Soot and Flame Structures in Turbulent Partially Premixed Jet Flames of Pre-Evaporated Diesel Surrogates with Admixture of OMEn
Abstract
1. Introduction
2. Experimental Setup
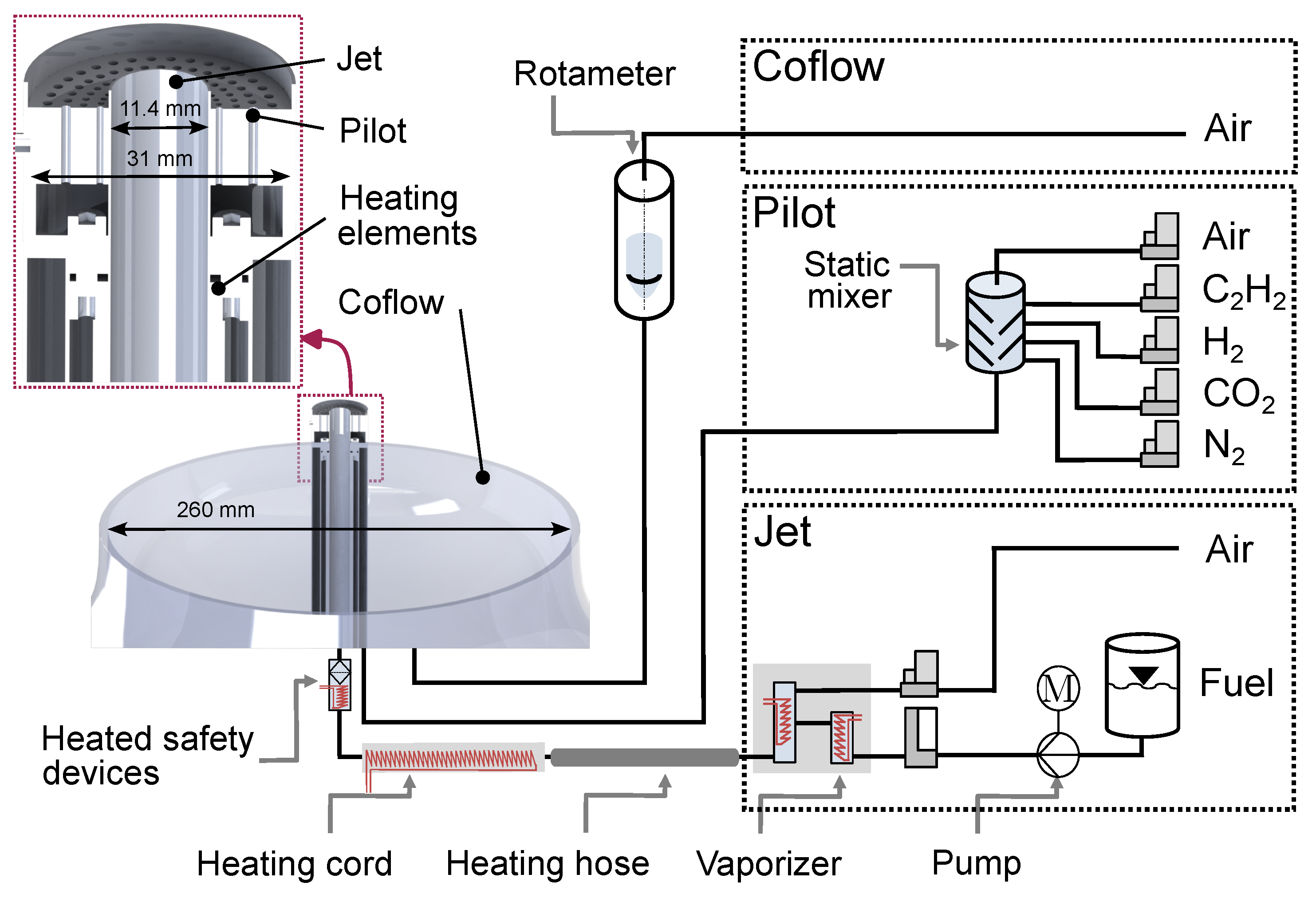
2.1. Temperature-Controlled Jet Burner
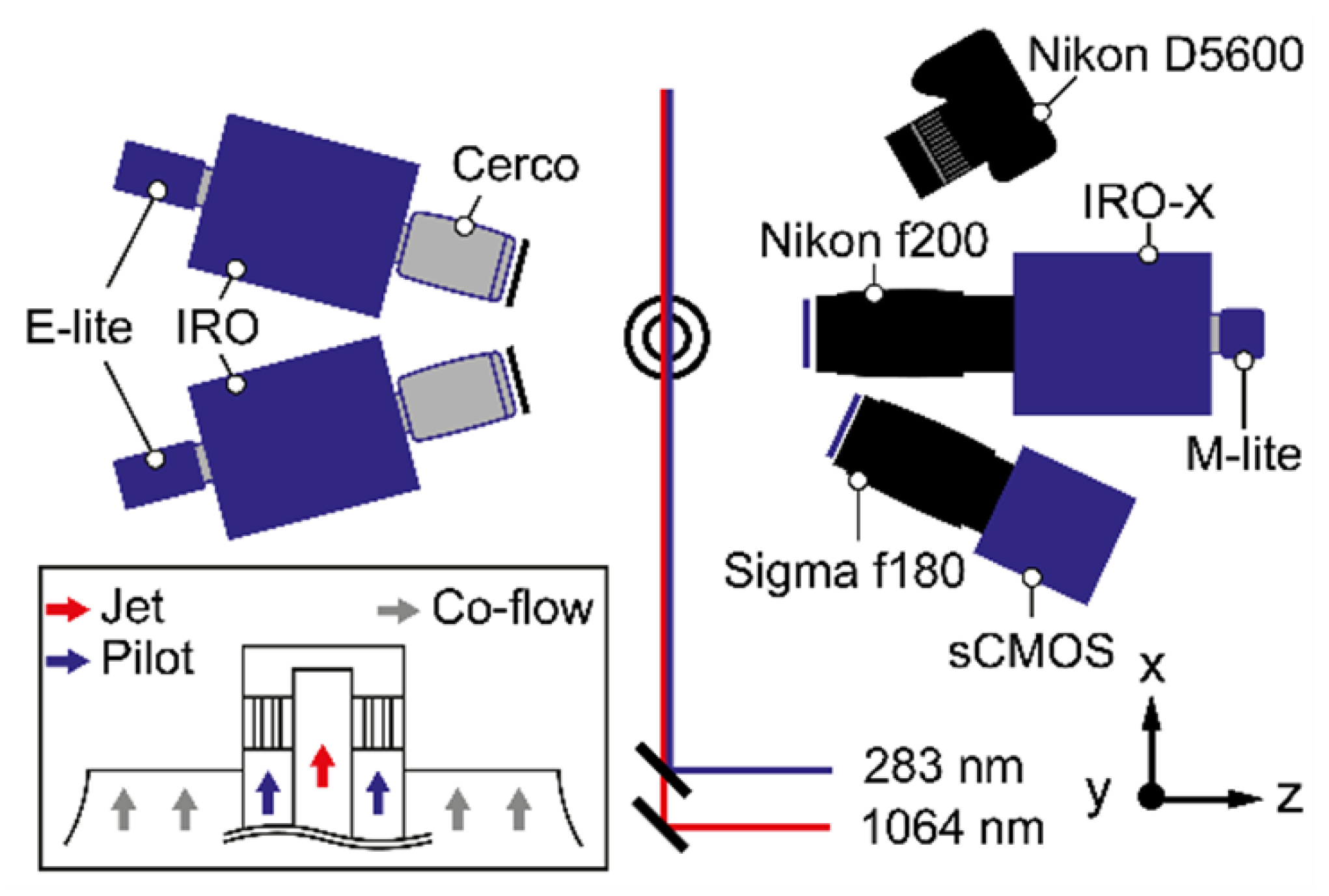
2.2. Simultaneous OH-LIF, PAH-LIF and LII Measurements
2.3. Chemiluminescence Imaging
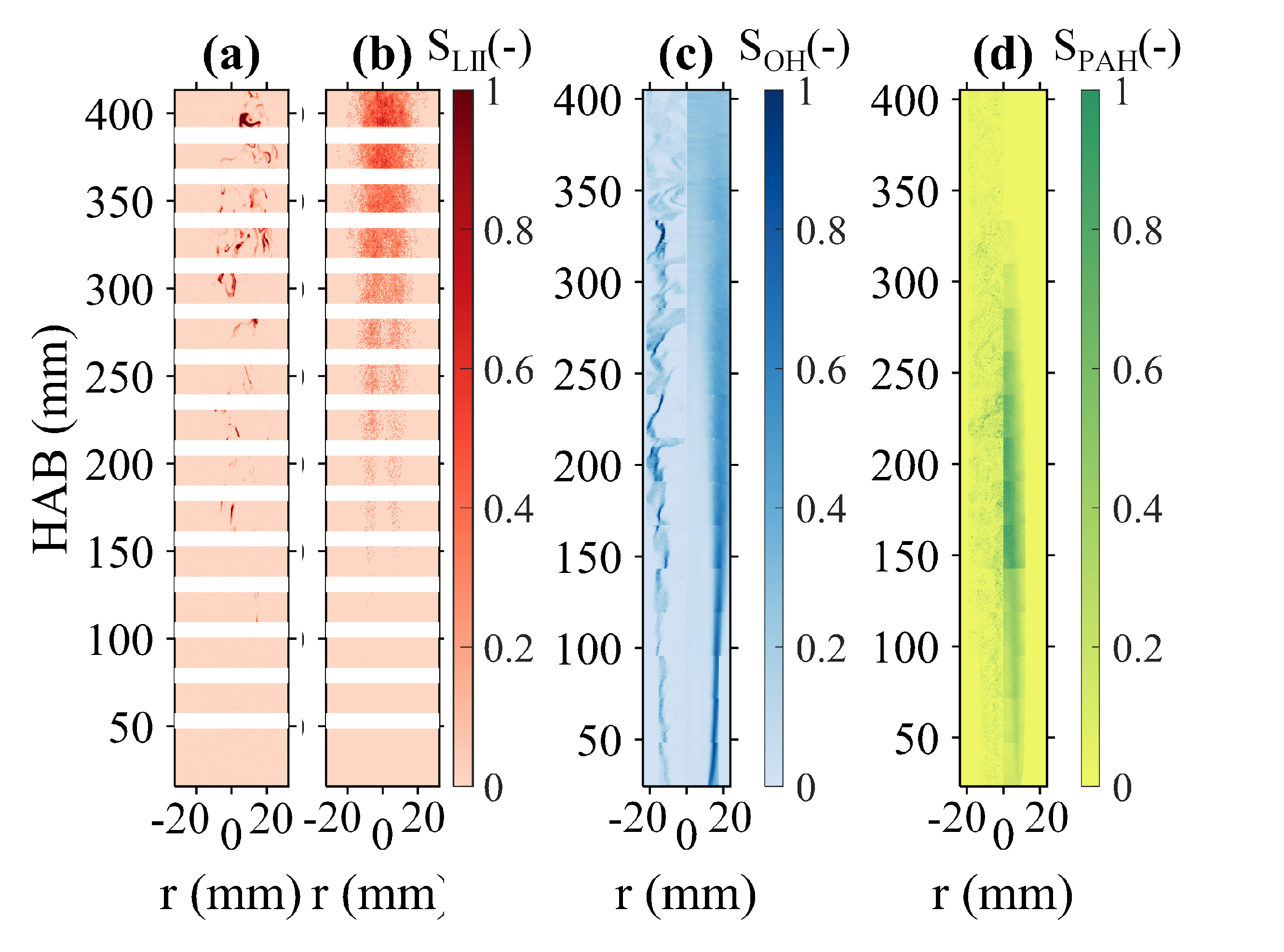
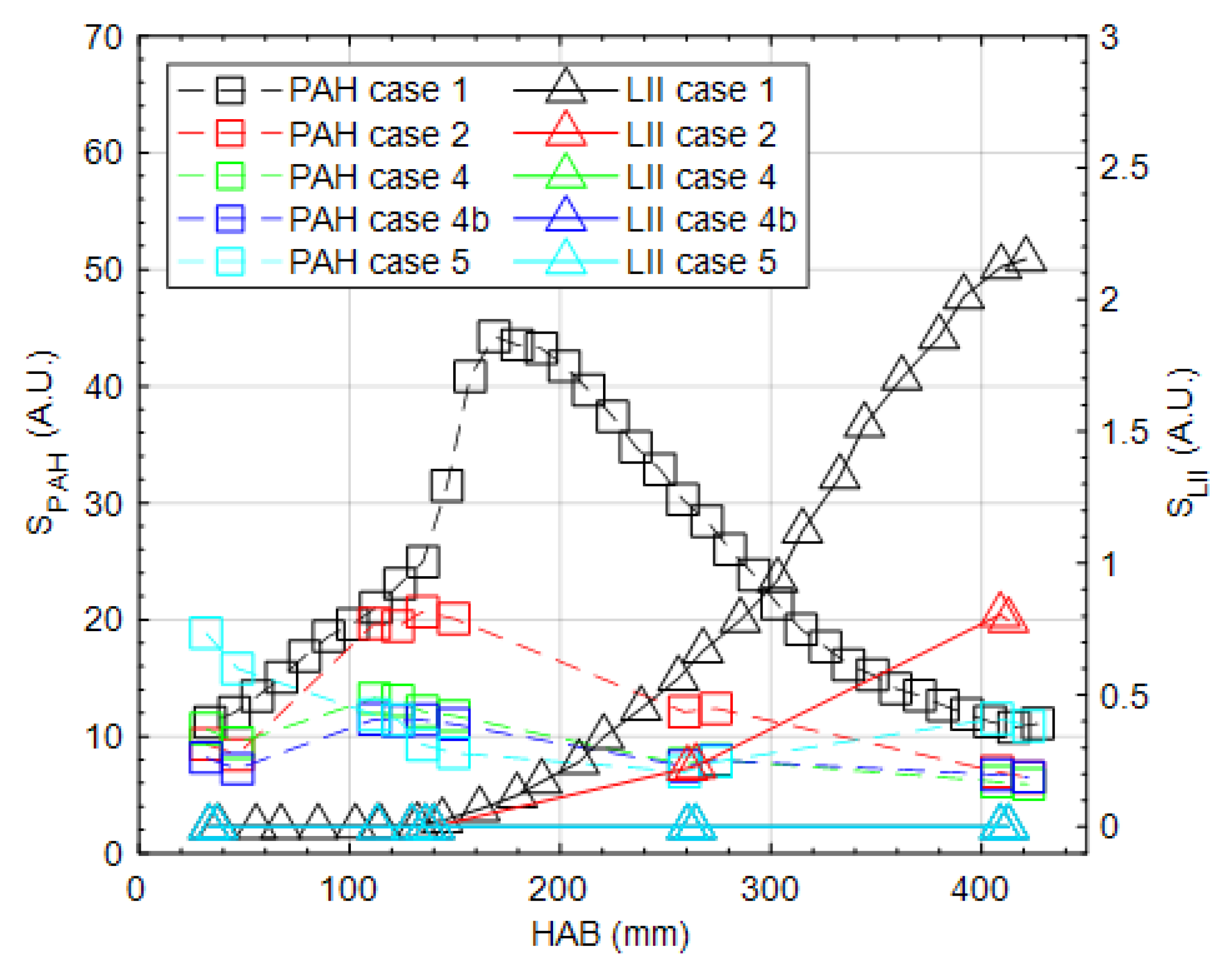
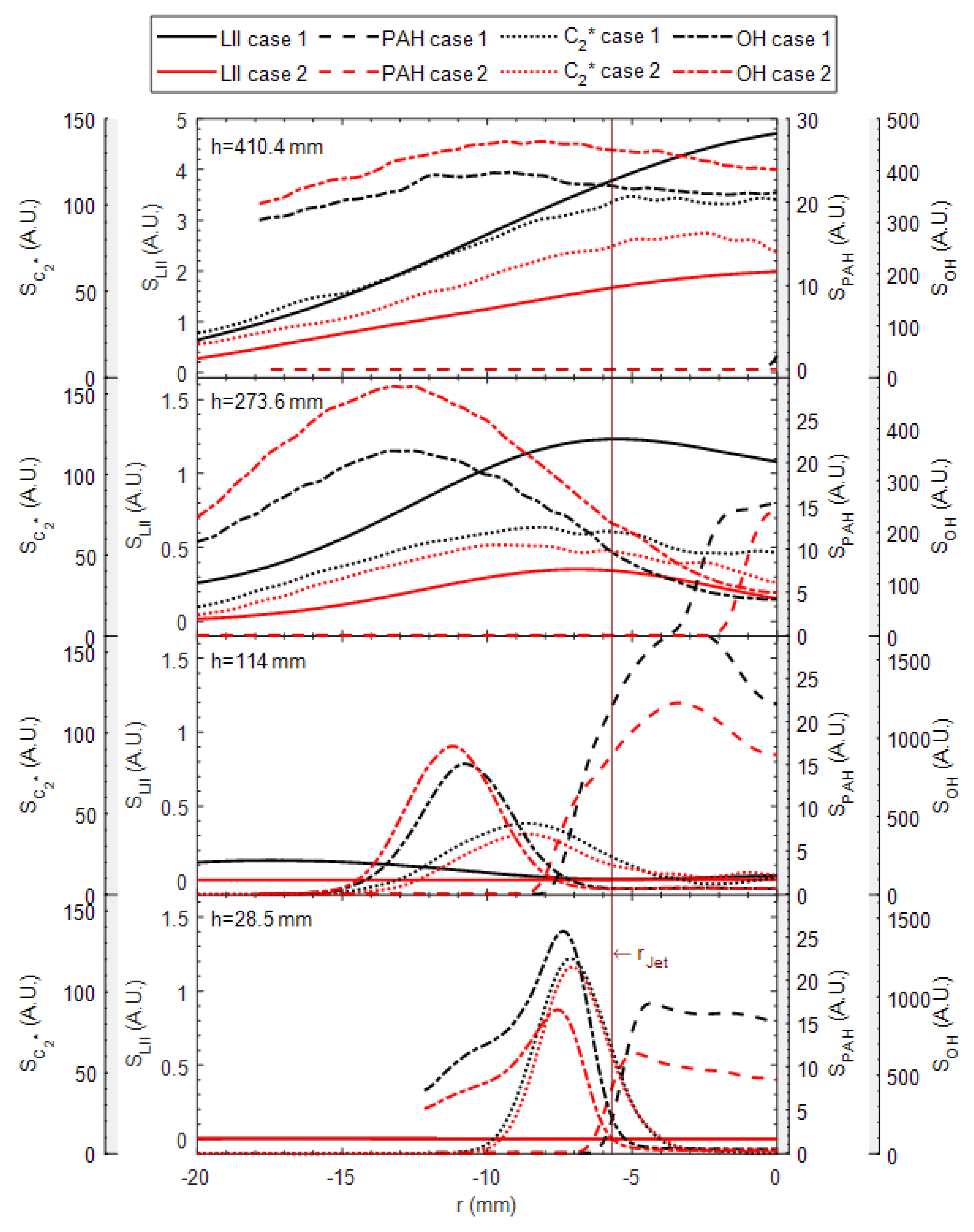
3. Results and Discussion
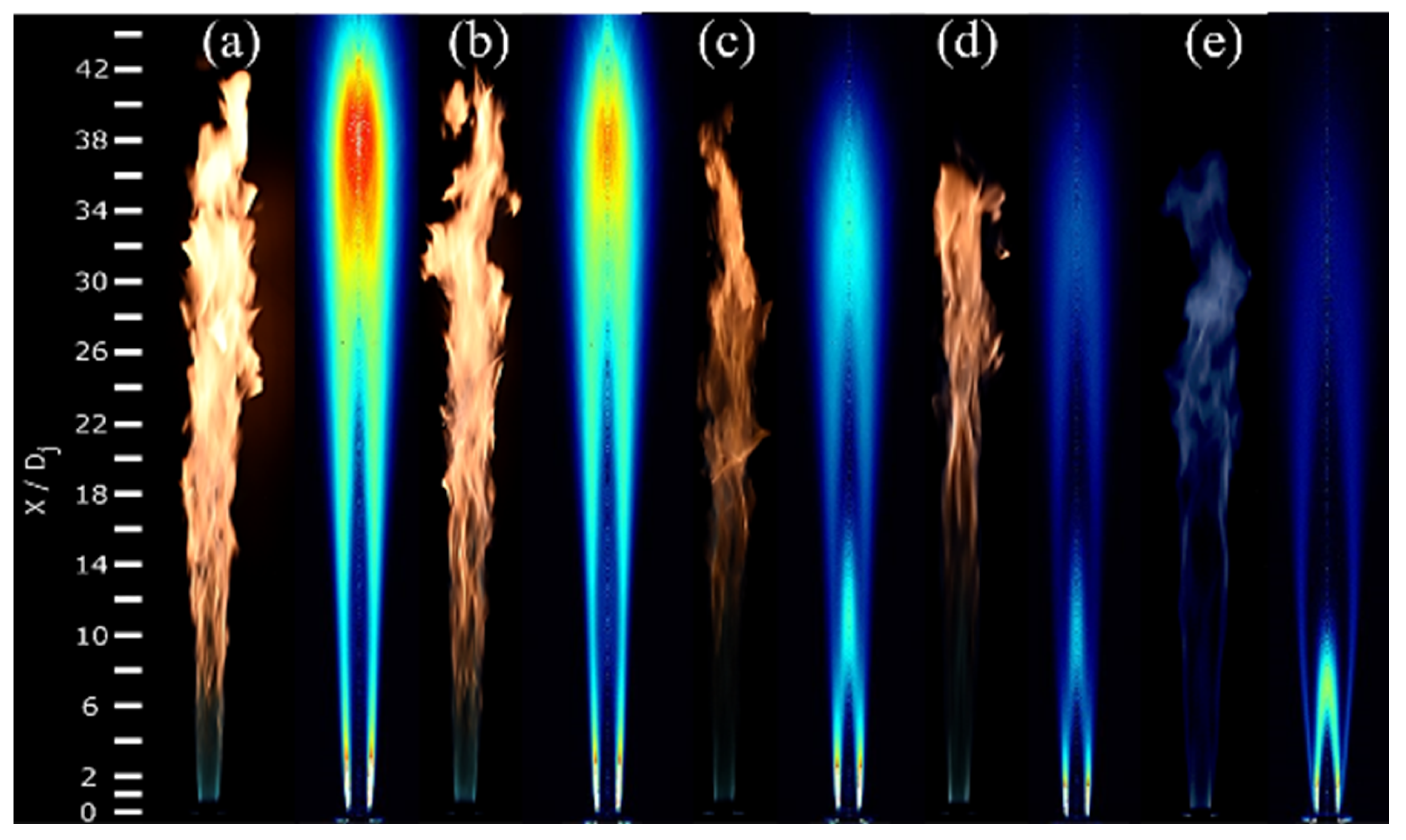
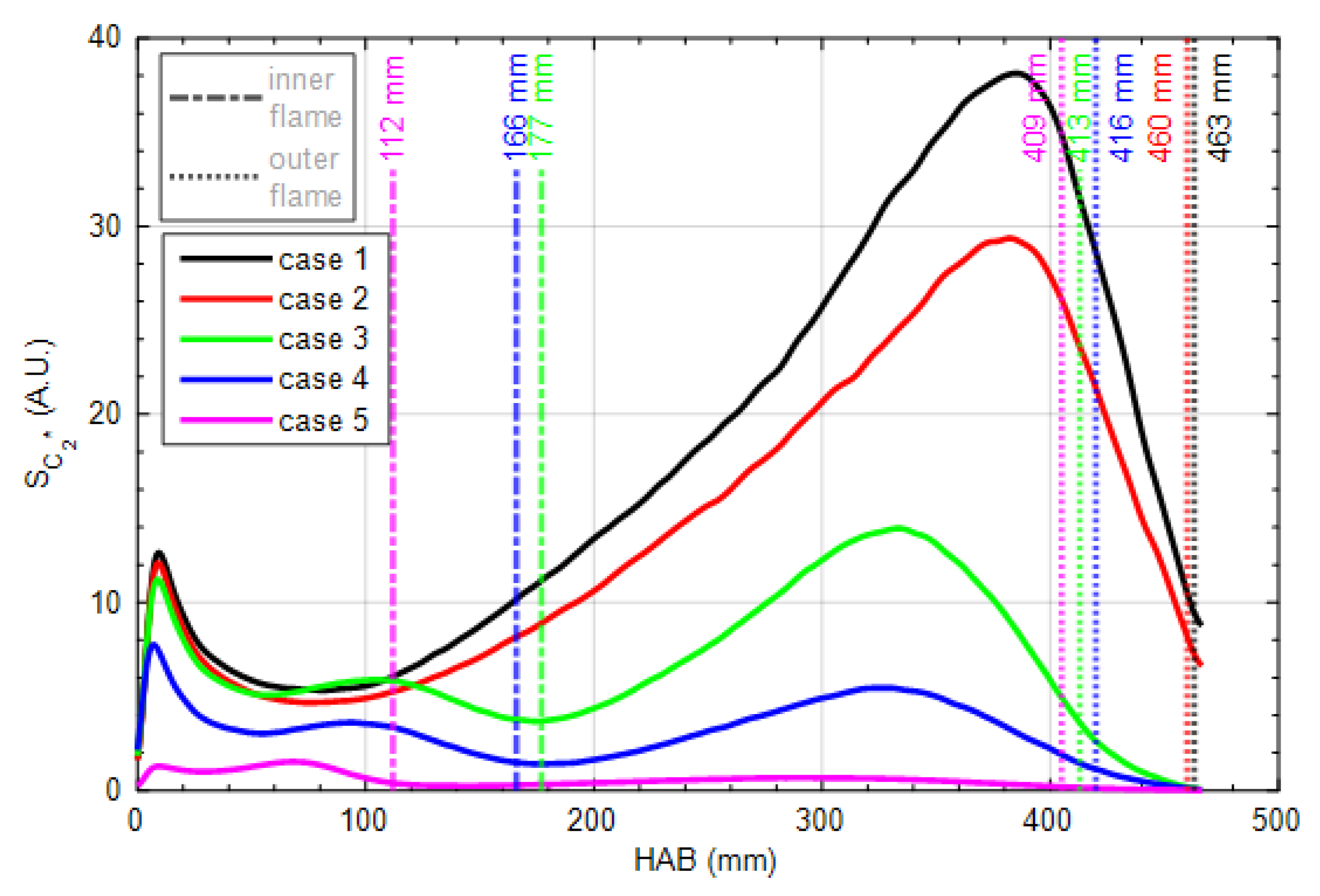
3.1. Global Flame Structure and Flame Length
3.2. OH, PAH and Soot Structures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMNL | -methylnaphthalene |
| A.U. | Arbitrary Units |
| C2* | carbon radicals |
| CCD | charge-coupled device |
| CL | chemiluminescence |
| CMOS | complementary metal-oxide-semiconductor |
| DSLR | digital single-lens reflex camera |
| FOV | field of view |
| FWHM | full width at half maximum |
| GaAsP | gallium arsenide phosphide |
| HAB | height above the burner |
| IR | infra-red |
| IRO | intensified relay optics |
| JP-8 | Jet Propellant 8 |
| LII | laser-induced incandescence |
| MFC | mass flow controller |
| Nd:YAG | neodymium-doped yttrium aluminum garnet |
| OH | hydroxyl radical |
| OH-LIF | planar laser-induced fluorescence of OH |
| OME | poly(oxymethylene) dimethyl ether |
| PAH | polycyclic aromatic hydrocarbon |
