Foam Based Fracturing Fluid Characterization for an Optimized Application in HPHT Reservoir Conditions
Abstract
:1. Introduction
- Have high viscosity to carry and place proppant into the fractures. As more proppants are transported and settled over a long distance, a more conductive pathway is created for oil and gas to flow;
- Be compatible with formation rock, reservoir fluid and the designed additives and proppant;
- Can generate a wide fracture by creating a high-pressure drop along the fracture;
- Achieve low viscosity after main treatment so that the fracturing fluid can easily flow back to the surface before hydrocarbon is produced;
- Be cost-effective and environmentally friendly.
1.1. The Basics of Foam-Based Fracturing Fluids
1.2. Advantages and Limitations of Foam-Based Fracturing Fluids
2. Rheological Characteristics of Foam-Based Fluids
2.1. Foam Rheology
2.2. Factors That Affect Foam Rheology
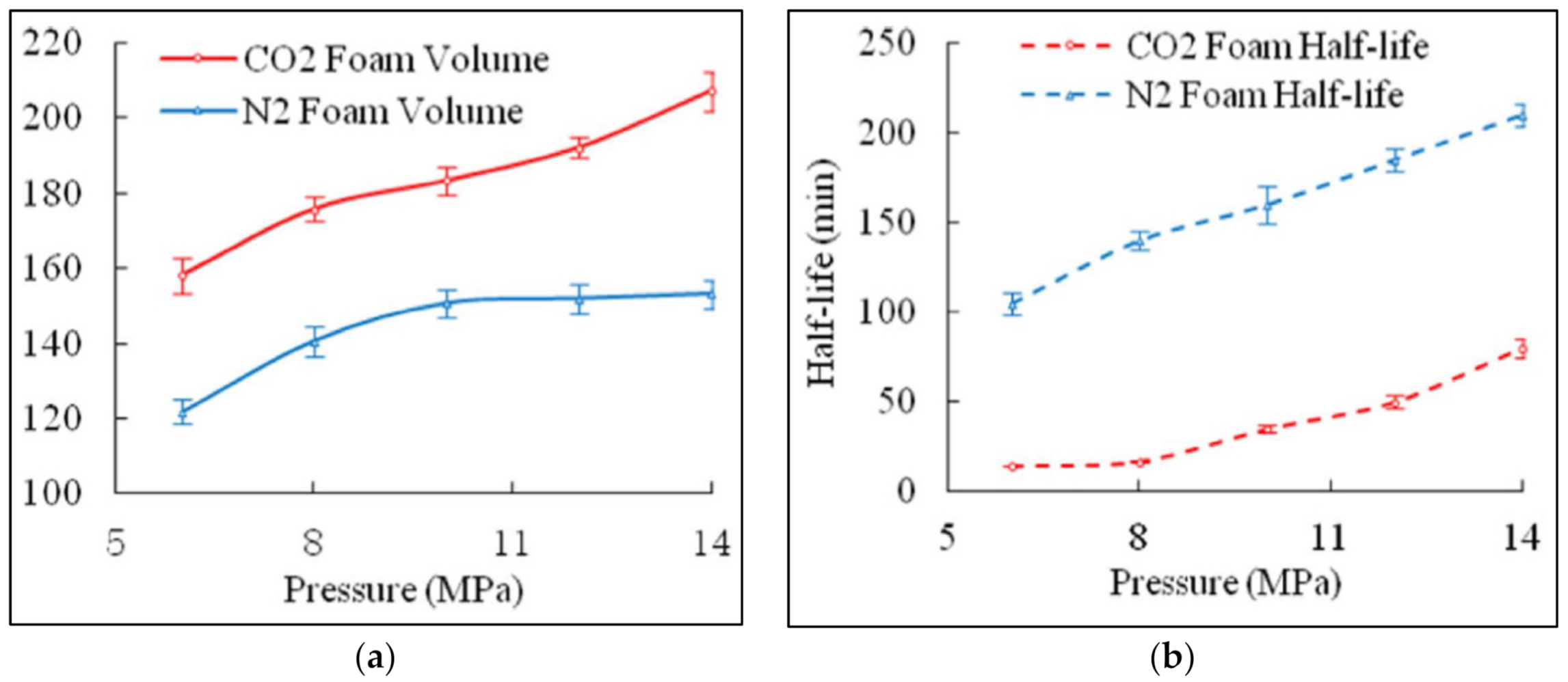
2.2.1. Effect of Pressure on Foam Rheology
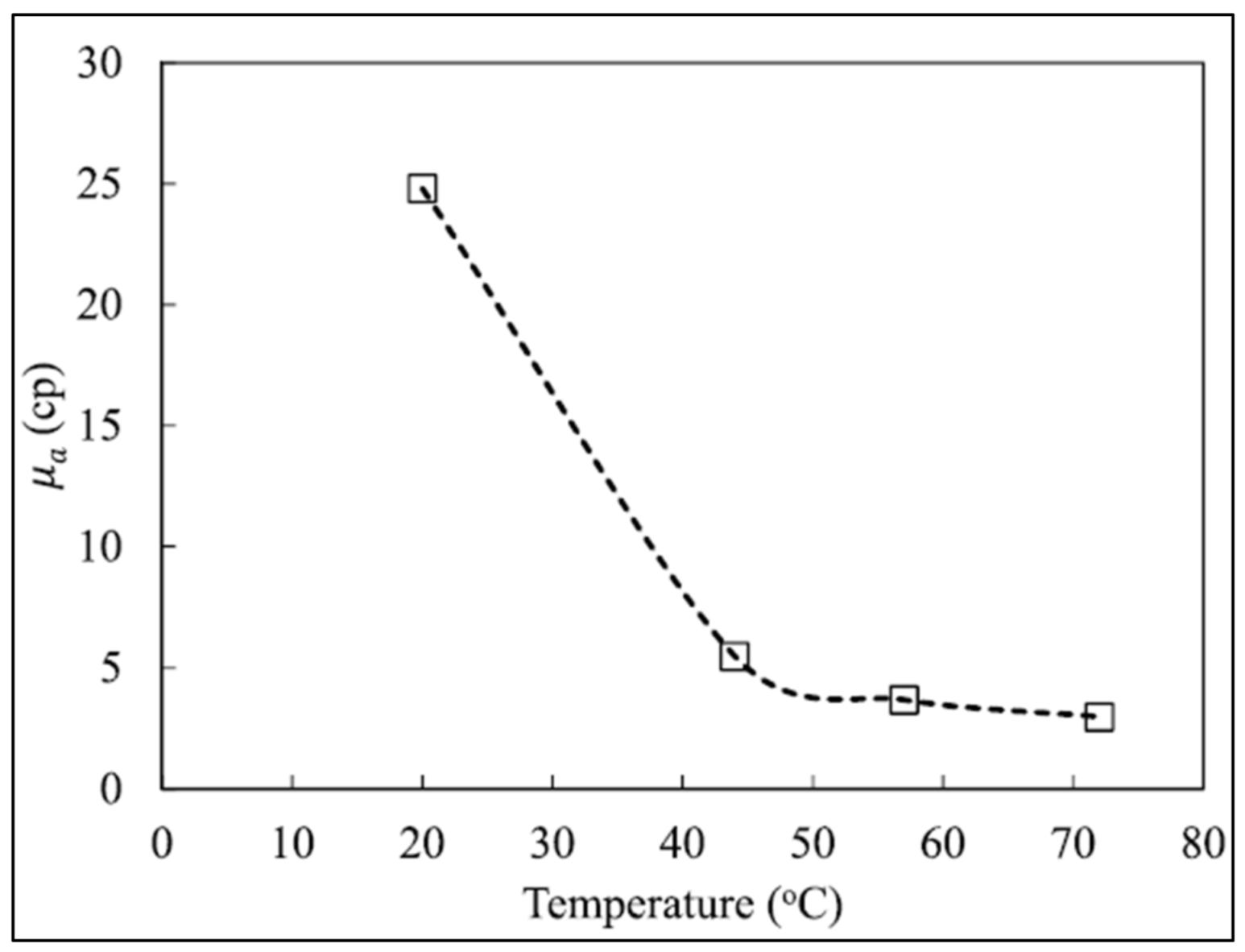
2.2.2. Effect of Temperature on Foam Rheology
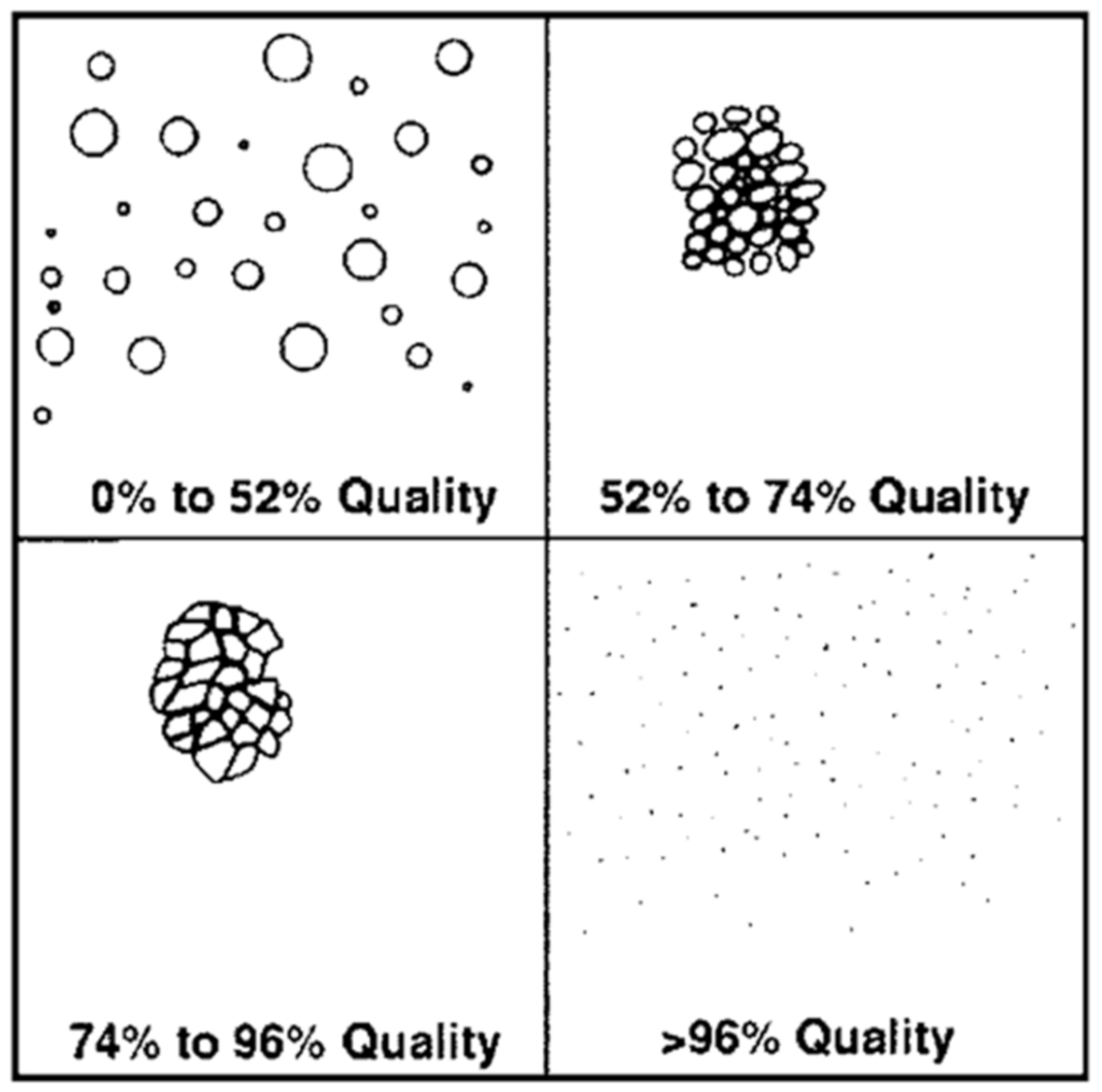
2.2.3. Effect of Foam Quality on Rheology
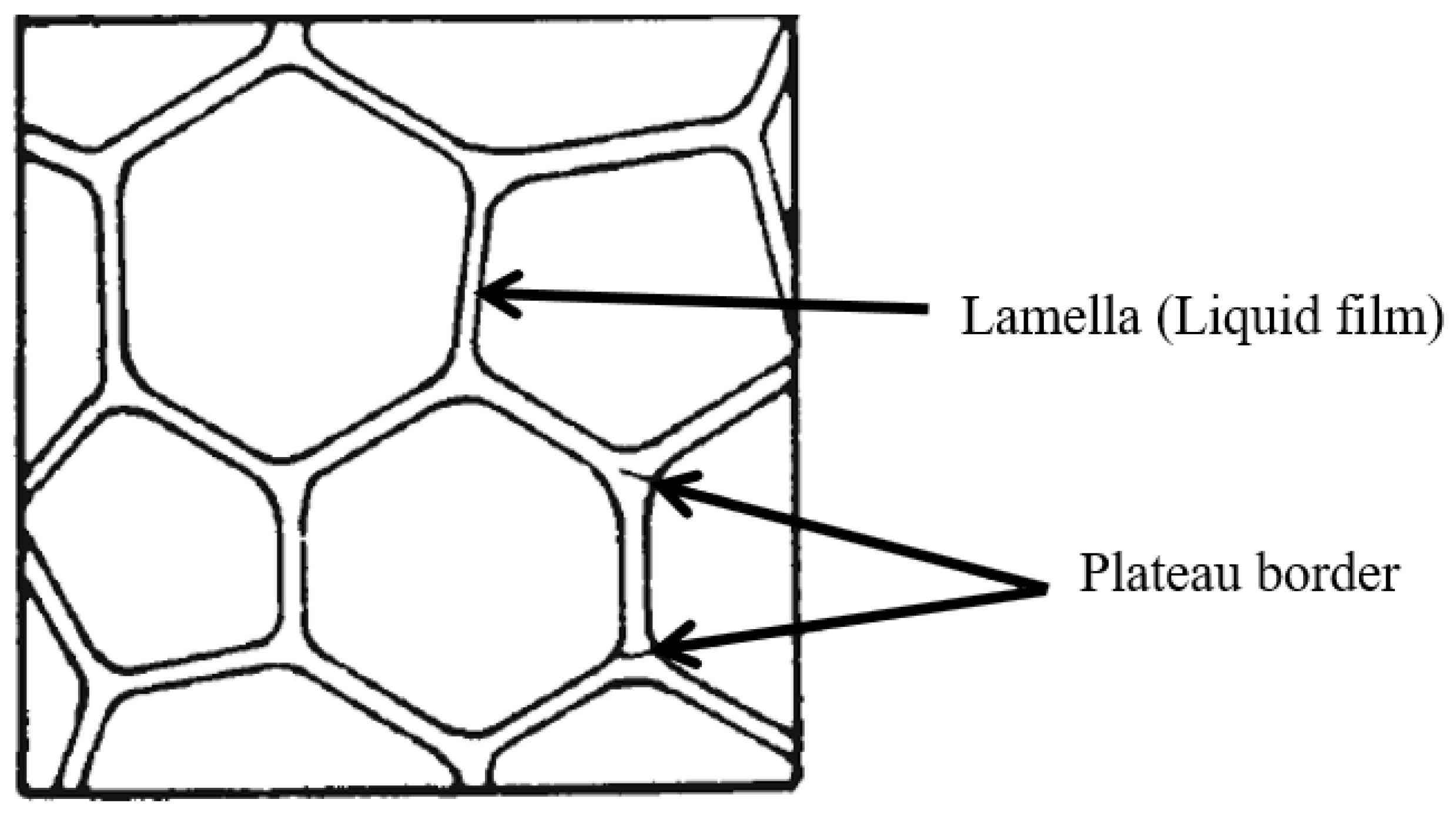
3. Foam Stability
3.1. Foam Drainage
3.2. Foam Coarsening
3.3. Foam Coalescence
3.4. Factors That Affect Foam Stability
3.4.1. Effect of Pressure on Foam Stability
3.4.2. Effect of Temperature on Foam Stability
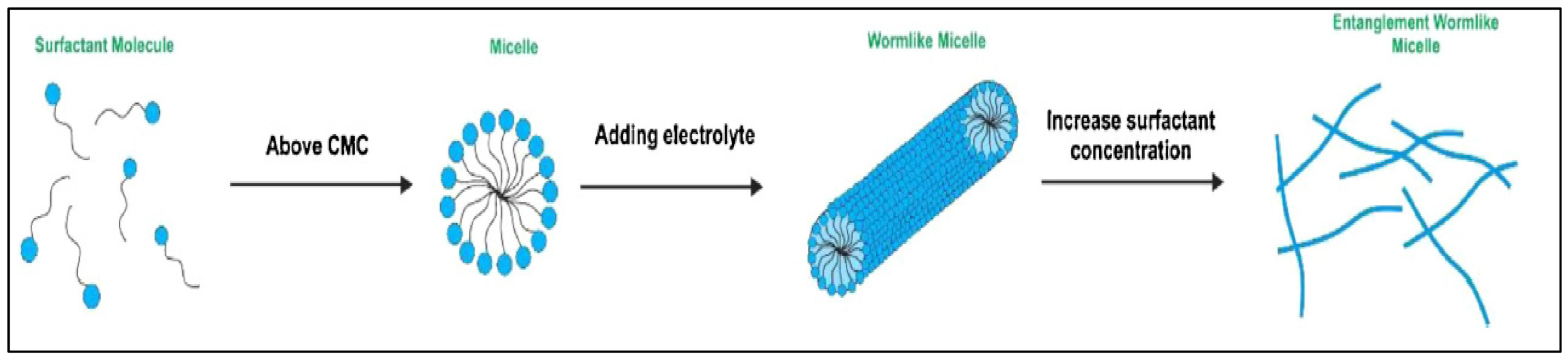
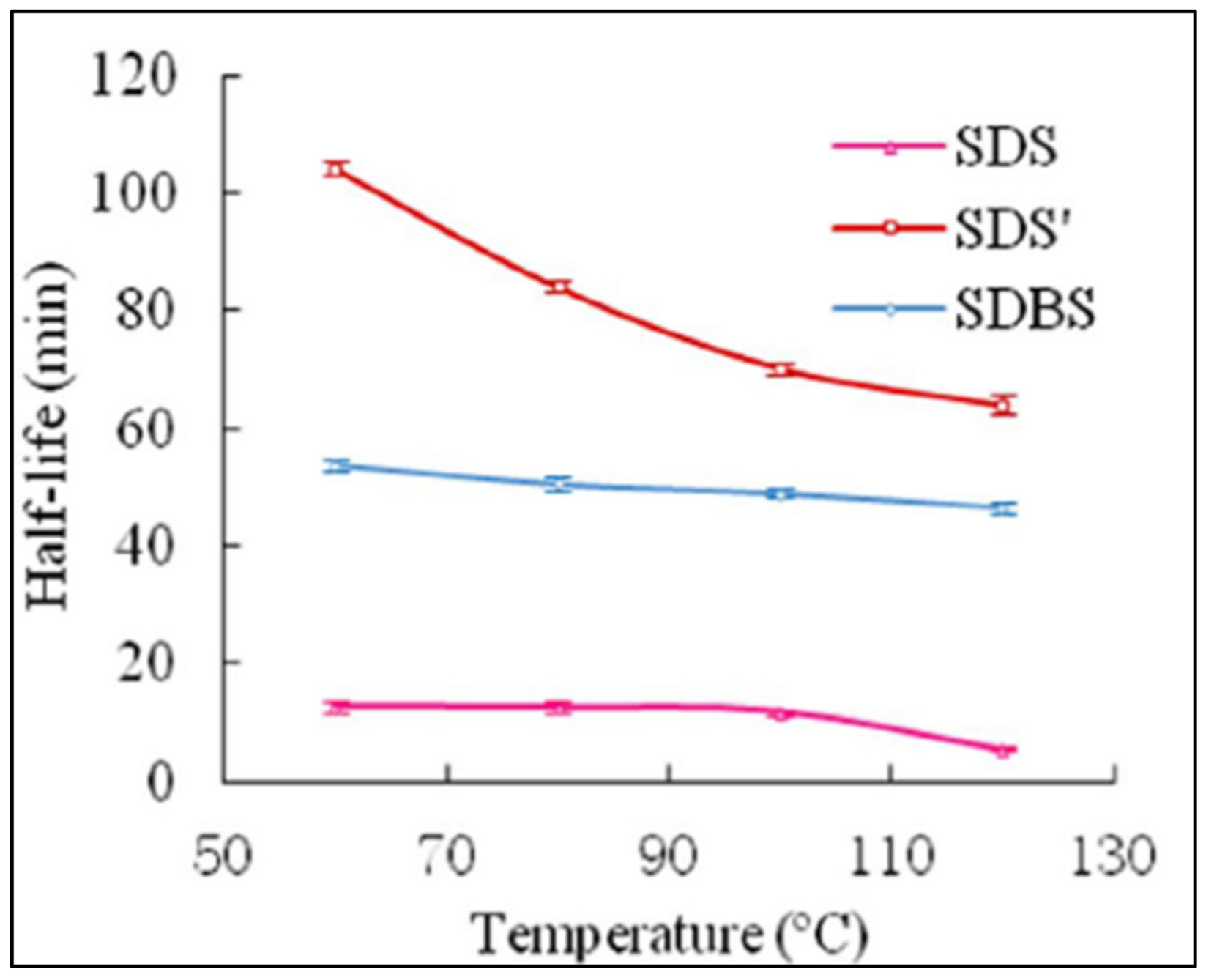
3.4.3. The Effect of Surfactants on Foam Stability
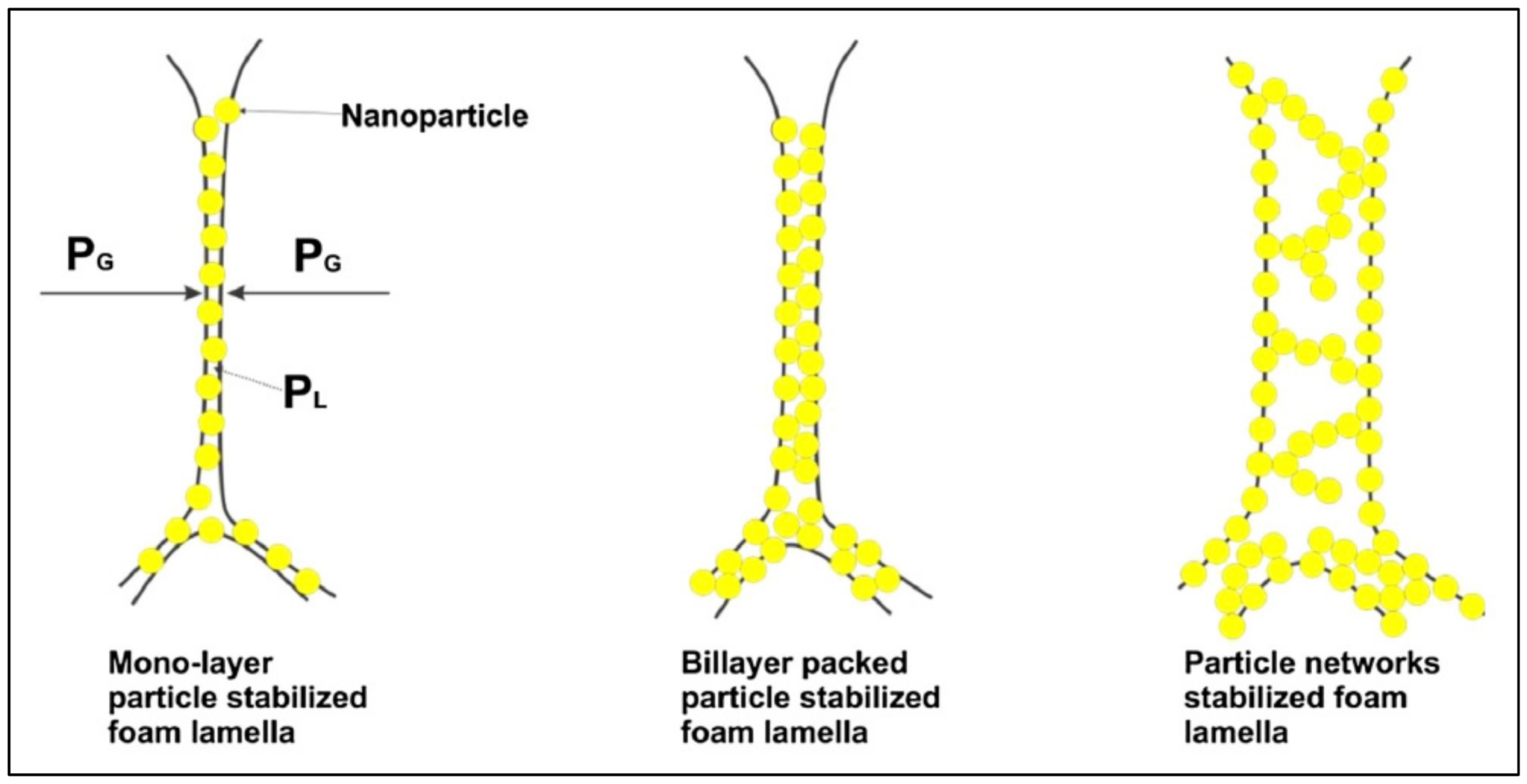
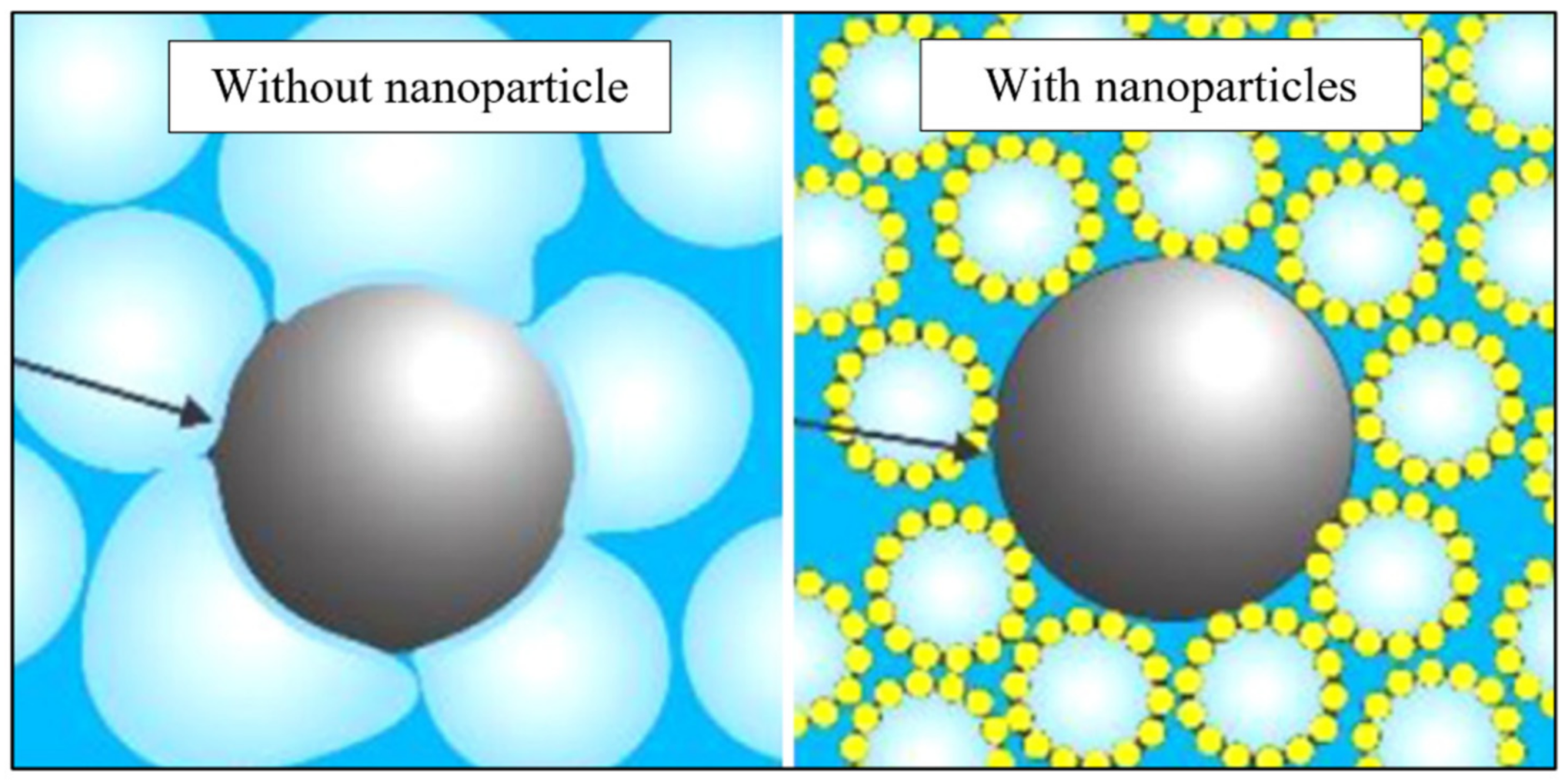
4. Nanoparticle as a Foam Stabilizing Agent
4.1. Mechanisms of Foam Stability
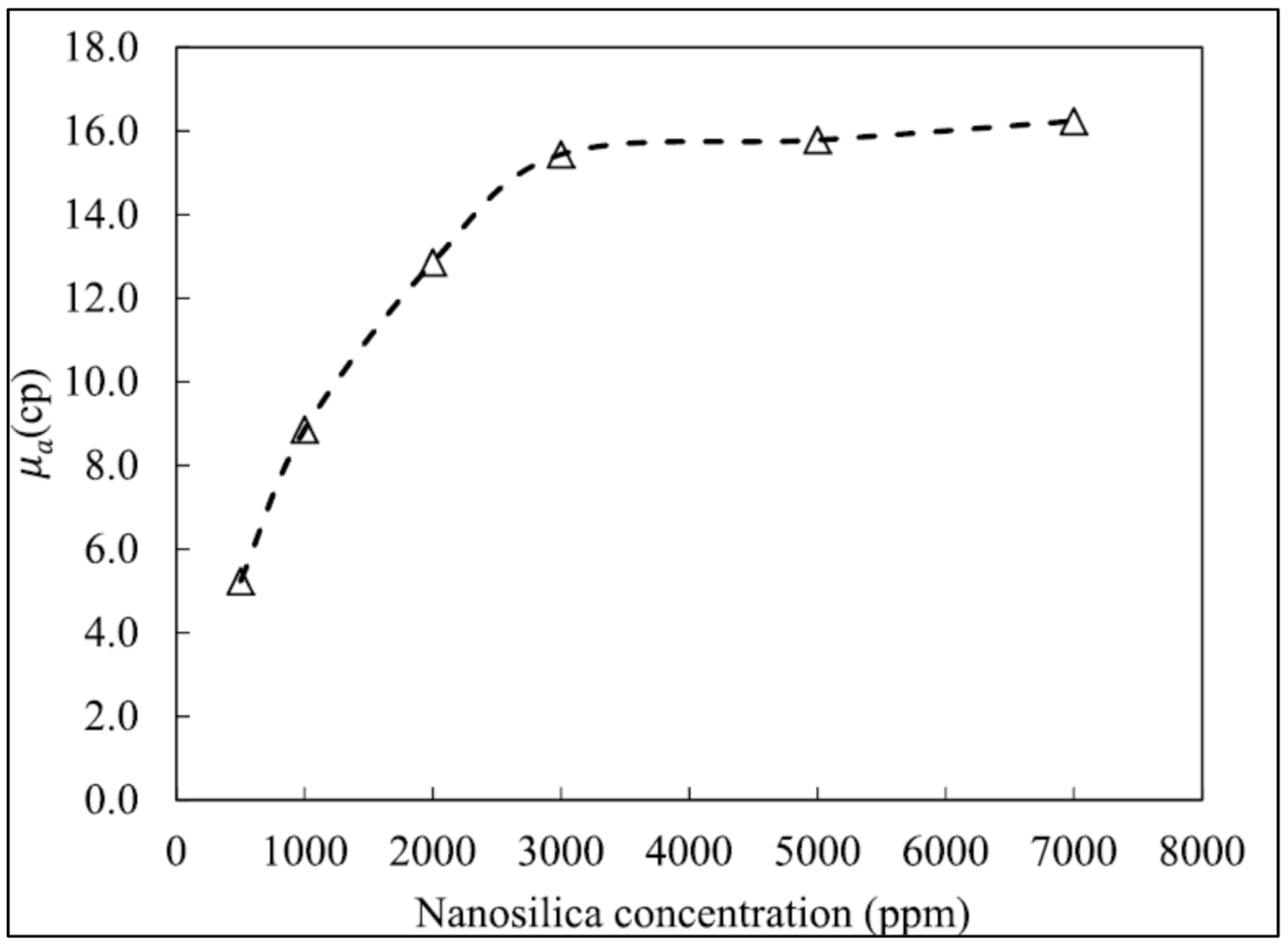
4.2. Nanoparticle Effect of Foam Rheology
4.3. Proppant Transportability of Nanoparticle-Stabilized Foam
5. Conclusions
- Foam viscosity appears to increase with increasing pressure and decrease with increasing temperature. However, the apparent increase in viscosity due to pressure is attributed to the expansion of gas in the foam, which increases gas quality, thus causing increased viscosity;
- The increase in foam quality causes the foam viscosity to continually increase up to a breaking point. Then, the foam becomes too dry, causing the viscosity to start rapidly declining. Hence, since the increase in pressure induces the increase in gas quality, the question arises whether there exists a point in pressure that will also become a breaking point that leads to a rapid decline in viscosity;
