Abstract
Cancer is one of the most prevalent and disruptive diseases affecting the population, and as such, is the subject of major research efforts. Recently, these efforts have been put towards understanding the role that exosomes can play in the progression of cancer. Exosomes are small extracellular vesicles ranging from 40–150 nm in size that carry bioactive molecules like proteins, DNA, RNA, miRNA, and surface receptors. One of the most important features of exosomes is their ability to easily travel throughout the body, extending the reach of parent cell’s signaling capabilities. Cancer derived exosomes (CDEs) carry dangerous cargo that can aid in the metastasis, and disease progression through angiogenesis, promoting epithelial to mesenchymal transition, and immune suppression. Exosomes can transport these molecules to cells in the tumor environment as well as distant premetastatic locations making them an extremely versatile tool in the toolbelt of cancer. This review aims to compile the present knowledge and understanding of the involvement of exosomes in the progression of cancer as well as current production, isolation, and purification methods, with particular interest on flow perfusion bioreactor and microfluidics systems, which allow for accurate modeling and production of exosomes.
1. Introduction
Cancer constitutes a large number of diseases, displaying uncontrollable cell growth and the capability to infiltrate and damage healthy tissues. Tens of millions of people are diagnosed with cancer every year, and with more than 9 million projected deaths in 2019 alone, cancer necessitates major research efforts to reduce mortality [1,2]. Exosomes are extracellular nanovesicles, ranging from 50–140 nm that can carry specific surface markers and various proteins, ribonucleic acids (RNA) and micro-RNAs (miRNA) [3]. The release of exosomes occurs when multivesicular bodies (MVBs) fuse with the plasma membrane and the contents are then released extracellularly becoming exosomes [4,5,6]. Exosomes are excreted by nearly every cell type in the body and can be found in almost every bodily fluid. These extracellular vesicles can play a pivotal role in signaling, and in the case of cancer, disease progression. Exosomes have been implicated in tumor growth, metastasis, angiogenesis, and immunomodulatory effects [3]. As a result, cancer derived exosomes (CDES) have attracted attention in the development of new therapeutic approaches for cancer. Hence, it is important to better understand the role that CDEs play in the regulation of the tumor environment and immune evasion. Yet, CDEs have been investigated for their role in immune suppression and cancer metastasis for only the last 15–20 years [7].
1.1. Exosome Biogenesis
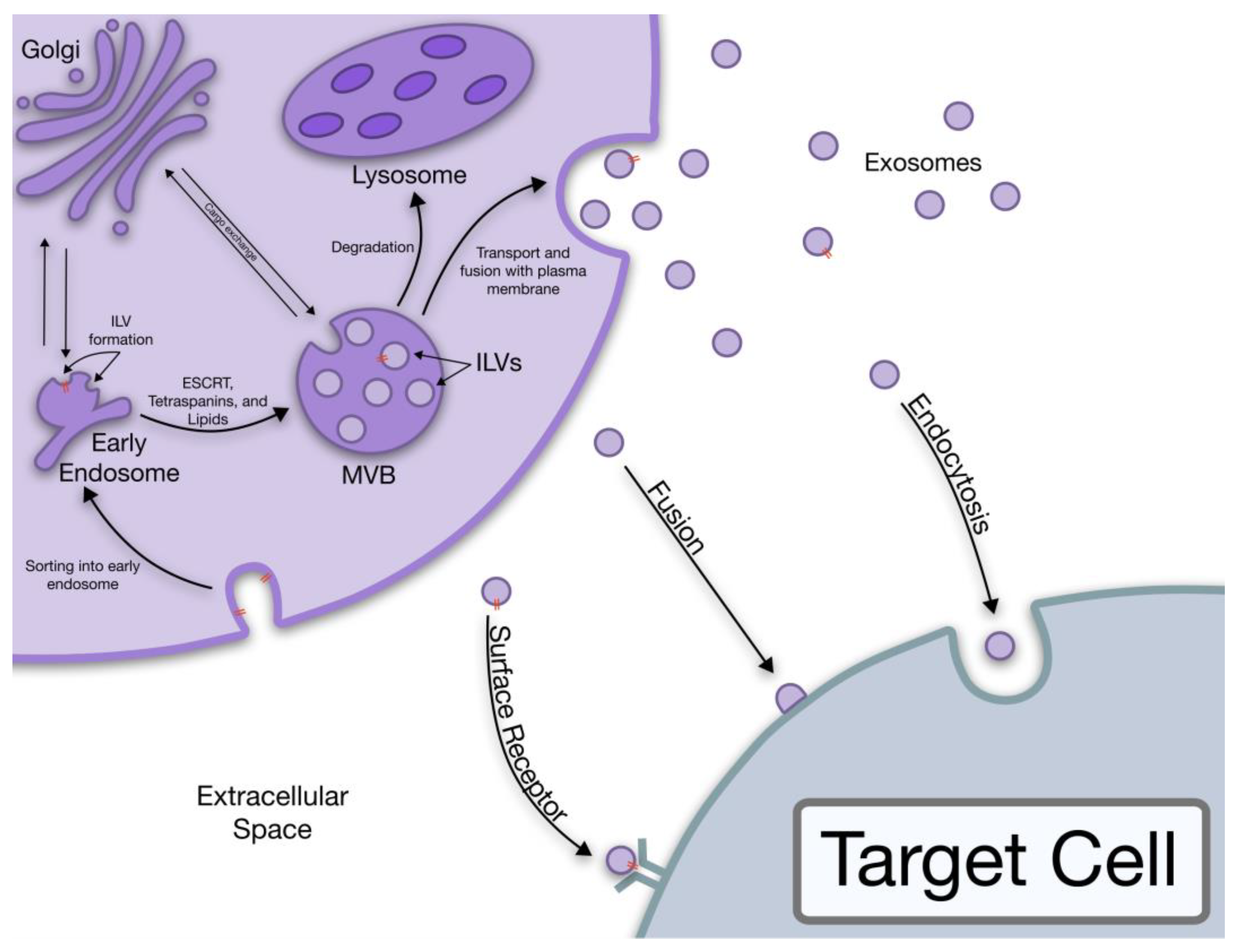
The term “exosome” was coined in 1987 by Rose Johnstone as the formation process seemed to be similar to a reversal of endocytosis [8,9]. However, we now have more understanding of the biogenesis of exosomes. The stepwise process of exosome release starts with the formation of intraluminal vesicles (ILVs) in MVBs, continues with the transportation of MVBs to the plasma membrane where they fuse to the membrane, and ends with the exosome release outside the cell [5,10]. This pathway was first observed in rat reticulocytes during the loss of the transferrin receptor (TfR) in the plasma membrane [10]. It was observed that gold labeled TfR after being endocytosed was subsequently released when the MVBs were bound to the plasma membrane. This released TfR was associated with small internal bodies about 50 nm in size [10]. It is now known that exosomes are excreted through two main pathways, the ESCRT dependent and the ESCRT independent pathway [3,6,11,12].
The ESCRT is a complicated protein “machinery” responsible for the formation and sorting of the ILVs that are destined for degradation or exosome release in the ESCRT dependent pathway. Additionally, this machinery is responsible for the sorting of the MVB cargo [3,12,13]. A few key protein complexes play a vital role in this pathway, namely the ESCRT-0, I, II, and III complexes as well as the protein exosome marker Alix [6,12]. The Alix protein has been found to be involved in endosomal membrane budding and exosome cargo selection [3,6,12,14].
The ESCRT independent pathway is reliant mostly upon lipids and membrane proteins like tetraspanins [15,16,17]. Specific sections of the lipid bilayer known as lipid rafts, or areas enriched in cholesterol, sphingolipids, and other membrane proteins, are important in the ESCRT independent pathway allowing for ceramide formation and the induction of ILV budding in MVBs [15,17]. The general process of exosome biogenesis is illustrated in Figure 1.
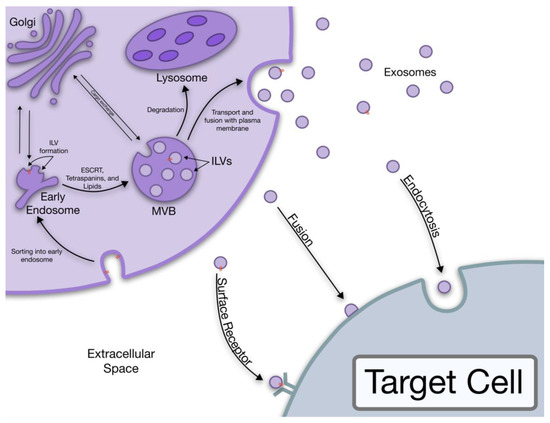
Figure 1.
The biogenesis of exosomes all the way to the uptake by target cells.