| PAH-LIF | planar laser-induced fluorescence of PAH |
| PLIF | planar laser-induced fluorescence |
| TCJB | temperature-controlled jet burner |
| UV | ultra-violet |
References
- Burger, J.; Siegert, M.; Ströfer, E.; Hasse, H. Poly(oxymethylene) dimethyl ethers as components of tailored diesel fuel: Properties, synthesis and purification concepts. Fuel 2010, 89, 3315–3319. [Google Scholar] [CrossRef]
- Lahaye, J. Soot in Combustion Systems and Its Toxic Properties; Volume 7, Nato Conference Ser; Springer: New York, NY, USA, 1983. [Google Scholar]
- de Ras, K.; Kusenberg, M.; Thybaut, J.W.; van Geem, K.M. Unraveling the carbene chemistry of oxymethylene ethers: Experimental investigation and kinetic modeling of the high-temperature pyrolysis of OME-2. Proc. Combust. Inst. 2023, 39, 125–133. [Google Scholar] [CrossRef]
- Svensson, K.I.; Richards, M.J.; Mackrory, A.J.; Tree, D.R. Fuel Composition and Molecular Structure Effects on Soot Formation in Direct-Injection Flames Under Diesel Engine Conditions. SAE Trans. 2005, 114, 594–604. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Pitz, W.J.; Curran, H.J. Chemical kinetic modeling study of the effects of oxygenated hydrocarbons on soot emissions from diesel engines. J. Phys. Chem. A 2006, 110, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, X.; Miao, H.; Yang, X.; Huang, Z. Effect of dimethoxy-methane and exhaust gas recirculation on combustion and emission characteristics of a direct injection diesel engine. Fuel 2011, 90, 1731–1737. [Google Scholar] [CrossRef]
