1. Introduction
Ultrasound technology has found a wide range of applications in various industries such as oil and gas, chemical processing, and more. The main reason for the positive impact of ultrasound on these processes is its ability to enhance heat and mass transfer and change the structure and properties of dispersed phases. This is achieved through the generation of acoustic waves, which propagate through the medium and produce second-order effects, such as changes in the water/hydrocarbon contact boundaries and the colloidal structure of oil [
1,
2].
One of the most notable effects of ultrasound is the phenomenon of cavitation. During ultrasonic exposure, the powerful acoustic vibrations cause the liquid to be subjected to high stress, which can lead to the formation of bubbles [
3,
4]. Inhomogeneities in the treated medium reduce the resistance of the liquid to collapse, leading to the formation of microbubbles of gases and hydrophobic solid particles. The collapse of these bubbles results in the formation of a shock wave, which has a number of important effects.
One of the key benefits of cavitation is that it can greatly accelerate diffusion processes. This occurs because the shock wave generated by the collapse of cavitation bubbles can disperse solid materials, ensuring that they are more evenly distributed throughout the medium. Additionally, the high pressure and high energy generated by the shock wave can result in the rupture of chemical bonds, making it easier to extract and emulsify substances [
5].
Overall, the use of ultrasound has been shown to have a significant impact on a wide range of technological processes. By intensifying heat and mass transfer, enhancing the properties of dispersed phases, and accelerating diffusion processes, ultrasound has the potential to optimize and streamline a wide range of industrial operations. As such, it has become an increasingly important tool in many industries and is likely to continue to play a significant role in the future [
6,
7].
Ultrasound is widely used in the oil production industry for the extraction, transportation, and processing of crude oil. In a review article by Hamidi et al. [
8], various aspects of ultrasound application in oil production to increase production volume and permeability of rocks are discussed, including examples of practical use in existing oil fields in Russia. Practical experience using ultrasound technology in oil production is mainly presented in works by Abramov et al. [
9] and Mullakaev et al. [
10]. The use of ultrasonic treatment of varying frequencies increased oil production volume by 40% to 100%, depending on the well’s characteristics, with the effectiveness lasting up to 6 months after treatment. The article’s review of laboratory experiments attributes the increase in oil production to improvements in parameters such as viscosity, the size of heavy component aggregates, and the permeability of porous rocks. Additionally, emulsification on contact with other liquids, increased efficiency of chemical treatment for enhanced production, and destruction of rocks with the accumulation of micro-cracks and removal of fine particles from clogged pores were noted. The article provides a detailed overview of the effects of frequency, duration, and power of treatment on rocks [
8]. In a study by Marfin et al. [
11], the use of lower sound frequencies of 8 kHz increased production volume by 11.8% and restored injection wells.
In this review, we have deliberately narrowed our focus mainly to the chemical transformation of oil under the influence of ultrasound due to the lack of structured information on this topic to advance this area of research.
2. Mechanism
The physical explanation of the phenomena observed in the application of ultrasound technology to oil processing involves several complex mechanisms that take place during the process. The primary mechanism responsible for the effects of ultrasound on oil is the phenomenon of cavitation, which occurs due to the generation of acoustic waves that produce second-order effects such as changes in the water/hydrocarbon contact boundaries and the colloidal structure of oil [
12,
13].
Cavitation is the formation, growth, and implosive collapse of gas or vapor-filled bubbles in a liquid. During ultrasonic exposure, the powerful acoustic vibrations cause the liquid to be subjected to high stress, which can lead to the formation of bubbles [
14]. Inhomogeneities in the treated medium reduce the resistance of the liquid to collapse, leading to the formation of microbubbles of gases and hydrophobic solid particles. The collapse of these bubbles results in the formation of a shock wave, which has several important effects.
One of the key benefits of cavitation is that it can greatly accelerate diffusion processes. This occurs because the shock wave generated by the collapse of cavitation bubbles can disperse solid materials, ensuring that they are more evenly distributed throughout the medium. Additionally, the high pressure and high energy generated by the shock wave can result in the rupture of chemical bonds, making it easier to extract and emulsify substances [
15].
Another mechanism through which ultrasound can affect oil processing is the generation of localized heating. The collapse of cavitation bubbles results in the formation of high-pressure hot spots, which can lead to localized heating of the liquid. This heating can have significant effects on the properties of the oil, such as viscosity and surface tension [
16].
In addition to the physical mechanisms of cavitation and heating, ultrasound can also affect oil processing through other mechanisms, such as acoustic streaming and radiation forces. Acoustic streaming refers to the motion of the fluid caused by the propagation of sound waves through it, which can lead to enhanced mixing and mass transfer [
17]. Radiation forces refer to the transfer of momentum from the sound wave to the medium, which can result in the movement and separation of particles [
18].
Overall, the physical explanation of the effects of ultrasound on oil processing is complex and involves several mechanisms, including cavitation, heating, acoustic streaming, and radiation forces. Understanding these mechanisms is essential for optimizing the use of ultrasound technology in oil processing and improving the efficiency and effectiveness of industrial processes.
Powerful acoustic vibrations in a medium can cause various nonlinear effects, also known as second-order effects [
19,
20], which are particularly prevalent in liquids. These effects are dependent on the amplitude of the acoustic wave and can give rise to secondary phenomena such as flotation, rectified diffusion, shock waves, and others, which have a significant impact on chemical and technological processes in the acoustic field. The formation of cavitation vapor–gas bubbles is one of the most noteworthy effects, and these bubbles, in turn, produce shock waves [
21].
Acoustic waves play a critical role in changing the structure and properties of dispersed phases, increasing the surface area of interfacial interaction, and intensifying processes such as dissolution, extraction, and emulsification [
22,
23]. The transition from low-amplitude acoustic oscillations to high-amplitude oscillations, where the liquid’s continuity breaks and cavitation bubbles form, results from the nonlinear characteristics of the medium caused by the presence of cavitation bubbles. As cavitation develops, periodic hydrodynamic ruptures propagate in the form of a wave front of collapsing bubbles.
Cavitation bubbles serve as secondary high-amplitude sources, producing ponderomotor forces in their vicinity, micro-flows in the acoustic field, and shock waves that propagate faster than acoustic waves. The collapse of cavitation bubbles in the liquid results in pressures of several tens of thousands of atmospheres, which can cause significant damage to almost any material [
24,
25,
26]. When a cavitation bubble reaches a liquid–solid interface, its sphericity is disrupted, leading to slight deformation when it closes. This deformation creates micro-jets of liquid that move at high speeds of tens and hundreds of meters per second, leading to destructive impacts on solid surfaces.
The use of ultrasonic vibrations intensifies physico-chemical processes in the liquid, resulting in increased temperature, mixing, and mass transfer [
27]. This vibration also disperses the solid phase (catalyst) and regenerates the surface of the catalyst, ultimately intensifying mass transfer processes in the medium. The presence of an acoustic field in a liquid can lead to the intensification of physico-chemical processes, resulting in increased temperature, mixing, and mass transfer. However, the formation of cavitation bubbles, shock waves, and micro-jets can have both positive and negative consequences. Therefore, it is essential to study and understand the effects of ultrasonic vibrations in detail.
3. Effects on Emulsions
Ultrasonic waves are an effective and widely used tool in various industrial and scientific fields. When they propagate in any medium, they cause cycles of compression and rarefaction of the medium. During the rarefaction cycle, there is a local decrease in pressure, leading to the separation of medium molecules, which form vapor–gas cavities or bubbles. These bubbles absorb energy from the ultrasonic wave during alternating compression and rarefaction cycles [
28,
29].