1.2. Exosome Structure
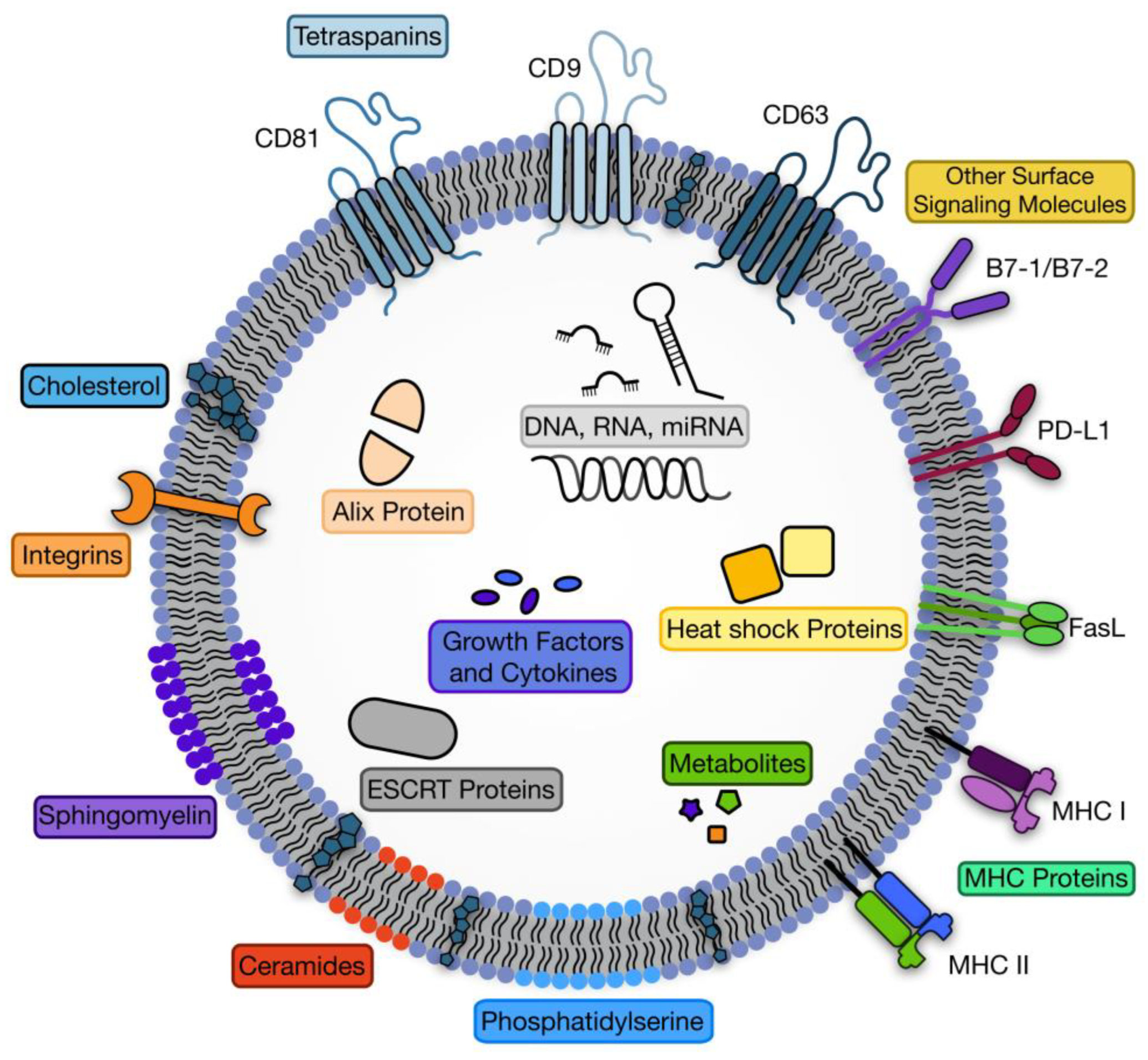
Exosomes contain biomolecules characteristic of their origin including ribonucleic acids, proteins, and specific lipids [6,11]. Although some proteins and RNAs are characteristic of the parent cell, many conserved surface protein molecules allow for exosome identification. Membrane bound proteins generally used in exosome identification include CD9, CD63, and CD81 [18]. The basic exosome structure is a lipid bilayer membrane containing minor amounts of cytosol and no organelles [3]. The lipid membrane contains comparably higher amounts of lipids such as sphingomyelin, phosphatidylcholine and Bis(monoacylglycero)phosphate (BMP), some of which indicate the exosomes endosomal origin [12]. The structure of exosomes is consistent with other endosomal vesicles, expressing proteins like tetraspanins and endosomal sorting complex required for transport (ESCRT) proteins [4,11,15]. Exosomes also contain common membrane bound proteins such as heat shock proteins (Hsp) and parent cell specific Major histocompatibility complex (MHC) Class I and II surface proteins [3,4]. The general structure and carried cargo can be seen in Figure 2.
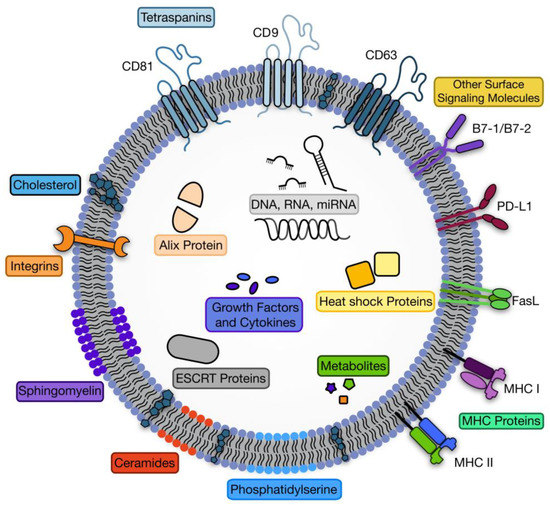
Figure 2.
The structure of exosomes including common cargo components.
1.3. Exosome Cargo
Exosomal cargo can vary based on the origin cell, but a sizable portion of the cargo appears conserved [3]. Proteins makie up a large portion of this cargo, with DNA, RNA, non-encoding miRNA, and lipids, making up the rest [3,11,18,19]. Common proteins sorted into exosomes include membrane proteins, cytoskeletal proteins, and metabolic enzymes [6,19]. The composition of these proteins varies based on the cell type and condition of the parent cell. One of the more common proteins found in exosomes is heat shock proteins, Hsp70 and Hsp90, that can facilitate peptide loading onto MHC class I and II proteins [11,20]. The MHC proteins and other exosomal membrane biomolecules are important in recognition as well as in immunomodulation [21,22,23]. Additionally, the tetraspanin family of membrane proteins can be found in copious quantities in exosomes and are characteristic markers of exosomes [6,11,12,15]. This group of tetraspanins includes CD9, CD63, CD81, and CD82 [6,11,12,15].
The lipid membranes of exosomes contain larger amounts of cholesterol and sphingomyelin which likely contribute to the characteristic membrane rigidity of exosomes. These lipids can also factor into exosome recognition and internalization [19]. The genetic content carried in exosomes has the potential to interact with distant cells after internalization. The mRNA present in exosomes is mostly degraded and not transcribable, however, there are tiny amounts of full-length mRNAs [6,19]. Aside from mRNA, exosomes also contain miRNAs, which are small non-encoding RNA sequences that can regulate gene expression through targeted RNA interference [11,15]. The specific gene targeting of miRNA is performed by complementary binding pairs at the seed, or the 2–8 nucleotide residues at the 5′ end of the miRNA guide [24,25]. The binding between miRNA and the target RNA varies in the degree of complementary base pairing, and in animals, this base pairing is most commonly a perfect match at the seed, but not throughout the entire sequence [24]. For mature miRNA to perform translation inhibition the strand must be contained within an Argonaute (AGO) protein, most commonly Ago2 [25]. The AGO-miRNA combination helps to functionalize the miRNA and facilitate translation inhibition [24,25]. This is accomplished through multiple mechanisms, but the most prominent appears to be the facilitation of mRNA degradation by the recruitment of mRNA cleaving proteins. Less commonly, the AGO-miRNA complex can block ribosome scanning and protein expression [25]. Additionally, the miRNA packaged in exosomes appears to be preferentially sorted, however, this mechanism is not fully understood [19].
2. Cancer Derived Exosome Cargo
Exosomes derived from cancer cells can provide insights into the state of the parent cell. Consistent with healthy cell-derived exosomes, CDEs contain mostly proteins and genetic fragments. The miRNA in CDEs play arguably the biggest role in disease progression compared to other carried items due to their direct effect on protein expression [26]. There is a multitude of miRNAs that are expressed in CDEs, a brief list can be seen in Table 1. These vary based on the type and state of the parent cell however, most act on recipient cells through down-regulation of specific genes [27]. This is done post transcriptionally by interfering with specific mRNA to prevent protein production through translation [24,25]. The miRNA acts as a guide binding to complementary targeted mRNA sequences [25]. Generally, cancer exhibits a downregulation of miRNAs selecting to express mainly oncogenes, as miRNA can be both oncogenes (oncomirs) and tumor suppressors [25]. Regulation of miRNA expression is thought to be controlled by DNA methylation and other environmental factors such as hypoxia and hormones [24,25]. The miRNAs found in CDEs are also preferentially and actively sorted, allowing cancer to include specific oncomirs in the excreted exosomes [14]. Exosomes also contain trace amounts of DNA fragments that can be full length oncogenes [3,28]. Such DNA was found to be mostly double stranded DNA and packaged at a higher rate in CDEs compared to healthy cell derived exosomes [28,29]. Though the presence of DNA in CDEs is well known, the packaging mechanisms are not yet known [30].

Table 1.
Common cancer promoting exosomal miRNA and their functions.
Although miRNA seems to be the most active CDE cargo, oncoproteins can still play a significant role. Oncoproteins interact with receiving cells in a variety of ways, as they are all slightly different in their mechanisms. One of the most extensively studied models of oncoproteins is the human papilloma virus oncoproteins in the causation of cervical cancer [47,48]. The proteins released through CDEs also tend to change as the cancer progresses in severity. Proteins released around the onset of cancer are reduced and replaced with proteins more indicative of metastasis as the disease progresses [49]. Common membrane proteins and surface makers on CDEs can influence neighboring cells as well. Surface markers have been shown to affect the state of, and promote metastasis in less advanced neighboring tumor cells through exosome communication [50]. Similarly, the MHC class I and II proteins present on the surface of CDEs can influence the T cells of the immune system directly or indirectly by use of antigen presenting cells [3]. Exosomal surface markers can provide clues into the origins of the CDEs, allowing for early cancer detection, diagnosis, and prognosis.
3. Cancer Exosomes Role in Immune Suppression
Cancer cells can modulate the immune system, reducing the severity of the antitumor response. The mechanisms at play in this process have long been investigated as the basis for immunotherapy, or the attempt to restore the patient’s natural antitumor immune response. One of the main problems plaguing the field of cancer immunotherapy is the lack of long-term remission and survival. Previously, traditional signaling methods were explored as potential reasons for the lack of immunotherapeutic success. Recently, CDEs have been also implicated in immunosuppression. CDEs can travel much quicker and easier than cancer cells to promote pro-cancerous signals and support immune suppression [51]. Cancers can utilize CDEs as extensions of themselves by expressing similar surface markers to their origin cell, and by carrying molecules that negatively affect immune cells [51]. Of these, the most important surface markers are common immune checkpoint inhibitors. These surface markers are important immunomodulators in the case of exaggerated or self-responses, acting as a brake for the immune system [52]. Although necessary for normal immune function, cancerous tissues can exploit the immune checkpoint inhibitors, reducing the anti-tumor response [53]. The most common immune checkpoint inhibitors in the context of cancer include programmed death protein 1 (PD-1), cytotoxic T-lymphocyte associated protein 4 (CTLA4), and Fas ligand [54]. While others are available for targeting, these were emphasized due to their prevalence in clinical trials and literature.
