exploring the Molecular Mechanism of lncRNA-miRNA-mRNA Networks in Non-Syndromic Cleft Lip with or without Cleft Palate. Int. J. Gen. Med. 2021, 14, 9931–9943. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, Z.H.; Jiang, H.; Wang, Y.H.; Yang, J.Y.; Li, L.M.; Wang, W.T.; Chen, J.; Li, D.H.; Long, S.Y.; et al. Prevalence and plasma exosome-derive microRNA diagnostic biomarker screening of adolescent idiopathic scoliosis in Yunnan Province, China. Front. Pediatr. 2024, 12, 1308931. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum. Dev. 2015, 91, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M. Calcium signalling in early embryos. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 1401–1418. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fu, S.; Lin, X.; Zheng, J.; Pu, J.; Gu, Y.; Deng, W.; Liu, Y.; He, Z.; Liang, W.; et al. miR-92b-3p Functions As A Key Gene In Esophageal Squamous Cell Cancer As Determined By Co-Expression Analysis. OncoTargets Ther. 2019, 12, 8339–8353. [Google Scholar] [CrossRef]
- Du, Y.; Miao, Z.; Wang, K.; Lv, Y.; Qiu, L.; Guo, L. Expression levels and clinical values of miR-92b-3p in breast cancer. World J. Surg. Oncol. 2021, 19, 239. [Google Scholar] [CrossRef]
- Long, M.; Zhan, M.; Xu, S.; Yang, R.; Chen, W.; Zhang, S.; Shi, Y.; He, Q.; Mohan, M.; Liu, Q.; et al. miR-92b-3p acts as a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol. Cancer 2017, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, Z.; Gu, X.; Feng, L.; Xu, M.; Zhang, X. miR-92b-3p Regulates Cell Cycle and Apoptosis by Targeting CDKN1C, Thereby Affecting the Sensitivity of Colorectal Cancer Cells to Chemotherapeutic Drugs. Cancers 2021, 13, 3323. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, L.Y.; Yang, R.H.; Zhou, Y.; Zhou, P.; Kong, L. Identification of reciprocal microRNA-mRNA pairs associated with metastatic potential disparities in human prostate cancer cells and signaling pathway analysis. J. Cell. Biochem. 2019, 120, 17779–17790. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Duan, S.; Zhao, L.; Jiang, J. Hsa-miR-92b-3p Targeting FHL2 to Enhance Radiosensitivity of Nasopharyngeal Carcinoma. Biochem. Genet. 2024. [Google Scholar] [CrossRef] [PubMed]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorram Khorshid, H.R. MicroRNA profiling in spermatozoa of men with unexplained asthenozoospermia. Andrologia 2019, 51, e13284. [Google Scholar] [CrossRef] [PubMed]
- von Grothusen, C.; Frisendahl, C.; Modhukur, V.; Lalitkumar, P.G.; Peters, M.; Faridani, O.R.; Salumets, A.; Boggavarapu, N.R.; Gemzell-Danielsson, K. Uterine fluid microRNAs are dysregulated in women with recurrent implantation failure. Hum. Reprod. 2022, 37, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Polytarchou, C.; Hatziapostolou, M.; Yau, T.O.; Christodoulou, N.; Hinds, P.W.; Kottakis, F.; Sanidas, I.; Tsichlis, P.N. Akt3 induces oxidative stress and DNA damage by activating the NADPH oxidase via phosphorylation of p47(phox). Proc. Natl. Acad. Sci. USA 2020, 117, 28806–28815. [Google Scholar] [CrossRef]
- Nonoguchi, K.; Tokuchi, H.; Okuno, H.; Watanabe, H.; Egawa, H.; Saito, K.; Ogawa, O.; Fujita, J. Expression of Apg-1, a member of the Hsp110 family, in the human testis and sperm. Int. J. Urol. 2001, 8, 308–314. [Google Scholar] [CrossRef]
- Son, W.Y.; Han, C.T.; Hwang, S.H.; Lee, J.H.; Kim, S.; Kim, Y.C. Repression of hspA2 messenger RNA in human testes with abnormal spermatogenesis. Fertil. Steril. 2000, 73, 1138–1144. [Google Scholar] [CrossRef]