However, when the bubbles reach a critical size, they can no longer effectively absorb energy, and they collapse during the next compression cycle. This collapse of bubbles is what is known as ultrasonic cavitation and is the source of several physical and chemical effects. For example, when bubbles collapse, they release heat, causing the temperature near the collapsed bubbles to reach temperatures as high as 3000–5000 °C and the pressure to reach up to 300 MPa. The intense release of heat and pressure leads to the formation of a thin emulsion between immiscible phases and the formation of radicals, which are chemically active species [
30,
31].
The efficiency of many technological processes in liquids under the influence of ultrasound (such as the destruction of surface films, ultrasonic emulsification and dispersion, ultrasonic cleaning, etc.) is mainly due to the pressure and temperature of steam and gas in the bubble at the final stage of collapse. Ultrasonic dispersion (emulsification) is a widely used technique for obtaining highly dispersed, almost homogeneous, and chemically pure emulsions [
32].
The mechanism of formation of emulsion droplets under the action of cavitation is not yet well understood. There are only a few hypotheses, one of which suggests that a cavitation cavity forms at the location of inhomogeneity, such as the interface of two phases, and at the stage of collapse, it carries away and tears off droplets from the other liquid. Another hypothesis explains the formation of emulsions by the disintegration of cumulative jets formed during the asymmetric collapse of cavitation cavities [
33].
The threshold intensity of ultrasound required for emulsification decreases if it occurs near the surface of the solid phase that initiates the formation of cavitation. As the time of exposure to ultrasonic vibrations increases, the concentration of the emulsion increases until it reaches a certain limit value. This limit is due to the simultaneous flow of the opposite process—acoustic coagulation—and a change in the conditions for cavitation [
34].
In addition to its use in forming emulsions, ultrasonic treatment can also be used to improve the properties of existing emulsions. For example, when bitumen emulsions are processed with ultrasound, their structural and technological properties improve, leading to a decrease in conditional viscosity by 37.5%, an increase in uniformity, and better storage stability. The decrease in viscosity allows for better mobility, which expands the range of applications of these emulsions [
35,
36].
Ultrasonic waves and their effects, specifically ultrasonic cavitation and emulsification, have a wide range of industrial and scientific applications. Further research into the detailed mechanisms of these processes can lead to further improvements and advancements in these fields.
In their study, Hassanshahi et al. [
37] determined the optimal parameters to increase the stability of oil emulsions using Cold Lake Blend (CLB) crude oil. The optimal vibration parameters for preparing an emulsion with water, with NaCl dissolved in it at a concentration of 15 g/L and pH 8.3, were as follows: power of 76–80 W and processing time of 16 min. The energy required for emulsification was 60–70 kJ. Increasing or decreasing the processing time or power had a negative effect on the emulsion stability.
In their review article, Agi et al. [
38,
39] analyzed practical research on the effect of ultrasound on emulsion particle size. They also observed that coagulation occurs during the process of grinding emulsion particles. Additionally, Agi et al. analyzed the influence of the time, power, and frequency of ultrasonic treatment. The results were dependent on the material being processed, but in general, intermittent ultrasonic processing had a positive effect, and an increase in power resulted in reduced viscosity.
Afanasenko et al. [
40] evaluated the effect of mechanical agitation on emulsification. By simulating the effect of cavitation flows through mechanical mixing with liquid movement in various installations, they observed an increase in emulsion formation efficiency not only due to an increase in the area of the phase boundary during mixing but also when superimposing waves during mechanical mixing and ultrasonic cavitation, which increases the amplitude, allowing for more efficient formation of small emulsion droplets.
Ultrasonication can also be used to clean membranes in ultrafiltration from adhering emulsion particles. Augustine et al. [
41] conducted experiments on the purification of oil-in-water emulsions using various membranes and concluded that the use of 40 kHz ultrasound with a power of 500 W during filtration improves the quality and flow rate of filtration. Additionally, the degree of purification (rejection ratio) with the use of ultrasound increases from 45% to 90%. The authors concluded that cavitation processes and Brownian motion under ultrasonic action effectively destroy formed crusts and reduce concentration polarization on the membrane surface.
4. Ultrasound Application in Desulfurization
Sulfur compounds are one of the most common contaminants found in petroleum, and a significant amount of these compounds are transferred to fuels during the refining process. The presence of these contaminants in fuels can lead to various environmental and health problems, as well as reduced fuel efficiency. Therefore, removing these contaminants from fuels is a critical step in the refining process. Ultrasound is one of the most promising methods for purifying fuels from these contaminants. In this method, an ultrasound probe is used to create cavities in the liquid fuel, which can improve the kinetics of the oxidative desulfurization process. This process is performed step-by-step in the presence of a catalyst and an oxidizer, followed by the removal of the purified fuel product [
42].
The use of ultrasound in the oxidative desulfurization process has been recognized as a highly effective method for removing sulfur from fuels. This method has gained more recognition than the hydrodesulfurization (HDS) process due to its high selectivity and mild operating conditions [
43,
44,
45]. Furthermore, the recent introduction of the ultrasound-assisted oxidative desulfurization (UADO) method has revolutionized the petrochemical industry. The UADO method has several advantages over the conventional mixing-assisted oxidative desulfurization process. The use of an ultrasound probe in the UADO process increases the rate of desulfurization due to its smoother dispersion capabilities, which results in better reaction kinetics. The reaction rate and mass transfer are also enhanced due to the simultaneous physical and mechanical effects of the ultrasound probe [
46,
47,
48].
Gildo et al. conducted a study on the ultrasound-assisted oxidative desulfurization of simulated fuel using an activated carbon-supported phosphotungstic acid catalyst. The simulated fuel oil contained sulfur-containing compounds, and hydrogen peroxide was added as an oxidizing agent. The experiment was optimized using the 24 factorial design and face-centered method. The study found that 94.74% of the sulfur was removed from the simulated fuel oil under the optimized conditions of 25.52 wt.% hydrogen peroxide concentration, 983.9 mg of catalyst loading, and 76.36 min of sonication time. The study also found that 99% of the sulfur was removed from kerosene oil using the optimized UADO parameters in a 4-cycle extraction process [
49].
Similarly, Jalali et al. applied ultrasound irradiation in the oxidative desulfurization process of gas oil. In this study, hydrogen peroxide was used as an oxidant, and formic acid was used as a catalyst. The experiment was optimized using the response surface methodology (RSM) and Box–Behnken design. The study investigated the effects of various reaction parameters, such as sonication time, temperature, peroxide to sulfur molar ratio, formic acid to oxidant molar ratio, and sonication power per gas oil volume. The study found that maximum desulfurization was achieved under the optimum conditions of 50 °C temperature, 19.81 min of sonication time, 46.36 oxidant to sulfur molar ratio, 3.22 formic acid to oxidant molar ratio, and 7.78 W/mL sonication power. The sulfur removal efficiency obtained was 96.2% after the fourth extraction cycle using acetonitrile as a solvent. The study also found that the UADO method resulted in higher desulfurization efficiency within a shorter reaction time compared to the traditional mixing oxidative desulfurization process [
50].