- Pellegrini, L.; Marchionna, M.; Patrini, R.; Florio, S. Emission Performance of Neat and Blended Polyoxymethylene Dimethyl Ethers in an Old Light-Duty Diesel Car; SAE Technical Paper; SAE: Warrendale, PA, USA, 2013. [Google Scholar] [CrossRef]
- Liu, H.y.; Wang, Z.; Wang, J.X. Performance, Combustion and Emission Characteristics of Polyoxymethylene Dimethyl Ethers (PODE 3-4 )/ Wide Distillation Fuel (WDF) Blends in Premixed Low Temperature Combustion (LTC). SAE Int. J. Fuels Lubr. 2015, 8, 298–306. [Google Scholar] [CrossRef]
- Pitsch, H.; Goeb, D.; Cai, L.; Willems, W. Potential of oxymethylene ethers as renewable diesel substitute. PRogress Energy Combust. Sci. 2024, 104, 101173. [Google Scholar] [CrossRef]
- Karataş, A.E.; Gülder, Ö.L. Soot formation in high pressure laminar diffusion flames. Prog. Energy Combust. Sci. 2012, 38, 818–845. [Google Scholar] [CrossRef]
- Mercier, X.; Carrivain, O.; Irimiea, C.; Faccinetto, A.; Therssen, E. Dimers of polycyclic aromatic hydrocarbons: The missing pieces in the soot formation process. Phys. Chem. Chem. Phys. PCCP 2019, 21, 8282–8294. [Google Scholar] [CrossRef]
- Bejaoui, S.; Lemaire, R.; Therssen, E. Analysis of Laser-Induced Fluorescence Spectra Obtained in Spray Flames of Diesel and Rapeseed Methyl Ester Using the Multiple-Excitation Wavelength Laser-Induced Incandescence Technique with IR, UV, and Visible Excitations. Combust. Sci. Technol. 