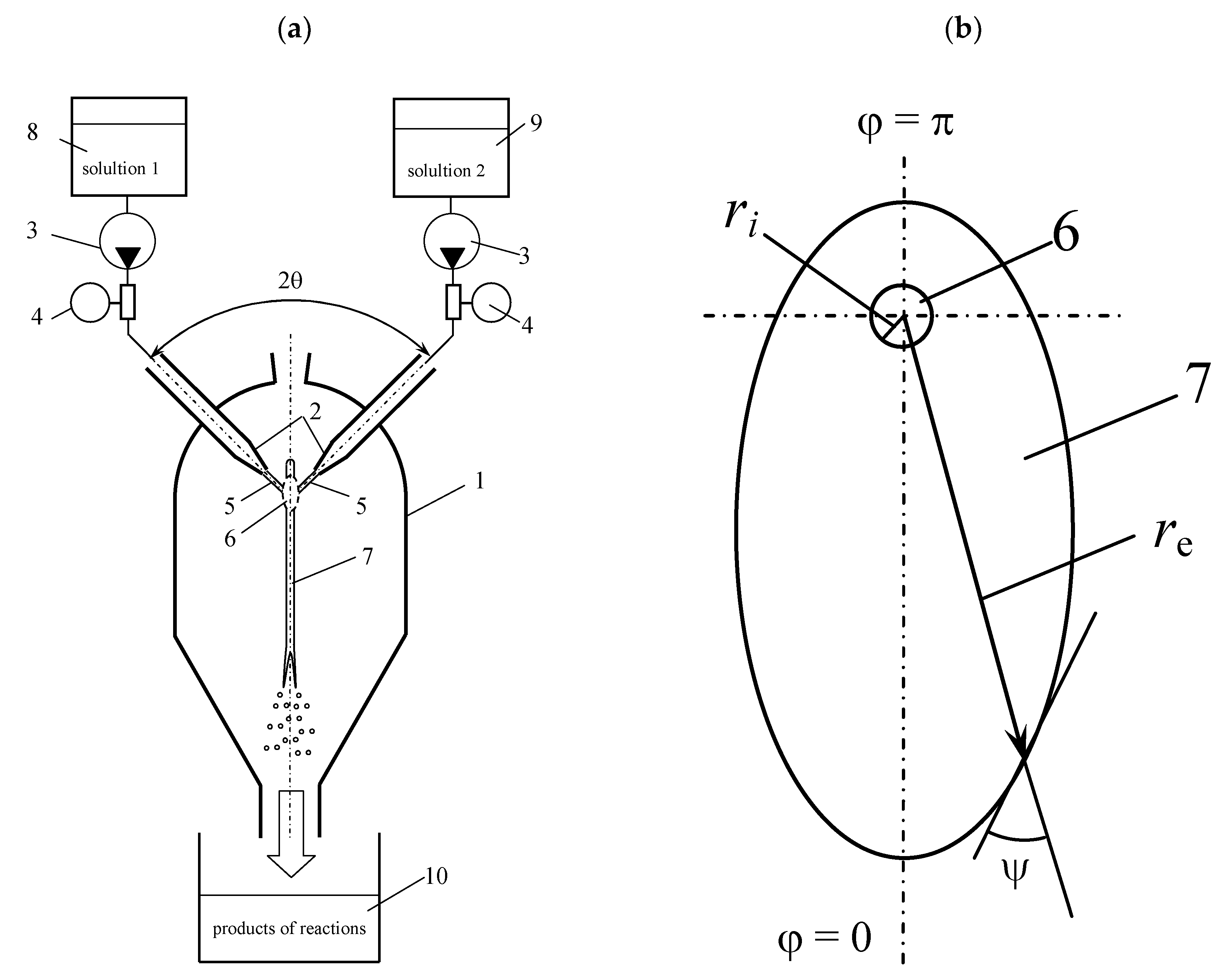
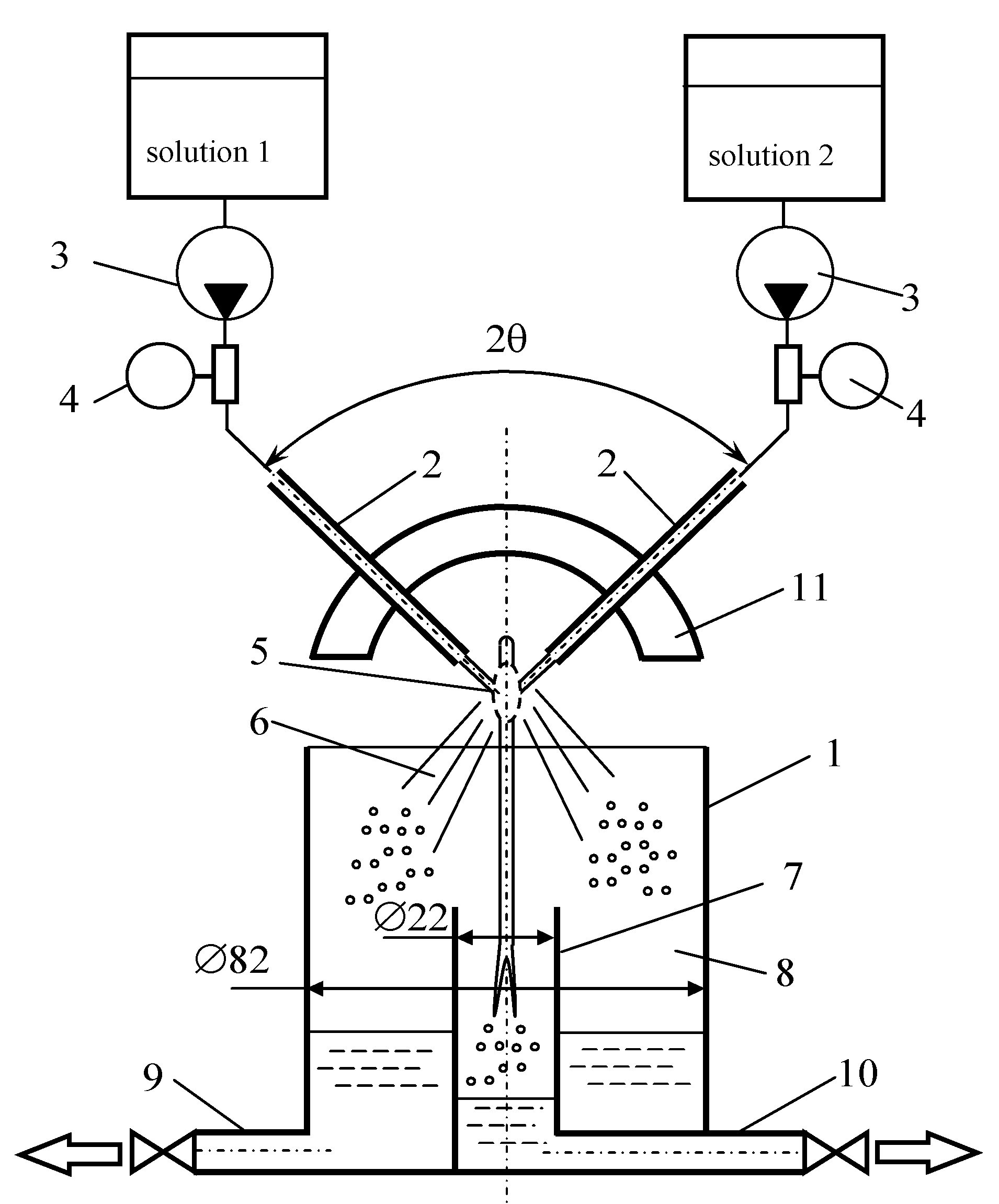
1. Introduction
The development of methods for the intensification of various processes of chemical technologies (Process Intensification) is one of the key directions in the development of modern chemistry, both in large-scale industries (typical examples are oil refining, petrochemistry, and metallurgy), and in “fine” chemistry—this includes fine organic synthesis and production of nanosized materials [
1].