- Foam rheology is a complex parameter because low-quality foam behaves as a Newtonian fluid, whereas foam with high-quality exhibits non-Newtonian behavior in which it possesses yield stress. However, the point in foam quality in which foam transforms from a Newtonian to non-Newtonian fluid could not be easily pinpointed as experiments conducted by multiple works of literature use various compositions of foam fracturing fluid, resulting in different outcomes;
- The most significant limitation with applying foam fracturing fluid is its instability under high temperatures. Hence, the addition of nanoparticles to foam formulation has been proposed by several authors to solve this problem;
- Many studies have proven that nanoparticle-stabilized foam shows improved resistance toward heat compared to conventional foam. However, most of the studies only tested the foam stability up to 90 °C when the temperature of the HPHT environment is at least 150 °C;
- There is still contradicting theories on the ideal combination of nanoparticles and type of surfactants. Further investigation is strongly recommended to fully utilize the synergistic effect of nanoparticles and surfactants. In addition to this, numerous research to date tends to focus only on the stability of nanoparticle-stabilized foam rather than the rheology. Yet, within the limited number of available studies, inconsistencies exist regarding nanoparticle-stabilized foam behavior toward stress. Therefore, future research should strategically examine the factor that produces the diversity of results regarding this matter;
- The findings presented in this paper are based on experimental data and no field applications. Other than that, some other factors that affect foam rheology and stability, such as salinity, were not discussed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montgomery, C.T.; Smith, M.B. Hydraulic fracturing: History of an enduring technology. J. Pet. Technol. 2010, 62, 26–40. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Liang, F.; Hull, K.L. Nanoparticle-enhanced hydraulic-fracturing fluids: A review. SPE Prod. Oper. 2017, 32, 186–195. [Google Scholar] [CrossRef]
- Gulrajani, S.N.; Nolte, K.; Economides, M. Fracture evaluation using pressure diagnostics. Reserv. Stimul. 2000, 1–9. [Google Scholar]
