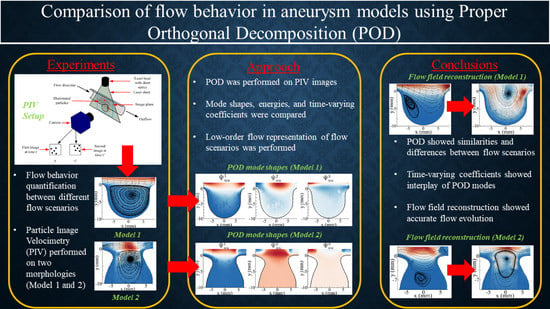
Comparison of Flow Behavior in Saccular Aneurysm Models Using Proper Orthogonal Decomposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aneurysm Models and Fluid
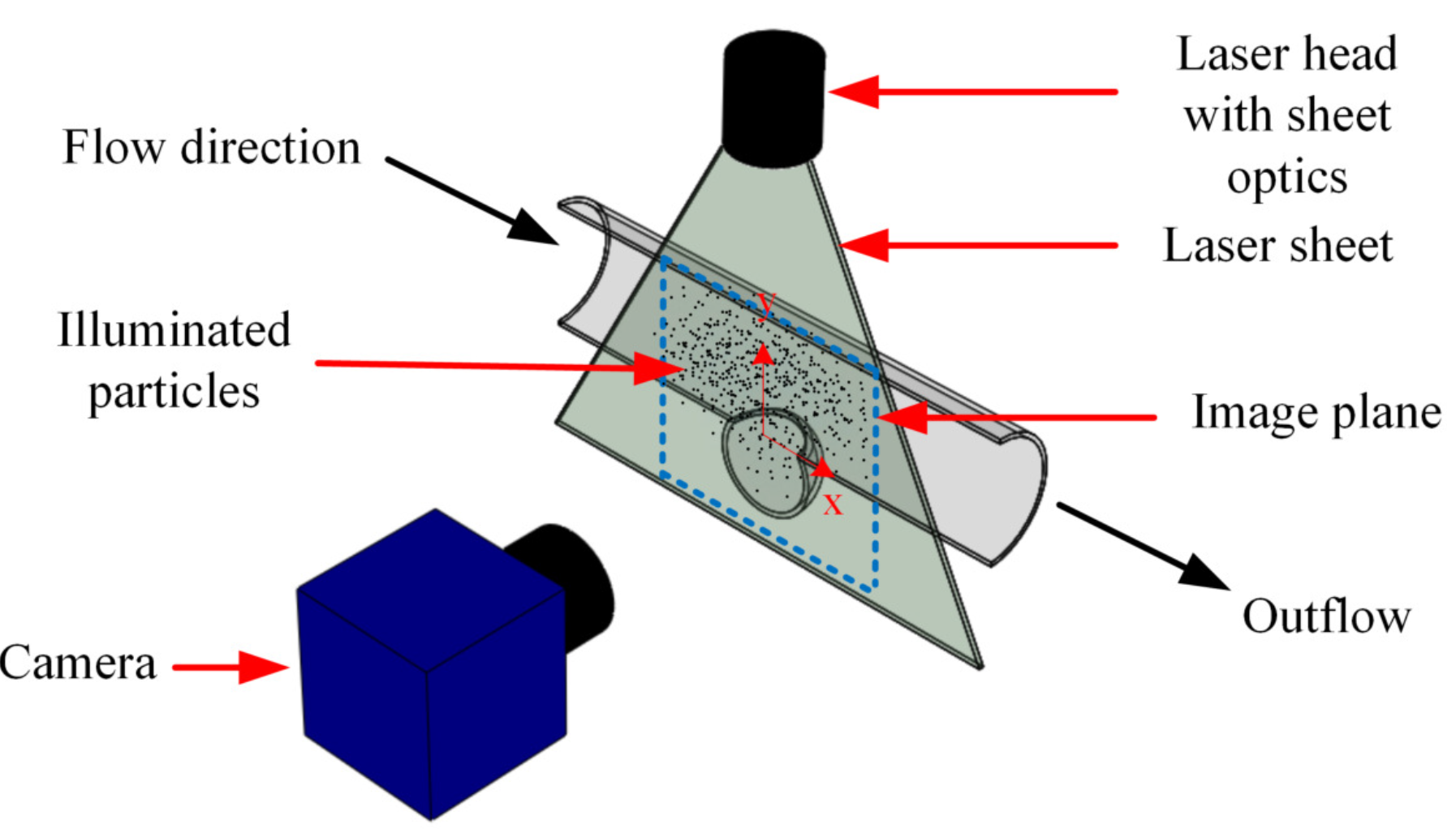
2.2. Velocity Field Measurements
2.3. Pump System
2.4. Test Conditions
2.5. POD
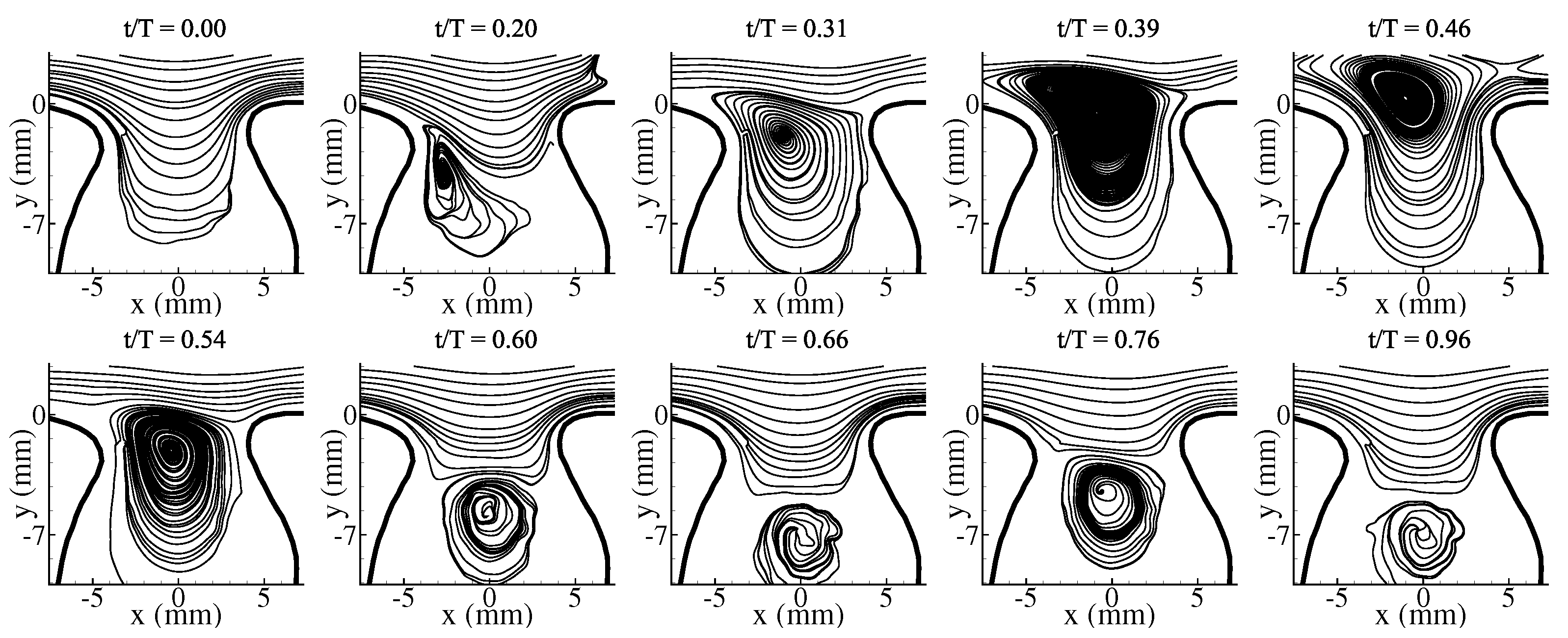
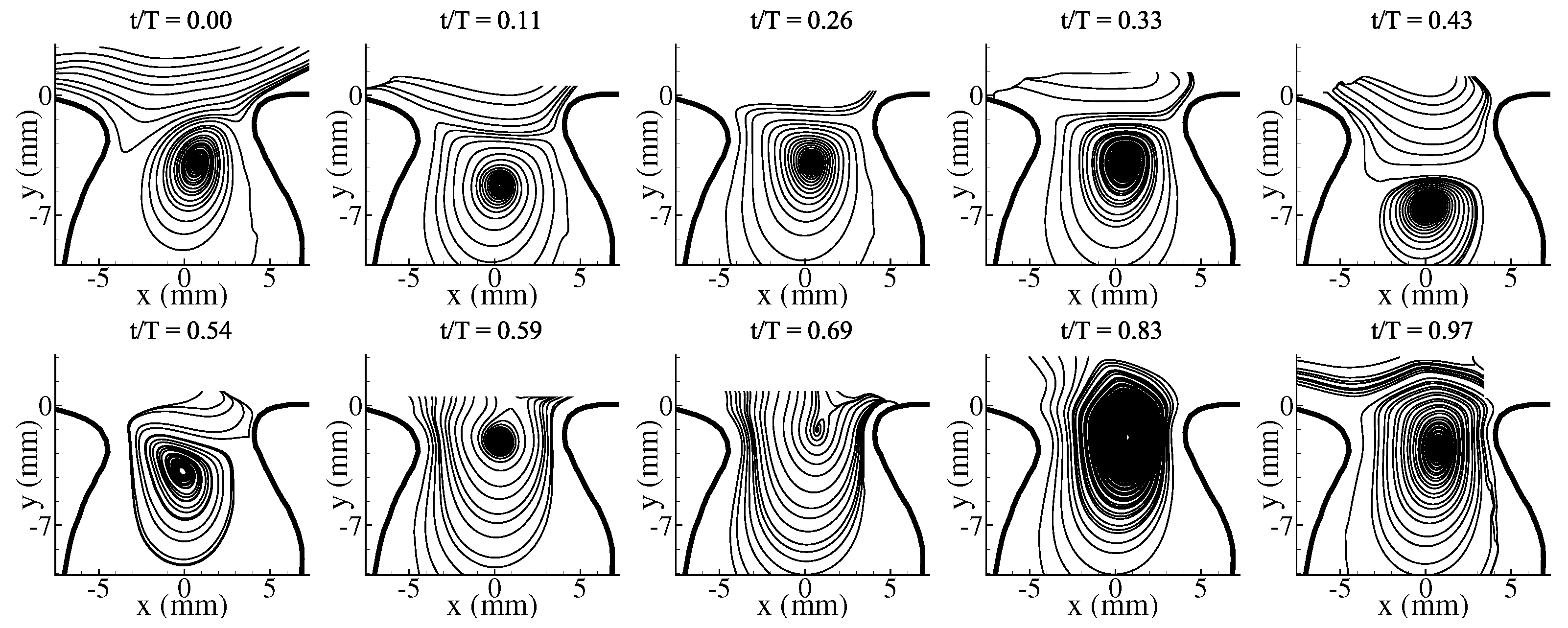
3. Results
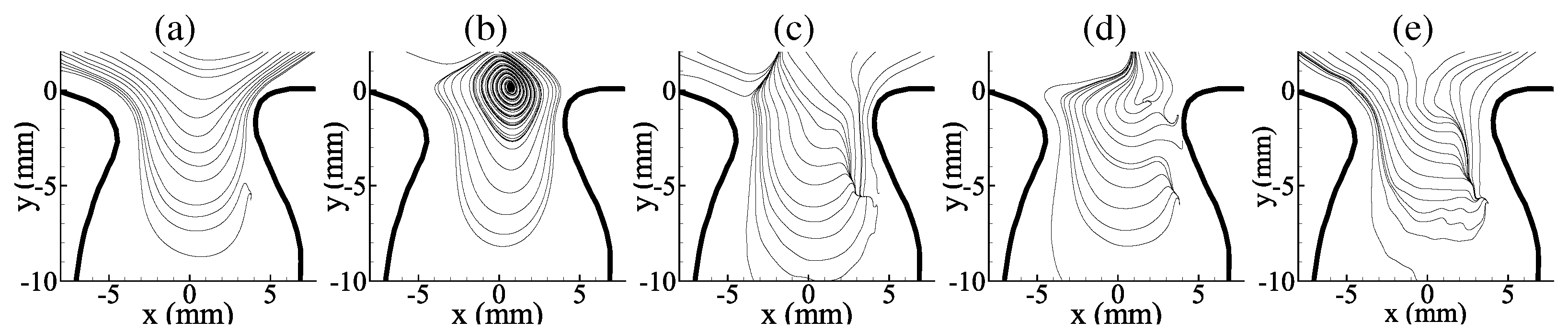
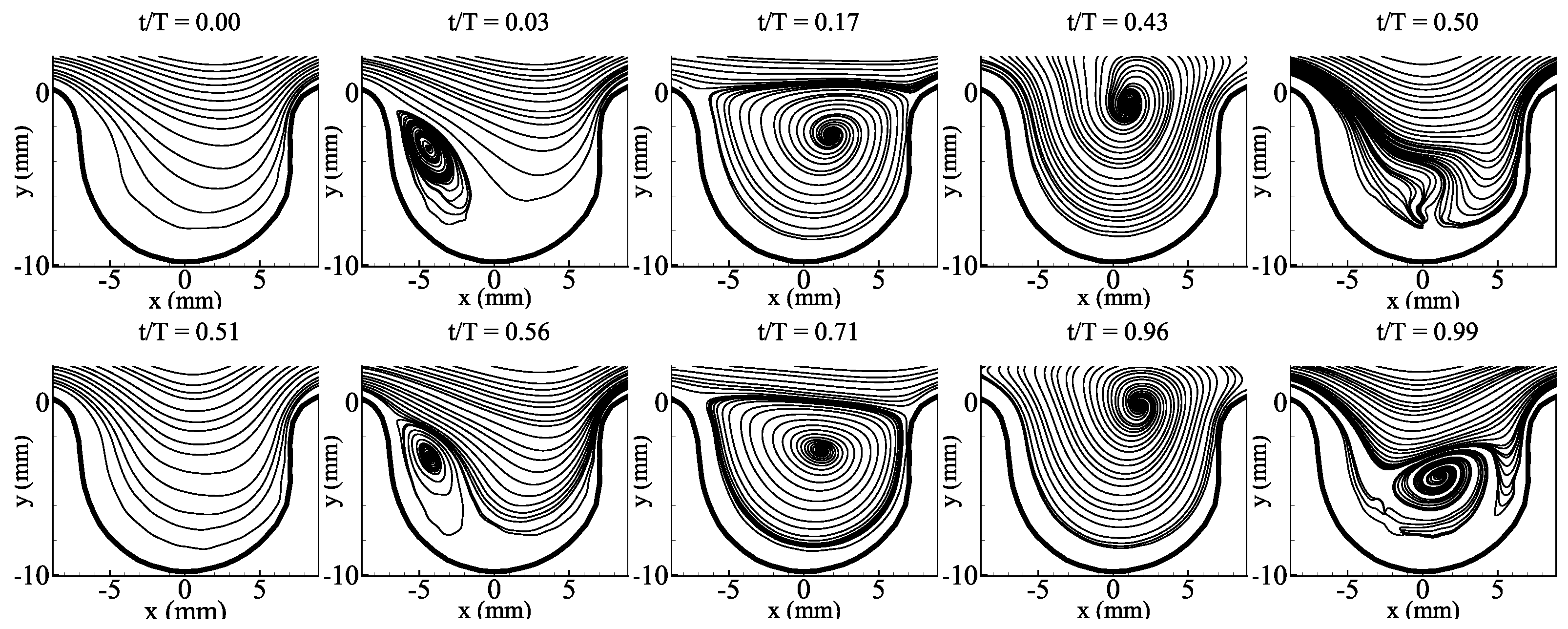
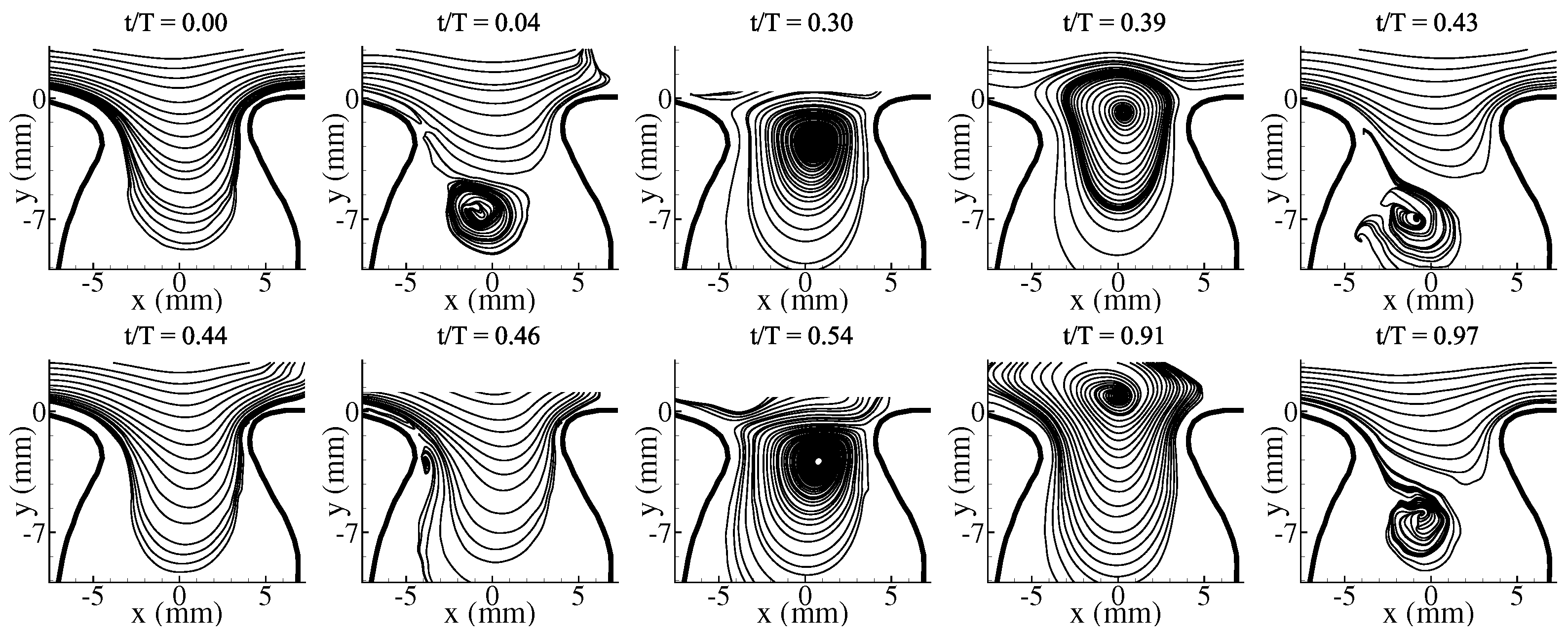
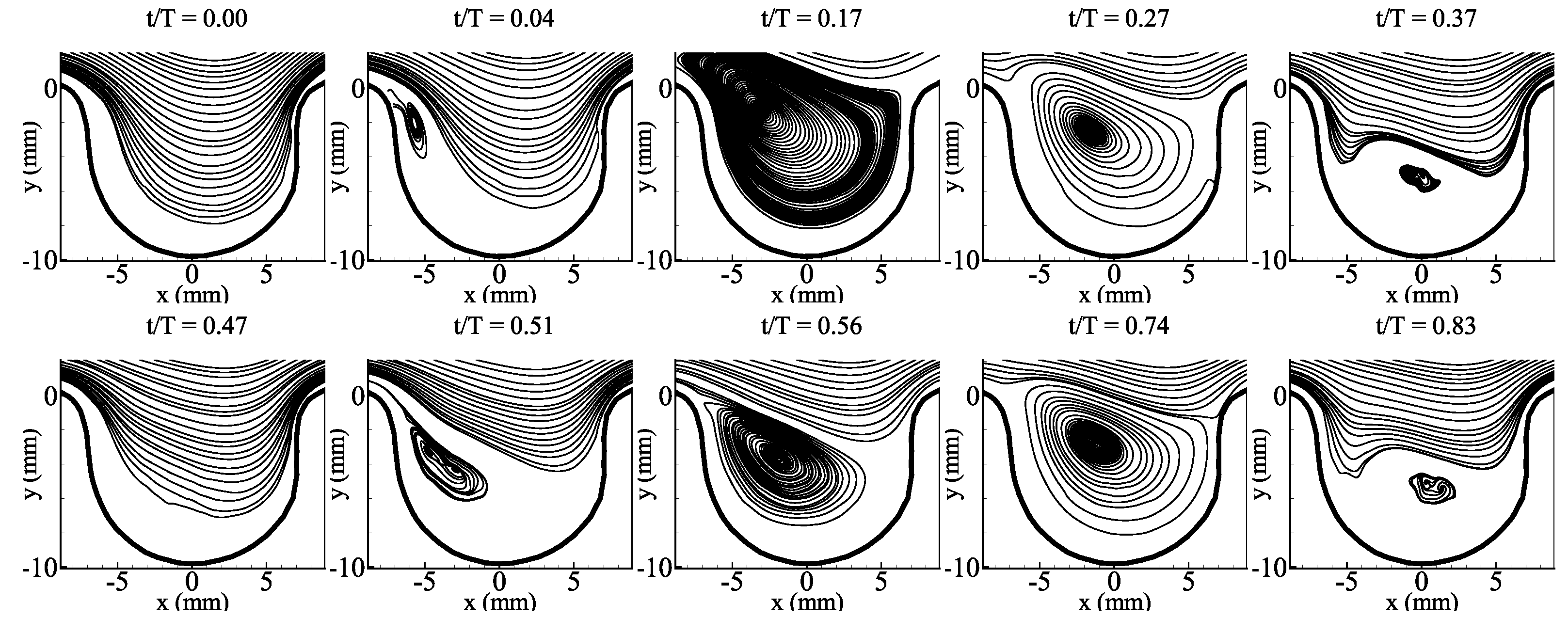
3.1. Average Flow Field
3.2. POD Modes
3.3. POD Energies
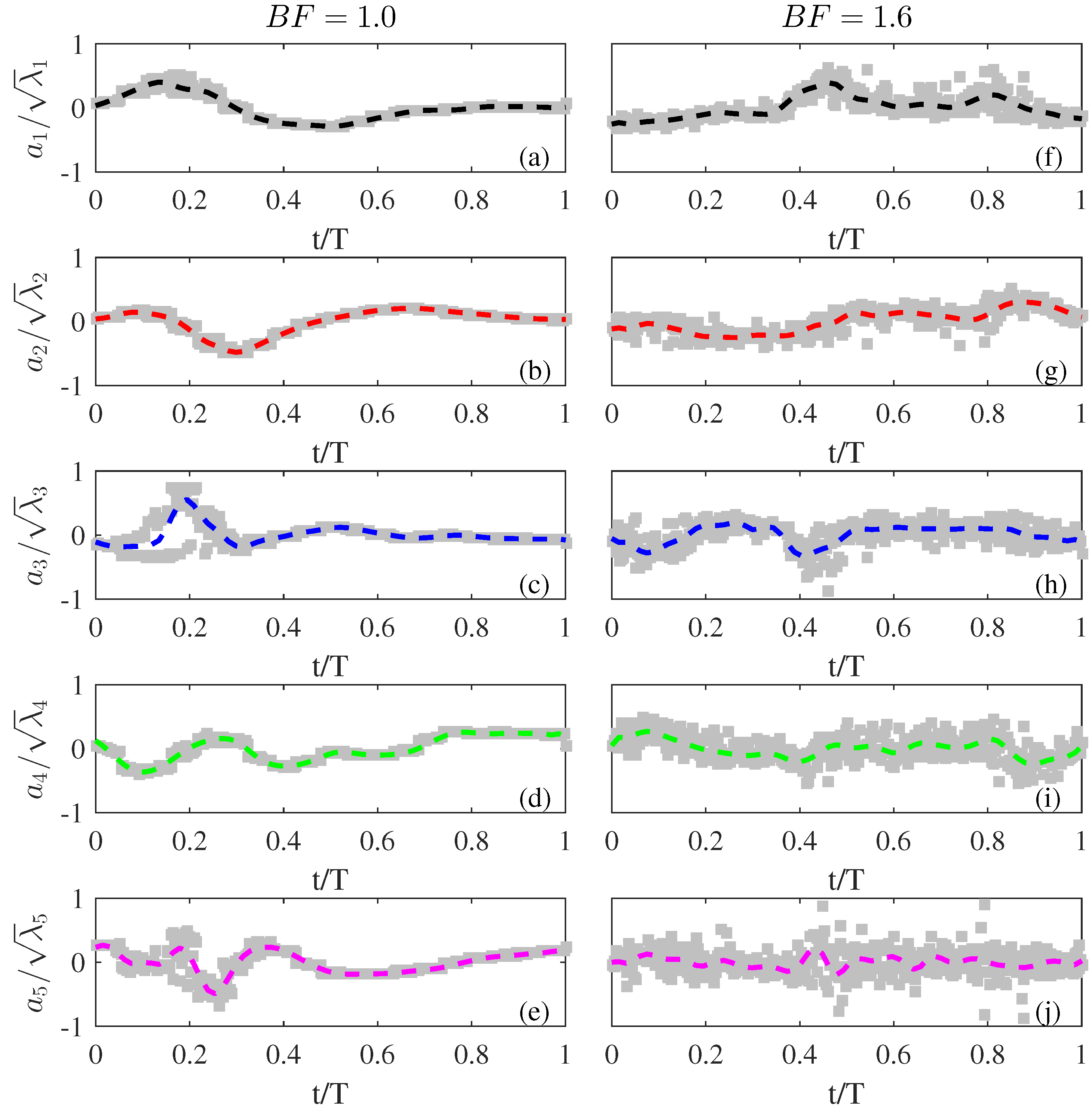
3.4. POD Time-Varying Coefficients
3.5. POD Low-Order Reconstruction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Computational Fluid Dynamics; | |
| Pipe diameter (m); | |
| Particle Image Velocimetry; | |
| Proper Orthogonal Decomposition; | |
| Spatial velocity correlation matrix; | |
| Peak Reynolds number; | |
| t | Time (s); |
| T | Time period (s); |
| Velocity vector; | |
| Velocity component in x-direction (m/s); | |
| Velocity component in y-direction (m/s); | |
| Maximum centerline velocity in the pipe (m/s); | |
| Cartesian coordinates; | |
| Womersley number; | |
| Blood kinematic viscosity (m2/s); | |
| Kinematic viscosity (m2/s); | |
| Blood density (kg/m3); | |
| Fluid density (kg/m3); | |
| Angular frequency (rad/s); | |
| ith POD mode; | |
| Streamwise component of ith POD mode; | |
| Transverse component of ith POD mode; | |
| Domain of interest; | |
| Energy captured by ith POD mode; | |
| ⊗ | Tensor product; |
| Ensemble averaging. |
References
- Wiebers, D.O.; Torner, J.C.; Meissner, I. Impact of unruptured intracranial aneurysms on public health in the United States. Stroke 1992, 23, 1416–1419. [Google Scholar] [CrossRef] [Green Version]
- Vega, C.; Kwoon, J.V.; Lavine, S.D. Intracranial aneurysms: Current evidence and clinical practice. Am. Fam. Physician 2002, 66, 601–610. [Google Scholar] [PubMed]
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef] [Green Version]
