Injectable Composite Systems of Gellan Gum:Alginate Microparticles in Pluronic Hydrogels for Bioactive Cargo Controlled Delivery: Optimization of Hydrogel Composition based on Rheological Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microparticle Production
2.3. Hydrogel and Microparticle–Hydrogel Composite System
2.4. Rheological Characterization
2.5. In Vitro Degradation of Pluronic
2.6. In Vitro Drug Release
3. Results and Discussion
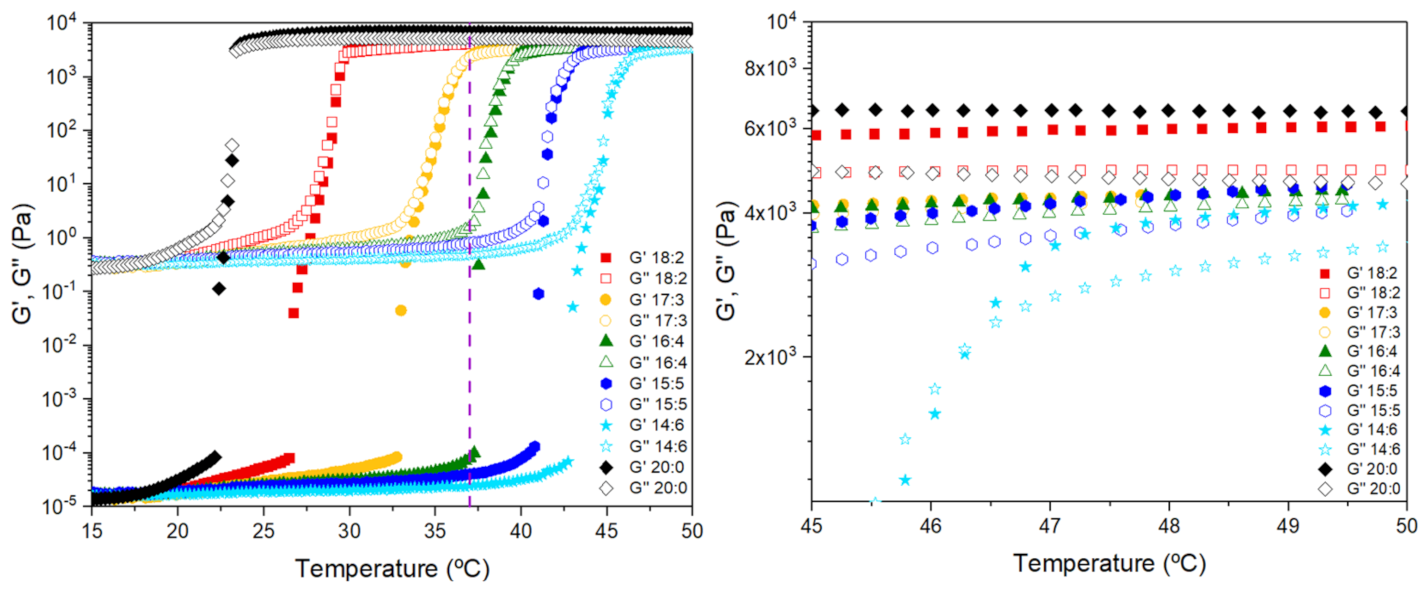
3.1. Sol–Gel Transition Temperature
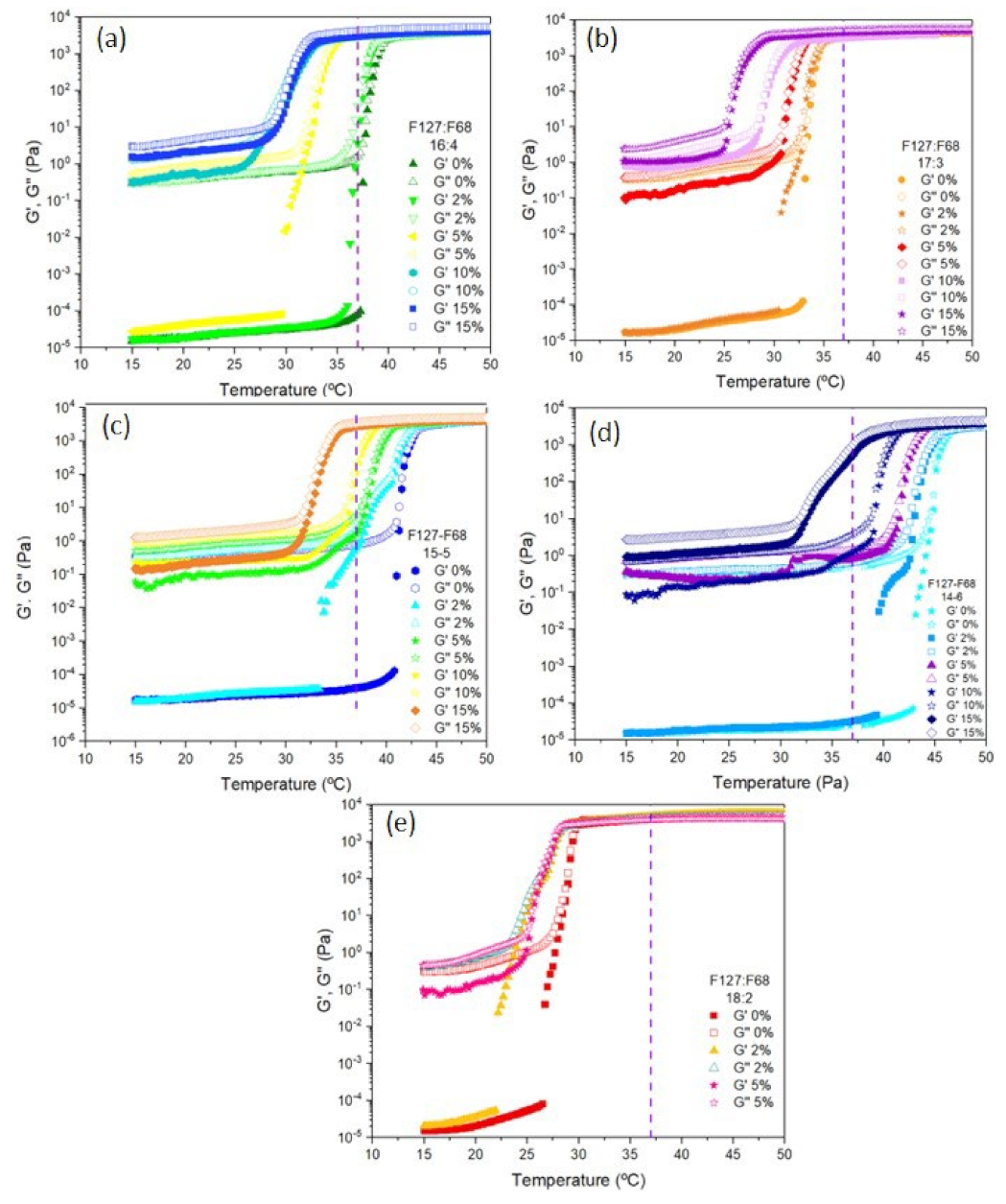
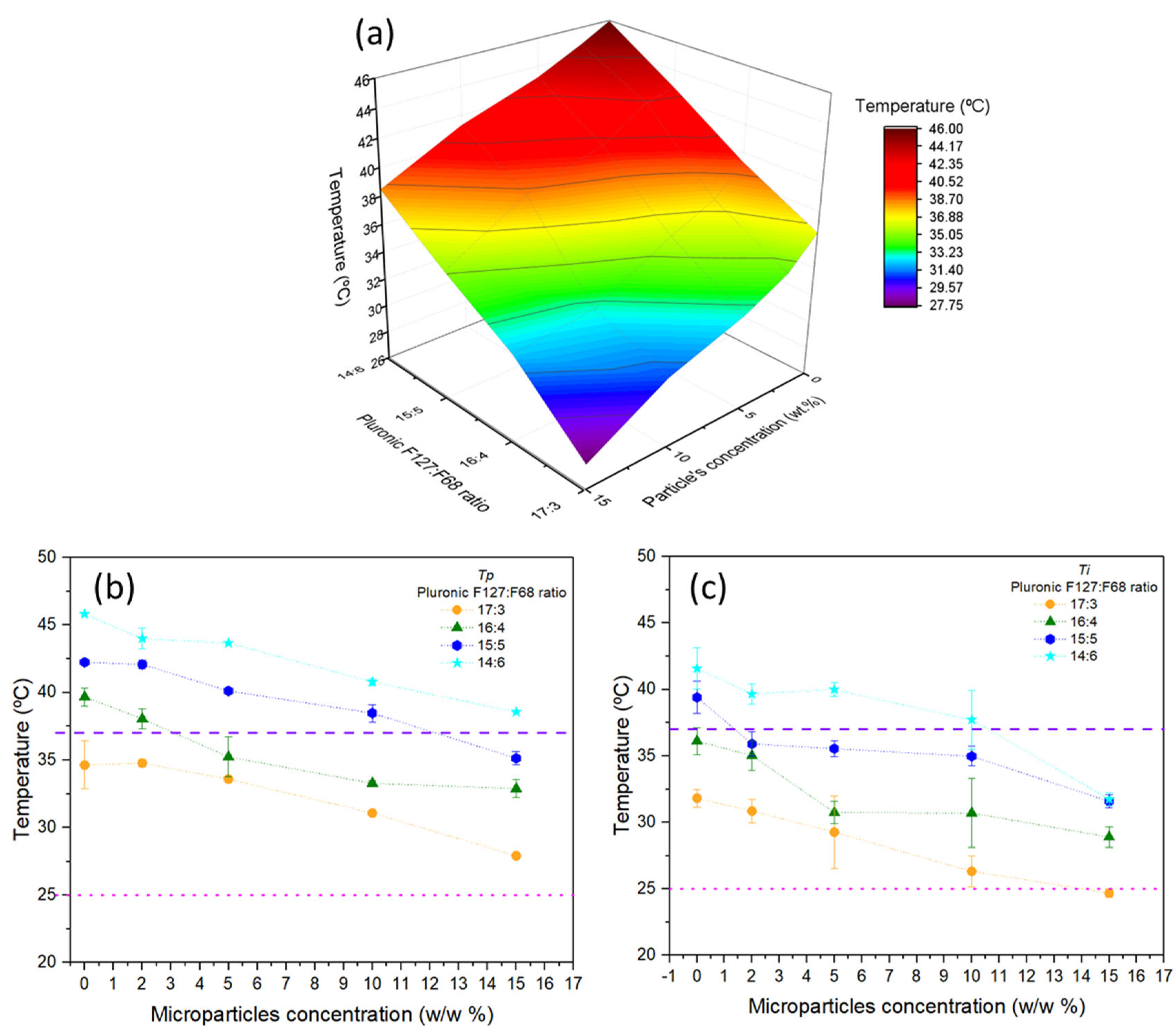
3.2. Sol–Gel Transition of the Microparticle–Hydrogel Composite System
Mathematical Fittings of the Transition Temperatures
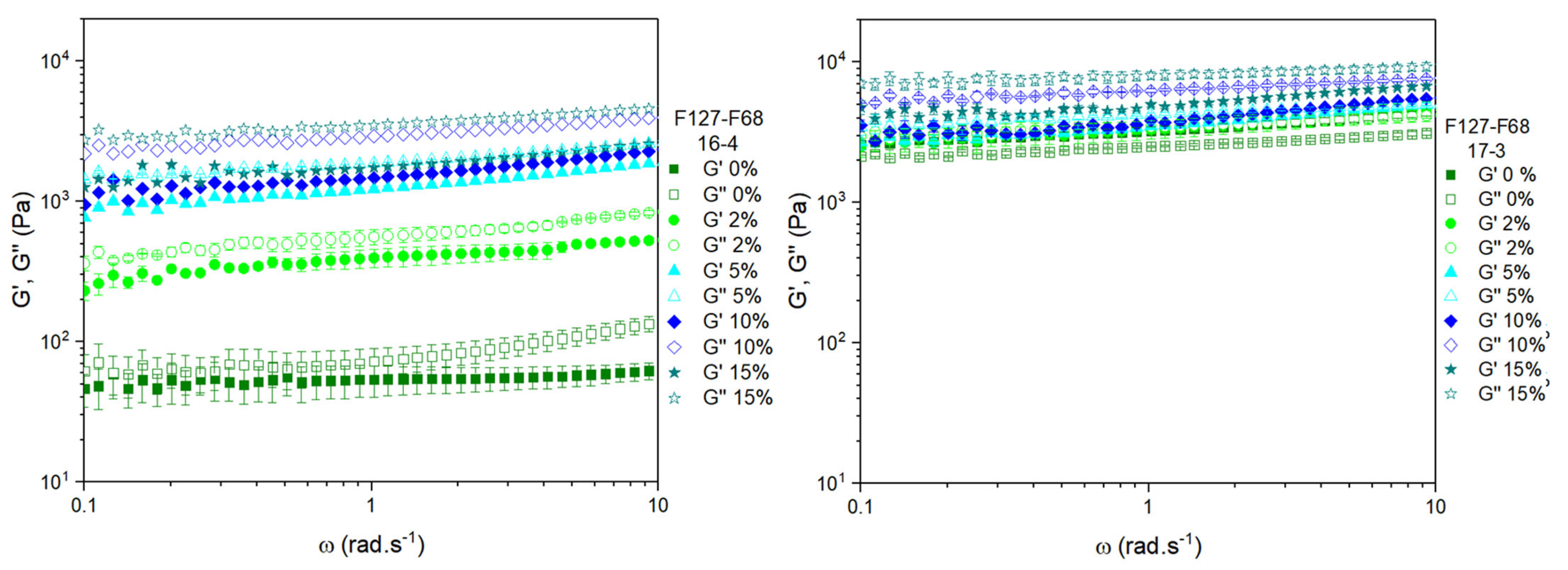
3.3. Frequency Sweeps at 37 °C
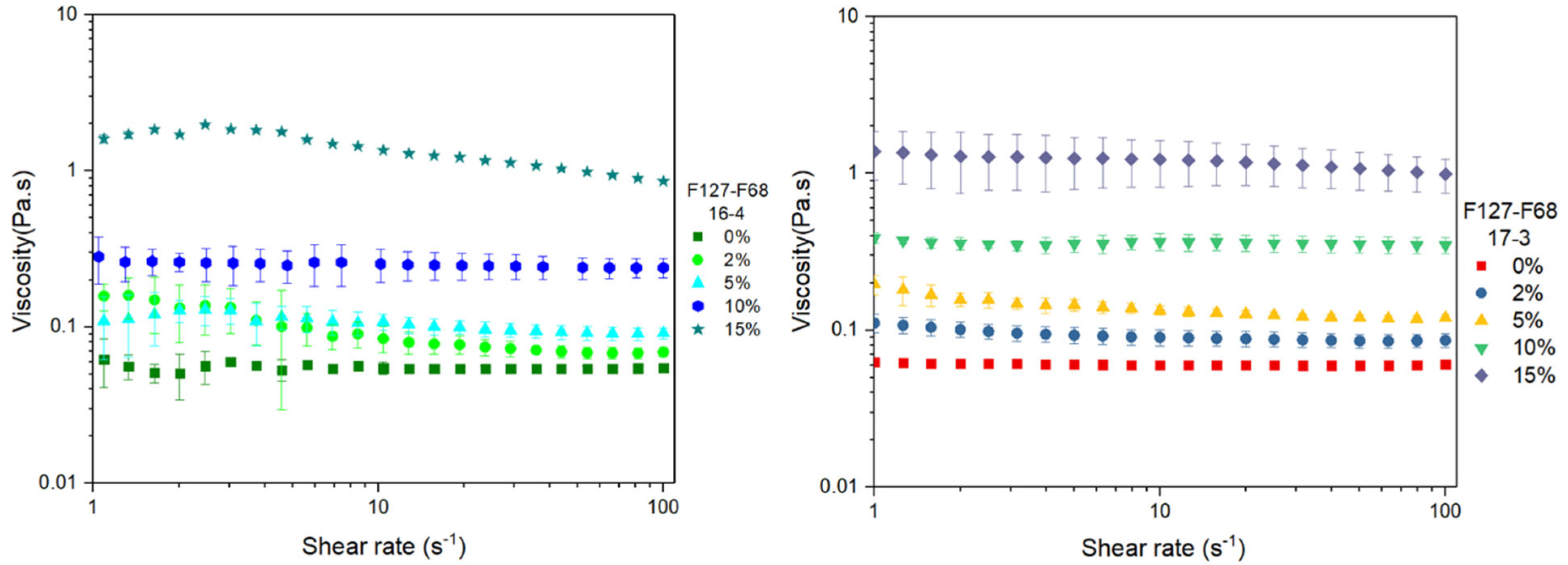
3.4. Flow Curves at 21 °C
3.5. Degradation of Pluronic Hydrogels
3.6. In Vitro MB-Release Profiles
3.7. Mathematical Fittings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Song, W.; He, Y.; Li, H. Multilayer Injectable Hydrogel System Sequentially Delivers Bioactive Substances for Each Wound Healing Stage. ACS Appl. Mater. Interfaces 2020, 12, 29787–29806. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, L.; Farhadi, F.; Xia, W.; Chang, J.; He, Y.; Li, H. An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 2018, 192, 149–158. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Hou, J.; Yang, G.; Zhou, S. A Time-Programmed Release of Dual Drugs from an Implantable Trilayer Structured Fiber Device for Synergistic Treatment of Breast Cancer. Small 2019, 16, 1902262. [Google Scholar] [CrossRef]