2015, 187, 906–924. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Jensen, K.A.; Choi, M.Y. Simultaneous laser-induced emission of soot and polycyclic aromatic hydrocarbons within a gas-jet diffusion flame. Combust. Flame 1997, 109, 399–414. [Google Scholar] [CrossRef]
- Schulz, C.; Kock, B.F.; Hofmann, M.; Michelsen, H.; Will, S.; Bougie, B.; Suntz, R.; Smallwood, G. Laser-induced incandescence: Recent trends and current questions. Appl. Phys. B 2006, 83, 333–354. [Google Scholar] [CrossRef]
- Gaydon, A.G. The Spectroscopy of Flames; Springer: Dordrecht, The Netherlands, 1974. [Google Scholar] [CrossRef]
- Aye, M.M.; Beeckmann, J.; Vanegas, A.; Peters, N.; Pitsch, H. Experimental Investigation of Diesel and Surrogate Fuels: Spray and Ignition Behavior; SAE Technical Paper Series; SAE: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Pei, Y.; Mehl, M.; Liu, W.; Lu, T.; Pitz, W.J.; Som, S. A Multicomponent Blend as a Diesel Fuel Surrogate for Compression Ignition Engine Applications. J. Eng. Gas Turbines Power 2015, 137, 192. [Google Scholar] [CrossRef]
- Mueller, C.J.; Cannella, W.J.; Bays, J.T.; Bruno, T.J.; DeFabio, K.; Dettman, H.D.; Gieleciak, R.M.; Huber, M.L.; Kweon, C.B.; McConnell, S.S.; et al. Diesel Surrogate Fuels for Engine Testing and Chemical-Kinetic Modeling: Compositions and Properties. Energy Fuels 2016, 30, 1445–1461. [Google Scholar] [CrossRef]