- Lasheras, J.C. The biomechanics of arterial aneurysms. Annu. Rev. Fluid Mech. 2007, 39, 293–319. [Google Scholar] [CrossRef] [Green Version]
- Cebral, J.R.; Raschi, M. Suggested connections between risk factors of intracranial aneurysms: A review. Ann. Biomed. Eng. 2013, 41, 1366–1383. [Google Scholar] [CrossRef] [PubMed]
- Burleson, A.C.; Strother, C.M.; Turitto, V.T. Computer modeling of intracranial saccular and lateral aneurysms for the study of their hemodynamics. Neurosurgery 1995, 37, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Tateshima, S.; Murayama, Y.; Villablanca, J.P.; Morino, T.; Takahashi, H.; Yamauchi, T.; Tanishita, K.; Viñuela, F. Intraaneurysmal flow dynamics study featuring an acrylic aneurysm model manufactured using a computerized tomography angiogram as a mold. J. Neurosurg. 2001, 95, 1020–1027. [Google Scholar] [CrossRef]
- Ujiie, H.; Tachi, H.; Hiramatsu, O.; Hazel, A.L.; Matsumoto, T.; Ogasawara, Y.; Nakajima, H.; Hori, T.; Takakura, K.; Kajiya, F. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery 1999, 45, 119–130. [Google Scholar]
- Sekhar, L.N.; Heros, R.C. Origin, growth, and rupture of saccular aneurysms: A review. Neurosurgery 1981, 8, 248–260. [Google Scholar] [CrossRef]
- Hoi, Y.; Meng, H.; Woodward, S.H.; Bendok, B.R.; Hanel, R.A.; Guterman, L.R.; Hopkins, L.N. Effects of arterial geometry on aneurysm growth: Three-dimensional computational fluid dynamics study. J. Neurosurg. 2004, 101, 676–681. [Google Scholar] [CrossRef]
- Ionita, C.N.; Hoi, Y.; Meng, H.; Rudin, S. Particle image velocimetry (PIV) evaluation of flow modification in aneurysm phantoms using asymmetric stents. In Proceedings of the Medical Imaging 2004: Physiology, Function, and Structure from Medical Images, San Diego, CA, USA, 15–17 February 2004; International Society for Optics and Photonics: Bellingham, WA, USA, 2004; Volume 5369, pp. 295–307. [Google Scholar]
- Bouillot, P.; Brina, O.; Ouared, R.; Lovblad, K.; Pereira, V.M.; Farhat, M. Multi-time-lag PIV analysis of steady and pulsatile flows in a sidewall aneurysm. Exp. Fluids 2014, 55, 1746. [Google Scholar] [CrossRef] [Green Version]