- Fei, Y. Experimental and Numerical Investigation of Nanotechnology on Foam Stability for Hydraulic Fracturing Application. Ph.D. Thesis, Australian School of Petroleum, North Terrace, Australia, 2017. [Google Scholar]
- Alotaibi, M.A.; Miskimins, J.L. Slickwater proppant transport in complex fractures: New experimental findings & scalable correlation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28 September 2015. [Google Scholar]
- Chang, O.; Dilmore, R.; Wang, J.Y. Model development of proppant transport through hydraulic fracture network and parametric study. J. Pet. Sci. Eng. 2017, 150, 224–237. [Google Scholar] [CrossRef]
- Jansen, T.; Zhu, D.; Hill, A.D. The effect of rock mechanical properties on fracture conductivity for shale formations. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 3 February 2015. [Google Scholar]
- Li, N.; Li, J.; Zhao, L.; Luo, Z.; Liu, P.; Guo, Y. Laboratory testing and numeric simulation on laws of proppant transport in complex fracture systems. In Proceedings of the SPE Asia Pacific Hydraulic Fracturing Conference, Beijing, China, 24 August 2016. [Google Scholar]
- Liu, Y.; Leung, J.Y.; Chalaturnyk, R.; Virues, C.J. Fracturing fluid distribution in shale gas reservoirs due to fracture closure, proppant distribution and gravity segregation. In Proceedings of the SPE Unconventional Resources Conference, Calgary, Canada, 15 February 2017. [Google Scholar]
- Shiozawa, S.; McClure, M. Simulation of proppant transport with gravitational settling and fracture closure in a three-dimensional hydraulic fracturing simulator. J. Pet. Sci. Eng. 2016, 138, 298–314. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Zhang, T.; Du, S.; Sepehrnoori, K. Numerical study of the effect of uneven proppant distribution between multiple fractures on shale gas well performance. Fuel 2015, 142, 189–198. [Google Scholar] [CrossRef]
- Zhang, G.; Li, M.; Gutierrez, M. Numerical simulation of proppant distribution in hydraulic fractures in horizontal wells. J. Nat. Gas Sci. Eng. 2017, 48, 157–168. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Hydraulic Fracturing; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Chai, Y.; Li, X.; Jing, D. Application of surfactants in hydraulic fracturing for enhanced. J. Oil Res. 2019, 5, 161. [Google Scholar]