- Weber, J.; Won, H.W.; Peters, N. Experimental Validation of a Surrogate Fuel for Diesel: Spray and Ignition Behavior; SAE Tehnical Paper; SAE: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Farrell, J.T.; Cernansky, N.P.; Dryer, F.L.; Law, C.K.; Friend, D.G.; Hergart, C.A.; McDavid, R.M.; Patel, A.K.; Mueller, C.J.; Pitsch, H. Development of an Experimental Database and Kinetic Models for Surrogate Diesel Fuels; SAE Technical Paper; SAE: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Nathan, G.J.; Alwahabi, Z.T.; Sun, Z.W.; Medwell, P.R.; Dally, B.B. The effect of exit Reynolds number on soot volume fraction in turbulent non-premixed jet flames. Combust. Flame 2018, 187, 42–51. [Google Scholar] [CrossRef]
- Bouvier, M.; Cabot, G.; Yon, J.; Grisch, F. On the use of PIV, LII, PAH-PLIF and OH-PLIF for the study of soot formation and flame structure in a swirl stratified premixed ethylene/air flame. Proc. Combust. Inst. 2021, 38, 1851–1858. [Google Scholar] [CrossRef]
- Köhler, M.; Geigle, K.P.; Blacha, T.; Gerlinger, P.; Meier, W. Experimental characterization and numerical simulation of a sooting lifted turbulent jet diffusion flame. Combust. Flame 2012, 159, 2620–2635. [Google Scholar] [CrossRef]
- Geigle, K.P.; O’Loughlin, W.; Hadef, R.; Meier, W. Visualization of soot inception in turbulent pressurized flames by simultaneous measurement of laser-induced fluorescence of polycyclic aromatic hydrocarbons and laser-induced incandescence, and correlation to OH distributions. Appl. Phys. B 2015, 119, 717–730. [Google Scholar] [CrossRef]