- Bluestein, D.; Niu, L.; Schoephoerster, R.; Dewanjee, M. Steady flow in an aneurysm model: Correlation between fluid dynamics and blood platelet deposition. J. Biomech. Eng. 1996, 118, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lieber, B.B.; Livescu, V.; Hopkins, L.; Wakhloo, A.K. Particle image velocimetry assessment of stent design influence on intra-aneurysmal flow. Ann. Biomed. Eng. 2002, 30, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Budwig, R.; Elger, D.; Hooper, H.; Slippy, J. Steady flow in abdominal aortic aneurysm models. J. Biomech. Eng. 1993, 115, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Matsuzawa, T.; Homma, T. Visualization and finite element analysis of pulsatile flow in models of the abdominal aortic aneurysm. Biorheology 1989, 26, 109–130. [Google Scholar] [CrossRef]
- Egelhoff, C. A model study of pulsatile flow regimes in abdominal aortic aneurysms. In Proceedings of the ASME FEDSM97-34-31, ASME Fluids Engineering Division Summer Division Meeting, Vancouver, BC, Canada, 22–26 June 1997. [Google Scholar]
- Taylor, T.W.; Yamaguchi, T. Three-dimensional simulation of blood flow in an abdominal aortic aneurysm—Steady and unsteady flow cases. J. Biomech. Eng. 1994, 116, 89–97. [Google Scholar] [CrossRef]
- Gobin, Y.; Counord, J.; Flaud, P.; Duffaux, J. In vitro study of haemodynamics in a giant saccular aneurysm model: Influence of flow dynamics in the parent vessel and effects of coil embolisation. Neuroradiology 1994, 36, 530–536. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, J. A steady flow analysis on the stented and non-stented sidewall aneurysm models. Med. Eng. Phys. 1999, 21, 133–141. [Google Scholar] [CrossRef]
- Le, T.B.; Borazjani, I.; Sotiropoulos, F. Pulsatile flow effects on the hemodynamics of intracranial aneurysms. J. Biomech. Eng. 2010, 132, 111009. [Google Scholar] [CrossRef]
- Yu, P.; Durgesh, V. Experimental Study of Large-Scale Flow Structures in an Aneurysm. In Proceedings of the Fluids Engineering Division Summer Meeting, Montreal, QC, Canada, 15–20 July 2018; Volume 51555, p. V001T02A009. [Google Scholar]