- Denney, D. Fluid selection for energized hydraulic fractures. J. Pet. Technol. 2010, 62, 42–44. [Google Scholar] [CrossRef]
- Friehauf, K.E.; Sharma, M.M. Fluid selection for energized hydraulic fractures. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4 October 2009. [Google Scholar]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. A review of recent advances in foam-based fracturing fluid application in unconventional reservoirs. J. Ind. Eng. Chem. 2018, 66, 45–71. [Google Scholar] [CrossRef]
- Fei, Y.; Gonzalez Perdomo, M.; Pokalai, K.; Haghighi, M. 3D simulation of hydraulic fracturing by foam based fluids using a fracture propagation model coupled with geomechanics in an unconventional reservoir the Cooper Basin, South Australia. In Proceedings of the International Conference on Geomechanics, Geo-energy and Geo-resources, Chengdu, China, 21–24 September 2018; pp. 21–24. [Google Scholar]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K.; Chauhan, P.S. Nanoparticles applications for hydraulic fracturing of unconventional reservoirs: A comprehensive review of recent advances and prospects. J. Pet. Sci. Eng. 2019, 178, 41–73. [Google Scholar] [CrossRef]
- Ahmed, S.; Hanamertani, A.S.; Hashmet, M.R. CO2 Foam as an Improved Fracturing Fluid System for Unconventional Reservoir. In Exploitation of Unconventional Oil and Gas Resources-Hydraulic Fracturing and Other Recovery and Assessment Techniques; IntechOpen: London, UK, 2019. [Google Scholar]
- Wanniarachchi, W.A.M.; Ranjith, P.; Perera, M.S.A.; Lashin, A.; Al Arifi, N.; Li, J. Current opinions on foam-based hydro-fracturing in deep geological reservoirs. Geomech. Geophys. Geo-Energy Geo-Resour. 2015, 1, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Bachman, R.; Peters, W. A comparison of the effectiveness of various fracture fluid systems used in multi-stage fractured horizontal wells: Montney formation, unconventional gas. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 4 February 2014. [Google Scholar]
- Zhou, J.; Ranjith, P.; Wanniarachchi, W. Different strategies of foam stabilization in the use of foam as a fracturing fluid. Adv. Colloid Interface Sci. 2020, 276, 102104. [Google Scholar] [CrossRef] [PubMed]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. Synergistic effects of nanoparticles and surfactants on n-decane-water interfacial tension and bulk foam stability at high temperature. J. Pet. Sci. Eng. 2019, 179, 814–830. [Google Scholar] [CrossRef]