Miniaturization of chemical engineering equipment allows for a qualitative leap in the space-time scales of objects in which the transformation of matter takes place [
2]. As a result of the extremely high density of the kinetic energy of the flow, dissipated in extremely small volumes of mixed solutions (on the order of microliters or even nanoliters), an unprecedented high quality of micromixing is achieved [
3,
4]. This leads to the production of particles (crystallite) with a size of 20–50 nm, and in some cases (when several products may be formed) to the production of a pure product in accordance with the stoichiometry employed (for example, formation of a pure bismuth orthoferrite BiFeO
3 in a microreactor synthesis without the formation of mullite Bi
2Fe
4O
9 or sillenite Bi
25FeO
39; the latter two products are formed during the hydrothermal synthesis [
3,
4]). It should be noted that the level of energy consumption in microreactors does not exceed units or tens of watts.
Thus, oxide materials in microreactors during the processes of synthesis exhibit the following advantages: (1) the ability to concentrate energy in small volumes at an energy level of no more than tens of watts to achieve high energy dissipation rate ε (up to 0.54·10
8 W/kg, see discussion in
Section 3.2; (2) an extremely high level of micromixing ensures a high uniformity of distribution of reactants in contacting solutions (the estimated time scale for micromixing in MRFIJ is from tens to hundreds of microseconds); (3) due to the unprecedented high level of micromixing, the production of pure products is achieved, and due to their short-term contact, the size of the formed particles can be controlled to a certain extent; (4) from a technological point of view, the continuity of the process achieved in flow-through devices is a valuable preference; (5) a significant reduction in the synthesis time of precursors in comparison with existing methods (mainly hydrothermal and their modifications)—from several tens of hours to several seconds and milliseconds.
One of the attractive options for the use of microreactor technologies for the synthesis of nanosized particles is a microreactor with free impinging jets (MRFIJ) [
5,
6]. MRFIJ w used earlier for the synthesis of CoFe
2O
4, BiFeO
3, GdFeO
3, LaPO
4, and a number of other compounds [
2,
3,
4,
5,
6,
7,
8,
9,
10] with a particle size of less than 50 nm. In essence, microreactor synthesis is a modification of the sol-gel method carried out with an extremely high degree of micromixing.
An important feature of MRFIJ is the level of energy dissipation rate, (up to 0.54·108 W/kg in the presented study), which is comparable to that obtained in ultrasound devices. In this case, the input energy in ultrasonic devices rapidly decays along the normal path to the emitter; in addition, in the volume of a sounding fluid, energy is dissipated mainly in the antinodes of standing waves.
The results obtained in previous works need to be explained from the point of view of the hydrodynamic conditions effect on the size of particles obtained immediately after the “wet” phase of the synthesis. In particular, it was necessary to find an explanation for factors such as the influence of the jets flow rate and the temperature of solutions on the size of the formed particles [
8].
The relevant complex task global research (which goes beyond the subject of the paper) that should be carried out together with specialists in physical chemistry of nanostructured materials is to determine conditions that are favorable to obtain nanosized particles of a given composition and structure using the following steps: (1) the microreactor mixing of reagents; (2) separation of the formed product from the liquid phase; (3) product washing; (4) heat treatment of the product. The characteristics of the final product are obviously influenced by each stage of the process; for this reason, it is necessary to investigate the role of each of the stages. The complexity and variety of factors affecting the formation of nanoparticles, ranging from the fine hydrodynamics during micromixing of solutions, nucleation conditions, particle aggregation and crystallite growth including the effect of the concentration of reagents, pH of solutions, temperature and rate of temperature change, process duration, chemical composition of the solutions, and other conditions of phase formation determine the complex nature of the problem as a whole. For each stage of the synthesis process, it is necessary to identify the most significant factors and study their influence on the characteristics of intermediate products and, ultimately, on the properties of the target product. This paper concerns only the first step of the process, linked with the micromixing.
In the published literature, the systematic studies of micromixing characteristic in MRFIJ as well as their influence on the properties of the resulting chemicals are scarcely presented.
The aim of this work is to study the influence of hydrodynamic conditions on the characteristics of micromixing in microreactors with impinging jets. Currently, to determine the characteristics of micromixing, the method of model reactions is most often used, which includes at least two parallel or consequently-parallel reactions, one of which is fast and the other is almost instantaneous [
11].
As a result of the examination of the literature [
11,
12,
13,
14,
15,
16,
17], it was revealed that there are two most suitable methods for the analysis of micromixing in MRFIJ with high values of ε: (1) the catalytic reaction of hydrolysis of 2,2-dimethoxypropane (DMP) [
5,
17]; (2) iodide-iodate method [
11,
12,
13,
14,
15,
16]. The second one has been selected as being more suitable for extended experimental studies.
The iodide-iodate technique proposed in the 1990s [
12] is described in more detail in [
13,
14,
15,
16] and is based on parallel competing Villermaux–Dushman reactions—neutralization (1) and redox (2) reactions:
The method is based on measuring the mixing-sensitive iodine yield, which depends on the competition between acid neutralization and its role in the release of iodine in the Dushman reaction. Reaction (R2) is fast and has the same time scale as mixing, but is much slower than reaction (R1).
The iodide-iodate technique implies the following steps [
13,
15]:
A mixture of solutions of iodide I− and iodate IO3− in a H2BO3−/H3BO3 buffer solution, as well as a solution of sulfuric acid which is the supplier of H+ protons in reactions (1) and (2), is preliminarily prepared. The acid concentration must be fixed to ensure a stoichiometric deficiency of protons in relation to borate ions.
In the case of ideal mixing, the acid is consumed only by the first reaction (1), which proceeds much faster than the redox reaction (2). In this case, the second reaction cannot proceed due to the stoichiometric lack of sulfuric acid.
In the case of poor mixing, the distribution time of sulfuric acid in the stirred volume may be longer than the characteristic time of the redox reaction. As a result, there is a local oversaturation of some reactor volumes with sulfuric acid, which, after participating in the reaction (1) with borate ions, is capable of reacting with iodide and iodate ions, causing the formation of iodine I2.
Thus, iodine formation is a measure of fluid segregation. In this system, iodine can further react with iodide, forming triiodide ions I
3−:
Data on the reaction rates and other details of the experimental procedure are given in [
13,
15,
16].
The concentration of triiodide can easily be measured by means of UV/vis-spectrophotometry at a wavelength of 353 nm, and is defined by the law Bouguer–Lambert–Beer law:
where
OD is the optical density of rel. units; ε
353 is the attenuation coefficient of waves in triiodide at a wavelength of 353 nm (ε
353 = 26,047 L/(mol cm) [
15]);
L is the optical path length of the measuring cell.