- Yu, P.; Durgesh, V.; Xing, T.; Budwig, R. Application of Proper Orthogonal Decomposition to Study Coherent Flow Structures in a Saccular Aneurysm. J. Biomech. Eng. 2021, 143, 061008. [Google Scholar] [CrossRef]
- Yu, P.; Durgesh, V. Application of Dynamic Mode Decomposition to Study Temporal Flow Behavior in a Saccular Aneurysm. J. Biomech. Eng. 2022, 144, 51002. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, H.; Borazjani, I. A non-dimensional parameter for classification of the flow in intracranial aneurysms. I. Simplified geometries. Phys. Fluids 2019, 31, 031904. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, H.; Asadi, H.; Meng, H.; Borazjani, I. A non-dimensional parameter for classification of the flow in intracranial aneurysms. II. Patient-specific geometries. Phys. Fluids 2019, 31, 031905. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, H.; Borazjani, I. Effects of Reynolds and Womersley numbers on the hemodynamics of intracranial aneurysms. Comput. Math. Methods Med. 2016, 2016, 7412926. [Google Scholar] [CrossRef] [Green Version]
- Lumley, J.L. The structure of inhomogeneous turbulent flows. In Atmospheric Turbulence and Radio Wave Propagation; Yaglom, A.M., Tartarsky, V.I., Eds.; Nauka: Tokyo, Japan, 1967; pp. 166–177. [Google Scholar]
- Rowley, C.; Colonius, T.; Murray, R. POD based models of self-sustained oscillations in the flow past an open cavity. In Proceedings of the 6th Aeroacoustics Conference and Exhibit, Lahaina, HI, USA, 12–14 June 2000; p. 1969. [Google Scholar]
- Berkooz, G.; Holmes, P.; Lumley, J.L. The proper orthogonal decomposition in the analysis of turbulent flows. Annu. Rev. Fluid Mech. 1993, 25, 539–575. [Google Scholar] [CrossRef]
- Graftieaux, L.; Michard, M.; Grosjean, N. Combining PIV, POD and vortex identification algorithms for the study of unsteady turbulent swirling flows. Meas. Sci. Technol. 2001, 12, 1422. [Google Scholar] [CrossRef]
- Chen, H.; Selimovic, A.; Thompson, H.; Chiarini, A.; Penrose, J.; Ventikos, Y.; Watton, P.N. Investigating the influence of haemodynamic stimuli on intracranial aneurysm inception. Ann. Biomed. Eng. 2013, 41, 1492–1504. [Google Scholar] [CrossRef]