- Kulikowski, D.; Amrouch, K.; Cooke, D. Geomechanical modelling of fault reactivation in the Cooper Basin, Australia. Aust. J. Earth Sci. 2016, 63, 295–314. [Google Scholar] [CrossRef]
- Smithson, T. HPHT wells. Oilfield Rev. 2016, 1028. [Google Scholar]
- Karadkar, P.; Bataweel, M.; Bulekbay, A.; Alshaikh, A.A. Energized Fluids for Upstream Production Enhancement: A Review. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23 April 2018. [Google Scholar]
- Hutchins, R.D.; Miller, M.J. A circulating foam loop for evaluating foam at conditions of use. SPE Prod. Facil. 2005, 20, 286–294. [Google Scholar] [CrossRef]
- Fei, Y.; Gonzalez, M.; Haghighi, M. Free drainage of foam mixed with proppants in the presence of nanoparticles. APPEA J. 2018, 58, 710–714. [Google Scholar] [CrossRef]
- Fink, J. Hydraulic Fracturing Chemicals and Fluids Technology; Gulf Professional Publishing: Waltham, MA, USA, 2013. [Google Scholar]
- Abel, J.T.; Idris, M.; Choon Hoong, C.; Simbolon, R. Energized Acid Fracturing in Deep Carbonate Reservoirs. CO2 versus N2. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 15 March 2019. [Google Scholar]
- Tran, T.H.M.; Perdomo, M.E.G.; Wilk, K.; Kasza, P.; Amrouch, K. Performance evaluation of synthetic and natural polymers in nitrogen foam-based fracturing fluids in the Cooper Basin, South Australia. APPEA J. 2020, 60, 227–241. [Google Scholar] [CrossRef]
- Tamayo, H.C.; Lee, K.; Taylor, R.S. Enhanced aqueous fracturing fluid recovery from tight gas formations: Foamed CO2 pre-pad fracturing fluid and more effective surfactant systems. J. Can. Pet. Technol. 2008, 47. [Google Scholar] [CrossRef]
- Davis, M.; Hille, E.; McDonnell, J.; Mearns, D. Basic Physics of Foam Stability and Collapse. Engineering 2012. [Google Scholar]
- Stevenson, P. Foam Engineering: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ahmed, S.; Elraies, K.A.; Hashmet, M.R.; Hanamertani, A.S. Viscosity models for polymer free CO2 foam fracturing fluid with the effect of surfactant concentration, salinity and shear rate. Energies 2017, 10, 1970. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.; Perdomo, M.E.G.; Haghighi, M.; Amrouch, K. Study of the synergistic effects between different surfactant types and silica nanoparticles on the stability of liquid foams at elevated temperature. Fuel 2022, 315, 122818. [Google Scholar] [CrossRef]