The index of segregation
XS [
15] is used as an indicator of the quality of micromixing, the values of which can vary from
XS = 0 (ideal micromixing) to
XS = 1 (complete segregation). In general, the segregation index is calculated by using the equation
where
Y is the selectivity for the analyte (in this case, iodine), while the “
ST” index corresponds to complete segregation.
For the iodide-iodate method, the selectivity values are determined by the following equations:
where
Vr is the volume of the reactor un m
3;
Vinj is the volume of the injected acid solution in m
3.
The numerator of Formula (6) is the number of moles which corresponds to the number of moles of acid consumed in the reaction (R2) (taking into account that part of I2 that was transformed into I3− in the reaction (R3)), the denominator is the total number of acid moles injected into the apparatus.
The numerator of Formula (7) is the number of moles corresponding to the number of moles of acid consumed in reaction (R2), the denominator is the total number of moles of acid consumed in reactions (R2) and (R1).
Essentially, the segregation index XS reflects the conversion of the acid injected into the reactor, taking into account its participation in reactions (R2) and (R3), expressed in terms of the concentration of iodine I2 and triiodide I3−.
Another indicator of the micromixing quality is the relative volume of ideal mixing [
18]:
where
VPM is the perfectly mixing volume in m
3;
VST is the complete segregation volume in m
3 (
VPM +
VST =
Vr).
Obviously, α→∞ as the ideal micromixing is approached; for complete segregation, α = 0.
This article has the following structure: the theoretical section describes the existing approaches describing the geometry of liquid sheet formed in MRFIJ at the collision of the jets and sheet hydrodynamics; further experimental apparatus and the experimental conditions are described as well (including photographs and characteristics of liquid sheet linked with the results of iodide-iodate technique for a wide range of jet flow rates). Further studies were carried out by using differential sampling in the central zone and on the periphery of the apparatus, followed by the results of the experiments and their analysis. For the entire investigated range of jet flow rates (from 100 to 2000 mL/min for each pump), the values of the Reynolds and Weber numbers, the dissipated power in watts, and the specific rate of energy dissipation in watts per kilogram were calculated.
4. Results and Discussion
4.1. Dependences of the Triiodide Concentration in the Mixture on the Flow Jets
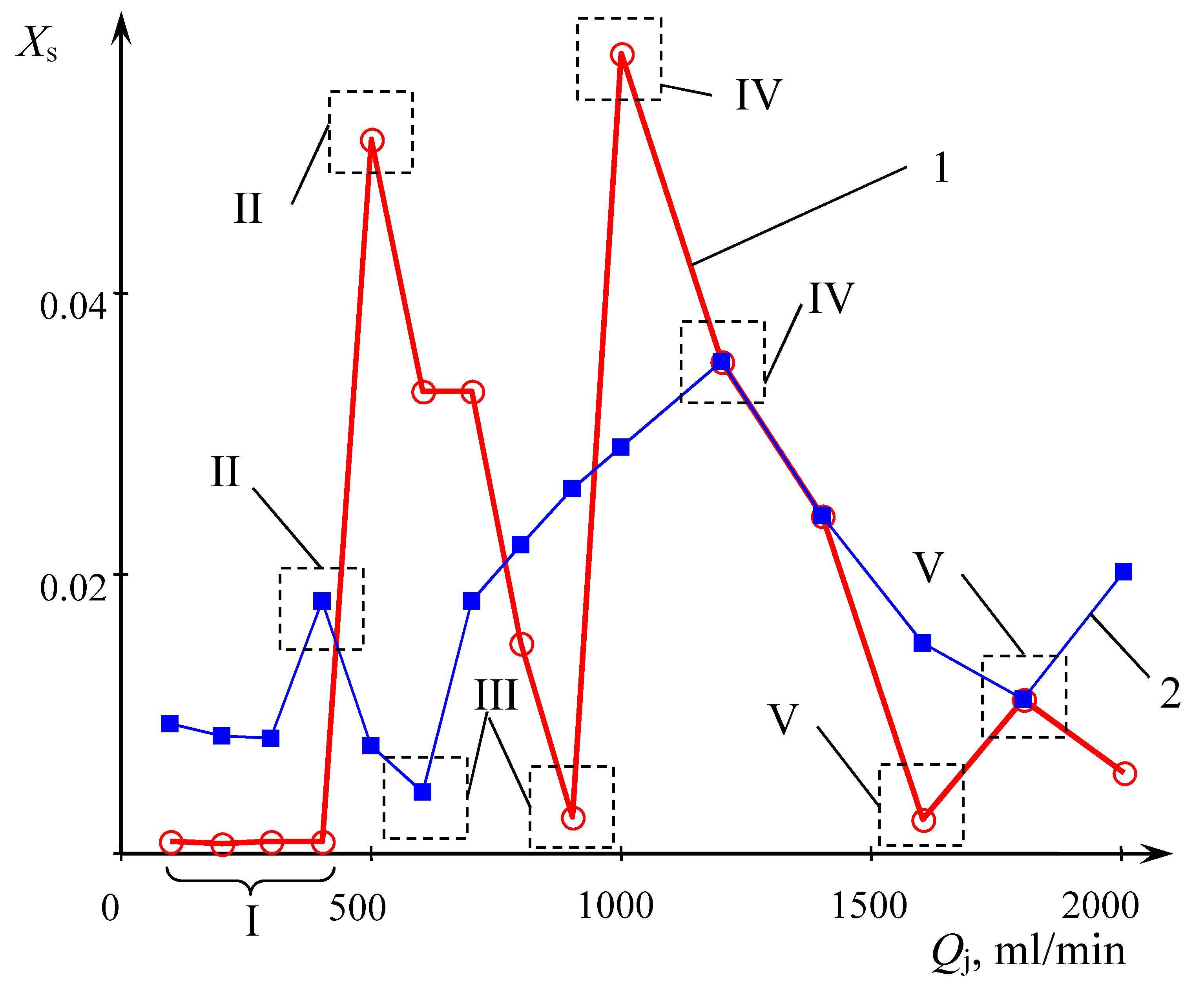
The dependence of the triiodide concentration of (I
3−) in the solution obtained at the outlet of the MRFIJ (weighted average concentration) in a wide range of flow rates, recalculated in segregation coefficient is shown in
Figure 4. Line 1 corresponds to distilled water, while line 2 corresponds to deionized water used to prepare solutions. The values of the Reynolds and Weber numbers corresponding to the indicated flow rates are given in
Table 3.
4.2. Analysis of the Influence of the Reynolds Number on the Behavior of the Liquid Sheet and Concentration in the Mixture
To analyze the obtained data, it is convenient to distinguish five characteristic zones (I-V) on lines 1 and 2 (
Figure 4), which are quite close one to the other, in terms of flow rates.
Table 3 shows the results of calculating some parameters of the flow in the liquid sheet (the calculation formulas are given in
Section 2).
Despite the fact that the absolute values of the concentrations have some differences, in general, the nature of the dependences is qualitatively the same.
In zone I (at flow rates
Q1 =
Q2 = 100–300 mL/min), the triiodide concentration is rather low (about 5·10
−6 for distilled water, 5·10
−5 for deionized water), which indicates good micromixing. As could be seen in
Figure 4, region I corresponds to flow rates
Q1 =
Q2 = 100–300 mL/min and the values of the numbers
Rej = 2122–6366. According to the data of [
22] (obtained on water in nozzles with a diameter of 0.4 mm), at
Rej < 3000–3500, the flow regime in a liquid sheet passes through the stages of “pre-sheet”, laminar flow in the sheet, the formation of waves in it, and at
Rej = 3000–3500 turns into turbulent.