- Erata, G.O.; Kocak Toker, N.; Durlanik, O.; Kadioglu, A.; Aktan, G.; Aykac Toker, G. The role of heat shock protein 70 (Hsp 70) in male infertility: Is it a line of defense against sperm DNA fragmentation? Fertil. Steril. 2008, 90, 322–327. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Z.; Huang, X.; Xu, M.; Lu, L.; Xu, Z.; Li, J.; Sha, J. Expression of a novel DnaJA1 alternative splicing in human testis and sperm. Int. J. Androl. 2004, 27, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, D.F.; Kai, H.; Zhao, L.M.; Mao, J.M.; Zhuang, X.J.; Ma, L.L.; Hui, J. miRNA-mediated regulation of heat shock proteins in human ejaculated spermatozoa. Turk. J. Med. Sci. 2015, 45, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Yin, T.; He, F.; Ding, J.; Shang, Y.; Yang, J. CaMK4 promotes abortion-related Th17 cell imbalance by activating AKT/mTOR signaling pathway. Am. J. Reprod. Immunol. 2020, 84, e13315. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.D.; Urriola-Munoz, P.; Lagos-Cabre, R. The emerging role of matrix metalloproteases of the ADAM family in male germ cell apoptosis. Spermatogenesis 2011, 1, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, T.A.; Yu, J.; Feng, W.; Zhang, Y.; Zhou, H.; Paulo, J.A.; Gygi, S.P.; Moazed, D. Genomic context- and H2AK119 ubiquitination-dependent inheritance of human Polycomb silencing. Sci. Adv. 2024, 10, eadl4529. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Mruk, D.D.; Cheng, C.Y. Mitogen-activated protein kinases in male reproductive function. Trends Mol. Med. 2009, 15, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, W.; Ji, G.X.; Gu, A.H.; Qu, J.H.; Song, L.; Wang, X.R. Genetic variants in TP53 and MDM2 associated with male infertility in Chinese population. Asian J. Androl. 2012, 14, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yamamoto, T.; Abe, S.I. IGF-I, IGF-II and insulin promote differentiation of spermatogonia to primary spermatocytes in organ culture of newt testes. Int. J. Dev. Biol. 1999, 43, 343–347. [Google Scholar]
- Raimondo, S.; Gentile, T.; Gentile, M.; Morelli, A.; Donnarumma, F.; Cuomo, F.; De Filippo, S.; Montano, L. p53 Protein Evaluation on Spermatozoa DNA in Fertile and Infertile Males. J. Hum. Reprod. Sci. 2019, 12, 114–121. [Google Scholar] [CrossRef]
- Selvaraju, S.; Parthipan, S.; Somashekar, L.; Kolte, A.P.; Krishnan Binsila, B.; Arangasamy, A.; Ravindra, J.P. Occurrence and functional significance of the transcriptome in bovine (Bos taurus) spermatozoa. Sci. Rep. 2017, 7, 42392. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Tyrosine protein kinases and spermatogenesis: Truncation matters. Mol. Reprod. Dev. 2006, 73, 399–403. [Google Scholar] [CrossRef]
- Xu, W.M.; Chen, J.; Chen, H.; Diao, R.Y.; Fok, K.L.; Dong, J.D.; Sun, T.T.; Chen, W.Y.; Yu, M.K.; Zhang, X.H.; et al. Defective CFTR-dependent CREB activation results in impaired spermatogenesis and azoospermia. PLoS ONE 2011, 6, e19120. [Google Scholar] [CrossRef]
- Bains, R.; Adeghe, J.; Carson, R.J. Human sperm cells express CD44. Fertil. Steril. 2002, 78, 307–312. [Google Scholar] [CrossRef]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef]
- Angeles-Floriano, T.; Roa-Espitia, A.L.; Baltierrez-Hoyos, R.; Cordero-Martinez, J.; Elizondo, G.; Hernandez-Gonzalez, E.O. Absence of aryl hydrocarbon receptor alters CDC42 expression and prevents actin polymerization during capacitation. Mol. Reprod. Dev. 2016, 83, 1015–1026. [Google Scholar] [CrossRef]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef]