- Yu, H.; Ning, N.; Meng, X.; Chittasupho, C.; Jiang, L.; Zhao, Y. Sequential Drug Delivery in Targeted Cancer Therapy. Pharmaceutics 2022, 14, 573. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- de Farias, A.L.; Meneguin, A.B.; Barud, H.D.S.; Brighenti, F.L. The role of sodium alginate and gellan gum in the design of new drug delivery systems intended for antibiofilm activity of morin. Int. J. Biol. Macromol. 2020, 162, 1944–1958. [Google Scholar] [CrossRef]
- Mun, S.; Kim, H.C.; Yadave, M.; Kim, J. Graphene oxide–gellan gum–sodium alginate nanocomposites: Synthesis, characterization, and mechanical behavior. Compos. Interfaces 2015, 22, 249–263. [Google Scholar] [CrossRef]
- Rosas-Flores, W.; Ramos-Ramírez, E.G.; Salazar-Montoya, J.A. Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii using alginate and gellan gum. Carbohydr. Polym. 2013, 98, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Chen, K.-N.; Kuo, Y.-T. Optimal thermotolerance ofBifidobacterium bifidum in gellan–alginate microparticles. Biotechnol. Bioeng. 2007, 98, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Das, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded unsaturated esterified alginate/gellan gum microspheres: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2013, 57, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Cidade, M.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable Hydrogels Based on Pluronic/Water Systems Filled with Alginate Microparticles for Biomedical Applications. Material 2019, 12, 1083. [Google Scholar] [CrossRef]