- Byrne, G.; Mut, F.; Cebral, J. Quantifying the large-scale hemodynamics of intracranial aneurysms. Am. J. Neuroradiol. 2014, 35, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Daroczy, L.; Abdelsamie, A.; Janiga, G.; Thevenin, D. State Detection and Hybrid Simulation of Biomedical Flows. In Proceedings of the Tenth International Symposium on Turbulence and Shear Flow Phenomena, Chicago, IL, USA, 7–9 July 2017; Begel House Inc.: Danbury, CT, USA, 2017. [Google Scholar]
- Janiga, G. Quantitative assessment of 4D hemodynamics in cerebral aneurysms using proper orthogonal decomposition. J. Biomech. 2019, 82, 80–86. [Google Scholar] [CrossRef]
- Bluestein, D.; Moore, J.E. Biofluids educational issues: An emerging field aims to define its next generation. Ann. Biomed. Eng. 2005, 33, 1674–1680. [Google Scholar] [CrossRef]
- Cebral, J.R.; Castro, M.A.; Burgess, J.E.; Pergolizzi, R.S.; Sheridan, M.J.; Putman, C.M. Characterization of cerebral aneurysms for assessing risk of rupture by using patient-specific computational hemodynamics models. Am. J. Neuroradiol. 2005, 26, 2550–2559. [Google Scholar] [PubMed]
- Shojima, M.; Oshima, M.; Takagi, K.; Torii, R.; Nagata, K.; Shirouzu, I.; Morita, A.; Kirino, T. Role of the bloodstream impacting force and the local pressure elevation in the rupture of cerebral aneurysms. Stroke 2005, 36, 1933–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateshima, S.; Tanishita, K.; Omura, H.; Villablanca, J.; Vinuela, F. Intra-aneurysmal hemodynamics during the growth of an unruptured aneurysm: In vitro study using longitudinal CT angiogram database. Am. J. Neuroradiol. 2007, 28, 622–627. [Google Scholar] [PubMed]
- Bluestein, D.; Dumont, K.; De Beule, M.; Ricotta, J.; Impellizzeri, P.; Verhegghe, B.; Verdonck, P. Intraluminal thrombus and risk of rupture in patient specific abdominal aortic aneurysm–FSI modelling. Comput. Methods Biomech. Biomed. Eng. 2009, 12, 73–81. [Google Scholar] [CrossRef]