3.1. Exosomal PD-L1 Protein
Tumors can use various mechanisms to fly under the radar and avoid detection by the immune system. One such mechanism is the over expression of programmed death ligand 1 (PD-L1) on the cell membrane [55]. PD-L1 is the ligand for the PD-1 receptor present on the majority of immune cells, specifically activated T cells and natural killer cells [52]. One example of cancer hijacking this checkpoint inhibitor is present in cancer associated fibroblasts (CAF), which can release exosomes that promote PD-L1 expression in associated breast cancer. The CAF exosomes increase the expression of miR-92, which can then downregulate large tumor suppressor kinase 2 (LATS2), in turn increasing the nuclear translocation of yes1 associated translational receptor (YAP1). YAP1 can then bind to the enhancer region of PD-L1, increasing the expression of this protein [56]. Another study found that CDE expressed PD-L1 maintained the same membrane topology as cell expressed PD-L1, retaining its binding capability [21]. The authors also found an increase in PD-L1 expression when tumor cells were in the presence of interferon gamma (IFN-γ), secreted by activated T cells [21]. In a murine prostate cancer model the level of PD-L1 present on the surface of CDEs was directly correlated with lymph node disease state showing that CDE carried PD-L1 can make its way to lymph nodes, which inhibits T cell activation [57]. Likewise, melanoma derived exosomes were shown to decrease T cell proliferation and induce apoptosis in over 50% of co-cultured T cells in a dose-dependent manner [58]. This was partially attributed to the higher levels of PD-L1 expression on the melanoma derived exosomes [58]. PD-L1 is hugely involved in the immune suppression of cancers and has been the subject of much investigation.
PD-L1, being an important protein in the immune suppression of cancers, has become a prominent target for therapies. A common approach to counteract the effects of PD-L1/PD-1 interaction is to use a blocking antibody. Usually, this antibody is either anti-PD-L1 or anti-PD-1 [59]. This therapy has shown mixed results, with some patients demonstrating successful remission and others with high levels of CDEs expressing PD-L1 showing a weaker tumor response. The lack of response to anti-PD-1/PD-L1 treatment could be due to several factors. The prominent level of CDEs expressing PD-L1 could exhaust the T cells to a point where PD-L1 blocking therapies no longer have a great enough effect to reignite the antitumor immune response [21]. Another explanation for the lack luster effect is the ability of CDEs expressing PD-L1 to compete with the anti-PD-L1 antibodies preventing binding to cell surface PD-L1 wasting a portion of the immunotherapeutic antibodies [60]. It was also suggested that CDEs carrying PD-L1 can reach targets that the antibodies are not able to find [60]. The most promising combination therapy with anti-PD-1/PD-L1 immunotherapy appears to be the use of therapeutic approaches that minimize the secretion of CDEs. By removing the effect of CDE PD-L1 the antibodies can provide much higher response levels [60]. Similarly, the drug pembrolizumab can block all PD-L1 and PD-1 interaction allowing it to bypass the CDE mediated PD-L1 effects [21]. However, this was only successful in patients whose T cells were not pushed too far past exhaustion as previously mentioned [21].
3.2. Exosomal B7 Proteins
CTLA4 is another common immune checkpoint molecule that cancer can take advantage of to decrease the anti-tumor response. This protein is expressed on activated CD4+, CD8+, and regulatory T cells (Tregs) [61]. It functions in an analogous manner to PD-1, inhibiting the immune response of these cells by decreasing proliferation and activation levels [62]. It binds to CD80 (B7-1) and CD86 (B7-2), competing with CD28, which is an immune upregulation receptor. CTLA4 shows a higher affinity for B7 protein binding compared to CD28 [62]. This provides a target for cancer to evade the immune system by surface protein expression. In fact, cancers do express B7 proteins to deregulate T cells. In the TC-1 murine lung cancer model, the importance of CD80 expression in immunosuppression was confirmed by a knockout of the CD80 gene. Removing the CD80 allowed for tumor T cell infiltration due to the absence of the CD80/CTLA4 interaction. These knockout cancer mice showed reduced and delayed tumor growth [63]. Additionally, other cancers express the B7 proteins in a comparable manner as a part of the immune suppression cocktail present in aggressive tumors (prostate, breast, colorectal) [64,65,66]. Therefore, due to the nature of exosome biogenesis it can be reasonably assumed that the exosomes derived from these cancers will express this protein in some manner. This was verified in glioblastoma derived exosomes with CD80 being weakly expressed in isolated CDEs [67]. However, a weak expression could be more immunosuppressive as the B7 binding to CTLA4 is stronger than binding to CD28, and in the presence of low B7 availability it would lead to predominantly CTLA4/B7 pairs that are immunosuppressive, rather than CD28/B7 pairs that are immunostimulating. As a result, a mostly negative immune regulation is achieved, leading to a preferable response for tumors [63,68].
Like PD-1/PD-L1, CTLA4 has been investigated as a potential target for cancer therapies. The target of one such potential therapy is the Treg cells that are heavily recruited to the tumor environment [69,70]. Treg cells have high expression levels of CTLA-4, thus CTLA-4 can be taken advantage of by cancer cells to further suppress the antitumor response. Anti-CTLA-4 antibodies were shown to block the CTLA-4/B7 interactions resulting in the depletion of Tregs from the tumor environment. This allowed for greater CD8+ response [70]. However, clinical trials with tremelimumab, an anti-CTLA-4 blocking antibody, have been disappointing as no significant survival benefits have been found [71], apart from few recent combination therapies [72].
3.3. Exosomal FasL Protein
The Fas receptor is expressed in T cells and other immune cells and binds to the Fas ligand (FasL). This ligand is expressed in many cancer cells and when bound to the Fas receptor of immune cells can cause their apoptosis [73]. The apoptotic pathway is initiated through caspase cascade activation [73]. Colorectal cancer (CRC) derived exosomes demonstrated expression of FasL and had a direct effect on the apoptosis of CD8+ T cells [74]. FasL was also found on exosomes secreted by melanoma cells and directly affected the levels of apoptosis seen in Jurkat cells (CD8- T cell line) [75]. FasL initiated apoptosis is yet another avenue of attack that cancer cells can deploy as a mechanism to further their survival. Although preliminary studies have been done to show FasL expression on CDEs, more work should be done to determine the efficacy of blocking this interaction as a potential therapeutic target.
4. Cancer Exosomes and Metastasis Progression
CDEs have been thought of as another mechanism in the toolbelt of cancer to evade and proliferate. They are believed to help progress the cancer severity and aid in metastasis in a multitude of ways [3,10]. Specifically, the miRNA in CDEs can contribute the most to the disease state. These miRNAs can affect the tumor environment through cell uptake, causing cells to become cancerous or lead to autophagy [3,76]. The catalogue of miRNA packaged into CDEs is different for each cell type, yet most cancers express miRNA that can promote angiogenesis and cell proliferation. This is accomplished through multiple different pathways and mechanisms.
4.1. Tumor Microenvironment
Understanding exactly how CDEs can affect the tumor environment is still being extensively researched, however, recent studies have begun to illuminate the mechanisms employed by CDEs and their respective miRNA in transforming their environment. In the case of hepatocellular carcinoma derived exosomes, the transfer of miR-210 to vascular endothelial cells can promote angiogenesis through the mothers against decapentaplegic (SMAD) 4 and signal transducer and activator of transcription (STAT) 6 pathways [37,38]. The A549 lung cancer cell line can output exosomes containing miR-494 promoting angiogenesis by targeting phosphatase and tensin homolog (PTEN) and activating the protein kinase B/endothelial nitric oxide synthase (Akt/eNOS) pathway [37,40]. The miR-494 was also shown to protect the cells from apoptosis and increase proliferation by lowering caspase 2 (CASP2) expression [27]. CRC is associated with a group of miRNAs that can promote angiogenesis and tumor environment remodeling [31]. One such miRNA, miR-1229, can regulate homeodomain interacting protein kinase 2 (HIPK2) expression which can promote angiogenesis. HIPK2 is responsible for inhibiting Akt and vascular endothelial growth factor (VEGF) A in CRC cells, therefore, a downregulation of HIPK2 can promote angiogenesis in the CRC tumor environment [31,32]. Another miRNA, miR-25-3p, found in CRC derived exosomes can promote angiogenesis by regulating the expression of the vascular endothelial factor receptor (VEGFR) 2, zonula occludens-1 (ZO-1), occludin, and Claudin5 in regional endothelial cells through targeting Krüppel-like factor (KLF) 2 and KLF4. This can also promote vascular leakiness and therefore, metastatic potential [36]. The CDEs containing this miR-25-3p were found to create distant metastatic niches in preferential locations through the targeting of similar mechanisms [36]. The exploration of the therapeutic potential of targeting miR-25-3p to prevent metastasis has shown the ability to generate therapeutics by direct targeting of miRNAs [36]. Yet, again, the SMAD signaling pathway is targeted by CRC derived miR-1246 by direct regulation of the promyelocytic leukemia (PML) nuclear body (subnuclear body rich in RNA [77]) RNA causing an activation of the SMAD 1, 5, and 8 signaling pathways that in turn stimulates angiogenesis [33]. Although extensively studied in CRC, this phenomenon persists across multiple cancer types. Glioblastoma derived exosomes containing miR-9-5p can affect endothelial cells increasing angiogenesis through the downregulation of regulator of G protein signaling (RGS) 5, SRY box (SOX) 7, and ATP binding cassette subfamily B member 1 (ABCB1) genes [46]. A large barrier for researchers to hurdle is the tissue dependent effects of miRNA, such as miR-125b promoting and inhibiting breast cancer simultaneously [31]. Another factor affecting the miRNA released in CDEs is the hypoxic nature of the environment. Hypoxia is known to promote cancer metastasis and is an indicator of cancer progression [78]. As a result, CDEs are influenced by the hypoxic tumor environment. Using miR-23a, CDEs can block natural killer cells of the immune system, however, miR-23a is only present in CDEs in hypoxic environments. The regional natural killer cells are blocked by miR-23a through limiting the CD107a mRNA expression [41]. This shows the importance of hypoxia in the regulation of the tumor environment. Prostate cancer cells exhibit similar behavior in hypoxic environments, releasing exosomes with higher levels of oncoproteins, and unique signaling molecules [79]. Hypoxia not only affects the cells, but by virtue, affects the CDEs released which can shape the tumor environment to promote metastasis and disease development.