- Calero, N.; Santos, J.; Echevarría, C.; Muñoz, J.; Cidade, M.T. Time-dependent behavior in analyte-, temperature-, and shear-sensitive Pluronic PE9400/water systems. Colloid Polym. Sci. 2018, 296, 1515–1522. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L.; Kan, C.-W. Thermoresponsive Hydrogels and Their Biomedical Applications: Special Insight into Their Applications in Textile Based Transdermal Therapy. Polymers 2018, 10, 480. [Google Scholar] [CrossRef]
- Jalaal, M.; Cottrell, G.; Balmforth, N.; Stoeber, B. On the rheology of Pluronic F127 aqueous solutions. J. Rheol. 2017, 61, 139–146. [Google Scholar] [CrossRef]
- Tipa, C.; Cidade, M.T.; Vieira, T.; Silva, J.C.; Soares, P.I.P.; Borges, J.P. A New Long-Term Composite Drug Delivery System Based on Thermo-Responsive Hydrogel and Nanoclay. Nanomaterials 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic® mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Rio, L.G.-D.; Diaz-Rodriguez, P.; Landin, M. New tools to design smart thermosensitive hydrogels for protein rectal delivery in IBD. Mater. Sci. Eng. C 2020, 106, 110252. [Google Scholar] [CrossRef]
- Carrêlo, H.; Soares, P.I.P.; Borges, J.P.; Cidade, M.T. Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery. Gels 2021, 7, 147. [Google Scholar] [CrossRef]
- Carrêlo, H.; Cidade, M.T.; Borges, J.P.; Soares, P.I.P. Gellan gum/alginate microparticles as drug delivery vehicles: DOE production optimization and drug delivery. Submited 2022. [Google Scholar]
- Nastase, I.; Croitoru, C.V.; Vartires, A.; Tataranu, L. Indoor Environmental Quality in Operating Rooms: An European Standards Review with Regard to Romanian Guidelines. Energy Procedia 2016, 85, 375–382. [Google Scholar] [CrossRef]
- Li, T.; Bao, Q.; Shen, J.; Lalla, R.V.; Burgess, D.J. Mucoadhesive in situ forming gel for oral mucositis pain control. Int. J. Pharm. 2020, 580, 119238. [Google Scholar] [CrossRef]