- Watton, P.; Ventikos, Y. Modelling evolution of saccular cerebral aneurysms. J. Strain Anal. Eng. Des. 2009, 44, 375–389. [Google Scholar] [CrossRef]
- Torii, R.; Oshima, M.; Kobayashi, T.; Takagi, K.; Tezduyar, T.E. Influencing factors in image-based fluid–structure interaction computation of cerebral aneurysms. Int. J. Numer. Methods Fluids 2011, 65, 324–340. [Google Scholar] [CrossRef]
- Usmani, A.Y.; Muralidhar, K. Flow in an intracranial aneurysm model: Effect of parent artery orientation. J. Vis. 2018, 21, 795–818. [Google Scholar] [CrossRef]
- Li, Z.; Hu, L.; Chen, C.; Wang, Z.; Zhou, Z.; Chen, Y. Hemodynamic performance of multilayer stents in the treatment of aneurysms with a branch attached. Sci. Rep. 2019, 9, 10193. [Google Scholar] [CrossRef] [Green Version]
- Saqr, K.M.; Rashad, S.; Tupin, S.; Niizuma, K.; Hassan, T.; Tominaga, T.; Ohta, M. What does computational fluid dynamics tell us about intracranial aneurysms? A meta-analysis and critical review. J. Cereb. Blood Flow Metab. 2020, 40, 1021–1039. [Google Scholar] [CrossRef]
- Fahrig, R.; Nikolov, H.; Fox, A.; Holdsworth, D. A three-dimensional cerebrovascular flow phantom. Med. Phys. 1999, 26, 1589–1599. [Google Scholar] [CrossRef]
- Hopkins, L.; Kelly, J.; Wexler, A.; Prasad, A. Particle image velocimetry measurements in complex geometries. Exp. Fluids 2000, 29, 91–95. [Google Scholar] [CrossRef]
- Ugron, Á.; Farinas, M.I.; Kiss, L.; Paál, G. Unsteady velocity measurements in a realistic intracranial aneurysm model. Exp. Fluids 2012, 52, 37–52. [Google Scholar] [CrossRef]
- Geoghegan, P.; Buchmann, N.; Spence, C.; Moore, S.; Jermy, M. Fabrication of rigid and flexible refractive-index-matched flow phantoms for flow visualisation and optical flow measurements. Exp. Fluids 2012, 52, 1331–1347. [Google Scholar] [CrossRef]
- Le, T.B.; Troolin, D.R.; Amatya, D.; Longmire, E.K.; Sotiropoulos, F. Vortex phenomena in sidewall aneurysm hemodynamics: Experiment and numerical simulation. Ann. Biomed. Eng. 2013, 41, 2157–2170. [Google Scholar] [CrossRef]