4.2. Epithelial to Mesenchymal Transition
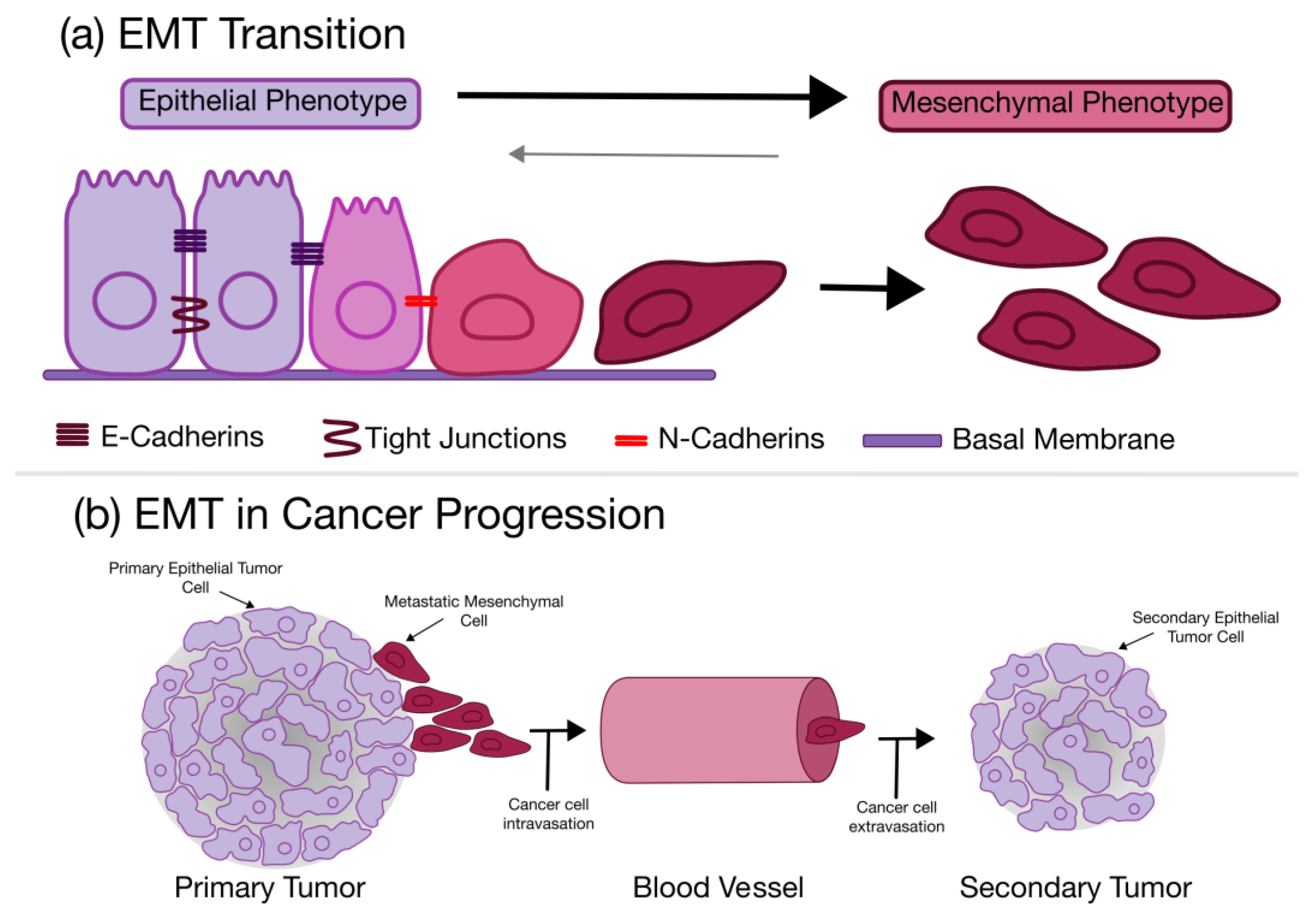
The epithelial to mesenchymal transition (EMT) is a process in which a polarized epithelial cell can undergo a phenotypic change resulting in a more mesenchymal phenotype [80]. This biochemical change creates a cell with elevated migratory potential, resistance to apoptosis, and much higher ECM production [80]. It has been hypothesized that this complex process is critical in embryogenesis and wound healing, defining the importance of the EMT in tissue and organ generation and repair [80]. The EMT process can be seen in Figure 3.
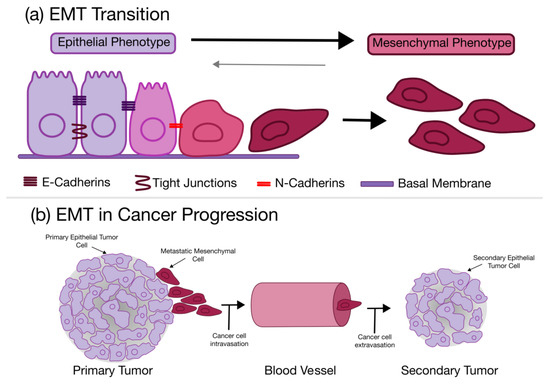
Figure 3.
The epithelial to mesenchymal transition (EMT) as it relates to cancers. (a) General overview of the transition and cell phenotype during the EMT; (b) The role of the EMT in cancer metastasis and progression.
The EMT is marked by a loss of cell–cell attachment, specifically E-cadherin in favor of N-cadherin, as well as an increase in vimentin [81]. This is accompanied by an upregulation of zinc finger protein (SNAIL) 1 and 2 transcription factors [81,82], causing an increase in phospholipase D2 (PLD2) protein that can contribute to mobility, viability and chemoresistance [83,84]. As cancer cells transition to a more mesenchymal phenotype, they begin to take on stem cell characteristics, and as such, have been dubbed cancer stem cells (CSCs). Cells having undergone the EMT showed more stemness and were equipped with stem cell traits [85,86]. The EMT bolsters cancer cells, providing higher chemoresistance, better mobility, and greater tumorigenesis potential [85,86]. This transition is present in many different cancers, but is especially apparent in prostate, lung, liver, pancreatic, and breast, some of the most aggressive cancers. It has been found that the miRNA packaged in CDEs can kickstart this process to the surrounding tissue.
One such miRNA, miR-335-5p, was displayed in exosomes released by highly metastatic SW620 CRC cells. When these CDEs were endocytosed by primary cell derived CRC cells it was found to promote CRC cell invasion and metastasis by facilitating EMT through the RAS p21 protein activator 1 (RASA1) [34]. The CDEs from more aggressive cancer cells can drive less invasive cancer cells to the same aggressive phenotype, promoting enhanced tumor spreading. Similar behavior is seen in the hepatocellular carcinoma cell line (Hep3B). Hep3B cells that had undergone the EMT produced exosomes containing miR-374-5p, which promoted proliferation, invasion, and migration in non-transitioned cells [39]. This effect was however, reversed by inhibiting miR-374a-5p demonstrating the huge role of miRNA in CDEs signaling [39].
4.3. Premetastatic Niche Formation
CDEs have been found to play a significant role in the precancerous niche formation at distant locations from the primary tumor. They not only affect the local tumor environment but can travel to preferable locations and prepare a new niche for metastatic cancer cells. To create this niche CDEs employ similar techniques involved in the original tumor site, including remodeling the secondary site, angiogenesis, and the recruitment of bone marrow-derived cells (BMDC) [87]. Recruiting BMDC is a complicated process. In the case of B16 melanoma, the CDEs released displayed an inhibition of the differentiation of the recruited BMDCs which could signal to the bone marrow to send more BMDCs [88]. Similar effects on BMDCs have also been seen in breast cancer, multiple myeloma, and colon carcinoma [87,89]. Another mechanism of BMDC recruitment involves the stimulation of the Toll-like receptor (TLR) 3 in lung epithelial cells [90]. Activation of TLR3 changes the chemokine secretion and can recruit neutrophils to the secondary tumor area. The recruitment of neutrophils to the secondary tumor site can create an inflammatory response that is pro-metastatic, changing expression profiles and again recruiting BMDCs [90]. It was also proposed that CDEs can target secondary organs in a controlled way by changing the integrins expressed on their membrane surface [90].
CDEs at the metastatic site can create similar angiogenic effects as experienced in the primary tumor environment. Again, this is facilitated by miRNA contained in CDEs which regulate and directly affect the gene expression in target cells. Pancreatic cancer cells can generate angiogenesis through release of miR-27a in exosomes to down regulate BTG anti-proliferation factor 2 (BTG2) increasing the proliferation and invasion of these cells [43]. Angiogenesis can be started by the release of CDEs from hypoxic tumor environments, which is a hallmark of metastatic cancer, containing miRNA like miR-135b, miR-210, miR-21, miR-30b, miR-30c, and miR-424. These miRNAs promote angiogenesis through the stabilization of the hypoxia inducible factor (HIF) 1 which increases the release of proangiogenic factors [91]. A higher level of expression of membrane like metalloproteinases on the surface of CDEs can also lead to higher levels of angiogenesis at metastatic sites [91,92,93]. The angiogenic potential of CDEs can be dependent on the state of the tumor environment at the time of release. A higher angiogenicity exists in CDEs from hypoxic environments. Hypoxic HCC tumor cells can produce exosomes rich in miR-23a increasing angiogenesis in endothelial cells at primed locations [42].
5. Exosomes and Flow
One of the most interesting and important characteristics of exosomes is their ability to travel throughout the body. This grants them the capacity to affect downstream tissues and extends the reach of cell signaling. The efficient transportation of exosomes has been implicated in cancer progression and metastasis, but also in additional groups of diseases, primarily viral. In recent times, this implication has been investigated in SARS-CoV-2, or COVID-19, showing the incredibly broad influences of exosomes and the obvious need for further understanding of their function [94]. Exosomes have been examined in cancers as a newly found mechanism for cell signaling and metastasis promotion, in addition, they have been identified as potential tools for the diagnosis and treatment of cancer [6]. The use of exosomes as a tool for diagnosis or treatment necessitates their precise and efficient isolation and characterization. Traditional exosome isolation methods involve a long series of differential centrifugations and ultracentrifugation to pellet only exosomes. These are extremely time-consuming processes, requiring enhanced manual labor with unclear scale-up potential. These processes also generate less than desirable exosome yields with the presence of significant impurities [95]. As a result, a clear need for improved exosome isolation methodologies exists, including processes that can lead to exosome production in good manufacturing practice (GMP) manufacturing facilities.