In zone II (at Q1 = Q2 = 400–500 mL/min), there is an increase of iodine concentrations (in distilled water, there is more substantial growth) and in zone III (Q1 = Q2 = 600 mL/min and 900 mL/min for the distilled and deionized water, respectively), there is a new decrease of iodine concentrations to a level approximately corresponding to the zone I.
In zone IV (Q1 = Q2 = 1100 mL/min and 1200 mL/min for distilled and deionized water, respectively), an increase in iodine concentration is observed—the quality of micromixing deteriorates. Finally, in zone V (Q1 = Q2 = 1700 mL/min and 1800 mL/min for distilled and deionized water, respectively), the iodine concentration again significantly decreases.
The reason for this influence of the flow rates of the supplied solutions on the quality of micromixing, in our opinion, may be a change in the hydrodynamic situation in the MRFIJ with an increase in the flow rates Q1 and Q2.
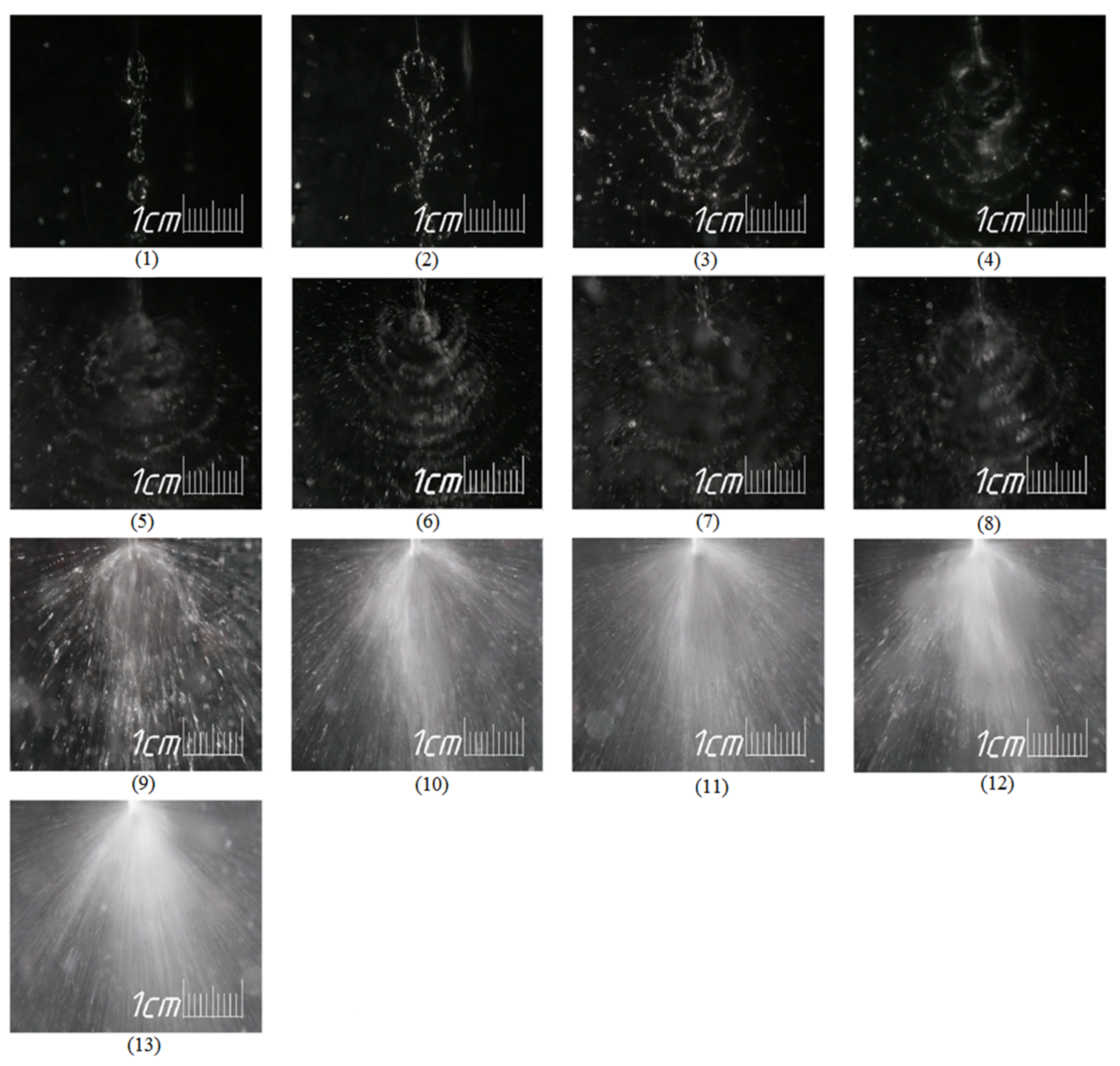
At low flow rates—from 100 mL/min to 300 mL/min (zone I)—liquid sheet retains its integrity (photographs 1–3 in
Figure 5), and the entire mixing process occurs in the sheet, and this leads to a good micromixing. As follows from the
Table 3, in the zone of impingement the energy dissipation level is sufficiently high (ε
i = 1.35·10 − 3.65·10
5 W/kg), which is higher than in the best types of micro-reactors studied in [
13]. According to [
13,
14], the highest values for ε ≈ 10
3 − 10
5 W/kg were obtained in the following devices: Starlam IMM microreactors (ε ≈ 9·10
2 − 6·10
3 W/kg) [
27] and Caterpillar micromixer IMM (ε ≈ 7·10
2 − 9·10
4 W/kg) [
28]. In this case, the maximum values ε were obtained in the micro-reactor with quite thin layers, of which the internal structure of the device has been formed with as little as 50 microns.
In zone II, splashes appear (photo 4–5 in
Figure 5), which fly off from the collision area to the reactor wall, forming a liquid film on it (thin film, with breaks along the surface), where some part of the solutions are mixed (the second part is mixed directly in the volume of jets collision). This mixing is rather poor, which leads to a deterioration in the general mixing rates.
With a further increase in the Reynolds number (zone III), the velocity of the splashes becomes so high that the mixing process in the film formed on the walls of the micro-reactor is intensified.
The subsequent increase in the consumption of solutions and the Reynolds number (zone IV) is accompanied by an increase in the thickness of the film on the walls of the reactor, and mixing in it slows down.
Finally, an increase in the speed of the jets to 35–38 m/s (see
Table 3), which corresponds to zone V, contributes to the intensified bombardment of the thick film by secondary jets (splashes, ligaments) and decreases the kinetic energy, which is high enough to ensure intensive mixing even in a thick film. Note that, according to the Bernoulli equation, the velocity in the liquid sheet is equal to the velocity of the jets (it was experimentally proven [
22] that the velocity variations in the liquid sheet at φ = 0°, i.e., vertically downward, do not exceed 30% of the velocity of the jets, and at φ = 90°, i.e., in the horizontal direction they are about 10% more than the speed of the jets). Thus, it can be expected that the velocity of the generated splashes slightly higher than the velocity in the liquid sheet is of the order of the velocity of the jets with an accuracy of a coefficient exceeding unity, i.e., can be estimated as
wspl ≈ 2
wj.