Studies [
51,
52,
53,
54] have shown that ultrasonic-assisted oxidative desulfurization (UAOD) is a highly effective method for reducing the sulfur content of fuel oil. Unlike traditional desulfurization methods that require harsh reaction conditions, UAOD can be performed at mild temperatures, making it a safer and more environmentally friendly alternative. An ultrasonic device with a low power of 70 W was used to oxidize model sulfur compounds in the fuel oil using a hydrogen peroxide–peracetic acid oxidizing system [
55]. The results of the studies were highly promising, indicating that UAOD could be a valuable tool for improving the quality of fuel oil and reducing the negative impact it has on the environment. Moreover, the use of low-power ultrasonic technology makes this process accessible and cost-effective for a variety of industries.
The ability to remove sulfur from fuel oil not only helps to reduce air pollution but also improves engine performance and prolongs the life of equipment. In addition, desulfurized fuel oil has a higher heating value, which can result in more efficient energy utilization. Given these benefits, it is expected that UAOD will continue to gain traction as a valuable method for improving the quality of fuel oil. Further research is needed to optimize the process and make it more widely applicable, but the initial results are very promising and suggest that UAOD could play a key role in creating cleaner, more sustainable energy solutions for the future.
A recent study [
56] focused on the ultrasound- and mixing-assisted oxidative desulfurization of synthetic oil containing sulfur compounds, such as benzothiophene and dibenzothiophene, using polyoxometalate catalysts, hydrogen peroxide as an oxidant, and a phase transfer agent. The results indicated that this method is comparable to the conventional oxidative desulfurization technique but shows a better performance in terms of oxidation efficiency, reaction rate, and activation energy.
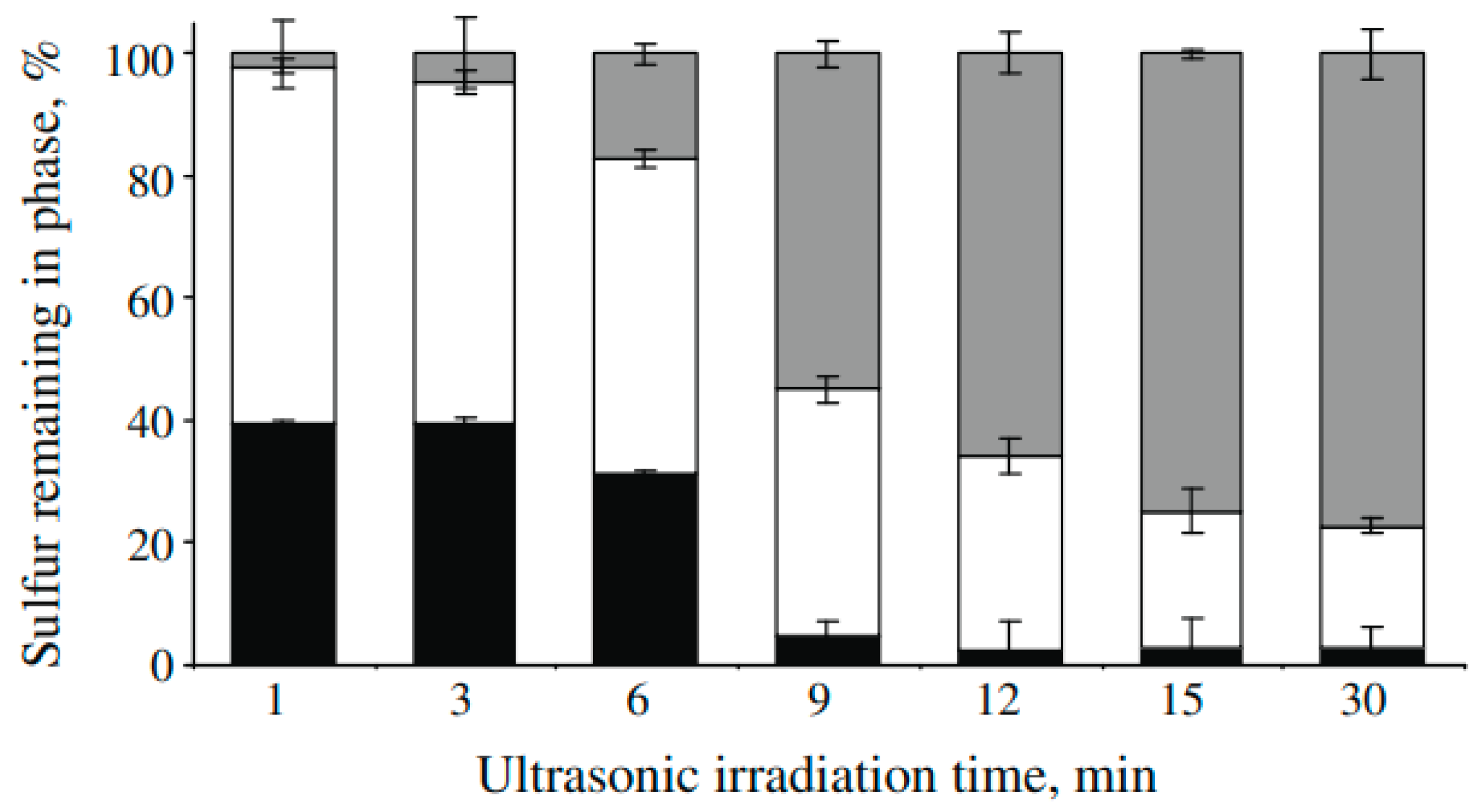
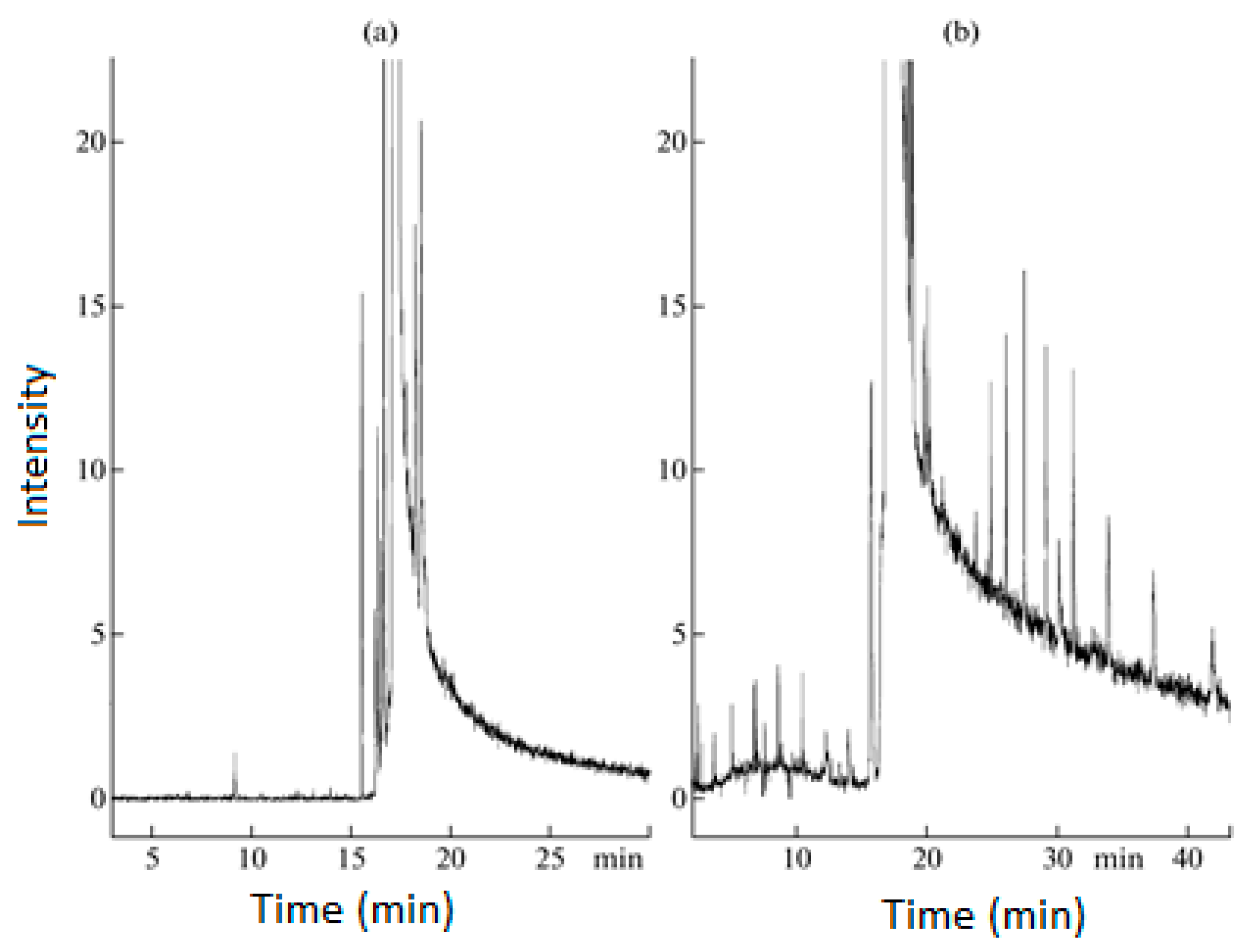
Under optimized conditions, ultrasound-assisted oxidative desulfurization achieved a sulfur removal rate of approximately 95% after 9 min of ultrasonic irradiation, with a frequency of 20 kHz and a power of 750 W, running at 40%. The process was carried out in the presence of hydrogen peroxide and acetic acid, and the final step involved extraction with methanol (
Figure 1).