- Yong, K.W.; Janmaleki, M.; Pachenari, M.; Mitha, A.P.; Sanati-Nezhad, A.; Sen, A. Engineering a 3D human intracranial aneurysm model using liquid-assisted injection molding and tuned hydrogels. Acta Biomater. 2021, 136, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Budwig, R. Refractive index matching methods for liquid flow investigations. Exp. Fluids 1994, 17, 350–355. [Google Scholar] [CrossRef]
- Bai, K.; Katz, J. On the refractive index of sodium iodide solutions for index matching in PIV. Exp. Fluids 2014, 55, 1704. [Google Scholar] [CrossRef]
- Miller, P.; Danielson, K.; Moody, G.; Slifka, A.; Drexler, E.; Hertzberg, J. Matching index of refraction using a diethyl phthalate/ethanol solution for in vitro cardiovascular models. Exp. Fluids 2006, 41, 375–381. [Google Scholar] [CrossRef]
- Adrian, R.J. Particle-imaging techniques for experimental fluid mechanics. Annu. Rev. Fluid Mech. 1991, 23, 261–304. [Google Scholar] [CrossRef]
- Boillot, A.; Prasad, A. Optimization procedure for pulse separation in cross-correlation PIV. Exp. Fluids 1996, 21, 87–93. [Google Scholar] [CrossRef]
- Meinhart, C.D.; Wereley, S.T.; Santiago, J.G. A PIV algorithm for estimating time-averaged velocity fields. J. Fluids Eng. 2000, 122, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Ku, D.N. Blood flow in arteries. Annu. Rev. Fluid Mech. 1997, 29, 399–434. [Google Scholar] [CrossRef]
- Ma, B.; Harbaugh, R.E.; Raghavan, M.L. Three-dimensional geometrical characterization of cerebral aneurysms. Ann. Biomed. Eng. 2004, 32, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.; Poll, A.; Liepsch, D.; Reulen, H.J. Haemodynamic stress in lateral saccular aneurysms. Acta Neurochir. 1987, 86, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Womersley, J.R. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol. 1955, 127, 553–563. [Google Scholar] [CrossRef]
- Steiger, H.J.; Poll, A.; Liepsch, D.; Reulen, H.J. Basic flow structure in saccular aneurysms: A flow visualization study. Heart Vessel. 1987, 3, 55–65. [Google Scholar] [CrossRef]
- Liou, T.M.; Liou, S.N. A review on in vitro studies of hemodynamic characteristics in terminal and lateral aneurysm models. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1999, 23, 133. [Google Scholar]