- Lemaire, R.; Faccinetto, A.; Therssen, E.; Ziskind, M.; Focsa, C.; Desgroux, P. Experimental comparison of soot formation in turbulent flames of Diesel and surrogate Diesel fuels. Proc. Combust. Inst. 2009, 32, 737–744. [Google Scholar] [CrossRef]
- Wang, Y.; Jain, A.; Schweizer, C.; Kulatilaka, W.D. OH, PAH, and sooting imaging in piloted liquid-spray flames of diesel and diesel surrogate. Combust. Flame 2021, 231, 111479. [Google Scholar] [CrossRef]
- Zhang, J.; Shaddix, C.R.; Schefer, R.W. Design of “model-friendly” turbulent non-premixed jet burners for C2+ hydrocarbon fuels. Rev. Sci. Instrum. 2011, 82, 074101. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Oliveira, M.H.; Olofsson, N.E.; Johnsson, J.; Bladh, H.; Lantz, A.; Li, B.; Li, Z.S.; Aldén, M.; Bengtsson, P.E.; Luijten, C.; et al. Soot, PAH and OH measurements in vaporized liquid fuel flames. Fuel 2013, 112, 145–152. [Google Scholar] [CrossRef]
- Kruse, S.; Medwell, P.; Davidovic, M.; Sun, Z.; Ye, J.; Pitsch, H.; Dally, B.B. The effect of fuel composition and Reynolds number on soot formation processes in turbulent non-premixed toluene jet flames. Proc. Combust. Inst. 2021, 38, 1395–1402. [Google Scholar] [CrossRef]
- Trabold, J.; Hartl, S.; Walther, S.; Johchi, A.; Dreizler, A.; Geyer, D. Fuel Effects in Turbulent Premixed Pre-vaporised Alcohol/Air Jet Flames. Flow Turbul. Combust. 2021, 106, 547–573. [Google Scholar] [CrossRef]