- Suman, K.; Sourav, S.; Joshi, Y.M. Rheological signatures of gel–glass transition and a revised phase diagram of an aqueous triblock copolymer solution of Pluronic F127. Phys. Fluids 2021, 33, 073610. [Google Scholar] [CrossRef]
- Costanzo, S.; Di Sarno, A.; D'Apuzzo, M.; Avallone, P.R.; Raccone, E.; Bellissimo, A.; Auriemma, F.; Grizzuti, N.; Pasquino, R. Rheology and morphology of Pluronic F68 in water. Phys. Fluids 2021, 33, 043113. [Google Scholar] [CrossRef]
- Lee, M.H.; Shin, G.H.; Park, H.J. Solid lipid nanoparticles loaded thermoresponsive pluronic-xanthan gum hydrogel as a transdermal delivery system. J. Appl. Polym. Sci. 2017, 135, 46004. [Google Scholar] [CrossRef]
- Delgado, B.; Carrêlo, H.; Loureiro, M.V.; Marques, A.C.; Borges, J.P.; Cidade, M.T. Injectable hydrogels with two different rates of drug release based on pluronic/water system filled with poly(ε-caprolactone) microcapsules. J. Mater. Sci. 2021, 56, 13416–13428. [Google Scholar] [CrossRef]
- Lin, H.-R.; Sung, K.C.; Vong, W.-J. In Situ Gelling of Alginate/Pluronic Solutions for Ophthalmic Delivery of Pilocarpine. Biomacromolecules 2004, 5, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2016, 66, 22–53. [Google Scholar] [CrossRef]
- Bek, M.; Gonzalez-Gutierrez, J.; Kukla, C.; Črešnar, K.P.; Maroh, B.; Perše, L.S. Rheological Behaviour of Highly Filled Materials for Injection Moulding and Additive Manufacturing: Effect of Particle Material and Loading. Appl. Sci. 2020, 10, 7993. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, Y.; Chen, J.; Ding, J.; Li, M.; Li, C.; Wang, J.