- White, F.M.; Corfield, I. Viscous Fluid Flow; McGraw-Hill: New York, NY, USA, 2006; Volume 3. [Google Scholar]
- Holman, R.; Utturkar, Y.; Mittal, R.; Smith, B.L.; Cattafesta, L. Formation criterion for synthetic jets. AIAA J. 2005, 43, 2110–2116. [Google Scholar] [CrossRef] [Green Version]
- Sirovich, L. Turbulence and the dynamics of coherent structures. I. Coherent structures. Q. Appl. Math. 1987, 45, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Holmes, P.; Lumley, J.L.; Berkooz, G.; Rowley, C.W. Turbulence, Coherent Structures, Dynamical Systems and Symmetry; Cambridge University Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Durgesh, V.; Naughton, J. Multi-time-delay LSE-POD complementary approach applied to unsteady high-Reynolds-number near wake flow. Exp. Fluids 2010, 49, 571–583. [Google Scholar] [CrossRef]




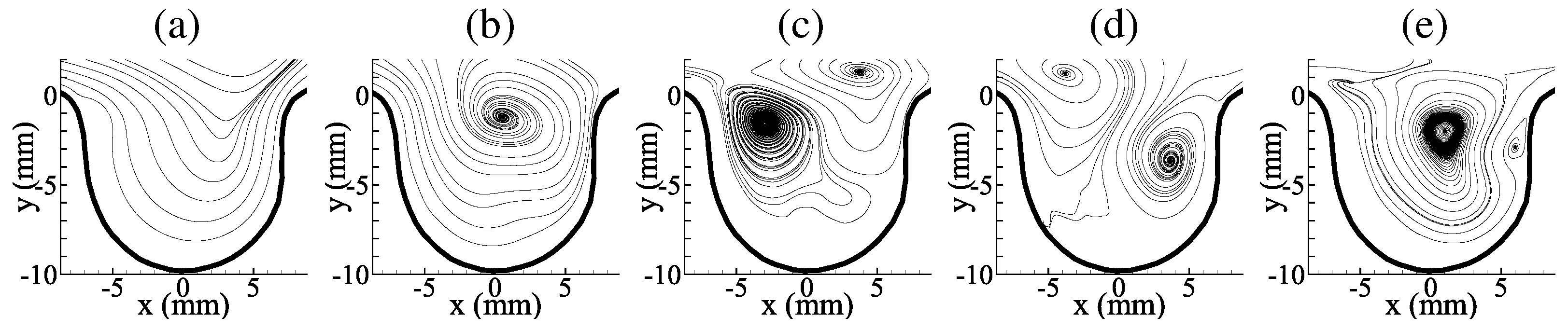


| BF | Re | PIV Images | PIV Frame Rate (Hz) | Pump Frequency (Hz) | |
|---|---|---|---|---|---|
| 1.0 | 50 | 2 | 500 | 1.17 | 0.4 |
| 1.0 | 270 | 2 | 500 | 1.17 | 0.4 |
| 1.0 | 50 | 5 | 500 | 1.17 | 2.4 |
| 1.0 | 270 | 5 | 500 | 1.17 | 2.4 |
| 1.6 | 50 | 2 | 500 | 1.17 | 0.4 |
| 1.6 | 270 | 2 | 500 | 1.17 | 0.4 |
| 1.6 | 50 | 5 | 500 | 1.17 | 2.4 |
| 1.6 | 270 | 5 | 500 | 1.17 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Durgesh, V. Comparison of Flow Behavior in Saccular Aneurysm Models Using Proper Orthogonal Decomposition. Fluids 2022, 7, 123. https://doi.org/10.3390/fluids7040123
Yu P, Durgesh V. Comparison of Flow Behavior in Saccular Aneurysm Models Using Proper Orthogonal Decomposition. Fluids. 2022; 7(4):123. https://doi.org/10.3390/fluids7040123
Chicago/Turabian StyleYu, Paulo, and Vibhav Durgesh. 2022. "Comparison of Flow Behavior in Saccular Aneurysm Models Using Proper Orthogonal Decomposition" Fluids 7, no. 4: 123. https://doi.org/10.3390/fluids7040123
APA StyleYu, P., & Durgesh, V. (2022). Comparison of Flow Behavior in Saccular Aneurysm Models Using Proper Orthogonal Decomposition. Fluids, 7(4), 123. https://doi.org/10.3390/fluids7040123