- Fei, Y.; Zhu, J.; Xu, B.; Li, X.; Gonzalez, M.; Haghighi, M. Experimental investigation of nanotechnology on worm-like micelles for high-temperature foam stimulation. J. Ind. Eng. Chem. 2017, 50, 190–198. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Shah, S.N. Emergence of nanotechnology in the oil and gas industry: Emphasis on the application of silica nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Gaydos, J.; Harris, P. Foam fracturing: Theories, procedures and results. In Proceedings of the SPE Unconventional Gas Recovery Symposium, Pittsburgh, PA, USA, 18 May 1980. [Google Scholar]
- Walstra, P. Principles of foam formation and stability. In Foams: Physics, Chemistry and Structure; Springer: Berlin/Heidelberg, Germany, 1989; pp. 1–15. [Google Scholar]
- Liew, M.; Danyaro, K.U.; Zawawi, N.A.W.A. A Comprehensive Guide to Different Fracturing Technologies: A Review. Energies 2020, 13, 3326. [Google Scholar] [CrossRef]
- McAndrew, J.J.; Fan, R.; Sharma, M.; Ribeiro, L. Extending the application of foam hydraulic fracturing fluids. In Proceedings of the Unconventional Resources Technology Conference, Denver, CO, USA, 25–27 August 2014; pp. 1421–1429. [Google Scholar]
- Fei, Y.; Pokalai, K.; Johnson, R.; Gonzalez, M.; Haghighi, M. Experimental and simulation study of foam stability and the effects on hydraulic fracture proppant placement. J. Nat. Gas Sci. Eng. 2017, 46, 544–554. [Google Scholar] [CrossRef]
- Kapetas, L.; Bonnieu, S.V.; Danelis, S.; Rossen, W.; Farajzadeh, R.; Eftekhari, A.; Shafian, S.; Bahrim, R. Effect of temperature on foam flow in porous media. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, BH, USA, 8 March 2015. [Google Scholar]
- Lv, Q.; Li, Z.; Li, B.; Li, S.; Sun, Q. Study of nanoparticle–surfactant-stabilized foam as a fracturing fluid. Ind. Eng. Chem. Res. 2015, 54, 9468–9477. [Google Scholar] [CrossRef]
- Weaire, D.; Hutzler, S.; Drenckhan, W.; Saugey, A.; Cox, S. The rheology of foams. In Smart Colloidal Materials; Springer: Berlin/Heidelberg, Germany, 2006; pp. 100–105. [Google Scholar]
- Xiao, C.; Balasubramanian, S.N.; Clapp, L.W. Rheology of viscous CO2 foams stabilized by nanoparticles under high pressure. Ind. Eng. Chem. Res. 2017, 56, 8340–8348. [Google Scholar] [CrossRef]
- Herzhaft, B. Rheology of aqueous foams: A literature review of some experimental works. Oil Gas Sci. Technol. 1999, 54, 587–596. [Google Scholar] [CrossRef]