5.1. Microfluidics and Exosomes
A promising approach to better understand exosome function is microfluidics. Many look to microfluidics as a cost effective and easy alternative to traditional culturing techniques. Microfluidics emerged as a methodology for the manipulation of tiny amounts of liquid in micron scale channel networks. Microfluidics has been expanded to biological liquid analysis, including molecular analysis, cell analysis, drug delivery, and cell sorting [96]. By using a miniaturized process, smaller samples can be used and interactions with multiple cell types can be investigated simultaneously (from “organ-on-a-chip” to “organism-on-a-chip”). In addition, lab-on-a-chip setups using microfluidics can accommodate biosensing modalities utilizing exosomes. Microfluidics has assisted new drug development by lowering costs in in vitro testing. In the case of cancer, microfluidics can provide a more specialized environment for chemotherapy treatment in vitro testing by allowing for efficient and cost-effective screening of therapeutics on patient specific cells. Cancer screening is a major part of cancer diagnosis, and recent research is pointing towards exosomes as potential diagnostic tools. The stage and state of the cancer cells influences the contents of the released CDEs, making these CDEs potential markers for cancer diagnosis [97].
Most immunoaffinity based microfluidics exosome isolation methodologies follow similar processes, employing a generic lateral flow assay style of analyte entrapment [98]. In this methodology, the base of the microfluidic device is treated with a capture antibody, usually antiCD63, which then attaches the exosomes to the surface so they can be collected and tested [99,100]. The ExoChip, one of the first proposed microfluidics devices, makes use of lateral flow entrapment in multiple channels allowing for testing of multiple samples. [3,101]. In a separate study, this process was proven effective for the isolation and analysis of Glioblastoma multiforme (GBM) derived exosomes [99,102]. As an alternative, Zhao et al. created a methodology for exosome isolation and testing dubbed ExoSearch. This technique involved conjugating ovarian cancer exosome capture antibodies CA-125, EpCAM, and CD24, to magnetic microbeads (2.8 um). These beads were then flown in one side of a Y-shaped mixer and patient plasma containing exosomes from the other, mixing the two and in turn binding the exosomes to the beads [103]. The exosomes/beads were then isolated by a magnet at the end of the chip allowing for further testing, or in the case Zhao et al., immunostaining [103]. This technique provided much more sensitive exosome detection than Western blotting, and gave a more cost-effective and efficient detection method, with accuracy and sensitivity comparable to current diagnostics [103].
Alternatives to immunoaffinity based microfluidics approaches allow for label free separation, making use of differing physical properties between heterogenous exosome liquid samples. A common simple approach to isolate exosomes is filtration. The two most prominent methods employed include nanomembranes and nanowires. These two methods provide an easier and more cost-effective isolation method when compared to immunoaffinity based approaches [104,105,106,107]. The use of a nanomembrane is not a novel concept, however, for this use case, it has been shown to work quite well. An example of its success can be seen in the study by Cho et al. where a porous membrane is used in combination with electrophoresis to improve upon the pressure driven diffusional exosome separation [108]. The combination of these two methodologies can improve the yield and purity of isolated exosomes by increasing the flux of proteins through the membrane and collecting exosomes safely on the membrane [108]. This methodology provided purities similar to ultra-centrifugation, but with much significantly higher yields comparable to isolation by precipitation [108]. Similarly, the novel approach to exosome membrane filtration utilized in the EXODUS system by Chen et al. showed promise, outperforming traditional methods in yields and purities, eliminating the trade-offs of these methodologies [106]. The EXODUS system utilizes an interior chamber formed by two porous membranes. These membranes allow for proteins to flow through while preventing exosome flow [106]. However, the novelty lies in the vibration of the membranes by a piezoelectric crystal [106]. This prevents exosome aggregation and membrane clogging while creating a pressure change at the membrane, forcing protein contaminants through more effectively [106]. As an auxiliary to membrane entrapment, nanowires can also provide a size-dependent exosome entrapment technique. An example of this is the device manufactured by Wang et al., which used nanowire coated micropillars to trap exosomes, allowing contaminants to pass through [109]. The nanowires consisted of a dissolvable porous silicone material that allowed the entrapped exosomes to be easily recovered after filtration [109]. The fabricated chip showed good size selectivity, with a relatively high recovery percentage of ~60% for exosomes in the 83–120 nm range, possibly improving clinical relevance [109]. Additionally, particle and fluid dynamics can be manipulated to separate exosomes through a methodology known as deterministic lateral displacement (DLD). DLD microfluidics approaches use micropillars arranged in a grid such that each row is offset by a known distance relative to the previous row [105,110]. This allows for particle separation such that particles of a smaller selected size will remain in the fluid flow path, but other larger particles will contact the pillars resulting in the lateral displacement of the particle, effectively sorting the particles to separate exit points [110]. Although this technique is used for exosome isolation, it is more effective for particles with diameters above 1 micron due to the need for proportionally longer channels as particle size decreases [110]. This may appear to reduce the DLD methodology’s applicability to exosome isolation, however, this methodology has been adapted for use in the nanoscale, and has shown its efficacy, with specific size isolation and high throughput levels [111]. Another approach to physical exosome isolation and purification is acoustic fluid separation. This technique uses acoustic waves to separate particles by size, as distinct size particles experience different acoustic forces. The proposed method can isolate exosomes from a whole blood sample, which is a huge step forward in ease of use and efficiency. This is done in a stepwise process by first removing larger particles like the red blood cells, white blood cells, and platelets [112,113]. Then, the final purification takes the sorted smaller bodies, filtering and recovering the exosomes with purity of ~98% [112]. Microfluidics is becoming increasingly important in the purification of exosomes as well as the diagnosis of cancers using exosomes.
Microfluidics can also open new possibilities in tumor modeling, providing a well-organized way to investigate CDEs biological processes. Just as exosomes can spread miRNA to promote the progression of cancer, engineered exosomes can be created to deliver therapeutic miRNA. As such, engineered exosomes are now under investigation as potential therapeutic vessels for their ability to integrate with cell membranes and carry endogenous genetic material or hydrophilic molecules into cells [114]. Microfluidic systems have the potential to expedite the screening process of potential therapeutic candidates, allowing for the identification of exosomal effects to take place in a fast and efficient way. One such study by Jeong et al., examined the therapeutic effects of engineering exosomes loaded with miR-497 on a non-small cell lung cancer (NSCLC) model. The model showed an effective reduction in the proliferation of the NSCLC cells, and a reduction in the angiogenic potential of co-cultured human umbilical vein cells (HUVECs) giving some credibility for the novel therapeutic approach [114]. Although microfluidics can be useful for tissue/tumor modeling, it is not without trade-offs. The very miniaturization that provides this methodology with its high efficiency and limited cost also produces its downsides. Due to the limited cell number used, microfluidics may generate signals that cannot be effectively or accurately measured. Larger cell numbers are often needed to generate adequate levels of signals for diagnostics, and in vitro tumor models that cannot be accommodated within the limited dimensions of microfluidic networks.
5.2. Bioreactors and Exosomes
As exosomes become increasingly investigated, the need for in vitro efficient production of exosomes has also increased. Exosome generation is not trivial with many barriers, including their isolation and purification from utilized cell culture media, variability in size and surface markers, and potential contamination from media serum supplements [115,116]. With in vitro yields hovering around 1 μg of protein per mL of media, and therapeutic doses ranging between 10–100 μg/mL, there is a clear need for improvements in production efficiency [117]. To solve these problems, bioreactor systems have been developed for controllable scaleup and production. Bioreactors can be designed with a wide variety of specialized tools that can improve exosome release and isolation, and furthermore, allow for straight forward scaleup whenever needed. Flow perfusion bioreactors in particular can accommodate adherent cancer cells on scaffolds, entrapped in gels, or even present in the form of tumor spheroids, so that only small particles like exosomes can be part of circulating medium. Bioreactors can also accommodate significant cancer cell numbers avoiding many of the issues that face microfluidics. A few prominent bioreactor methodologies can be found in the literature.
One bioreactor system used in exosome research is the hollow fiber bioreactor [115]. This bioreactor uses capillary hollow fibers that are semi-permeable allowing for the diffusion of nutrients and waste through the fiber walls. These fibers are then placed in a cylindrical chamber with cells on top or between them. Media is then flowed through these fibers allowing for nutrient and gas exchange. Since the cells are held stationary exosome isolation is simplified dramatically, as the need to filter out cells is removed. This bioreactor has become popular in exosome research, yielding 5–10 times more exosome production [115,118]. Serum free media has also been used to reduce the serum protein contamination of exosomes [115,116,118]. However, serum free media can cause cell stress, which affects the exosome content and calls into question the usefulness of exosomes generated in these conditions.
Additional bioreactor systems beyond the hollow fiber bioreactor have been created. One alternative is the seesaw motion bioreactor (SMB) developed by Wu et al. [119]. The SMB uses mechanical stimulation, a rocking back and forth motion over a pivot point, to increase the exosome output in certain cell types [119]. Similarly, vertical wheel bioreactors can provide this stimulation with shear forces, and in turn doubling the exosome yields [120]. Although exosome yields are increased in bioreactors the consistency of the produced exosomes has been a challenge. Exosome consistency has been investigated using the CELLine adherent bioreactor, which is like a 2D culture flask, but utilizes a cellulose membrane to separate the cells from the media. The consistency of the produced exosomes was verified by Western blotting, nanoparticle tracking analysis, and transmission electron microscopy [121]. It was verified that this bioreactor was generating increased yields as well as consistent products, but due to its simplicity, mimicking a 2D culture, addons like shear forces or other types of mechanical stimulation could not be incorporated [121]. Another bioreactor system, originally used for bone tissue engineering applications, that utilizes flow perfusion has been used by Karami et al. to study the silencing effect of CDEs on human CD8+ T cells that have been attached to a RGD functionalized porous polymeric scaffold [122]. Flow perfusion bioreactors hold great promise to generate more physiologically relevant tumor microenvironments, with better translation to in vivo experiments. While microfluidics can also accommodate flow perfusion and mechano-stimulation, their miniaturization and low cellularity capability affect the level of signals generated, as a result limiting the ability of the user to detect changes of biological importance. Moving forward, bioreactors are expected to play a crucial role in the production and biological experimentation of exosomes for cancer research and the screening of novel therapeutics, especially in the personalized therapeutics arena.