-C.; Chen, X. Injectable Hydrogel–Microsphere Construct with Sequential Degradation for Locally Synergistic Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 3487–3496. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.L.-I. Review of Applications and Future Prospects of Stimuli-Responsive Hydrogel Based on Thermo-Responsive Biopolymers in Drug Delivery Systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Zarandona, I.; Bengoechea, C.; Álvarez-Castillo, E.; de la Caba, K.; Guerrero, A.; Guerrero, P. 3D Printed Chitosan-Pectin Hydrogels: From Rheological Characterization to Scaffold Development and Assessment. Gels 2021, 7, 175. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Dolatyar, B.; Zeynali, B. In vitro study of alginate–gelatin scaffolds incorporated with silica NPs as injectable, biodegradable hydrogels. RSC Adv. 2021, 11, 16688–16697. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and mechanical behavior of milk protein composite gel for extrusion-based 3D food printing. LWT 2018, 102, 338–346. [Google Scholar] [CrossRef]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interface Sci. 2019, 565, 119–130. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, Y.; Bae, K.H.; Ahn, C.-H.; Park, T.G. Temperature/pH-Sensitive Hydrogels Prepared from Pluronic Copolymers End-Capped with Carboxylic Acid Groups via an Oligolactide Spacer. Macromol. Rapid Commun. 2007, 28, 1172–1176. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Xu, X.; Ansari, S.; Zadeh, H.H.; Marques, M.M.; Shi, S.; Moshaverinia, A. Pluronic F-127 hydrogel as a promising scaffold for encapsulation of dental-derived mesenchymal stem cells. J. Mater. Sci. Mater. Med. 2015, 26, 153. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.Y.; Noh, J.H.; Park, S.H.; Ji, Y.B.; Ju, H.J.; Kim, D.Y.; Lee, B.; Kim, M.S. An Injectable Click-Crosslinked Hydrogel that Prolongs Dexamethasone Release from Dexamethasone-Loaded Microspheres. Pharmaceutics 2019, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Prezotti, F.G.; Cury, B.S.F.; Evangelista, R.C. Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr. Polym. 2014, 113, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparision of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–124. [Google Scholar]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.-H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 1274–1325. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]


| Tp | |||
|---|---|---|---|
| F127:F68 Ratio | m (°C/(wt.%)) | ||
| 14:6 | –0.4658 | 45.55 | 0.9799 |
| 15:5 | –0.4762 | 42.66 | 0.9764 |
| 16:4 | –0.4564 | 38.74 | 0.8805 |
| 17:3 | –0.4668 | 35.38 | 0.9616 |
| Ti | |||
| F127:F68 Ratio | m (°C/(wt.%)) | (°C) | |
| 14:6 | –0.5886 | 41.91 | 0.8804 |
| 15:5 | –0.4182 | 38.16 | 0.8413 |
| 16:4 | –0.4646 | 35.27 | 0.8341 |
| 17:3 | –0.4900– | 31.72 | 0.9900 |
| Batches (g)/pH | 16:4 5% | 17:3 5% | 17:3 2% | Microparticles | ||||
|---|---|---|---|---|---|---|---|---|
| pH 6.5 | pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | ||
| KP Tlag | k | 21.150 | 22.533 | 20.995 | 20.710 | 23.236 | 21.849 | 30.191 |
| n | 0.349 | 0.309 | 0.331 | 0.298 | 0.312 | 0.325 | 0.240 | |
| Tlag | 1.938 | 2.923 | 3.868 | 3.896 | 3.959 | 2.957 | 2.957 | |
| R2adj | 0.929 | 0.956 | 0.965 | 0.956 | 0.955 | 0.961 | 0.955 | |
| PS Tlag | k1 | 10.316 | 12.253 | 9.105 | 7.820 | 8.011 | 11.780 | 13.744 |