- Sinha, V.; Ahmed, R.; Akhtar, T.; Shah, S.; Amani, M. Rheology and hydraulics of polymer-based foams at elevated temperatures. J. Pet. Sci. Eng. 2019, 180, 330–346. [Google Scholar] [CrossRef]
- Reidenbach, V.; Harris, P.; Lee, Y.; Lord, D. Rheological study of foam fracturing fluids using nitrogen and carbon dioxide. SPE Prod. Eng. 1986, 1, 31–41. [Google Scholar] [CrossRef]
- Jing, Z.; Feng, C.; Wang, S.; Xu, D. Effects of temperature and pressure on rheology and heat transfer among bubbles in waterless CO2-based foam fracturing fluid. J. Nat. Gas Sci. Eng. 2019, 63, 18–26. [Google Scholar] [CrossRef]
- Valkó, P.; Economides, M.J. Volume equalized constitutive equations for foamed polymer solutions. J. Rheol. 1992, 36, 1033–1055. [Google Scholar] [CrossRef]
- Akhtar, T.F.; Ahmed, R.; Elgaddafi, R.; Shah, S.; Amani, M. Rheological behavior of aqueous foams at high pressure. J. Pet. Sci. Eng. 2018, 162, 214–224. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Rheology and stability of nanoparticle-stabilized CO2 foam under reservoir conditions. J. Pet. Sci. Eng. 2021, 196, 107671. [Google Scholar] [CrossRef]
- Ahmed, S.; Elraies, K.A.; Forooozesh, J.; Shafian, S.R.B.M.; Hashmet, M.R.; Hsia, I.C.C.; Almansour, A. Experimental investigation of immiscible supercritical carbon dioxide foam rheology for improved oil recovery. J. Earth Sci. 2017, 28, 835–841. [Google Scholar] [CrossRef]
- Sun, X.; Liang, X.; Wang, S.; Lu, Y. Experimental study on the rheology of CO2 viscoelastic surfactant foam fracturing fluid. J. Pet. Sci. Eng. 2014, 119, 104–111. [Google Scholar] [CrossRef]
- Govindu, A.; Ahmed, R.; Shah, S.; Amani, M. Foam Stability-Does Well Inclination Matter? In Proceedings of the SPE/ICoTA Well Intervention Conference and Exhibition, The Woodlands, TX, USA, 16 March 2020. [Google Scholar]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Liu, Y.; Zhang, L.; Ren, S.; Lu, J.; Wang, X.; Fan, N. The stability study of CO2 foams at high pressure and high temperature. J. Pet. Sci. Eng. 2017, 154, 234–243. [Google Scholar] [CrossRef]
- Bayat, A.E.; Rajaei, K.; Junin, R. Assessing the effects of nanoparticle type and concentration on the stability of CO2 foams and the performance in enhanced oil recovery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 222–231. [Google Scholar] [CrossRef]
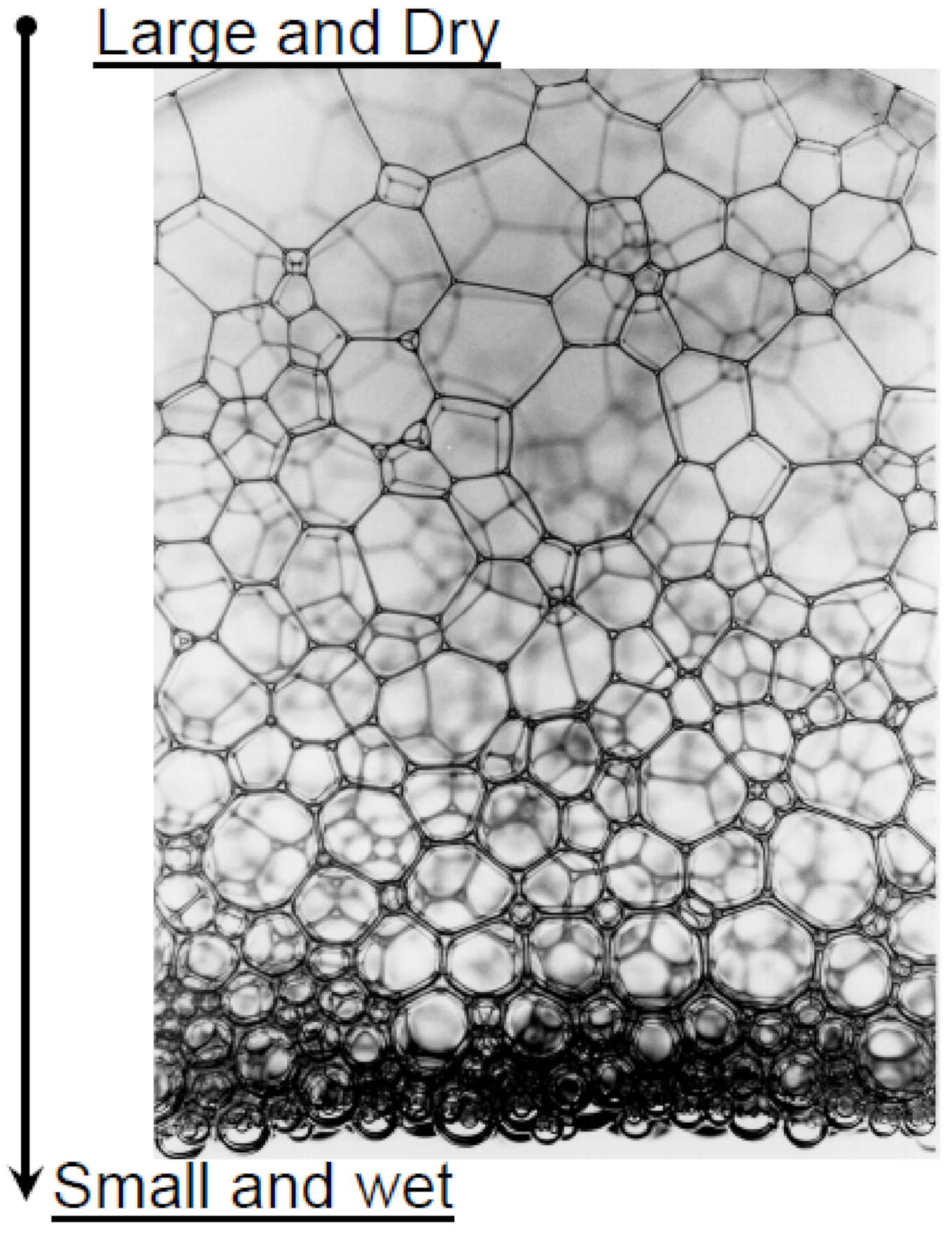