5.3. Flow Cytometry and Exosomes
Flow cytometry (FCM) is a useful tool for analyzing surface proteins on cells. FCM is extensively used in research and clinical applications, allowing for rapid and accurate characterization. This technique has size restrictions, with a lower limit ranging from 270–600 nm, which makes the analysis of exosomes impossible [123]. Although new methods of detection in FCM are being developed, there are still attempts to alleviate the size restrictions in traditional FCM. One such way around this restriction is to create exosome clusters to increase the size of these particles to detectable levels. In a study by Liu et al., creation of exosome clusters using pH sensitive diacyl lipid conjugated polymer was tested [123]. These clusters were about 1 μm in diameter, as determined by dynamic light scattering, and were detectable with conventional flow cytometry [123]. Conjugation is a common method for increasing the size of exosomes to allow for detection in FCM. Like the microfluidics technique, one suggested detection method is to link the exosomes to magnetic beads (~3 um), thus creating an easily detectable mass of exosomes allowing for traditional FCM surface protein analysis [124,125]. This approach allows for parallel use with the ExoSearch isolation and purification procedure, which leaves the exosomes already bound to magnetic beads [103].
As an alternative approach to conventional flow cytometry, imaging flow cytometry (iFCM) allows for sub-micron level particle detection. iFCM gives the same benefits as conventional FCM at a higher resolution. As an exosome analysis method, iFCM has been proven viable allowing for accurate, easy, and high throughput phenotypic characterization of exosomes [126,127]. An additional method of surface analysis is flow cytometry using photoacoustic (PA) signals. In conventional FCM and iFCM a laser excites a fluorophore attached to the analyte, but in PAFCM the laser vibrates the analyte generating a PA signal. This signal is then captured by an ultrasonic transducer rather than an image sensor. When using PAFCM a label free detection method can be used, detecting pristine exosomes. This method can be combined with iFCM or conventional FCM to achieve higher resolution and better detection limits [128]. The combination PAFCM and fluorescent FCM give researchers the ability to use complimenting detection peaks and enabled in vivo direct analysis of CDEs in mice [128]. FCM is an extremely crucial tool for the analysis of CDEs, although, the inability of conventional FCM to provide accurate repeatable results severely hinders the study of exosomes. However, new techniques and innovations on FCM can remove this roadblock in the future.
6. Conclusions and Future Directions
As exosomes become increasingly more important in cancer research, there is urgency in better understanding these small extra-cellular vesicles. Cancer is a complicated and constantly adapting disease, making use of any means possible to survive and grow within the body. Recently, the discovery of the role CDEs play in the self-preservation of cancer has shed a new light on the importance of these bioactive vesicles in cancer related cell signaling mechanisms. As many have observed, CDEs can affect cancer progression by injecting carried bioactive molecules into the local tumor environment as well as selected premetastatic sites. Additionally, CDEs can provide local and systemic immune suppression, preventing the ability of the immune system to eliminate cancer cells. As the study of exosomes evolves and progresses, the effects of CDEs will continue being elucidated. Of particular importance are the characteristics derived from tumor cells at different stages, and the influence of these CDEs on the tumor microenvironment, the immune system, and metastasis. The effects of CDEs on the immune system, in light of the development of immune checkpoint inhibitors as cancer therapeutics, can lead to improvements of these therapies and their wider use. Furthermore, the development of new microfluidics, and bioreactor technologies for in vitro tumor models and tumor diagnostics are expanding, and they are expected to be widely used in the near future. Technologies that can improve the isolation and characterization of exosomes are emerging, and they are also expected to assist in the better understanding of the role of CDEs in cancer growth and metastasis, and the development of successful cancer therapeutics.
Author Contributions
Conceptualization, P.B. and V.I.S.; investigation, P.B. and V.I.S.; writing—original draft preparation, P.B. and V.I.S.; writing—review and editing, P.B. and V.I.S.; funding acquisition, V.I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Presbyterian Health Foundation, Grant Number 220948.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, C.; Harikumar, K.B. The Origin and Functions of Exosomes in Cancer. Front. Oncol. 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Hessvik, N.P.; Llorente, A. Current Knowledge on Exosome Biogenesis and Release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Filipazzi, P.; Bürdek, M.; Villa, A.; Rivoltini, L.; Huber, V. Recent Advances on the Role of Tumor Exosomes in Immunosuppression and Disease Progression. Semin. Cancer Biol. 2012, 22, 342–349. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Johnstone, R.M. Revisiting the Road to the Discovery of Exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Frydrychowicz, M.; Kolecka-Bednarczyk, A.; Madejczyk, M.; Yasar, S.; Dworacki, G. Exosomes—Structure, Biogenesis and Biological Role in Non-Small-Cell Lung Cancer. Scand. J. Immunol. 2015, 81, 2–10. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, Biogenesis, and Mechanisms in Cancer Metastasis and Drug Resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- The ESCRT Pathway—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1534580711002073?via%3Dihub (accessed on 19 October 2022).
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef]
- Yue, B.; Yang, H.; Wang, J.; Ru, W.; Wu, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. Exosome Biogenesis, Secretion and Function of Exosomal MiRNAs in Skeletal Muscle Myogenesis. Cell Prolif. 2020, 53, e12857. [Google Scholar] [CrossRef]
- Babst, M. MVB Vesicle Formation: ESCRT-Dependent, ESCRT-Independent and Everything in Between. Curr. Opin. Cell Biol. 2011, 23, 452–457. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Mathivanan, S.; Fahner, C.J.; Reid, G.E.; Simpson, R.J. ExoCarta 2012: Database of Exosomal Proteins, RNA and Lipids. Nucleic Acids Res. 2012, 40, D1241–D1244. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Ding, W.-Q. Intercellular Communication by Exosome-Derived MicroRNAs in Cancer. Int. J. Mol. Sci. 2013, 14, 14240–14269. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Théry, C. Exosome Secretion: Molecular Mechanisms and Roles in Immune Responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, Z.; Wu, W.; Wang, Z.; Zhang, N.; Zhang, L.; Zeng, W.-J.; Liu, Z.; Cheng, Q. Regulatory Mechanisms of Immune Checkpoints PD-L1 and CTLA-4 in Cancer. J. Exp. Clin. Cancer Res. 2021, 40, 184. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Ding, Y.; Xue, Z.; Li, P.; Li, J.; Li, F. Roles of Exosomes as Drug Delivery Systems in Cancer Immunotherapy: A Mini-Review. Discov. Oncol. 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Gebert, L.F.R.; MacRae, I.J. Regulation of MicroRNA Function in Animals. Nat. Rev. Mol. Cell Biol 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of MiRNA-Mediated Gene Regulation from Common Downregulation to MRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhao, M.; Lin, H.; Wang, W.; Li, D.; Cui, W.; Zhou, C.; Zhong, J.; Huang, C. MiR-494 Acts as a Tumor Promoter by Targeting CASP2 in Non-Small Cell Lung Cancer. Sci. Rep. 2019, 9, 3008. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 275–280. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.-F.; Thakur, B.K. DNA in Extracellular Vesicles: From Evolution to Its Current Application in Health and Disease. Cell Biosci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Tang, Y.; Zong, S.; Zeng, H.; Ruan, X.; Yao, L.; Han, S.; Hou, F. MicroRNAs and Angiogenesis: A New Era for the Management of Colorectal Cancer. Cancer Cell Int. 2021, 21, 221. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Yu, C.-H.; Zhang, H.-H.; Zhang, S.-Z.; Yu, W.-Y.; Yang, Y.; Chen, Q. Exosomal MiR-1229 Derived from Colorectal Cancer Cells Promotes Angiogenesis by Targeting HIPK2. Int. J. Biol. Macromol. 2019, 132, 470–477. [Google Scholar] [CrossRef]
- Yamada, N.; Tsujimura, N.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Nakagawa, Y.; Naoe, T.; Akao, Y. Colorectal Cancer Cell-Derived Microvesicles Containing MicroRNA-1246 Promote Angiogenesis by Activating Smad 1/5/8 Signaling Elicited by PML down-Regulation in Endothelial Cells. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2014, 1839, 1256–1272. [Google Scholar] [CrossRef]
- Sun, X.; Lin, F.; Sun, W.; Zhu, W.; Fang, D.; Luo, L.; Li, S.; Zhang, W.; Jiang, L. Exosome-Transmitted MiRNA-335-5p Promotes Colorectal Cancer Invasion and Metastasis by Facilitating EMT via Targeting RASA1. Mol. Ther.-Nucleic Acids 2021, 24, 164–174. [Google Scholar] [CrossRef]
- Dai, W.; Zhou, J.; Wang, H.; Zhang, M.; Yang, X.; Song, W. MiR-424-5p Promotes the Proliferation and Metastasis of Colorectal Cancer by Directly Targeting SCN4B. Pathol.-Res. Pract. 2020, 216, 152731. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-Derived Exosomal MiR-25-3p Promotes Pre-Metastatic Niche Formation by Inducing Vascular Permeability and Angiogenesis. Nat. Commun. 2018, 9, 5395. [Google Scholar] [CrossRef]