Note that a doubling of flow rates solutions leads to an 8 times increase in consumed power
P and the energy dissipation rate ε
i (as is known,
P ∝
wj3), since the volume of the collision zone jets has been theorized [
22,
24] to be independent of the jet velocity.
4.3. Analysis of the Influence of the Solution Preparation Time and the Sampling Zone on the Quality of Micromixing
For micromixing studies by use of the iodide-iodate technique, the key issue is the “freshness” of the initial solutions. In the literature, this issue has not been separately considered, although for the resulting product (I
3−), the limit value is considered 30 min from the moment the acid is introduced into the solution [
15].
In addition, to determine the influence of the sampling zone on the quality of micromixing, differential sampling was carried out from the central and peripheral zones, as shown in
Figure 3.
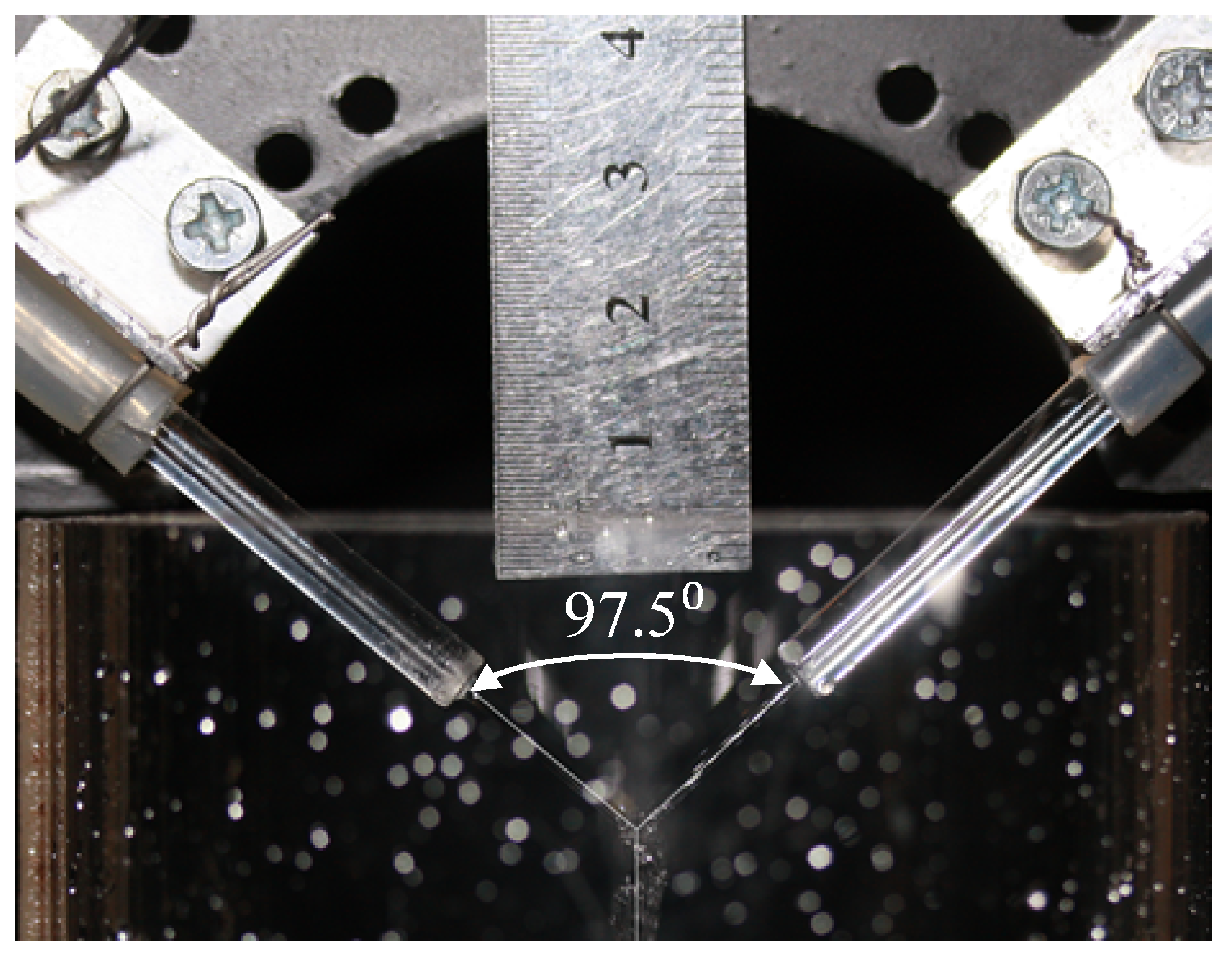
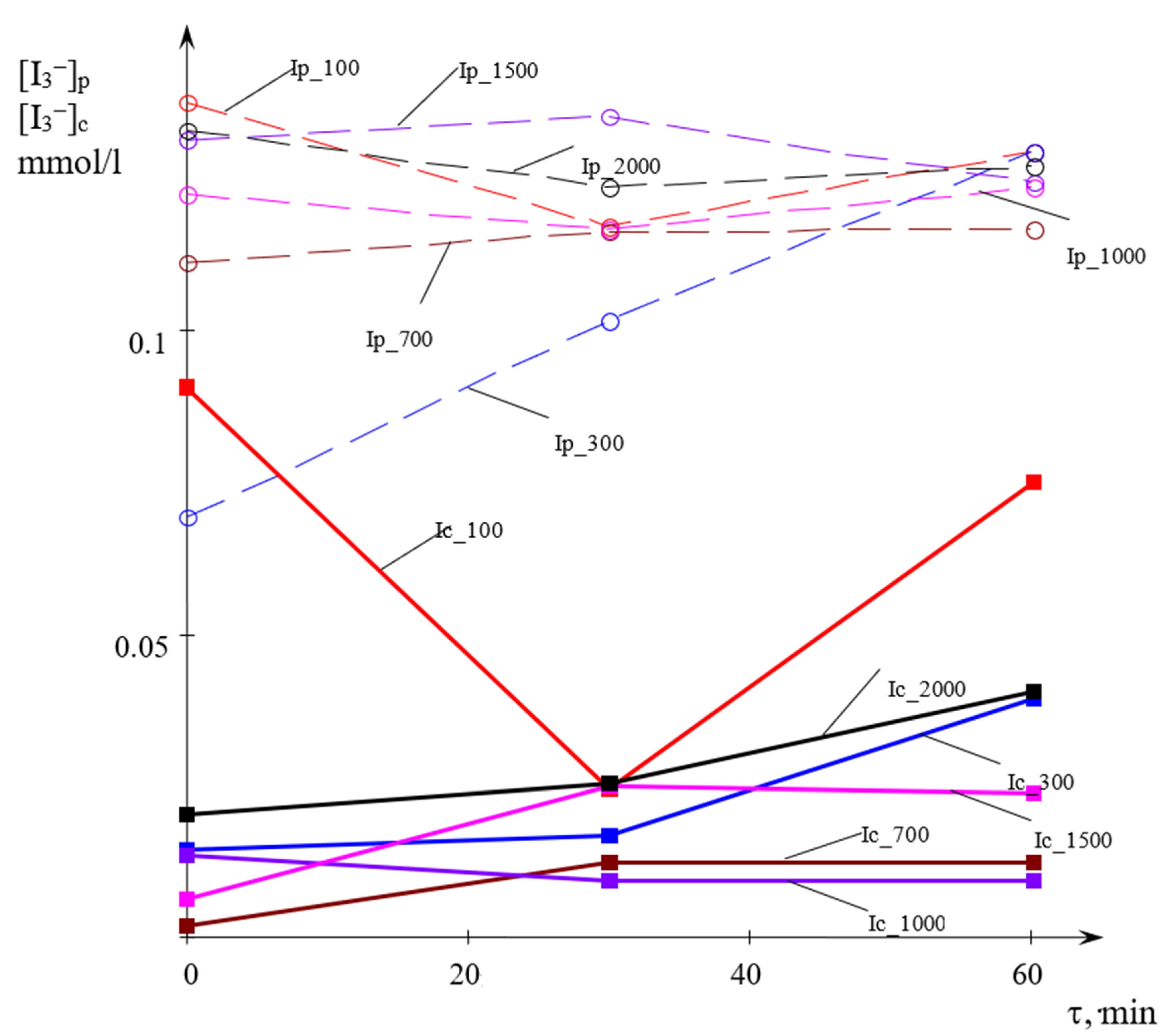
The studies were carried out at the flow rates of each of the jets Qj = 100, 300, 700, 1000, 1500, and 2000 mL/min. The temperature of the solutions was 21 °C, the angle between the jets was 97.5°. In each experiment, the sample to be taken was poured into five 10 mm thick quartz cuvettes, the concentration of triiodide [I3−] was measured by the use of SF-2000 spectrophotometer at a wavelength of 353 nm.