- Chhikara, N.; Tomar, A.K.; Datta, S.K.; Yadav, S. Proteomic changes in human spermatozoa during in vitro capacitation and acrosome reaction in normozoospermia and asthenozoospermia. Andrology 2023, 11, 73–85. [Google Scholar] [CrossRef]
- Friedlander, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Sticht, C.; De La Torre, C.; Parveen, A.; Gretz, N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE 2018, 13, e0206239. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, Q.; Qu, W.; Xu, Z.; Liu, X.; Li, X.; Li, S.; Ma, W.; Miao, Y.; Zhang, L.; et al. sRNAPrimerDB: Comprehensive primer design and search web service for small non-coding RNAs. Bioinformatics 2019, 35, 1566–1572. [Google Scholar] [CrossRef]

| High-Throughput Sequencing | Quantitative RT-PCR | ||||
|---|---|---|---|---|---|
| miRNA | Fold Change (FC) | Log2 (FC) | Probability (1-FDR) | FC | p-Value |
| Upregulated in iRPL | |||||
| hsa-miR-4454 | 2.888 | 1.530 | 0.997 | 6.487 | 0.0196 |
| hsa-miR-142-3p | 2.505 | 1.325 | 0.998 | 69.824 | 0.0538 |
| hsa-miR-145-5p | 2.501 | 1.322 | 0.995 | 14.070 | 0.0213 |
| hsa-miR-1290 | 2.377 | 1.249 | 0.999 | 20.551 | 0.0405 |
| hsa-miR-1246 | 2.371 | 1.245 | 1.000 | 109.297 | 0.0183 |
| hsa-miR-7977 | 2.319 | 1.213 | 0.999 | 8.282 | 0.0525 |
| hsa-miR-449c-5p | 2.153 | 1.106 | 0.981 | 13.951 | 0.0389 |
| hsa-miR-92b-3p | 2.074 | 1.053 | 0.994 | 17.185 | 0.0310 |
| Downregulated in iRPL | |||||
| hsa-miR-29c-3p | 0.495 | −1.015 | 1.000 | 0.056 | 0.1755 |
| hsa-miR-30b-5p | 0.489 | −1.031 | 1.000 | 0.971 | 0.4853 |
| hsa-miR-519a-2-5p | 0.485 | −1.043 | 0.981 | 0.114 | 0.0476 |
| hsa-miR-520b-5p | 0.485 | −1.043 | 0.981 | 0.374 | 0.2469 |
| High-Throughput Sequencing | Quantitative RT-PCR | |||
|---|---|---|---|---|
| Features | iRPL (n = 20) * | Controls (n = 20) * | iRPL (n = 10) * | Controls (n = 10) * |
| Age of donors (years) | 29.05 ± 3.2 | 32.0 ± 1.6 | 30.2 ± 3.7 | 30.6 ± 3 |
| Number of abortions in female partners | 4.15 ± 1.1 | - | 3.8 ± 2.0 | - |
| Sample volume (mL) | 2.72 ± 0.8 | 3.2 ± 0.9 | 3.1 ± 1.2 | 3.5 ± 1.3 |
| Sperm Count (million/mL) | 76.40 ± 44.5 | 117.30 ± 65.2 | 96.1 ± 25.1 | 113.6 ± 59.5 |
| Sperm Motility (%) | 71 ± 9.5 | 69.5 ± 8.4 | 63 ± 8.8 | 69.5 ± 7.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapliyal, A.; Tomar, A.K.; Naglot, S.; Dhiman, S.; Datta, S.K.; Sharma, J.B.; Singh, N.; Yadav, S. Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development. Non-Coding RNA 2024, 10, 41. https://doi.org/10.3390/ncrna10040041
Thapliyal A, Tomar AK, Naglot S, Dhiman S, Datta SK, Sharma JB, Singh N, Yadav S. Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development. Non-Coding RNA. 2024; 10(4):41. https://doi.org/10.3390/ncrna10040041
Chicago/Turabian StyleThapliyal, Ayushi, Anil Kumar Tomar, Sarla Naglot, Soniya Dhiman, Sudip Kumar Datta, Jai Bhagwan Sharma, Neeta Singh, and Savita Yadav. 2024. "Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development" Non-Coding RNA 10, no. 4: 41. https://doi.org/10.3390/ncrna10040041
APA StyleThapliyal, A., Tomar, A. K., Naglot, S., Dhiman, S., Datta, S. K., Sharma, J. B., Singh, N., & Yadav, S. (2024). Exploring Differentially Expressed Sperm miRNAs in Idiopathic Recurrent Pregnancy Loss and Their Association with Early Embryonic Development. Non-Coding RNA, 10(4), 41. https://doi.org/10.3390/ncrna10040041