- Li, B.; Cao, Y.; Sun, M.; Feng, H. Expression, Regulation, and Function of Exosome-Derived MiRNAs in Cancer Progression and Therapy. FASEB J. 2021, 35, e21916. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging Role of Exosomes in Cancer Progression and Tumor Microenvironment Remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Lin, Q.; Zhou, C.-R.; Bai, M.-J.; Zhu, D.; Chen, J.-W.; Wang, H.-F.; Li, M.-A.; Wu, C.; Li, Z.-R.; Huang, M.-S. Exosome-Mediated MiRNA Delivery Promotes Liver Cancer EMT and Metastasis. Am. J. Transl. Res. 2020, 12, 1080–1095. [Google Scholar]
- Mao, G.; Liu, Y.; Fang, X.; Liu, Y.; Fang, L.; Lin, L.; Liu, X.; Wang, N. Tumor-Derived MicroRNA-494 Promotes Angiogenesis in Non-Small Cell Lung Cancer. Angiogenesis 2015, 18, 373–382. [Google Scholar] [CrossRef]
- Berchem, G.; Noman, M.Z.; Bosseler, M.; Paggetti, J.; Baconnais, S.; Le Cam, E.; Nanbakhsh, A.; Moussay, E.; Mami-Chouaib, F.; Janji, B.; et al. Hypoxic Tumor-Derived Microvesicles Negatively Regulate NK Cell Function by a Mechanism Involving TGF-β and MiR23a Transfer. OncoImmunology 2016, 5, e1062968. [Google Scholar] [CrossRef]
- Sruthi, T.V.; Edatt, L.; Raji, G.R.; Kunhiraman, H.; Shankar, S.S.; Shankar, V.; Ramachandran, V.; Poyyakkara, A.; Kumar, S.V.B. Horizontal Transfer of MiR-23a from Hypoxic Tumor Cell Colonies Can Induce Angiogenesis. J. Cell. Physiol. 2018, 233, 3498–3514. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic Cancer Cell–Derived Exosomal MicroRNA-27a Promotes Angiogenesis of Human Microvascular Endothelial Cells in Pancreatic Cancer via BTG2. J. Cell. Mol. Med. 2020, 24, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes Derived from Hypoxic Oral Squamous Cell Carcinoma Cells Deliver MiR-21 to Normoxic Cells to Elicit a Prometastatic Phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, K.; Chen, L.; Du, M.; Qu, Z. Exosomal CircGDI2 Suppresses Oral Squamous Cell Carcinoma Progression Through the Regulation of MiR-424-5p/SCAI Axis. Cancer Manag. Res. 2020, 12, 7501–7514. [Google Scholar] [CrossRef] [PubMed]
- Lucero, R.; Zappulli, V.; Sammarco, A.; Murillo, O.D.; Cheah, P.S.; Srinivasan, S.; Tai, E.; Ting, D.T.; Wei, Z.; Roth, M.E.; et al. Glioma-Derived MiRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020, 30, 2065–2074.e4. [Google Scholar] [CrossRef] [PubMed]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The Human Papillomavirus Oncoproteins: A Review of the Host Pathways Targeted on the Road to Transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef]
- Chiantore, M.V.; Mangino, G.; Iuliano, M.; Zangrillo, M.S.; De Lillis, I.; Vaccari, G.; Accardi, R.; Tommasino, M.; Columba Cabezas, S.; Federico, M.; et al. Human Papillomavirus E6 and E7 Oncoproteins Affect the Expression of Cancer-Related MicroRNAs: Additional Evidence in HPV-Induced Tumorigenesis. J. Cancer Res. Clin. Oncol. 2016, 142, 1751–1763. [Google Scholar] [CrossRef]
- Konstantinell, A.; Bruun, J.-A.; Olsen, R.; Aspar, A.; Škalko-Basnet, N.; Sveinbjørnsson, B.; Moens, U. Secretomic Analysis of Extracellular Vesicles Originating from Polyomavirus-Negative and Polyomavirus-Positive Merkel Cell Carcinoma Cell Lines. Proteomics 2016, 16, 2587–2591. [Google Scholar] [CrossRef]
- Luga, V.; Zhang, L.; Viloria-Petit, A.M.; Ogunjimi, A.A.; Inanlou, M.R.; Chiu, E.; Buchanan, M.; Hosein, A.N.; Basik, M.; Wrana, J.L. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell 2012, 151, 1542–1556. [Google Scholar] [CrossRef]
- Hofmann, L.; Ludwig, S.; Vahl, J.M.; Brunner, C.; Hoffmann, T.K.; Theodoraki, M.-N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 4072. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer Immunotherapies Targeting the PD-1 Signaling Pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef]
- Sholl, L.M. Biomarkers of Response to Checkpoint Inhibitors beyond PD-L1 in Lung Cancer. Mod. Pathol. 2022, 35, 66–74. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. Pd-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- Dou, D.; Ren, X.; Han, M.; Xu, X.; Ge, X.; Gu, Y.; Wang, X. Cancer-Associated Fibroblasts-Derived Exosomes Suppress Immune Cell Function in Breast Cancer via the MiR-92/PD-L1 Pathway. Front. Immunol. 2020, 11, 2026. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef]
- Sharma, P.; Diergaarde, B.; Ferrone, S.; Kirkwood, J.M.; Whiteside, T.L. Melanoma Cell-Derived Exosomes in Plasma of Melanoma Patients Suppress Functions of Immune Effector Cells. Sci. Rep. 2020, 10, 92. [Google Scholar] [CrossRef]
- Araki, Y.; Mimura, T. The Mechanisms Underlying Chronic Inflammation in Rheumatoid Arthritis from the Perspective of the Epigenetic Landscape. J. Immunol. Res. 2016, 2016, e6290682. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.-C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-Tumor Immunity and Memory. Cell 2019, 177, 414–427.e13. [Google Scholar] [CrossRef]
- Chan, D.V.; Gibson, H.M.; Aufiero, B.M.; Wilson, A.J.; Hafner, M.S.; Mi, Q.-S.; Wong, H.K. Differential CTLA-4 Expression in Human CD4+ versus CD8+ T Cells Is Associated with Increased NFAT1 and Inhibition of CD4+ Proliferation. Genes Immun. 2014, 15, 25–32. [Google Scholar] [CrossRef]
- Xing, C.; Li, H.; Li, R.-J.; Yin, L.; Zhang, H.-F.; Huang, Z.-N.; Cheng, Z.; Li, J.; Wang, Z.-H.; Peng, H.-L. The Roles of Exosomal Immune Checkpoint Proteins in Tumors. Mil. Med. Res. 2021, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Vackova, J.; Polakova, I.; Johari, S.D.; Smahel, M. CD80 Expression on Tumor Cells Alters Tumor Microenvironment and Efficacy of Cancer Immunotherapy by CTLA-4 Blockade. Cancers 2021, 13, 1935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, Y.; Yu, H.; Lin, S.-H.; Tu, H.; Liang, D.; Chang, D.W.; Huang, M.; Wu, X. Immune Checkpoint-Related Serum Proteins and Genetic Variants Predict Outcomes of Localized Prostate Cancer, a Cohort Study. Cancer Immunol. Immunother. 2021, 70, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, W.; Zhang, L. The Overexpression of CD80 and ISG15 Are Associated with the Progression and Metastasis of Breast Cancer by a Meta-Analysis Integrating Three Microarray Datasets. Pathol. Oncol. Res. 2020, 26, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, Z.; Pirpour Tazehkand, A.; Mansoori, B.; Khaze, V.; Asadi, M.; Baradaran, B.; Samadi, N. The Impact of Nrf2 Silencing on Nrf2-PD-L1 Axis to Overcome Oxaliplatin Resistance as Well as Migration in Colon Cancer. Avicenna J. Med. Biotechnol. 2021, 13, 116–122. [Google Scholar] [CrossRef]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.; Rao, A.; Braganhol, E.; Whiteside, T.L. Molecular Profiles and Immunomodulatory Activities of Glioblastoma-Derived Exosomes. Neurooncol. Adv. 2020, 2, vdaa056. [Google Scholar] [CrossRef]
- Sansom, D.M. CD28, CTLA-4 and Their Ligands: Who Does What and to Whom? Immunology 2000, 101, 169–177. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-Regulatory Cells: Key Players in Tumor Immune Escape and Angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.A.; Haanen, J.B.; Marmol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III Randomized Clinical Trial Comparing Tremelimumab With Standard-of-Care Chemotherapy in Patients With Advanced Melanoma. JCO 2013, 31, 616–622. [Google Scholar] [CrossRef]
- Johnson, M.L.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Kim, S.-W.; Ursol, G.; Hussein, M.; Lim, F.L.; et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non–Small-Cell Lung Cancer: The Phase III POSEIDON Study. JCO 2022, JCO.22.00975. [Google Scholar] [CrossRef]
- Kugeratski, F.G.; Kalluri, R. Exosomes as Mediators of Immune Regulation and Immunotherapy in Cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Huber, V.; Fais, S.; Iero, M.; Lugini, L.; Canese, P.; Squarcina, P.; Zaccheddu, A.; Colone, M.; Arancia, G.; Gentile, M.; et al. Human Colorectal Cancer Cells Induce T-Cell Death Through Release of Proapoptotic Microvesicles: Role in Immune Escape. Gastroenterology 2005, 128, 1796–1804. [Google Scholar] [CrossRef]
- Andreola, G.; Rivoltini, L.; Castelli, C.; Huber, V.; Perego, P.; Deho, P.; Squarcina, P.; Accornero, P.; Lozupone, F.; Lugini, L.; et al. Induction of Lymphocyte Apoptosis by Tumor Cell Secretion of FasL-Bearing Microvesicles. J. Exp. Med. 2002, 195, 1303–1316. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, H.; Lv, C.; Lan, F.; Wang, Y.; Deng, Y. Exosomes and Exosomal MicroRNA in Non-Targeted Radiation Bystander and Abscopal Effects in the Central Nervous System. Cancer Lett. 2021, 499, 73–84. [Google Scholar] [CrossRef]
- Boisvert, F.-M.; Hendzel, M.J.; Bazett-Jones, D.P. Promyelocytic Leukemia (Pml) Nuclear Bodies Are Protein Structures That Do Not Accumulate RNA. J. Cell Biol. 2000, 148, 283–292. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. Hypoxic Control of Metastasis. Science 2016, 352, 175–180. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes Secreted under Hypoxia Enhance Invasiveness and Stemness of Prostate Cancer Cells by Targeting Adherens Junction Molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Kaufhold, S.; Bonavida, B. Central Role of Snail1 in the Regulation of EMT and Resistance in Cancer: A Target for Therapeutic Intervention. J. Exp. Clin. Cancer Res. 2014, 33, 62. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.R.; Hogg, K.; Suman, R.; Berney, D.M.; Bourgoin, S.; Maitland, N.J.; Rumsby, M.G. Phospholipase D2 in Prostate Cancer: Protein Expression Changes with Gleason Score. Br. J. Cancer 2019, 121, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Mallets, E.; Gomez-Cambronero, J. The Transcription Factors Slug (SNAI2) and Snail (SNAI1) Regulate Phospholipase D (PLD) Promoter in Opposite Ways towards Cancer Cell Invasion. Mol. Oncol. 2016, 10, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.-J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- May, C.D.; Sphyris, N.; Evans, K.W.; Werden, S.J.; Guo, W.; Mani, S.A. Epithelial-Mesenchymal Transition and Cancer Stem Cells: A Dangerously Dynamic Duo in Breast Cancer Progression. Breast Cancer Res. 2011, 13, 202. [Google Scholar] [CrossRef]