This study highlights the potential of ultrasound- and mixing-assisted oxidative desulfurization as a highly effective and efficient method for removing sulfur compounds from synthetic oil. The optimized conditions of this process can provide a fast and environmentally friendly approach to reduce the levels of sulfur in petroleum-based products, which can have harmful effects on both human health and the environment [
58,
59,
60].
The removal of sulfur from waste tires using ultrasonic-assisted oxidation and desulfurization (UAOD) is a promising solution for the recycling of waste tires and the elimination of environmental pollution. Chen et al. [
61] conducted a study to investigate the efficiency of the UAOD process in removing organic sulfur from waste tire pyrolysis oil (WTPO).
The process involved the use of a transitional metal catalyst, phosphotungstic acid (H3PW12O40), a solution of oxidant H2O2, and a biphasic agent tetraoctylammonium bromide. The reaction mixture was subjected to ultrasonic emission at a temperature of 88 °C, a frequency of 20 kHz, and an oxidation time of 20 min. This high-energy environment created by the ultrasonic emission promotes the formation, growth, and collision of microbubbles in the liquid medium, which leads to the release of excessive heat energy and pressure. These physical and chemical effects enhance the oxidation reaction rate by increasing the mass transfer rate between the hydrocarbon and oxidant phases.
After the oxidation process, the oxidized WTPO was extracted with acetonitrile and subjected to adsorption using Al2O3 as the adsorbent. The maximum desulfurization efficiency achieved was 89%. The study found that the degree of desulfurization was influenced by factors such as the amount of catalyst used, the duration of sonication, and the diameter of the adsorption column. An increase in the amount of transitional metal catalysts and a longer sonication time resulted in improved oxidation efficiency.
The economic benefits of the UAOD process were also analyzed by Chen et al. [
62]. They compared the cost and percentage of sulfur removal based on the use of one or two UAOD units. A single UAOD unit had a cost of USD 0.70 per gallon with a sulfur removal rate of 68%, while the cost and removal rate for two UAOD units connected in series were USD 1.39 per gallon and 90.91%, respectively. These results indicated that the continuous-flow UAOD process provides excellent economic benefits along with environmental benefits by eliminating high levels of SO
2 and sulfate PM emissions.
Waste tires pose a significant environmental and health hazard due to their high sulfur content. The sulfur in waste tires can lead to the release of harmful SO2 emissions, which can contribute to air pollution and acid rain. In addition, the high sulfur content in waste tires can result in the production of sulfate PM emissions, which are a major contributor to the formation of fine particulate matter in the atmosphere.
The UAOD process provides a solution to these environmental and health hazards by removing the organic sulfur from waste tires, thereby reducing their environmental impact. The use of ultrasonic technology in the process not only enhances the oxidation reaction rate but also provides an efficient and cost-effective solution for removing organic sulfur from waste tires.
The study conducted by Chen et al. highlights the potential of the UAOD process in removing organic sulfur from waste tires and the significant economic and environmental benefits that can be achieved through its implementation. The UAOD process provides a sustainable solution for the recycling of waste tires and the elimination of environmental pollution, making it a promising technology for the future.
Graphene oxide has become a popular choice in various applications due to its unique properties that allow it to be used as both a sorbent and an oxidizer [
63]. In particular, graphene oxide is capable of adsorbing oxidation products of sulfur-containing compounds, making it an attractive option for the desulfurization of petroleum products. The sonocatalytic desulfurization reaction has been shown to be a promising method for improving the quality of oil and petroleum products.
One of the by-products of the sonocatalytic desulfurization reaction is the reverse emulsion of water in the petroleum product [
64]. The water content in this emulsion does not have a linear relationship with the intensity of ultrasonic treatment but instead reaches a maximum. When separating this emulsion, it was found that a significant portion of the sulfur-containing compounds pass into the newly formed aqueous phase. The sulfur content in the hydrocarbon phase is comparable to that in refined petroleum products, with the water content in the combined hydrocarbon phase also being comparable to the water content in the source oil.
It is believed that the formation of diphilic molecules of oxidized sulfur-containing organic compounds, which are a result of sonocatalytic oxidation, are adsorbed on the surface of water droplets in the reverse emulsion due to the solvation of polar sulfoxide groups by the aqueous phase. On the other hand, nonpolar hydrocarbon radicals are converted into the hydrocarbon medium. When the reverse emulsion is separated into its aqueous and hydrocarbon phases, the molecules of sulfur-containing organic compounds pass into the aqueous phase, forming micelles [
65,
66].