The research results are shown in
Figure 6.
First of all, it should be noted that in almost all cases, the concentration of triiodide, with the exception of Ic_100 and Ip_300, i.e., at Qj ≥ 700 mL/min, weakly depends on the time from the moment of preparation of the solution. The reasons for the scatter in concentrations at Qj < 700 mL/min are discussed below. Thus, the experiments carried out showed that when the initial solutions were kept for up to 60 min, the triiodide concentration in the mixture remained practically unchanged.
As shown in
Figure 6 lines, at all flow rates, the concentration of triiodide in the center is 6–12 times less than in the peripheral zone. This result indicates that the liquid collected in the central zone is much better mixed at the microscale level than the liquid collected from the periphery of the apparatus.
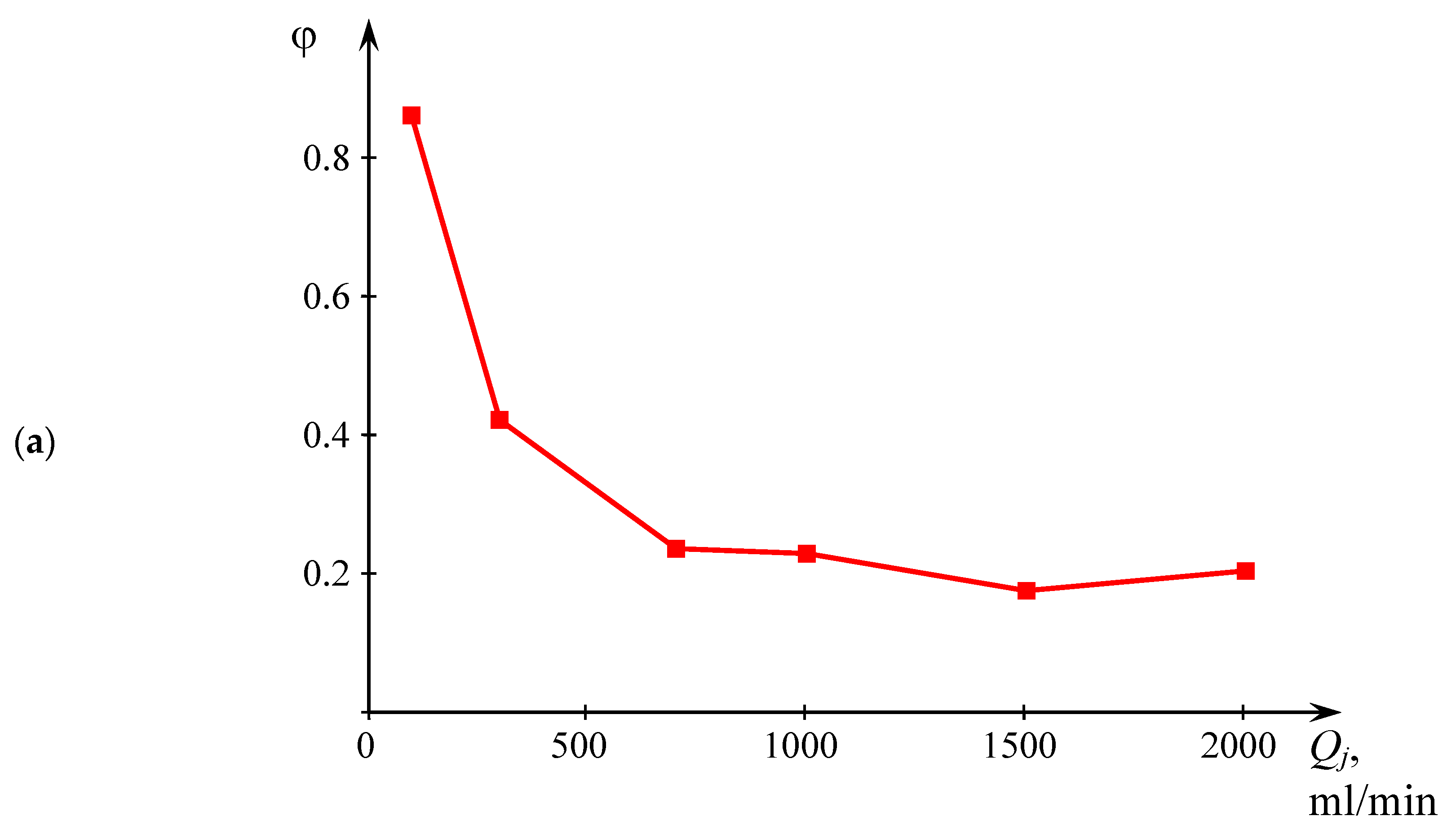
Figure 7a illustrates the weight distribution of liquid between the center and the periphery. The mass fraction of liquid sample collected in the center related to the total mass of the sample is designated as φ:
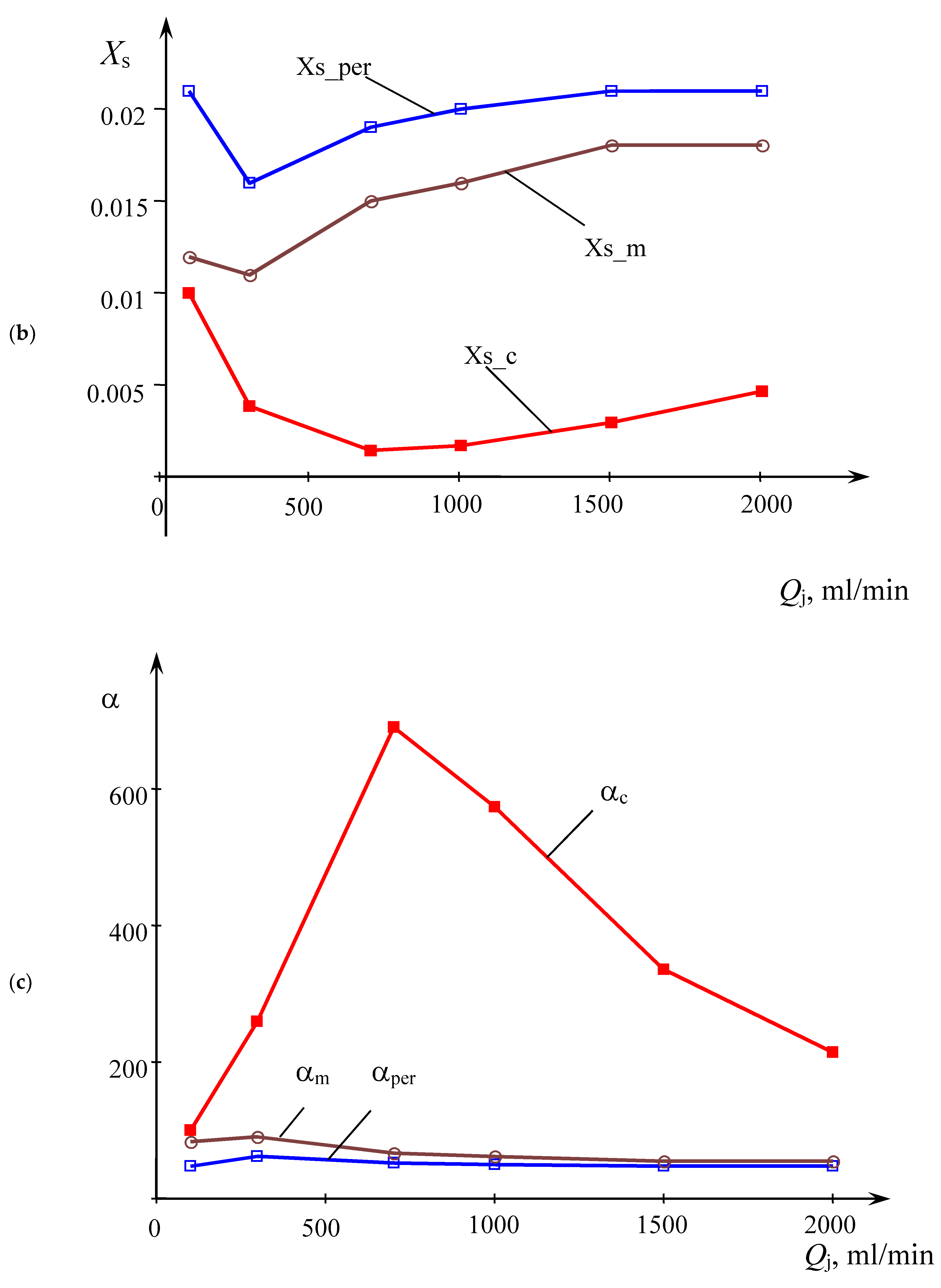
Figure 7b,c show the dependencies of segregation coefficient
Xs and the relative volume of ideal mixing α on the jets flow rates