- Lobb, R.J.; Lima, L.G.; Möller, A. Exosomes: Key Mediators of Metastasis and Pre-Metastatic Niche Formation. Semin. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, X.; Zhuang, X.; Zhang, S.; Liu, C.; Cheng, Z.; Michalek, S.; Grizzle, W.; Zhang, H.-G. Contribution of MyD88 to the Tumor Exosome-Mediated Induction of Myeloid Derived Suppressor Cells. Am. J. Pathol. 2010, 176, 2490–2499. [Google Scholar] [CrossRef]
- Wang, J.; De Veirman, K.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple Myeloma Exosomes Establish a Favourable Bone Marrow Microenvironment with Enhanced Angiogenesis and Immunosuppression. J. Pathol. 2016, 239, 162–173. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-Metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef]
- Olejarz, W.; Kubiak-Tomaszewska, G.; Chrzanowska, A.; Lorenc, T. Exosomes in Angiogenesis and Anti-Angiogenic Therapy in Cancers. Int. J. Mol. Sci. 2020, 21, 5840. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, J.; Liu, T.; Zhang, J.; Dai, Z.; Zhang, H.; Wang, D.; Tang, D. Small Extracellular Vesicles: From Mediating Cancer Cell Metastasis to Therapeutic Value in Pancreatic Cancer. Cell Commun. Signal. 2022, 20, 1. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-Type Matrix Metalloproteinases: Their Functions and Regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating Exosomes Are Strongly Involved in SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef]
- Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef]
- Tai, Y.; Chen, K.; Hsieh, J.; Shen, T. Exosomes in Cancer Development and Clinical Applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; López-Cobo, S.; Reyburn, H.T.; Costa-García, A.; López-Martín, S.; Yáñez-Mó, M.; Cernuda-Morollón, E.; Paschen, A.; Valés-Gómez, M.; Blanco-López, M.C. Development of a Rapid Lateral Flow Immunoassay Test for Detection of Exosomes Previously Enriched from Cell Culture Medium and Body Fluids. J. Extracell. Vesicles 2016, 5, 31803. [Google Scholar] [CrossRef]
- Chen, C.; Skog, J.; Hsu, C.-H.; Lessard, R.T.; Balaj, L.; Wurdinger, T.; Carter, B.S.; Breakefield, X.O.; Toner, M.; Irimia, D. Microfluidic Isolation and Transcriptome Analysis of Serum Microvesicles. Lab Chip 2010, 10, 505–511. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Xue, Y.; Qiao, L.; Yu, G.; Liu, Y.; Yu, S. Ultrasensitive Analysis of Exosomes Using a 3D Self-Assembled Nanostructured SiO2 Microfluidic Chip. ACS Appl. Mater. Interfaces 2022, 14, 14693–14702. [Google Scholar] [CrossRef]
- Kanwar, S.S.; Dunlay, C.J.; Simeone, D.M.; Nagrath, S. Microfluidic Device (ExoChip) for on-Chip Isolation, Quantification and Characterization of Circulating Exosomes. Lab Chip 2014, 14, 1891–1900. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Nagrath, S. Microfluidics and Cancer: Are We There Yet? Biomed. Microdevices 2013, 15, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yang, Y.; Zeng, Y.; He, M. A Microfluidic ExoSearch Chip for Multiplexed Exosome Detection towards Blood-Based Ovarian Cancer Diagnosis. Lab Chip 2016, 16, 489–496. [Google Scholar] [CrossRef]
- Davies, R.T.; Kim, J.; Jang, S.C.; Choi, E.-J.; Gho, Y.S.; Park, J. Microfluidic Filtration System to Isolate Extracellular Vesicles from Blood. Lab Chip 2012, 12, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Lu, Y.; Luo, X.; Huang, Y.; Xie, T.; Pilarsky, C.; Dang, Y.; Zhang, J. Microfluidic Technology for the Isolation and Analysis of Exosomes. Micromachines 2022, 13, 1571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Q.; Cheng, L.; Wang, Y.; Li, M.; Yang, Q.; Hu, L.; Lou, D.; Li, J.; Dong, X.; et al. Exosome Detection via the Ultrafast-Isolation System: EXODUS. Nat. Methods 2021, 18, 212–218. [Google Scholar] [CrossRef]
- Liu, F.; Vermesh, O.; Mani, V.; Ge, T.J.; Madsen, S.J.; Sabour, A.; Hsu, E.-C.; Gowrishankar, G.; Kanada, M.; Jokerst, J.V.; et al. The Exosome Total Isolation Chip. ACS Nano 2017, 11, 10712–10723. [Google Scholar] [CrossRef]
- Cho, S.; Jo, W.; Heo, Y.; Kang, J.Y.; Kwak, R.; Park, J. Isolation of Extracellular Vesicle from Blood Plasma Using Electrophoretic Migration through Porous Membrane. Sens. Actuators B Chem. 2016, 233, 289–297. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Fine, D.; Schmulen, J.; Hu, Y.; Godin, B.; Zhang, J.X.J.; Liu, X. Ciliated Micropillars for the Microfluidic-Based Isolation of Nanoscale Lipid Vesicles. Lab Chip 2013, 13, 2879–2882. [Google Scholar] [CrossRef]
- Abreu, C.M.; Caballero, D.; Kundu, S.C.; Reis, R.L. From Exosomes to Circulating Tumor Cells: Using Microfluidics to Detect High Predictive Cancer Biomarkers. In Microfluidics and Biosensors in Cancer Research: Applications in Cancer Modeling and Theranostics; Caballero, D., Kundu, S.C., Reis, R.L., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2022; pp. 369–387. ISBN 978-3-031-04039-9. [Google Scholar]
- Wunsch, B.H.; Smith, J.T.; Gifford, S.M.; Wang, C.; Brink, M.; Bruce, R.L.; Austin, R.H.; Stolovitzky, G.; Astier, Y. Nanoscale Lateral Displacement Arrays for the Separation of Exosomes and Colloids down to 20 Nm. Nat. Nanotech 2016, 11, 936–940. [Google Scholar] [CrossRef]
- Wu, M.; Ouyang, Y.; Wang, Z.; Zhang, R.; Huang, P.-H.; Chen, C.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of Exosomes from Whole Blood by Integrating Acoustics and Microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 2020, 16, 1903916. [Google Scholar] [CrossRef]
- Jeong, K.; Jun Yu, Y.; Young You, J.; Jong Rhee, W.; Ah Kim, J. Exosome-Mediated MicroRNA-497 Delivery for Anti-Cancer Therapy in a Microfluidic 3D Lung Cancer Model. Lab Chip 2020, 20, 548–557. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-Scale Production, Isolation, Drug Loading Efficiency, and Biodistribution and Uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lin, E.-Y.; Chiou, T.-W.; Harn, H.-J. Exosomes in Clinical Trial and Their Production in Compliance with Good Manufacturing Practice. Tzu. Chi Med. J. 2019, 32, 113–120. [Google Scholar] [CrossRef]
- Kim, H.; Kim, E.H.; Kwak, G.; Chi, S.-G.; Kim, S.H.; Yang, Y. Exosomes: Cell-Derived Nanoplatforms for the Delivery of Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 14. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient Production and Enhanced Tumor Delivery of Engineered Extracellular Vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef]
- Wu, J.; Wu, D.; Wu, G.; Bei, H.-P.; Li, Z.; Xu, H.; Wang, Y.; Wu, D.; Liu, H.; Shi, S.; et al. Scale-out Production of Extracellular Vesicles Derived from Natural Killer Cells via Mechanical Stimulation in a Seesaw-Motion Bioreactor for Cancer Therapy. Biofabrication 2022, 14, 045004. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Canonicco Castro, M.L.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling Human Mesenchymal Stromal Cell Production in a Novel Vertical-Wheel Bioreactor Enhances Extracellular Vesicle Secretion and Cargo Profile. Bioact. Mater. 2022. [Google Scholar] [CrossRef]
- Hisey, C.L.; Artuyants, A.; Guo, G.; Chang, V.; Reshef, G.; Middleditch, M.; Jacob, B.; Chamley, L.W.; Blenkiron, C. Investigating the Consistency of Extracellular Vesicle Production from Breast Cancer Subtypes Using CELLine Adherent Bioreactors. bioRxiv 2022. [Google Scholar] [CrossRef]
- Karami, D.; Srivastava, A.; Ramesh, R.; Sikavitsas, V.I. Investigating Cancerous Exosomes’ Effects on CD8+ T-Cell IL-2 Production in a 3D Unidirectional Flow Bioreactor Using 3D Printed, RGD-Functionalized PLLA Scaffolds. J. Funct. Biomater. 2022, 13, 30. [Google Scholar] [CrossRef]
- Liu, X.; Zong, Z.; Xing, M.; Liu, X.; Li, J.; Liu, D. PH-Mediated Clustering of Exosomes: Breaking Through the Size Limit of Exosome Analysis in Conventional Flow Cytometry. Nano Lett. 2021, 21, 8817–8823. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Hong, C.-S.; Donnenberg, V.S.; Donnenberg, A.D.; Whiteside, T.L. Evaluation of Exosome Proteins by On-Bead Flow Cytometry. Cytom. Part A 2021, 99, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Moura, S.L.; Martín, C.G.; Martí, M.; Pividori, M.I. Multiplex Detection and Characterization of Breast Cancer Exosomes by Magneto-Actuated Immunoassay. Talanta 2020, 211, 120657. [Google Scholar] [CrossRef] [PubMed]
- Mastoridis, S.; Bertolino, G.M.; Whitehouse, G.; Dazzi, F.; Sanchez-Fueyo, A.; Martinez-Llordella, M. Multiparametric Analysis of Circulating Exosomes and Other Small Extracellular Vesicles by Advanced Imaging Flow Cytometry. Front. Immunol. 2018, 9, 1583. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Rudy, C.K.; Etter, M.E.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging Flow Cytometry Elucidates Limitations of Microparticle Analysis by Conventional Flow Cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef]
- Nolan, J.; Sarimollaoglu, M.; Nedosekin, D.A.; Jamshidi-Parsian, A.; Galanzha, E.I.; Kore, R.A.; Griffin, R.J.; Zharov, V.P. In Vivo Flow Cytometry of Circulating Tumor-Associated Exosomes. Anal. Cell. Pathol. 2016, 2016, e1628057. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).