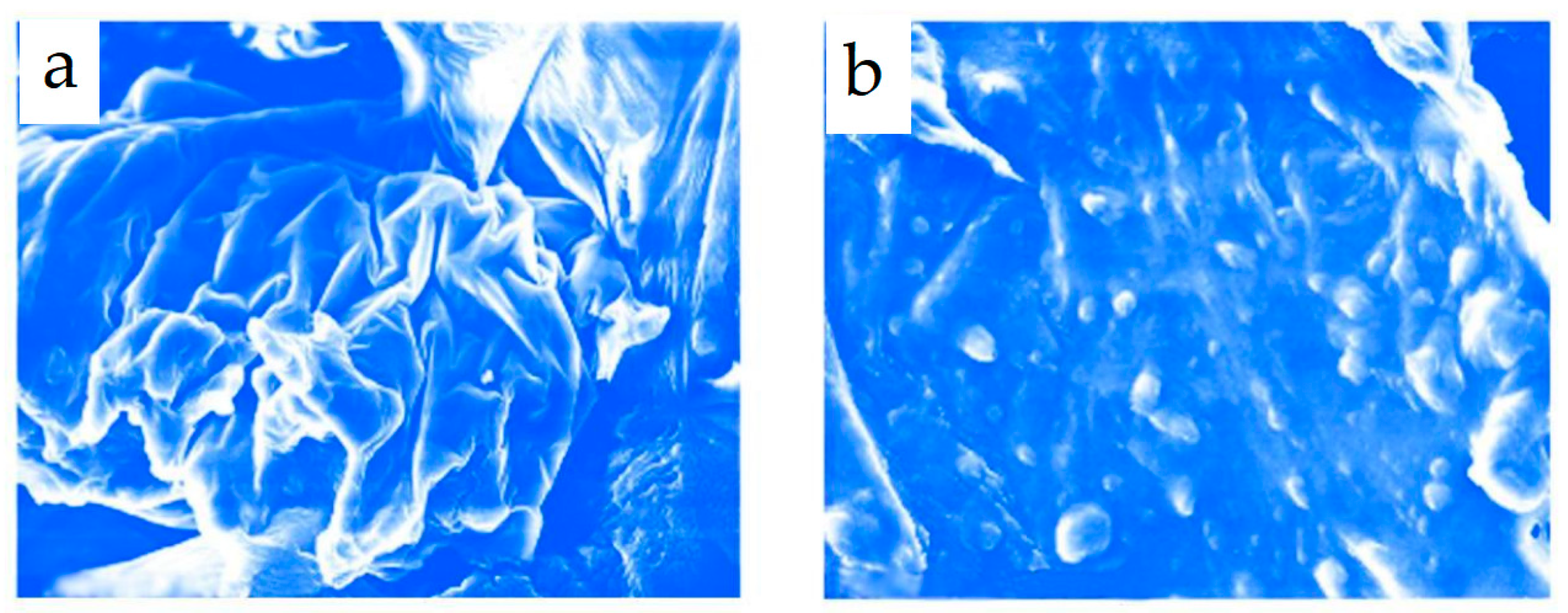
The sonocatalytic oxidative desulfurization of oil from the Zhanazhol field has been extensively studied, and the results show that the use of stepwise periodic processing in an ultrasonic field in the presence of a Ni-skeletal catalyst and oxidizer is an effective method of improving the quality of oil and petroleum products. This method allows for the intentional alteration of the chemical composition of these products, resulting in increased yields of light products such as gasoline and diesel fractions (from 15.6 to 21.9% and from 47.7 to 58.6%, respectively), as well as a reduction in the sulfur content of petroleum products to 49%. The study also noted a change in the morphology of catalyst particles under the influence of ultrasound (
Figure 2) [
67].
The sonocatalytic desulfurization reaction is a promising method for improving the quality of oil and petroleum products. The use of graphene oxide as a sorbent and oxidizer, along with the utilization of a Ni-skeletal catalyst, has been shown to be effective in reducing the sulfur content of petroleum products and increasing the yields of light products. The reverse emulsion that is formed as a by-product of the reaction can also be effectively separated into its aqueous and hydrocarbon phases, resulting in the formation of micelles of sulfur-containing organic compounds in the aqueous phase. The results of this study highlight the potential of the sonocatalytic desulfurization reaction in enhancing the quality of oil and petroleum products while reducing environmental pollution.
The optimization of ultrasonic exposure during oxidative desulfurization involves improving the reactor design to enhance the synergistic effects and accelerate mass transfer in the gas–liquid system. A surface-centered reactor with simultaneous ultrasound and UV irradiation was used to hydrotreat kerosene and achieved a desulfurization rate of 91.7% and a dearomatization rate of 48%. The effectiveness of ultrasonic exposure in the desulfurization process has been extensively studied and demonstrated in various studies [
68,
69].
The results of these studies show that ultrasonic exposure significantly reduces the content of organosulfur compounds in the hydrocarbon phase of the emulsion, making it comparable to the sulfur content in the hydrocarbon phase formed through ultrasonic treatment alone. These findings highlight the potential of ultrasonic exposure in improving the efficiency of oxidative desulfurization processes, reducing the sulfur content of hydrocarbon fuels, and enhancing their quality.
Moreover, the utilization of ultrasonic exposure in oxidative desulfurization not only reduces the environmental impact of hydrocarbon fuels but also offers economic benefits, as it allows for the recovery and reuse of valuable sulfur-containing compounds. Overall, ultrasonic exposure has emerged as a promising technology for optimizing oxidative desulfurization processes and improving the quality of hydrocarbon fuels.
5. The Impact of Ultrasound Technology on Paraffin Reduction in Oil and Gas Industries
Paraffin deposits are a common problem in the oil industry, making it difficult to extract, transport, and store oil. To remove these deposits, chemical, thermal, and mechanical treatments can be used, and these methods can be combined. However, they are often energy-consuming, and remote deposits are often left untreated [
70].
Ultrasonication is an advanced and effective technique for combating wax deposition. When combined with existing methods for cleaning paraffin deposits, this technique increases their effectiveness. Zhou and Wang [
71] conducted a study on the combined effects of ultrasound of various frequencies and powers and chemical treatment with 10% hydrochloric acid and 5% mud acid concentration. The study found that the core permeability recovery increased up to two times when using the combined treatment compared to only ultrasonic or only chemical treatment. The most effective frequencies for removing paraffin plugs were found to be around 20–25 kHz.
The effect of ultrasonic treatment on paraffins is combined, resulting in a change in structure under the influence of mechanical vibrations, chemical changes such as molecule rupture, the formation of free radicals, and heating. Ultrasonic heating can also be used for cleaning paraffin deposits [
72]. In this case, a combined mechanical and thermal effect occurs, and the oil near the resonator can reach temperatures of up to 50 °C. The authors propose the use of ultrasound primarily for cleaning tanks from sediments, but it can also be applied in oil production. The versatility of ultrasound for scale control lies in the fact that it is also an effective way to clean rocks from inorganic scales. Taheri-Shahib et al. studied the effect of ultrasound on the permeability of rocks saturated with KCl and found that ultrasound increased the solubility of KCl in water, leading to the appearance of pores and increased rock permeability [
73].