Qj, respectively.
At Qj ≤ 300 mL/min, the value of φ decreases from 0.86 (at 100 mL/min) and 0.42 (at 300 mL/min), and at Qj ≥ 700 mL/min, the mass fraction of liquid collected in the center practically stabilizes at the level of 17.5–23.5% from the total mass of the product.
In our opinion, the mechanism for dividing the mixture into two parts with high quality of micromixing (sample in the central zone for
Qj ≥ 700 mL/min) and with low quality of micromixing (sample in the peripheral zone for
Qj ≥ 700 mL/min) is as follows.
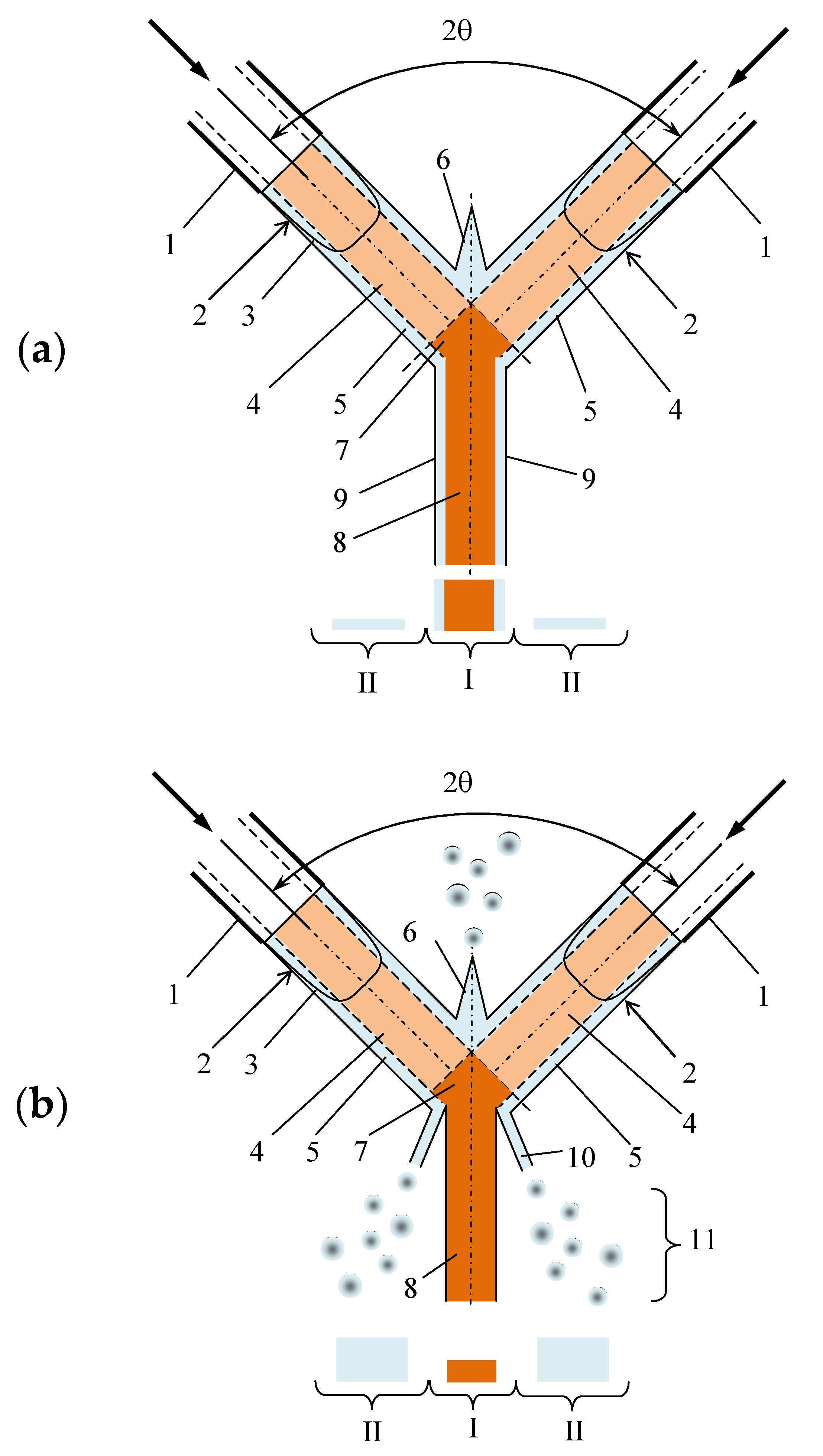
Figure 8 shows the scheme of the collision of the jets and the velocity profile. Upon exiting the jets from the nozzles, the velocity profile has a significant nonuniformity: near the walls there is thin laminar layer 5 with a local velocity on the wall
ww = 0, whereas in the central part of the jet, a flow core stream 4 with a maximum of speed on the jet axis is formed. As the jets leave the nozzles, the velocity profile begins to rebuild, but because of the short distance between the nozzles (the distance from the nozzle exit to the collision plane was
L = 17 mm), this evolution of the velocity profile does not have time to finalize.