- Schneider, C.; Dreizler, A.; Janicka, J.; Hassel, E.P. Flow field measurements of stable and locally extinguishing hydrocarbon-fuelled jet flames. Combust. Flame 2003, 135, 185–190. [Google Scholar] [CrossRef]
- Pretzier, G. A New Method for Numerical Abel-Inversion. Z. für Naturforschung A 1991, 46, 639–641. [Google Scholar] [CrossRef]
- Carbone, F.; Smolke, J.L.; Fincham, A.M.; Egolfopoulos, F.N. Comparative behavior of piloted turbulent premixed jet flames of C 1 C 8 hydrocarbons. Combust. Flame 2017, 180, 88–101. [Google Scholar] [CrossRef]
- Sun, W.; Wang, G.; Li, S.; Zhang, R.; Yang, B.; Yang, J.; Li, Y.; Westbrook, C.K.; Law, C.K. Speciation and the laminar burning velocities of poly(oxymethylene) dimethyl ether 3 (POMDME3) flames: An experimental and modeling study. Proc. Combust. Inst. 2017, 36, 1269–1278. [Google Scholar] [CrossRef]
- Tan, Y.R.; Botero, M.L.; Sheng, Y.; Dreyer, J.A.; Xu, R.; Yang, W.; Kraft, M. Sooting characteristics of polyoxymethylene dimethyl ether blends with diesel in a diffusion flame. Fuel 2018, 224, 499–506. [Google Scholar] [CrossRef]
- Singh, P.; Hui, X.; Sung, C.J. Soot formation in non-premixed counterflow flames of butane and butanol isomers. Combust. Flame 2016, 164, 167–182. [Google Scholar] [CrossRef]
- Mulla, I.A.; Renou, B. Simultaneous imaging of soot volume fraction, PAH, and OH in a turbulent n-heptane spray flame. Combust. Flame 2019, 209, 452–466. [Google Scholar] [CrossRef]