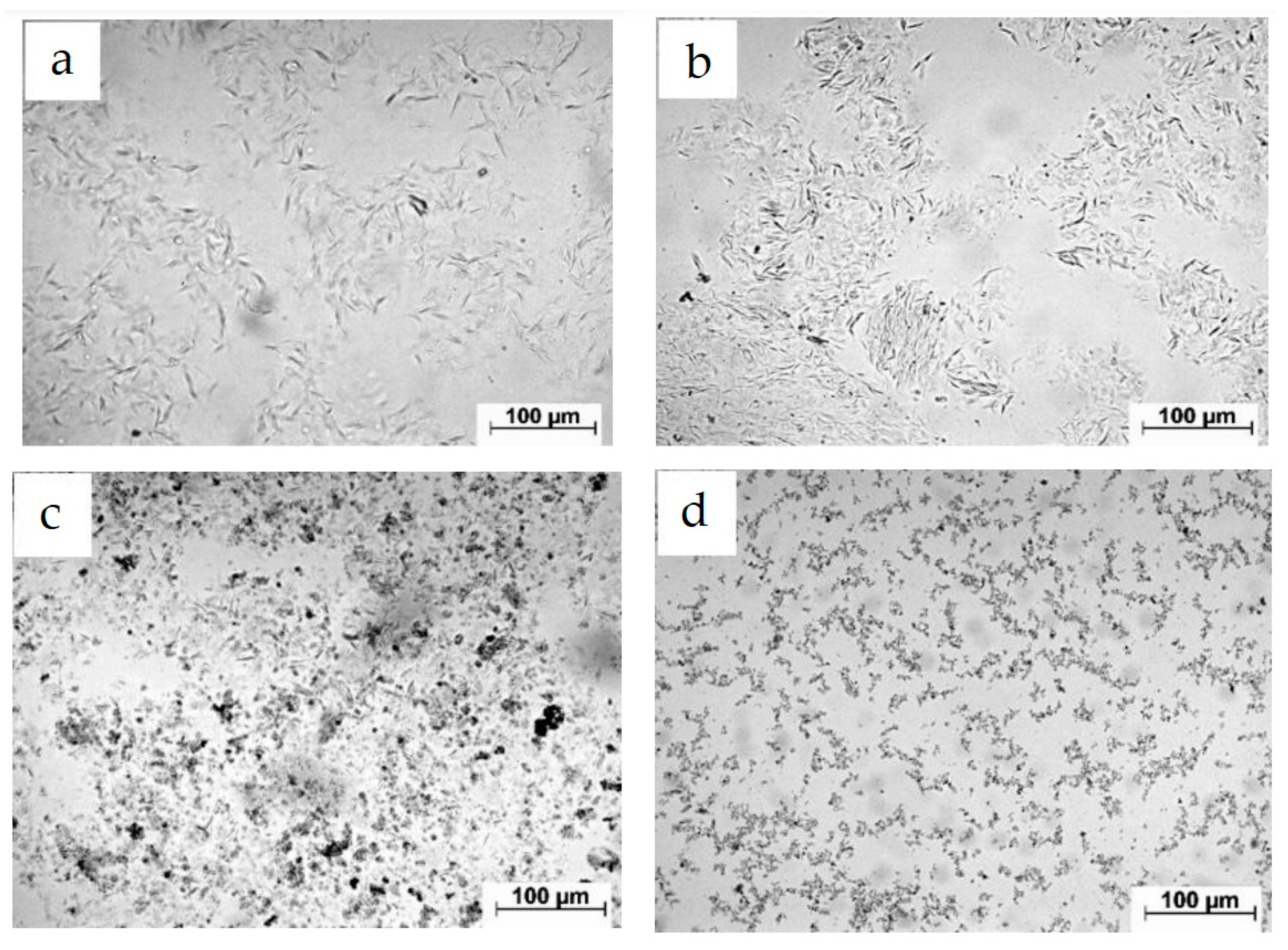
Experiments were conducted by Dong et al. [
74] to study the ultrasonic treatment of oil. The results of SEM microscopy revealed that ultrasonic treatment affects paraffin crystals, which have a layered and needle-shaped structure before treatment and easily adsorb heavy components, thereby affecting oil viscosity. However, after ultrasonic treatment, not only the size but also the shape of the crystals changes to spherical (
Figure 3).
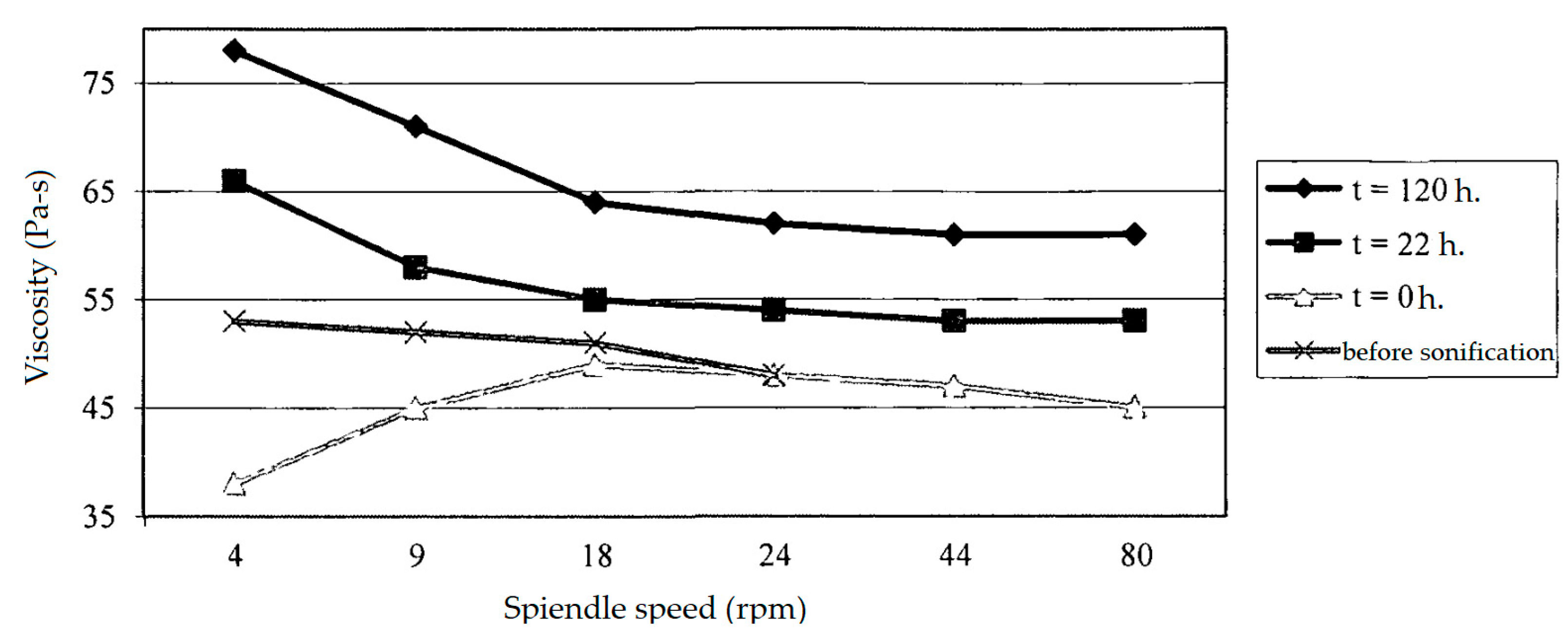
The impact of ultrasound on the colloidal structure of paraffin and resin-rich oils is particularly relevant from a practical standpoint. In a study conducted on Usinsk oil [
75], the impact of an acoustic field of 18 kHz and an intensity of 8 kW/m
2 for 30–60 min was investigated. It was found that the initial ultrasound exposure reduced viscosity at a low shear rate, but after relaxation, an increase in viscosity was observed (
Figure 4). The authors explained this by a change in the colloidal structure after destruction. Crude oil containing paraffins and resins has a periodic colloidal structure formed at elevated temperatures and pressures in reservoir conditions. Ultrasonic treatment destroys the colloidal structure, and a new structuring occurs under new conditions, which changes the colloidal particles themselves and increases the viscosity of the oil.
Specialists from the Ukhta State Technical University have proposed a technology for processing highly paraffinic oil that can significantly increase the relaxation time after ultrasonic treatment. This technique involves ultrasonic treatment of the oil, heating, and mixing with water. During the process, supramolecular structures are destroyed, followed by the dissociation of water and the filling of bonds formed during the destruction of paraffins [
76].
The presence of resins and asphaltenes has a significant influence on the viscosity of highly paraffinic oils after treatment. According to the research of Morozova A.V., during the destruction of crystalline formations of paraffins, adsorption of resins on crystals can occur, which prevents their growth [
77].
Figure 5 shows photographs of the crystal structure, which demonstrate an increase in the size of paraffin crystals after processing and relaxation. This increase in size causes an increase in viscosity. Additionally, the figure shows a change in the initial structure of the crystals when resins are introduced during processing, which limits the growth of crystals.