| k2 | –0.245 | –0.389 | –0.205 | –0.178 | –0.158 | –0.345 | –0.446 | |
| m | 0.691 | 0.593 | 0.658 | 0.670 | 0.730 | 0.606 | 0.557 | |
| Tlag | 0.835 | 1.746 | 2.330 | 1.791 | 1.872 | 1.894 | 0.893 | |
| R2adj | 0.995 | 0.994 | 0.997 | 0.996 | 0.989 | 0.991 | 0.990 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrêlo, H.; Escoval, A.R.; Soares, P.I.P.; Borges, J.P.; Cidade, M.T. Injectable Composite Systems of Gellan Gum:Alginate Microparticles in Pluronic Hydrogels for Bioactive Cargo Controlled Delivery: Optimization of Hydrogel Composition based on Rheological Behavior. Fluids 2022, 7, 375. https://doi.org/10.3390/fluids7120375
Carrêlo H, Escoval AR, Soares PIP, Borges JP, Cidade MT. Injectable Composite Systems of Gellan Gum:Alginate Microparticles in Pluronic Hydrogels for Bioactive Cargo Controlled Delivery: Optimization of Hydrogel Composition based on Rheological Behavior. Fluids. 2022; 7(12):375. https://doi.org/10.3390/fluids7120375
Chicago/Turabian StyleCarrêlo, Henrique, André R. Escoval, Paula I. P. Soares, João P. Borges, and Maria Teresa Cidade. 2022. "Injectable Composite Systems of Gellan Gum:Alginate Microparticles in Pluronic Hydrogels for Bioactive Cargo Controlled Delivery: Optimization of Hydrogel Composition based on Rheological Behavior" Fluids 7, no. 12: 375. https://doi.org/10.3390/fluids7120375
APA StyleCarrêlo, H., Escoval, A. R., Soares, P. I. P., Borges, J. P., & Cidade, M. T. (2022). Injectable Composite Systems of Gellan Gum:Alginate Microparticles in Pluronic Hydrogels for Bioactive Cargo Controlled Delivery: Optimization of Hydrogel Composition based on Rheological Behavior. Fluids, 7(12), 375. https://doi.org/10.3390/fluids7120375