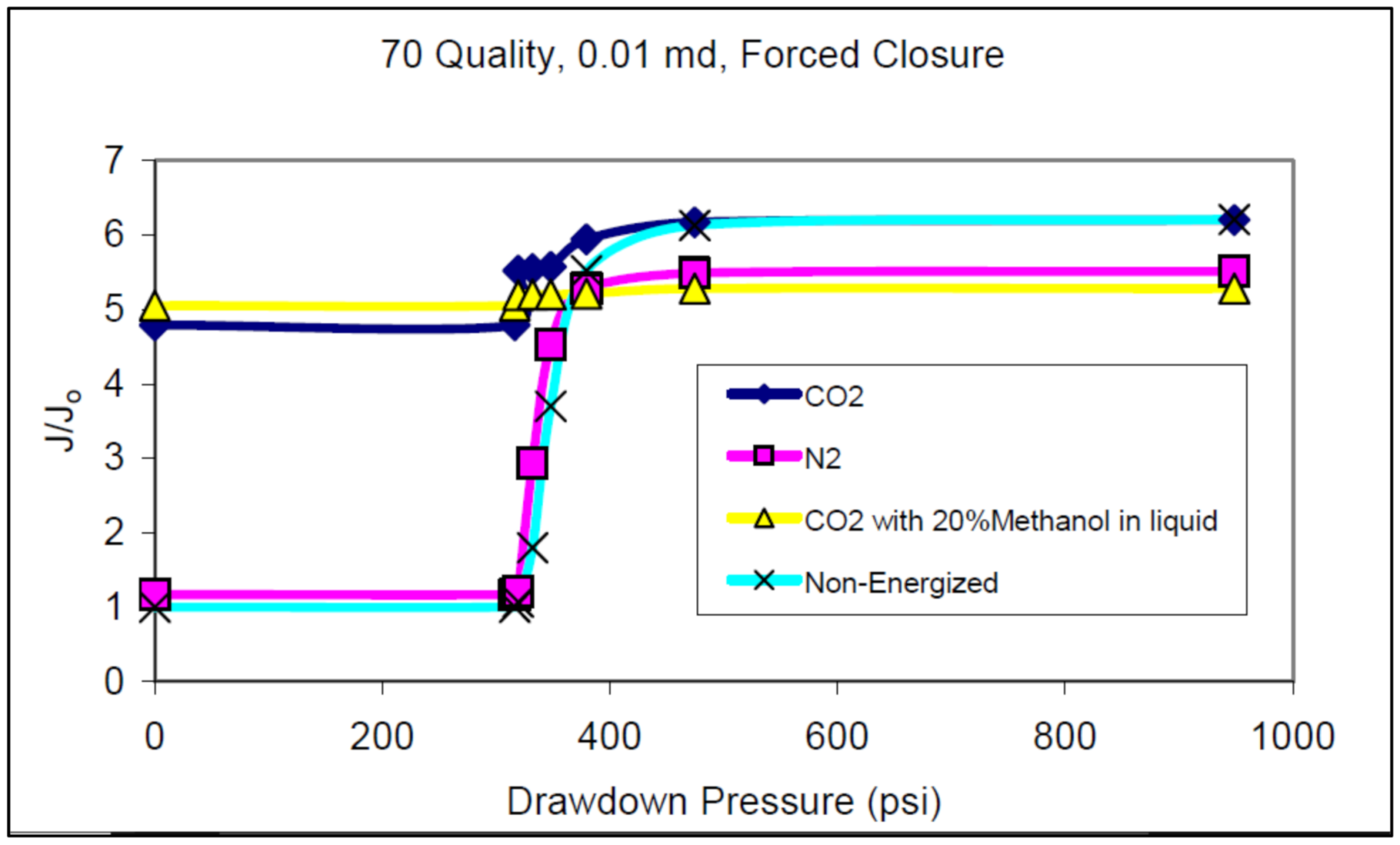
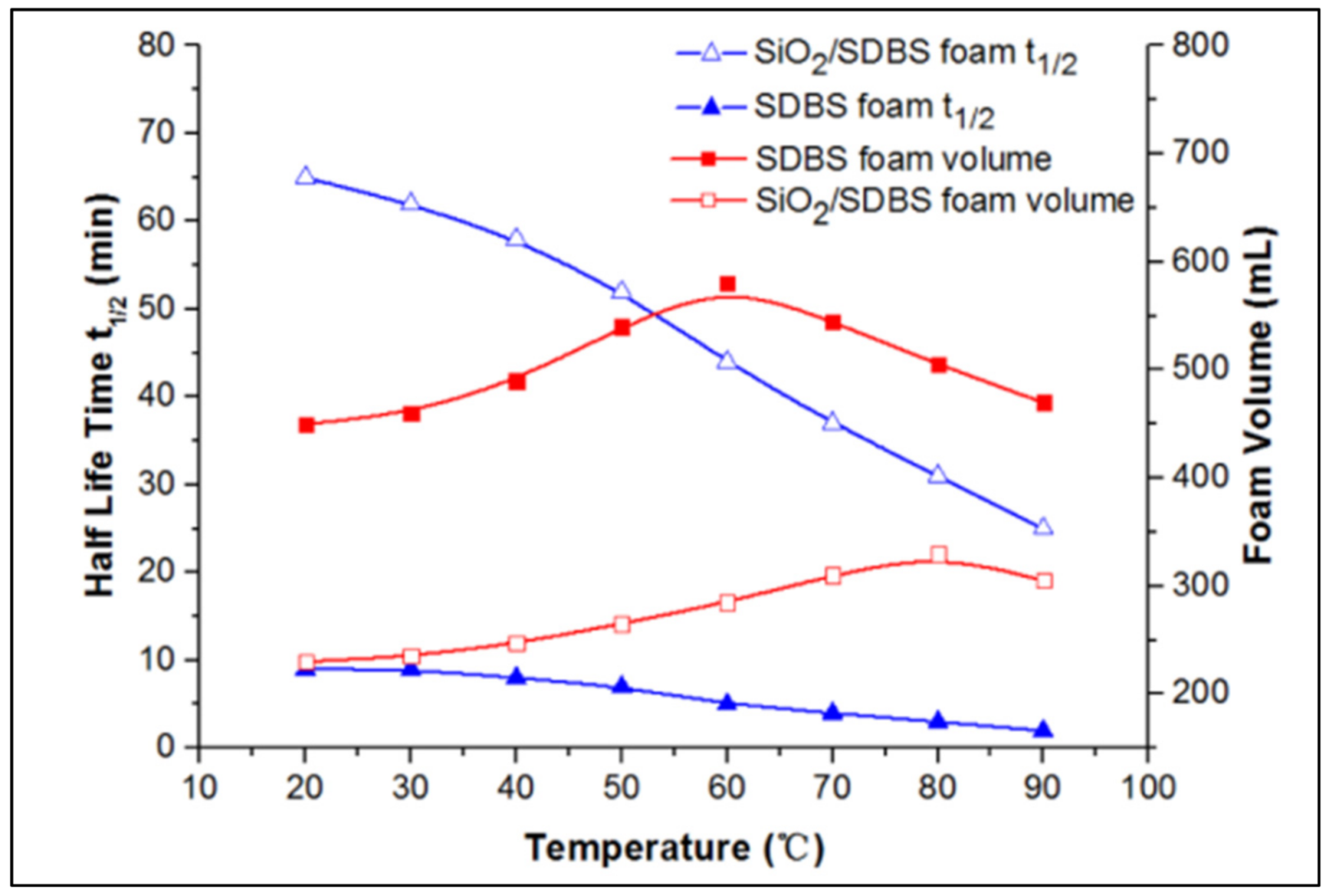

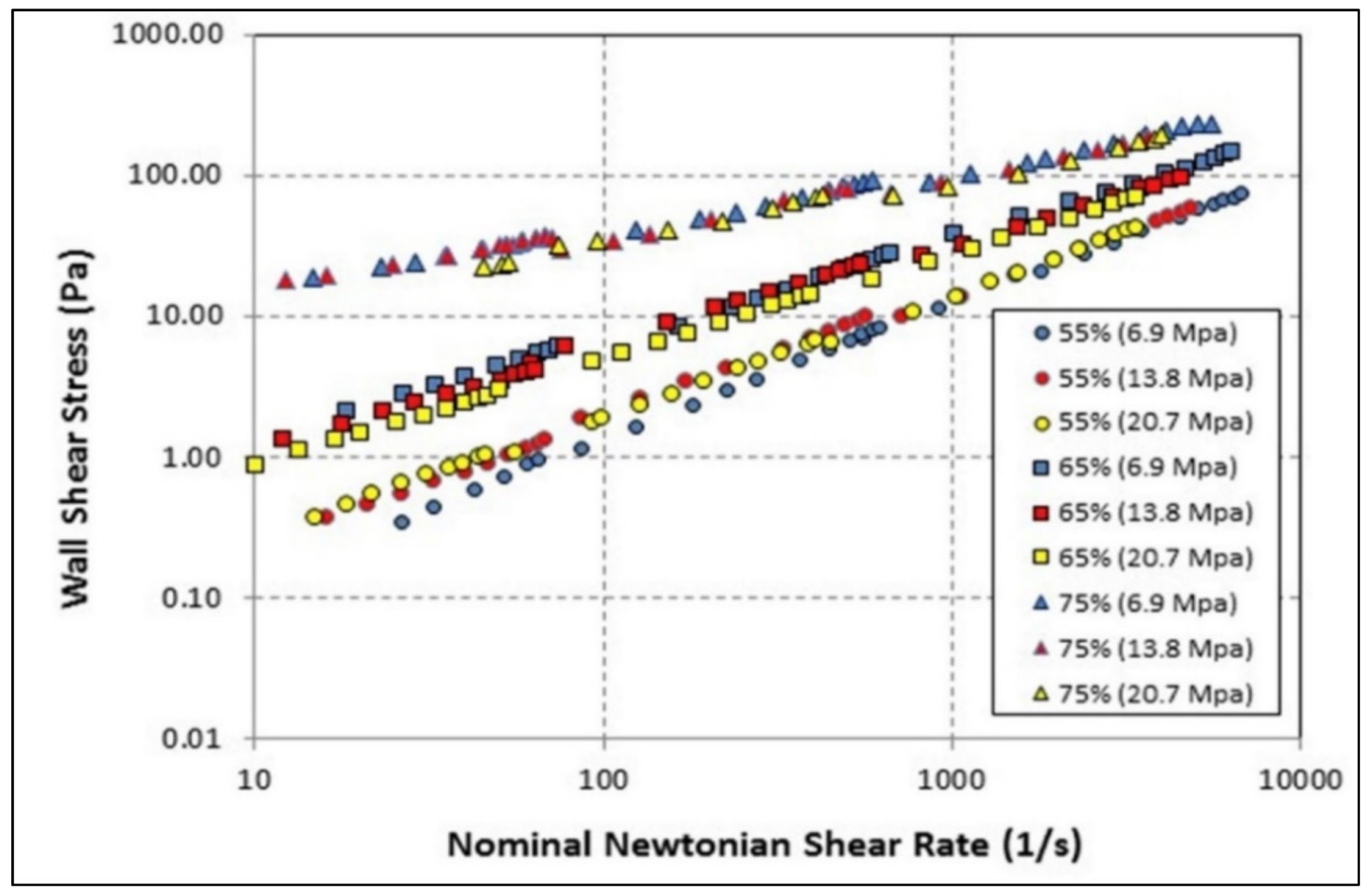
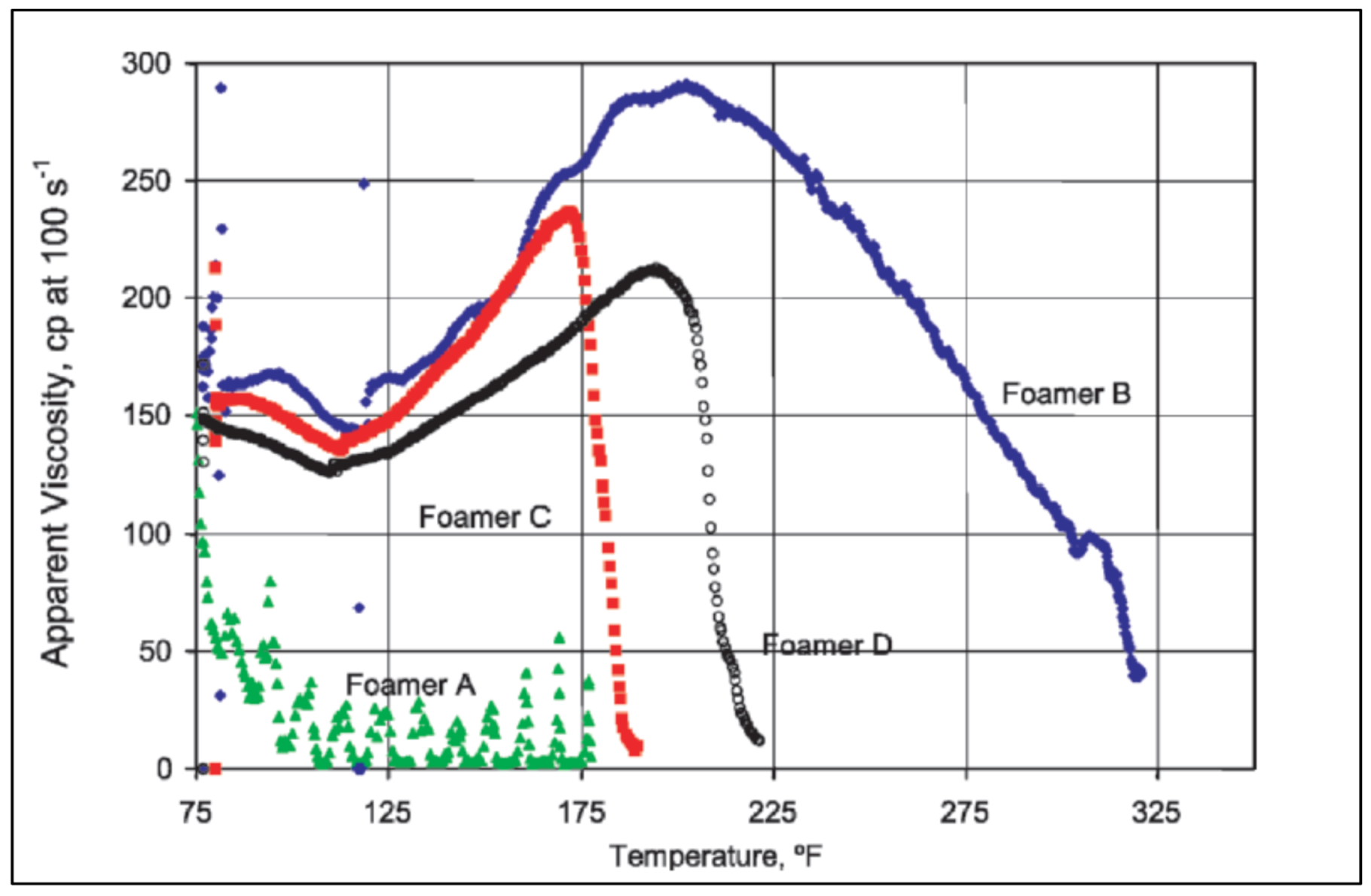
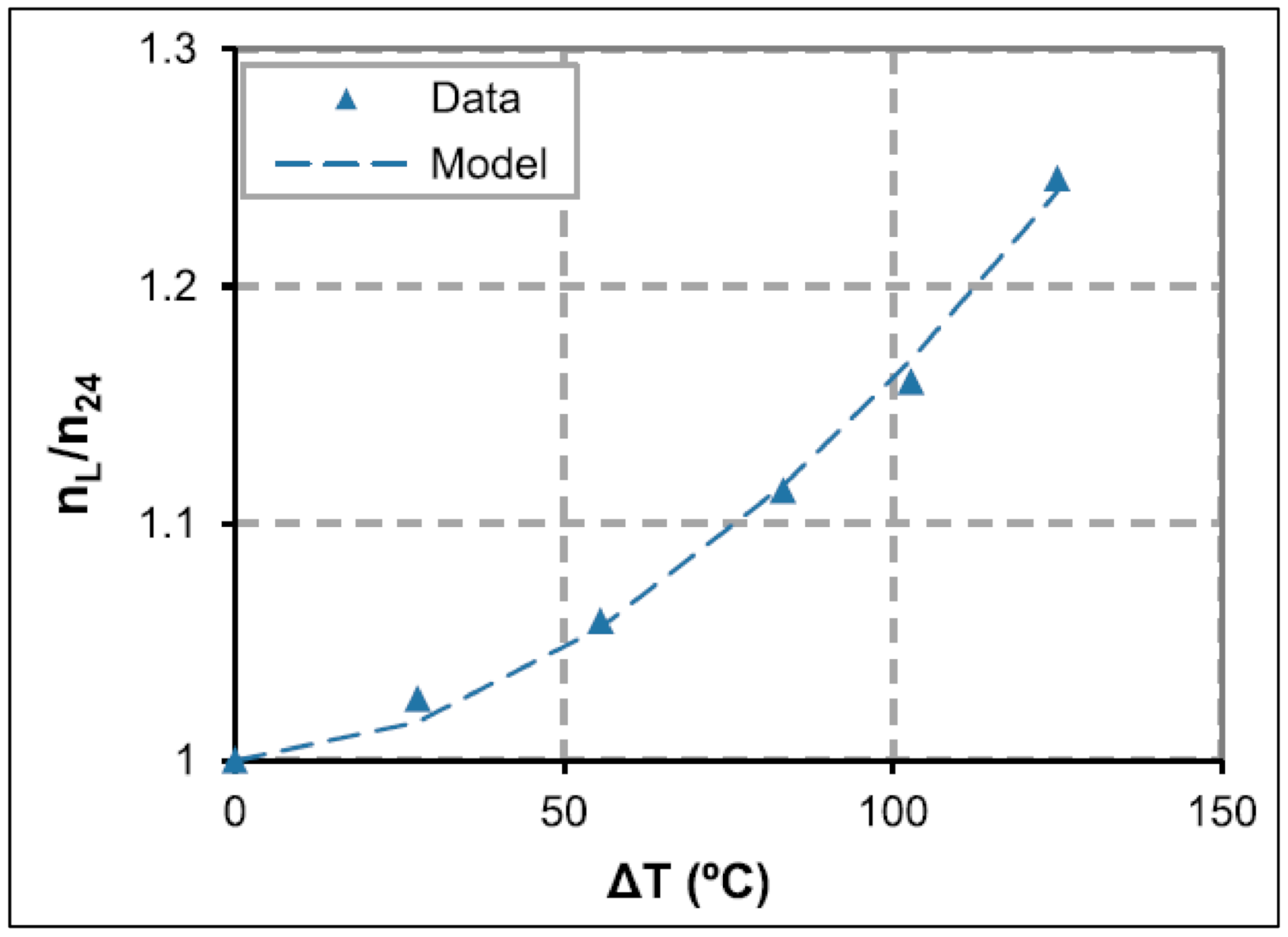
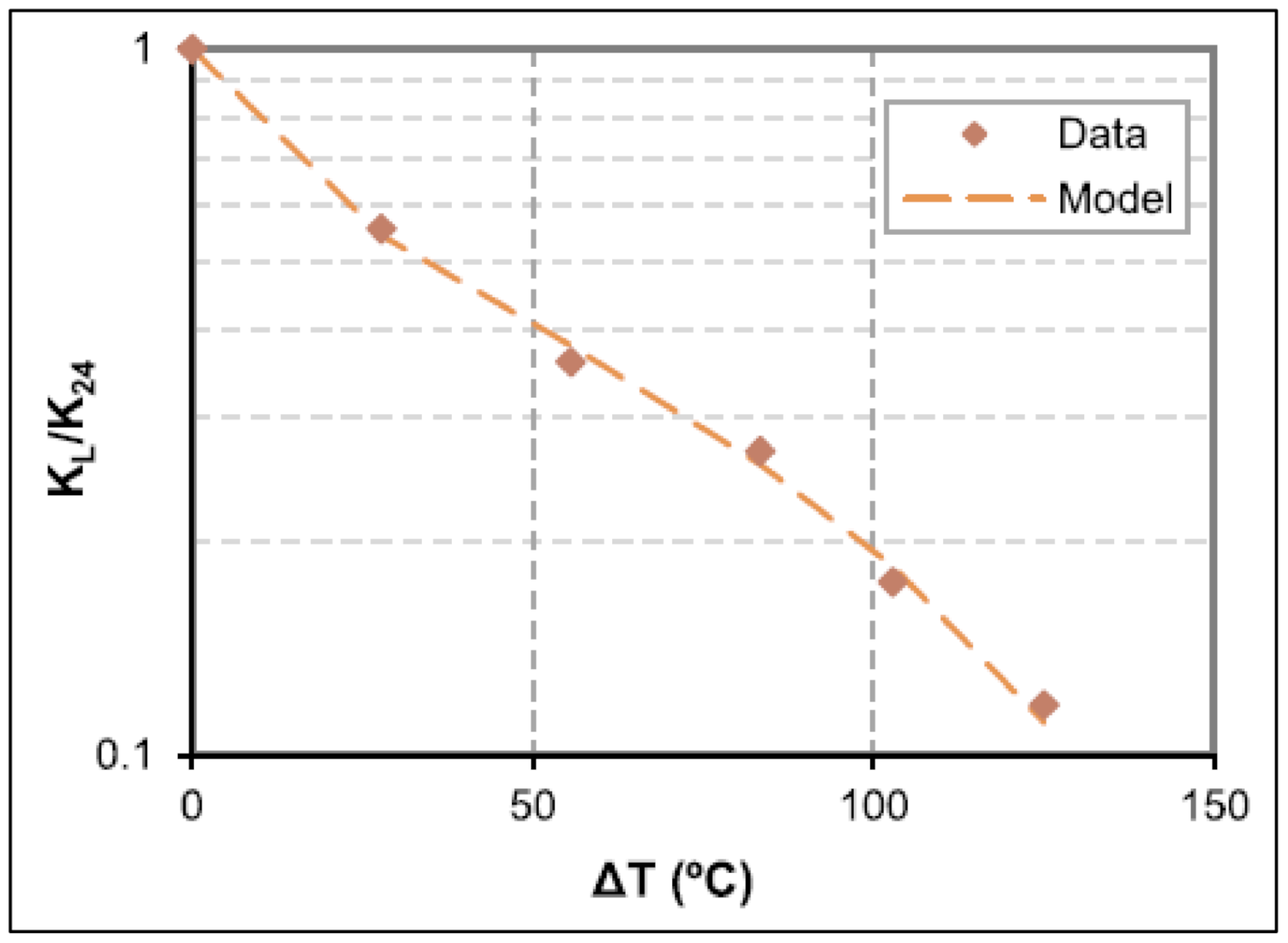

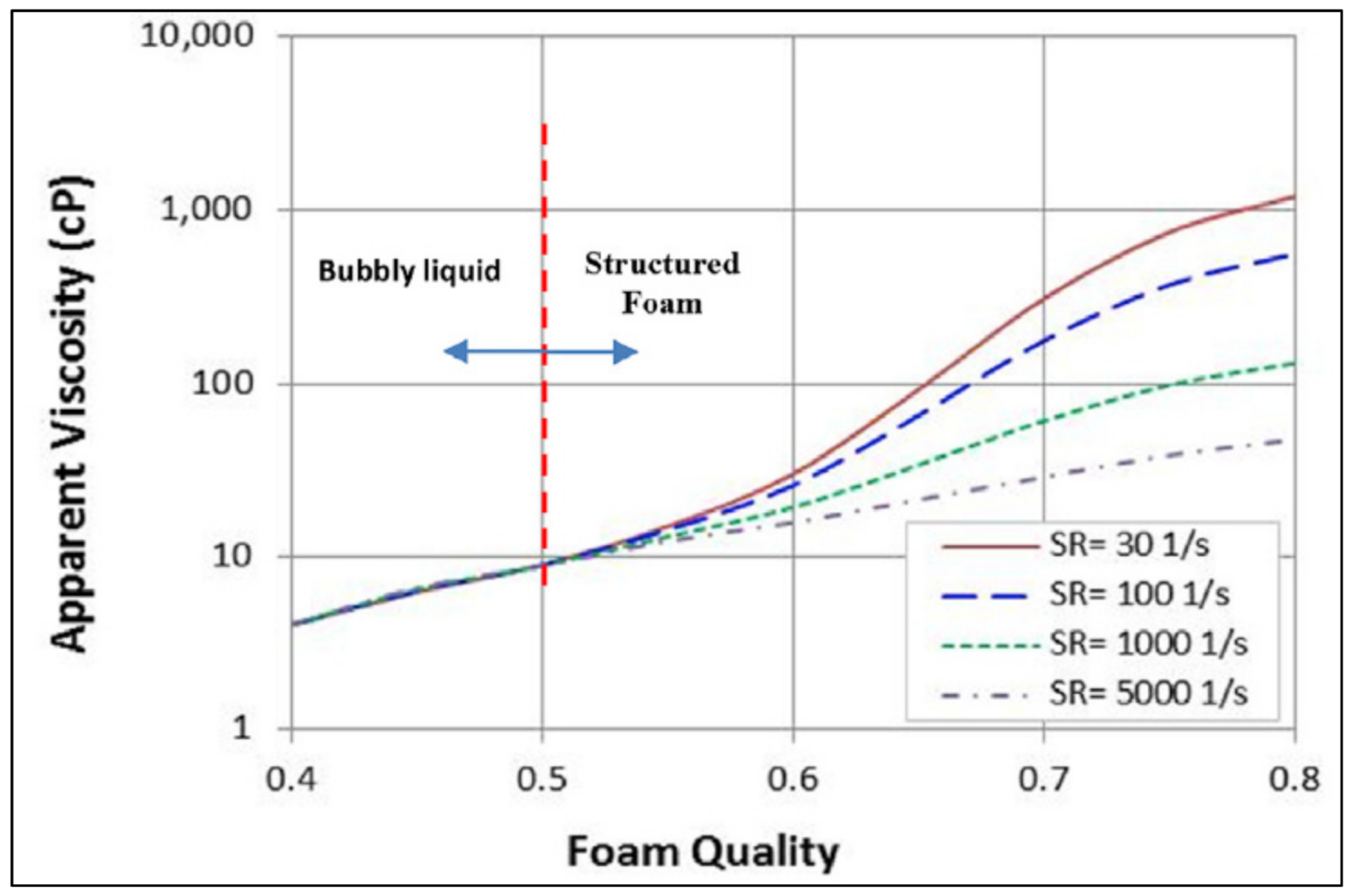

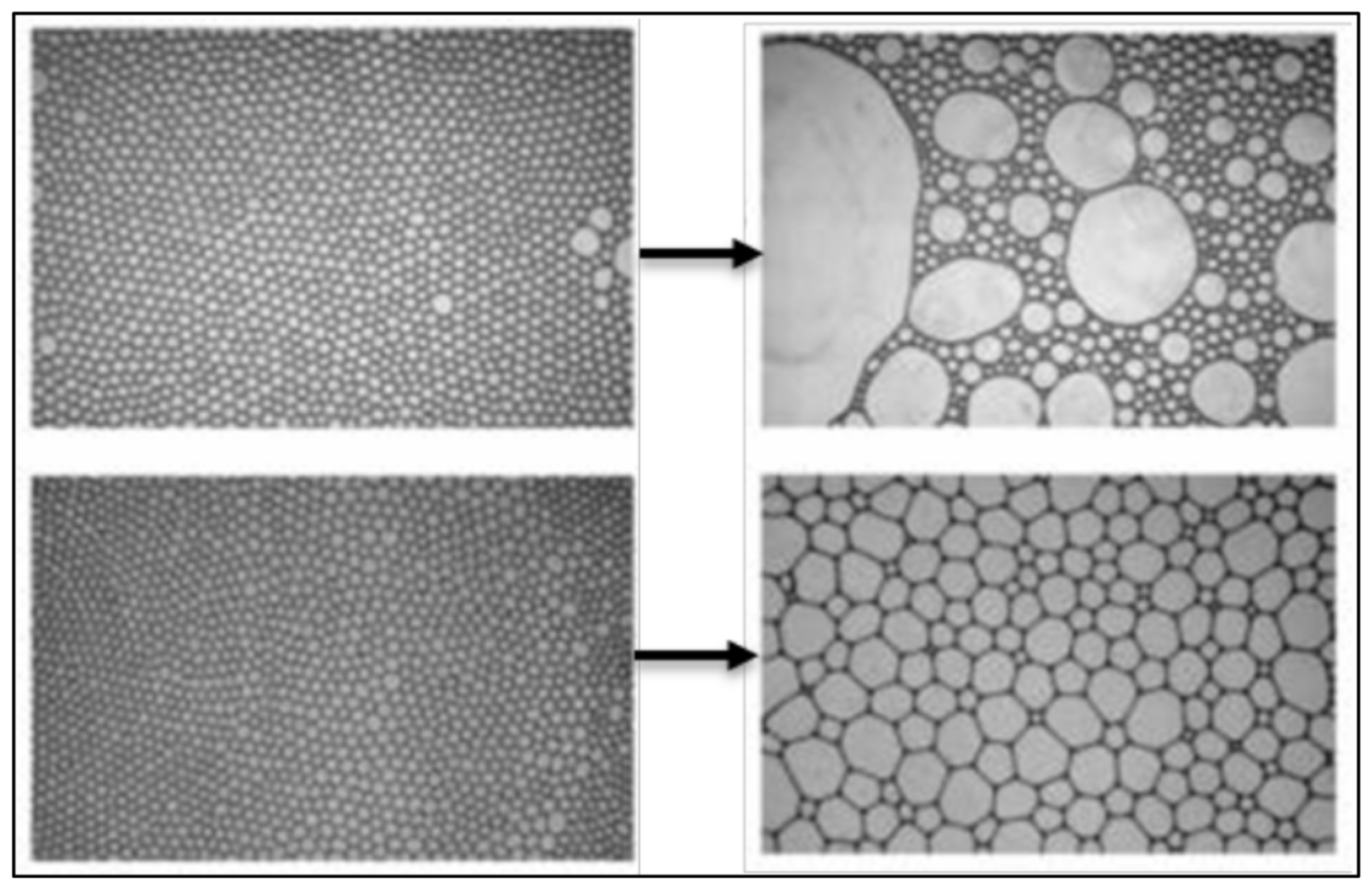
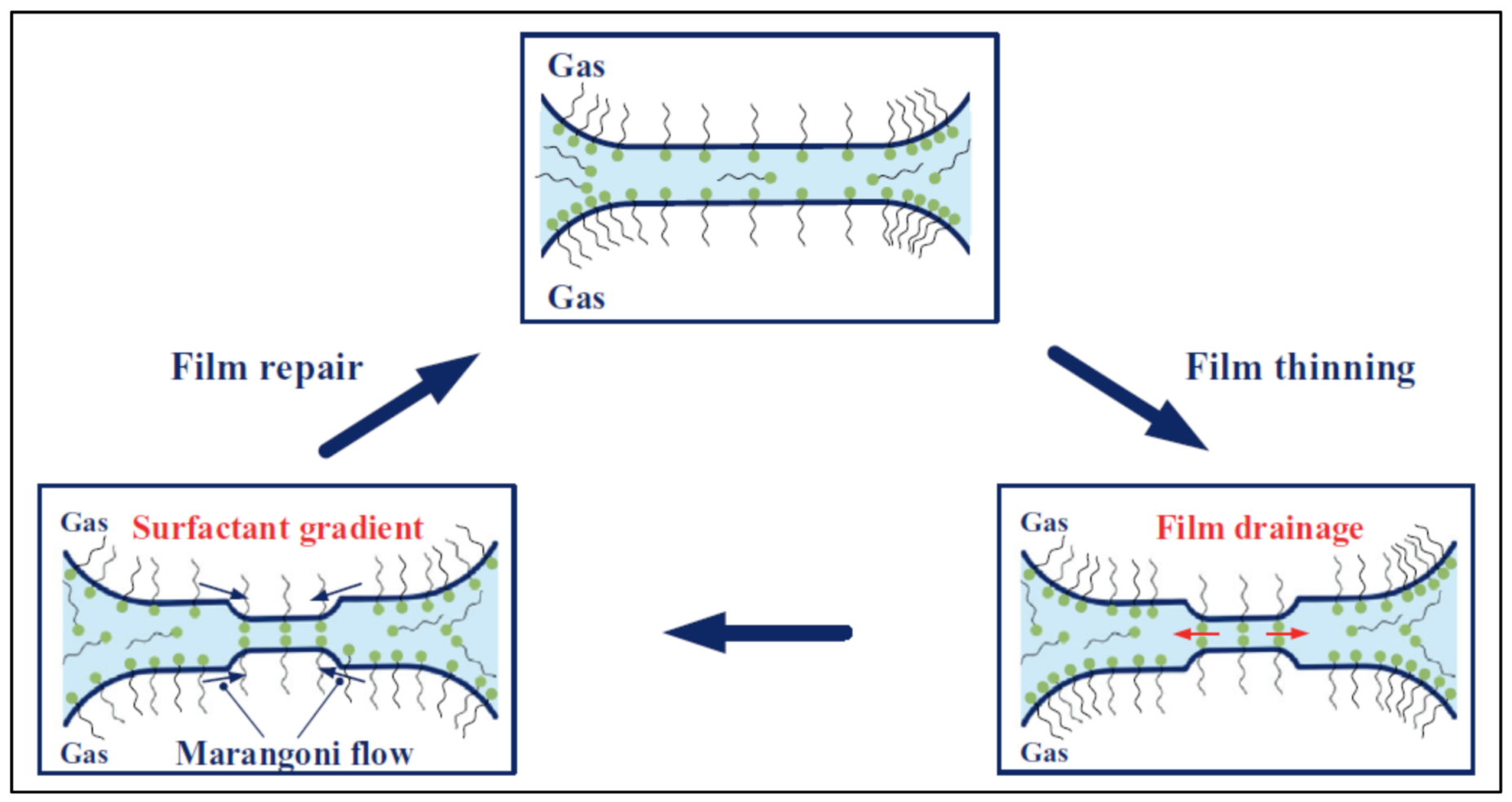
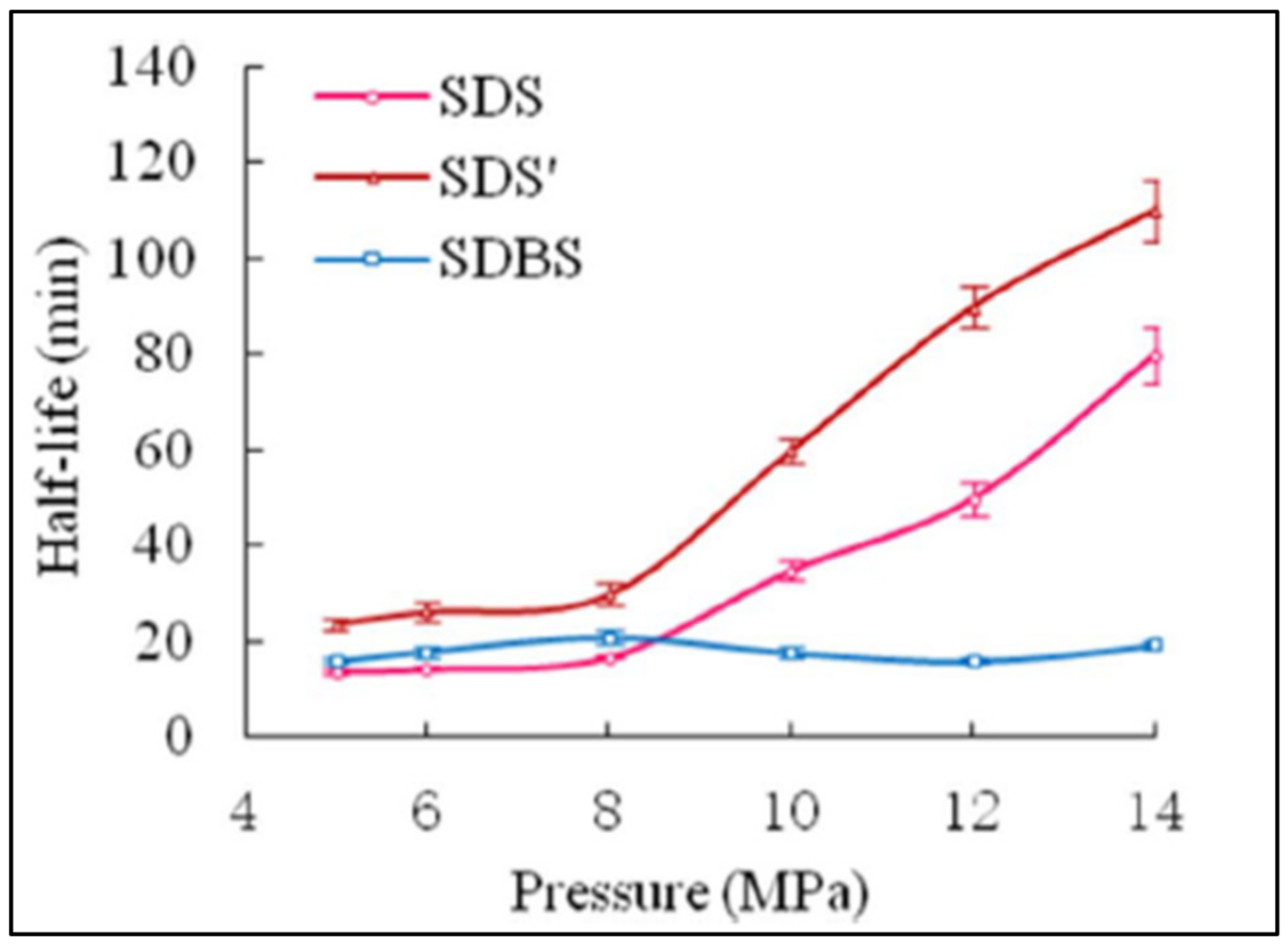
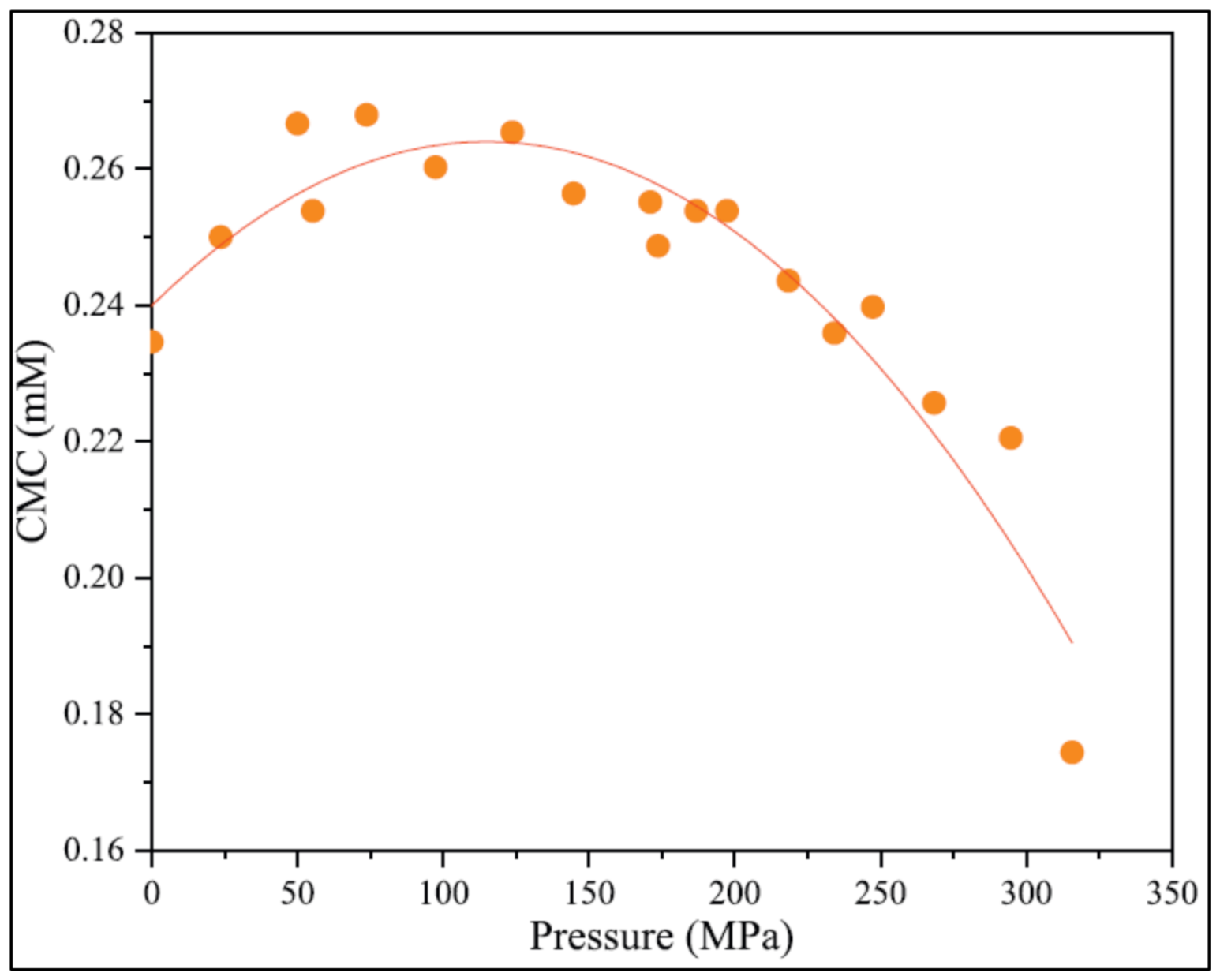

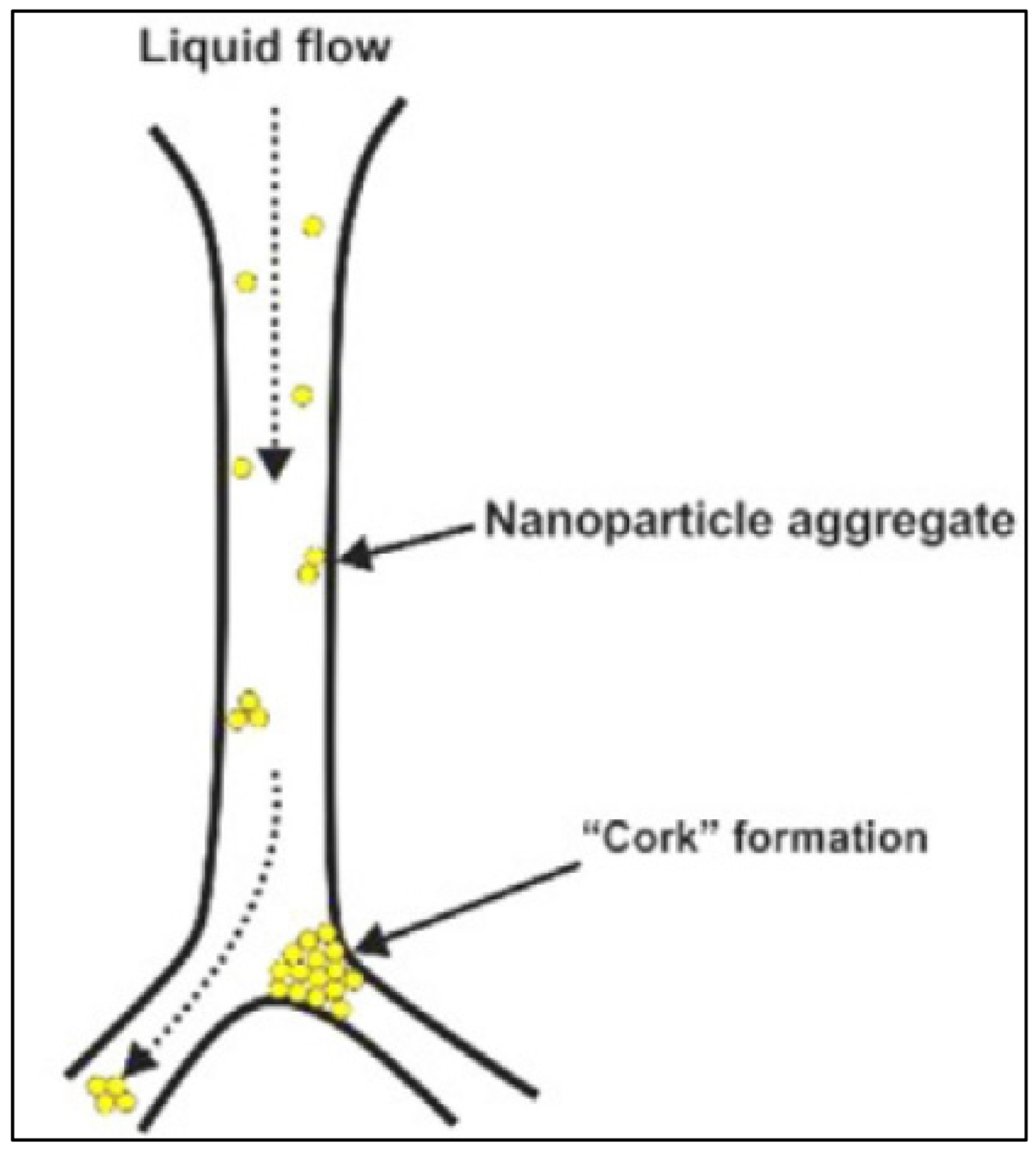
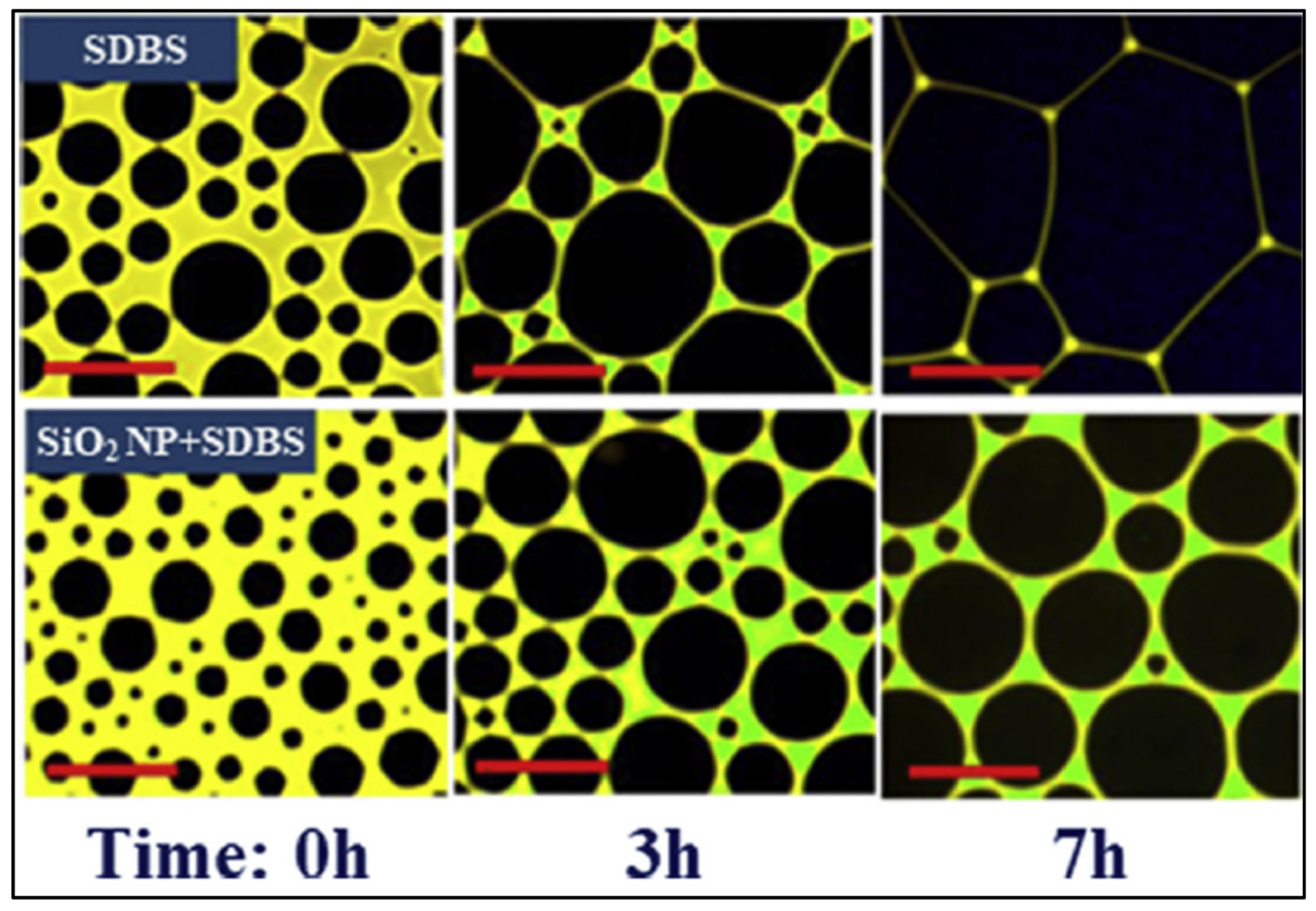
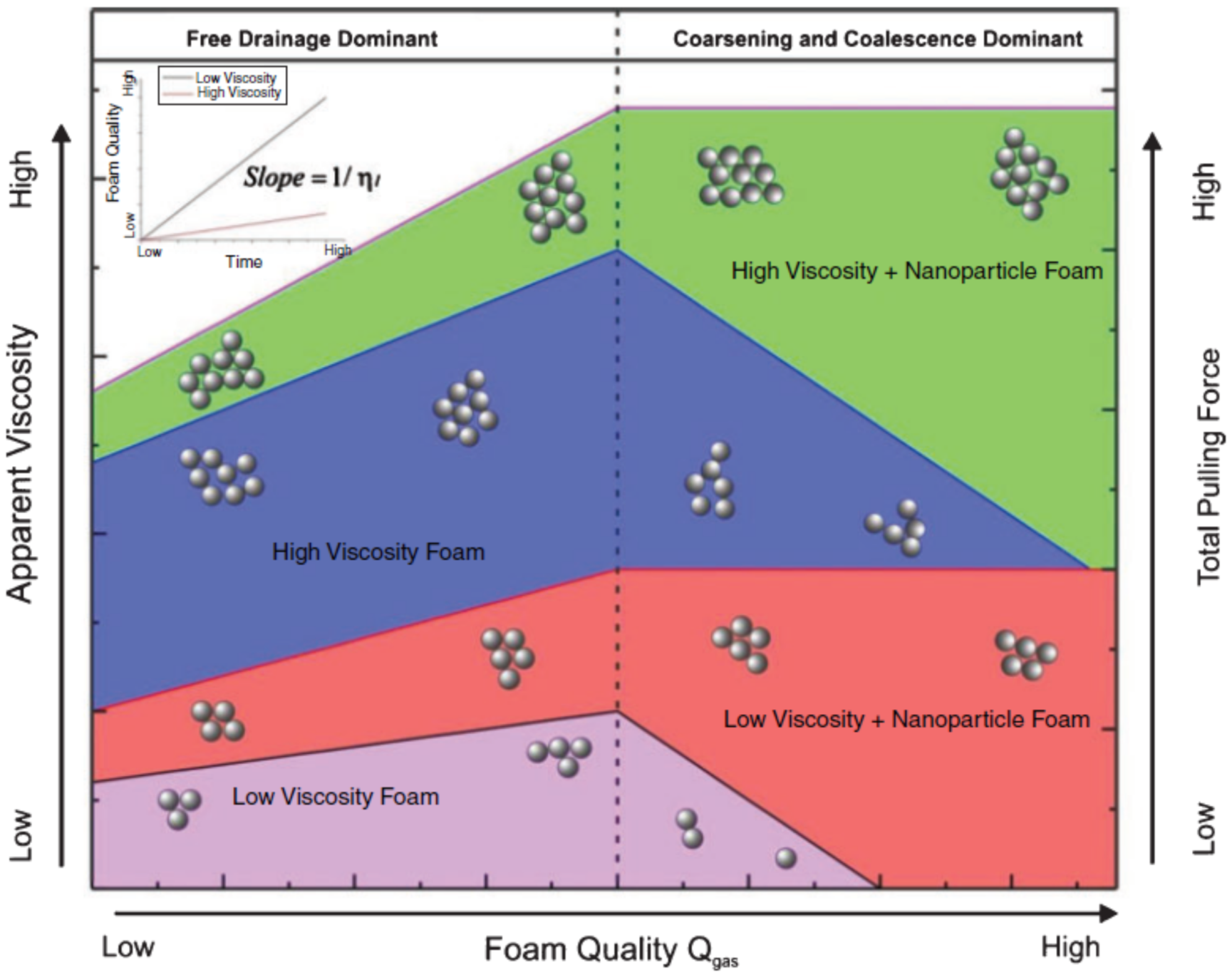
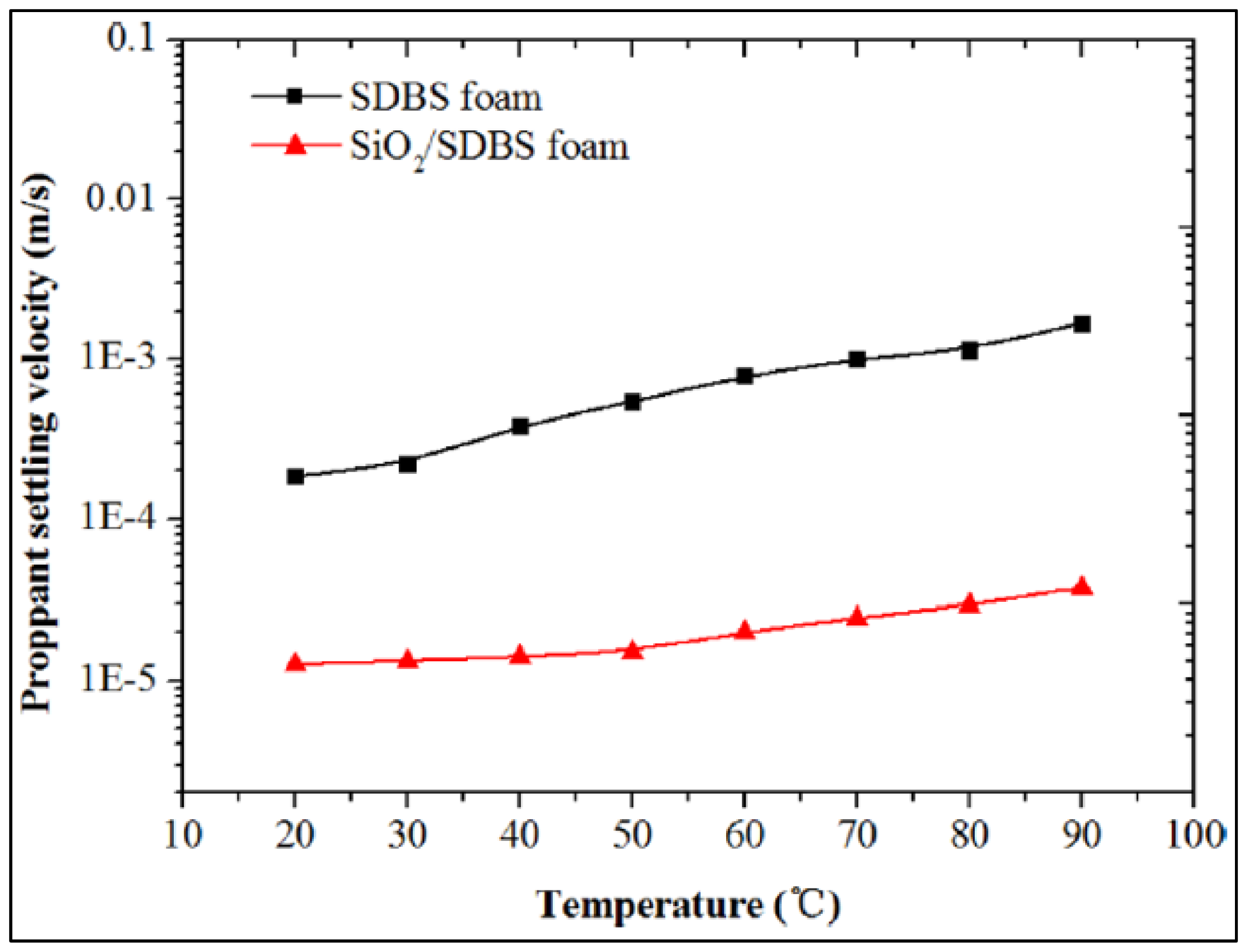
| Base Fluid | Gelant | Foamer Type | Test Type |
|---|---|---|---|
| 2% KCL, 15 to 25 vol % methanol, 2 gal per 1000-gal clay stabilizer | 20 to 40 lbm linear HPG | Amphoteric | Stability, mixing order |
| 2% KCL | 40 lbm linear guar or CMHPG | Anionic | Temperature limits, compare surfactants |
| 2 to 6% KCL, 20 vol% methanol, 0 to 2 gal per 1000-gal clay stabilizer, 0 to 2 gal TMCA/1000 gal | 40 lbm linear HPG | Amphoteric | Stability, mixing order |
| 2 KCL, 15 to 20 vol% methanol, 2 gal per 1000-gal clay stabilizer, 0 to 0.3 lbm biocide/1000 gal | 40 lbm linear HPG | Amphoteric | Stability, mixing order |
| Surfactants | Effective Concentration (%) | HLB | Average Molar Mass |
|---|---|---|---|
| Sodium dodecyl sulfate (SDS) | >90 | 40 | 288 |
| Sodium dodecyl sulfonate (SDS’) | >98 | 12.3 | 272 |
| Sodium dodecyl benzene sulfonate (SDBS) | >90 | 10.6 | 348.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Perdomo, M.E.; Wan Madihi, S. Foam Based Fracturing Fluid Characterization for an Optimized Application in HPHT Reservoir Conditions. Fluids 2022, 7, 156. https://doi.org/10.3390/fluids7050156
Gonzalez Perdomo ME, Wan Madihi S. Foam Based Fracturing Fluid Characterization for an Optimized Application in HPHT Reservoir Conditions. Fluids. 2022; 7(5):156. https://doi.org/10.3390/fluids7050156
Chicago/Turabian StyleGonzalez Perdomo, Maria E., and Sharifah Wan Madihi. 2022. "Foam Based Fracturing Fluid Characterization for an Optimized Application in HPHT Reservoir Conditions" Fluids 7, no. 5: 156. https://doi.org/10.3390/fluids7050156
APA StyleGonzalez Perdomo, M. E., & Wan Madihi, S. (2022). Foam Based Fracturing Fluid Characterization for an Optimized Application in HPHT Reservoir Conditions. Fluids, 7(5), 156. https://doi.org/10.3390/fluids7050156