However, heavy impurities can have a negative effect. For example, asphaltenes can become centers of crystal formation, which negatively affects the oil viscosity.
Ultrasound can also have a chemical effect on paraffin processing. Cui et al. [
78] noted that exposure to 20 kHz ultrasound on oil samples affects the saturated fraction of oil. After 20 min of exposure to ultrasound, a decrease in the amount of saturated fraction above C22 of 2% is observed, with an increase in the amount of lighter saturated hydrocarbons C12–C22 of 4%. The increase in the amount is partly due to the destruction of resins with the formation of aromatic and saturated hydrocarbons.
Kim et al. [
79] conducted ultrasonic (20 kHz) treatment of
n-hexadecane, resulting in the formation of two fractions, R1(<C16) and R2(>C16), with 4.46% n-hexadecane. The amount of R1 fraction after 60 min of treatment at 230 °C was 3.36%.
6. The Chemistry of Cavitation Phenomena
The mechanism of action of ultrasound on paraffin and highly paraffinic vacuum atmospheric residual (VAT) oils is a topic of ongoing research in the field of chemical engineering. This mechanism is based on a model developed by Syunyaev et al. [
80] that is centered around the concept of “supramolecular structures”. This model postulates that the composition of the oil determines the core of the complex structural unit in the oil. In highly paraffinic oils, the core is made up of paraffins, which are dispersed in an ultrasonic field, leading to an increase in specific surface area and surface energy. An example of such an effect was recorded in [
54] (
Figure 6).
The dispersal of the system in an acoustic field creates a state of excitement in which the system has excess energy. When the load is removed, the system tends to reduce the excess energy by reducing the specific surface area. For highly paraffinic oils with low content of resinous asphaltene components, the reduction in surface energy occurs as a result of the recrystallization of n-alkanes with the formation of a continuous structural grid. This process leads to an increase in the structural and mechanical parameters of the dispersed system.
On the other hand, in oils with a high content of resinous asphaltene components, the surface tension is reduced due to the adsorption of resins on paraffin crystals. This process prevents the aggregation of paraffin crystals, thereby reducing viscosity, pour point, and energy parameters. The study of the interaction of ultrasound and highly paraffinic VAT oils has important implications for the refining and processing of crude oil into petroleum products. The application of ultrasound to highly paraffinic VAT oils can lead to improvements in the structural and mechanical parameters of the oils, resulting in improved flow properties and energy efficiency [
81,
82].
The mechanism of action of ultrasound on paraffin and highly paraffinic VAT oils is a complex process that is still being researched and studied. However, the current understanding of this process provides valuable insights into the interactions between ultrasonic fields and highly paraffinic VAT oils and has important implications for the refining and processing of crude oil into petroleum products [
83].
The effect of ultrasonic exposure on the rheological properties of crude oil has been the subject of numerous studies in recent years [
84]. Ultrasonic exposure has been shown to be an effective method for inhibiting the flocculation and deposition of asphaltenes in oil, which can lead to improved flow characteristics and a reduction in pipeline blockages. The goal of this study is to explore the effects of ultrasonic exposure on the molecular structure of crude oil and to better understand the underlying processes involved.
It has been shown that short-term ultrasonic exposure of crude oil results in a decrease in viscosity. This is due to the destruction of intermolecular bonds in resins, which leads to their separation from asphaltene particles. However, after 10 min of exposure, an increase in viscosity begins to occur as a result of the destruction of asphaltene structures and the formation of smaller particles, which increases their solubility in the crude oil. The particle size of asphaltenes has been observed to decrease from 1.6 microns to 0.8 microns with the help of a microscope [
85].
Experiments have also shown that the destruction of asphaltene structures can lead to the reorganization of aggregates, which can result in the formation of stronger and larger aggregates. This phenomenon can be seen in the repeated effect of ultrasonic exposure on the molecular structure of a solution of asphaltenes and heptane in toluene. When heptane was added, the size of asphaltene structures was up to 500 nm. With up to the third repeated exposure to ultrasound, an increase in the size of molecular structures up to 600 nm was observed. However, subsequent cycles of ultrasound exposure have the potential to achieve stable structures of a smaller size. With the sixth exposure to ultrasound, the size of the structures decreases to 200 nm [
86].
It is important to note that the effect of ultrasonic exposure on the molecular structure of asphaltenes can vary greatly depending on the type of oil and the presence of other components in the crude. For example, the presence of polar or nonpolar solvents can greatly influence the effect of ultrasonic exposure on the stability and size of asphaltene structures.
In addition to the effects on asphaltene structures, ultrasonic exposure has also been shown to have an impact on the deposition of asphaltenes in pipelines and storage tanks. This is because the flocculation and precipitation of asphaltenes can cause serious problems in the transportation and storage of oil. The deposition of asphaltenes can lead to the formation of a thick layer of asphaltene residue on the walls of pipelines and storage tanks, which can lead to a reduction in the flow of oil and the formation of a bottleneck.
To mitigate the effects of asphaltene deposition, it is essential to understand the mechanism by which ultrasonic exposure affects the size and stability of asphaltene structures. This understanding can then be used to develop an effective strategy for preventing asphaltene deposition in pipelines and storage tanks.
One strategy that has been proposed to mitigate the effects of asphaltene deposition is the use of ultrasound in combination with other treatments. For example, it has been shown that the combination of ultrasonic exposure and heat treatment can greatly reduce the formation of asphaltene deposits. This is because the heat treatment can disrupt the intermolecular bonds in the asphaltenes, while the ultrasonic exposure can promote the dispersion of the asphaltenes in the oil.
Asphaltene deposits, which are formed by resinous asphaltene components as a result of physical or physico-mechanical action, can severely impact the quality and productivity of oil reservoirs. They can cause problems such as reduced flow capacity and reduced oil recovery, leading to substantial economic losses. The formation of asphaltene deposits in the oil film is a complex process that has been the subject of much research in the industry.
Studies have proposed a mechanism for the formation of asphaltene deposits in the oil film [
87]. It is believed that when the viscous and asphaltenic components (VAT) are destabilized in an oil reservoir, a well-defined proportion of precipitating asphaltenes can be located on the surface of the pore space, forming an adsorption-retained self-organizing system of associates. At the same time, another part of asphaltenes, structured in the form of aggregates and floccules, can form deposits in the form of a solid phase at the edges of oil films. A small portion of this solid phase can then move with the de-asphalted oil.
The ultrasonic treatment can have a significant impact on the structure of asphaltenes [
88]. The effect of the treatment depends on a number of factors, including the processing time, the physical parameters of ultrasound, temperature, and concentration of the solution. According to literature data [
89], ultrasonic cavitation has been shown to be effective in reducing the size of asphaltene clusters, thereby improving the characteristics of the oil. The ability of ultrasonic cavitation to block the precipitation of asphaltenes can be seen as a significant advantage in the oil industry, as it can help to prevent the formation of asphaltene deposits and improve the productivity of oil reservoirs.
A recent study was conducted to determine the most effective processing time for ultrasonic treatment of oil, with a focus on the kinematic viscosity of the oil over a 50 min period [
90]. The results showed that there were positive dynamics in the oil’s viscosity after ultrasonic treatment in the time interval from 10 to 20 min. The study aimed to understand the rheological behavior of the oil during each of these ranges.
In the first time range (up to 5 min), ultrasonic waves were observed to dissolve suspended particles in the crude oil, leading to an increase in the oil’s viscosity. This effect was due to the formation of free radicals resulting from the rupture of long chains and the destruction of large asphaltene associates. During the second time range, the decrease in viscosity was attributed to the rise in temperature and the dispersion of asphaltene clusters.