As a result, before the collision of the jets in the cross section of the jets, a central zone with a high velocity (a zone of intense micromixing) can be selected, and around it an annular zone can be selected with a velocity which is close to zero (a zone of poor micromixing). During the jets impingement in the zone 7 the flow cores collide one with the other (in case of a sufficiently accurate coincidence of the centers of the jets which could be easily checked by the position of the liquid sheet plane related to the plane of the jets; they should be normal from one to the other), the best micromixing occurs, because local velocities are maximal here; in the annular zone of the jets, micromixing is much worse, because the velocities in this zone are close to zero.
If velocity of jets is sufficiently small (
Figure 8a,
Qj < 700 mL/min to our experiments), the peripheral portion of the impinging jets is transformed into the outer layers 9 of the bottom half of the liquid sheet, moving together with the core 8 of the liquid sheet bottom part. Thus, at low flow rates, most of the peripheral portion of the jet falls into the central zones I of sampling. The fraction of liquid (1 − φ) entering zone II is quite small (14% at
Qj = 100 mL/min and 58% at
Qj = 300 mL/min).
If, on the contrary, the speed of the jets is high enough (
Figure 8b,
Qj ≥ 700 mL/min) during the collision of jets, the ligaments 10 and drops 11, formed during the collision of jets, mainly fall into the peripheral zone II of sampling. In this case apparently the central zone I of sampling is filled in predominantly by a mixture formed by mixing of the jets’ cores, and zone II preferably filled in by the liquid from the annular (laminar) layers of jets. This assumption is confirmed by the data presented in
Figure 7.
Thus, at high flow rates, the liquid from the annular layers of the jets, which is not well mixed enough, practically does not enter the central zone I of samples collection, but instead scatters in the form of ligaments and drops and falls mainly into the peripheral zone II of samples collection.
According to the mechanism described above, at low flow rates, the predominance of the collected liquid mass in the central sample I at Qj = 100 mL/min (86%) and its significant amount at Qj = 300 mL/min (42%) directly influences the measured quality of micromixing, inasmuch as a large amount of liquid falls into the central sample I, which contains a part of the mixture that did not took part in the intensive micromixing (shown as a blue color in the sample I) in addition to the well-mixed solutions (denoted as an orange color in sample I).
At higher flow rates (
Qj ≥ 700 mL/min), the mixture in the form of drops and ligaments, which has not undergone intensive micromixing, flies to the periphery (
Figure 8b), and therefore is practically only a product with a high micromixing quality, is collected in the central zone (orange color of sample I).
According to
Figure 7, the optimum flow rate for the central sampling area is about 700 mL/min (bottom line in
Figure 7b, upper line in
Figure 7c). The weighted average concentration of the sample [I
3−]
m has a local minimum at
Qj = 300 mL/min, but at higher flow rates monotonically grows up to the flow rate of 1500 mL/min, and then decreases slightly. This behavior of the curve [I
3−]
m indicates that the optimal conditions for micromixing, on average over the sample, are achieved at a flow rate of
Qj = 300 mL/min.
Interestingly, that relative volume of ideal mixing α for the central zone of sampling is up to 10.5 times than that for the weighted average (αc/αm = 10.5) at Qj = 700 mL/min. Along with low value of Xs (1.45·10−3), this fact demonstrates almost perfect mixing at 700 mL/min flow rate in the central zone.
Comparison of curves [I
3−]
c and [I
3−]
p in
Figure 7b shows that from the point of view of achieving the highest quality, it is advisable to take the product from the central zone I, where the level of micromixing is at its maximum. At the same time, from a practical point of view, such a process will lead to a significant decrease in the overall capacity of the apparatus and a decrease in the yield. Confrontation of the curves in
Figure 7 and
Figure 4 shows that the flow rate of 300 mL/min has a lower segregation coefficient (i.e., lower triiodide concentration [I
3−] values) compared to the flow rates of 100 mL/min and 700–1500 mL/min, i.e., there is a good correlation between curve 2 in
Figure 4 and curve Xs_m in
Figure 7.
5. Conclusions
An experimental study of micromixing in a microreactor with free impinging jets (MRFIJ) of 1 mm diameter at an angle between the jets 107° and 97.5° was performed. In the experimental part, the iodide-iodate technique was used.
The effect of “freshness” of solutions on the concentration of reaction products was studied. It was revealed that no significant change in properties occurs within 60 min from the moment of preparation of the solutions.
The differentiated sampling revealed nonuniform micromixing quality in the central and peripheral zones of the reactor: at moderate flow rates (700–1000 mL/min, the velocity of jets 15–21 m/s) samples collected in the central area have demonstrated a micromixing level that is 11–13 times better than those in the periphery.
It was revealed that as the jet velocity wj increases, the mass fraction of samples in the central zone φ decreases monotonically (from 86% at a flow rate of 100 mL/min), and then stabilizes at a level of 20–25% at a flow rate of ≥ 700 mL/min. This effect is associated with a more intense formation of ligaments and droplets upon collision of jets, their secondary mixing on the walls of the apparatus.
The data on the concentration [I
3−] in the samples and the segregation coefficient values were compared with the photographs reflecting the hydrodynamic situation in the apparatus (see
Figure 5), elucidating the effect hydrodynamics on the micromixing.
On the basis of mathematical modeling of the liquid sheet geometry, its volume, as well as the volume of the collision zone, the values of the specific rate of energy dissipation in these two volumes, εe and εi, were calculated. The calculated values of εi varied from 6.75·103 to 5.41·107 W/kg [at flow rates from 100 mL/min (wj = 2.12 m/s) to 2000 mL/min (wj = 42.4 m/s)].
In terms of the weighted average concentration, the best quality of micromixing is achieved at a flow rate of 300 mL/min, which corresponds to the conditions for the formation of an intensively mixed, but not disintegrated liquid sheet. As the flow rate of the jets increases to 700–1000 mL/min (and the velocity grows correspondingly, see
Table 3), the energy dissipation rate in the zone of jets collision ε
i increases, and the intensity of micromixing in this local zone improves, which is confirmed by the minimum on the segregation coefficient curve (lower line in
Figure 7b).
With a further increase in the jets velocity (flow rates over 1000 mL/min), the formation of splashes increases, and the mixing of solutions occurs not only in the zone of collision of the jets, but also on the walls of the reactor, where the mixing intensity is quite poor. This leads, apparently, to an increase in the average concentration [I
3−] (and segregation coefficient growth) in the samples, as observed in
Figure 4 and
Figure 7b, as the flow rate increases.
Thus, from a practical point of view the most favorable for the studied conditions (2θ = 107° and 97.5°, dj = 1.0 mm, L = 17 mm) is a flow rate of 300 mL/min, when the best micromixing quality is ensured by the weighted average concentration. If it is possible to take samples from the central zone, the highest quality of micromixing is achieved at a jet flow rate of 700–1000 mL/min; however, 75–80% of the mixture enters the peripheral zone, i.e., it becomes a “waste” of production; This approach can be used, for example, in scientific research when it is necessary to obtain a product with a particularly high quality of micromixing.