- Valencia, S.; Ruiz, S.; Manrique, J.; Celis, C.; Da Figueira Silva, L.F. Soot modeling in turbulent diffusion flames: Review and prospects. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 219. [Google Scholar] [CrossRef]

| Case | Surrogate vol% | OME35 vol% |
|---|---|---|
| 1 (base case) | 100 | 0 |
| 2 | 90 | 10 |
| 3 1 | 70 | 30 |
| 4 | 50 | 50 |
| 4b (OME4) | 50 | 50 2 |
| 5 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walther, S.; Li, T.; Geyer, D.; Dreizler, A.; Böhm, B. Soot and Flame Structures in Turbulent Partially Premixed Jet Flames of Pre-Evaporated Diesel Surrogates with Admixture of OMEn. Fluids 2024, 9, 210. https://doi.org/10.3390/fluids9090210
Walther S, Li T, Geyer D, Dreizler A, Böhm B. Soot and Flame Structures in Turbulent Partially Premixed Jet Flames of Pre-Evaporated Diesel Surrogates with Admixture of OMEn. Fluids. 2024; 9(9):210. https://doi.org/10.3390/fluids9090210
Chicago/Turabian StyleWalther, Steffen, Tao Li, Dirk Geyer, Andreas Dreizler, and Benjamin Böhm. 2024. "Soot and Flame Structures in Turbulent Partially Premixed Jet Flames of Pre-Evaporated Diesel Surrogates with Admixture of OMEn" Fluids 9, no. 9: 210. https://doi.org/10.3390/fluids9090210
APA StyleWalther, S., Li, T., Geyer, D., Dreizler, A., & Böhm, B. (2024). Soot and Flame Structures in Turbulent Partially Premixed Jet Flames of Pre-Evaporated Diesel Surrogates with Admixture of OMEn. Fluids, 9(9), 210. https://doi.org/10.3390/fluids9090210