In the third range, the process of reverse regeneration of free radicals into large molecules occurred, which caused an increase in viscosity. After 10 min of ultrasonic exposure, there was a tendency for the precipitation of asphaltenes to decrease. The researchers believed that the decrease in asphaltenes was due to an increase in the degree of dispersion of asphaltene aggregates, leading to improved sedimentation stability in the dispersion medium.
Another study looked at the effect of ultrasound on oils of various chemical natures and on the distillation residues of these oils with different depths of fraction selection [
91]. The results showed that ultrasonic vibrations caused the destruction of high-molecular molecules of a linear and branched structure, as well as alkylaromatic hydrocarbons with long side substituents. The destruction of these molecules occurred in places where the energy of the valence bond was less than the force acting on it. In normal alkanes, this was observed in the C-C bond located closer to the center, while in aromatic hydrocarbons with an alkyl substituent, the β-bond of the side chain was affected.
The result of the destruction was the formation of free radicals of different masses and structures. These radicals formed after ultrasonic treatment had increased activity and could enter initiation and recombination reactions with molecules and radicals of various hydrocarbons. During these reactions, high-molecular hydrocarbons could form in the system, which could lead to the formation of new centers of stability and energy (CSE). With the interaction of free radicals, it was also possible to form hydrocarbons with a lower molecular weight compared to the initial ones. After removing the ultrasonic load, the new hydrocarbon molecules formed could be part of both the dispersion medium and the solvate shell of the CSE.
This study has shown that ultrasonic treatment of oil has a significant impact on its viscosity, with positive dynamics observed in the time interval from 10 to 20 min. The study also demonstrated that ultrasonic vibrations caused the destruction of high-molecular molecules in the oil, leading to the formation of free radicals and new hydrocarbon molecules with a lower molecular weight. This research provides valuable insight into the potential benefits of ultrasonic treatment for improving the properties of oil and could have practical applications in the petroleum industry.
Cui et al. [
78] studied the viscosity reduction and structural change in crude oil treated with acoustic cavitation, and they noted that ultrasonic exposure can also affect heavy fractions, resulting in changes in the structure of the asphaltene fraction. This change is reflected in the increase in the aromatic hydrogen index (Iar) and the aliphatic side chain index (CH3/CH2), indicating structural changes in the asphaltene molecules.
7. Limitations of Ultrasound Assistance
Although ultrasound is highly efficient and relatively environmentally friendly, improving its energy efficiency remains an important issue. Agi et al. [
92] studied the catalytic effect of SiO
2 nanoparticles during ultrasonic treatment and found that the combined effect of SiO
2 and ultrasound reduces the viscosity of heavy oil by promoting thermal decomposition and the formation of free radicals around nanoparticles that effectively absorb ultrasound.
Using catalysts also enhances the destruction and chemical transformation of heavy fractions and saturated hydrocarbons. In a study by Cui et al. [
78], nickel oleate was used as a catalyst, which resulted in an increase in the content of saturated hydrocarbons and a decrease in the amount of paraffins in the oil after treatment.
The design of ultrasonic emitters can introduce restrictions on their use. Most of the research discussed in this context is based on magnetostrictive emitters, which convert magnetic field energy into mechanical vibrations of ultrasonic frequency. However, their significant drawback is the need for water cooling because magnetostrictive materials lose their magnetostrictive properties at high temperatures. This also limits their operating time at great depths [
93,
94]. Furthermore, magnetostrictive emitters have a relatively low efficiency of no more than 55% [
95].
One promising direction is the use of piezoceramic emitters, which convert electric field energy into mechanical vibrations of ultrasonic frequency with high efficiency, reaching values of 85–95% [
96]. However, piezoceramic radiators have limitations associated with both thermal and power characteristics. The field of piezoelectric emitters is actively developing, with new types of emitters such as lightweight lithium niobate emitters with a high operating temperature range (up to 1210 °C) being proposed in 2015 [
94].
In their review article [
94], Wang and Gu analyzed existing ultrasonic emitters used in oil production and highlighted problems with transmitting high-frequency electrical signals to the emitter. They also noted the active development of new types of emitters to increase the range of operation and efficiency.
Another method of increasing the efficiency of ultrasonic emitters is the simultaneous use of ultrasonic waves of different frequencies. Khavsky N.N. previously noted that the simultaneous action of ultrasonic waves of two different frequencies leads to a significant increase in the efficiency of cavitation compared to the linear summation of the action of each of the fields of different frequencies [
97]. This combined effect of different frequencies is also used in the dispersion of various substances in other areas such as pharmaceuticals. Ziylan-Yavas and Ince [
98] described the dispersion of ibuprofen in water and emphasized the high efficiency of simultaneous exposure to different frequencies of ultrasound. Lee and Oh [
99] also described the high efficiency of simultaneous exposure to low and high ultrasonic frequencies.
To date, there have not been many studies aimed at studying the application of the combined effect of several frequencies in the oil field, despite the fact that this type of treatment has a large number of advantages. For example, Fu et al. [
100] described the process of oil extraction from terrigenous reservoirs (surfactant extraction of oil sand) under the influence of ultrasound, while the authors compared the impact of single frequencies and double frequencies (28 kHz and 68 kHz simultaneously). They found that the performance when exposed to single frequencies reaches only 13%, while exposure to double frequencies increases it to 95%.
In addition, the distance of effective ultrasonic treatment raises questions. Obviously, high frequencies have a low wavelength, which limits the possibility of propagation in a porous medium. However, there are no studies in the literature aimed at identifying the impact distance of different frequencies in reservoir media. Increasing the distance of ultrasonic exposure can be achieved by increasing the power of ultrasound. However, too high power can lead to destruction near the emitter, which can result in the accumulation of sand and other debris near the well [
101]. Nevertheless, there are studies that evaluate the effective distance of the emitters when cleaning paraffin deposits. In the work of Bezymyannikov et al., calculations are provided for the required number of installations of emitters when cleaning standard tanks, and these calculations are supported by laboratory tests [
72].
8. Conclusions
In conclusion, ultrasound technology has been shown to have a significant impact on the kinematic viscosity of crude oils and oil residues. Through the application of ultrasonic cavitation, suspended particles in crude oil can be dissolved, leading to an increase in viscosity. However, in the time interval between 10 to 20 min, ultrasonic waves have been observed to cause the dispersion of asphaltene clusters and the formation of free radicals, leading to a decrease in viscosity. This reduction in viscosity can be attributed to the destruction of high-molecular-weight molecules of linear and branched structures, as well as alkylaromatic hydrocarbons.
The formation of free radicals after ultrasonic exposure has been shown to have increased activity, leading to initiation and recombination reactions with various hydrocarbons, resulting in the formation of new hydrocarbon molecules with lower molecular weight. This could have important implications for the refining industry, as reducing the viscosity of crude oils could lead to more efficient and cost-effective refining processes.
Moreover, the increased dispersion stability of asphaltene aggregates after ultrasonic treatment could also reduce the risk of precipitation and improve the overall quality of the oil system. In addition, ultrasound technology could potentially be used to modify the chemical composition of crude oils and improve their properties, making it a promising avenue for further research and development.
However, it is important to note that the results of this study are based on a limited time interval and may not necessarily hold true for all oil systems. Further studies are needed to fully understand the impact of ultrasound technology on crude oils and oil residues over longer times and under different conditions. Nevertheless, the positive dynamics observed in this study make it a promising tool for the refining industry and a valuable subject for continued investigation.