Comparative Studies of Hyaluronic Acid Concentration in Normal and Osteoarthritic Equine Joints
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Model NaHA Solutions
2.2. Collection of Synovial Fluid Samples from Healthy and Pathological Equine Joints
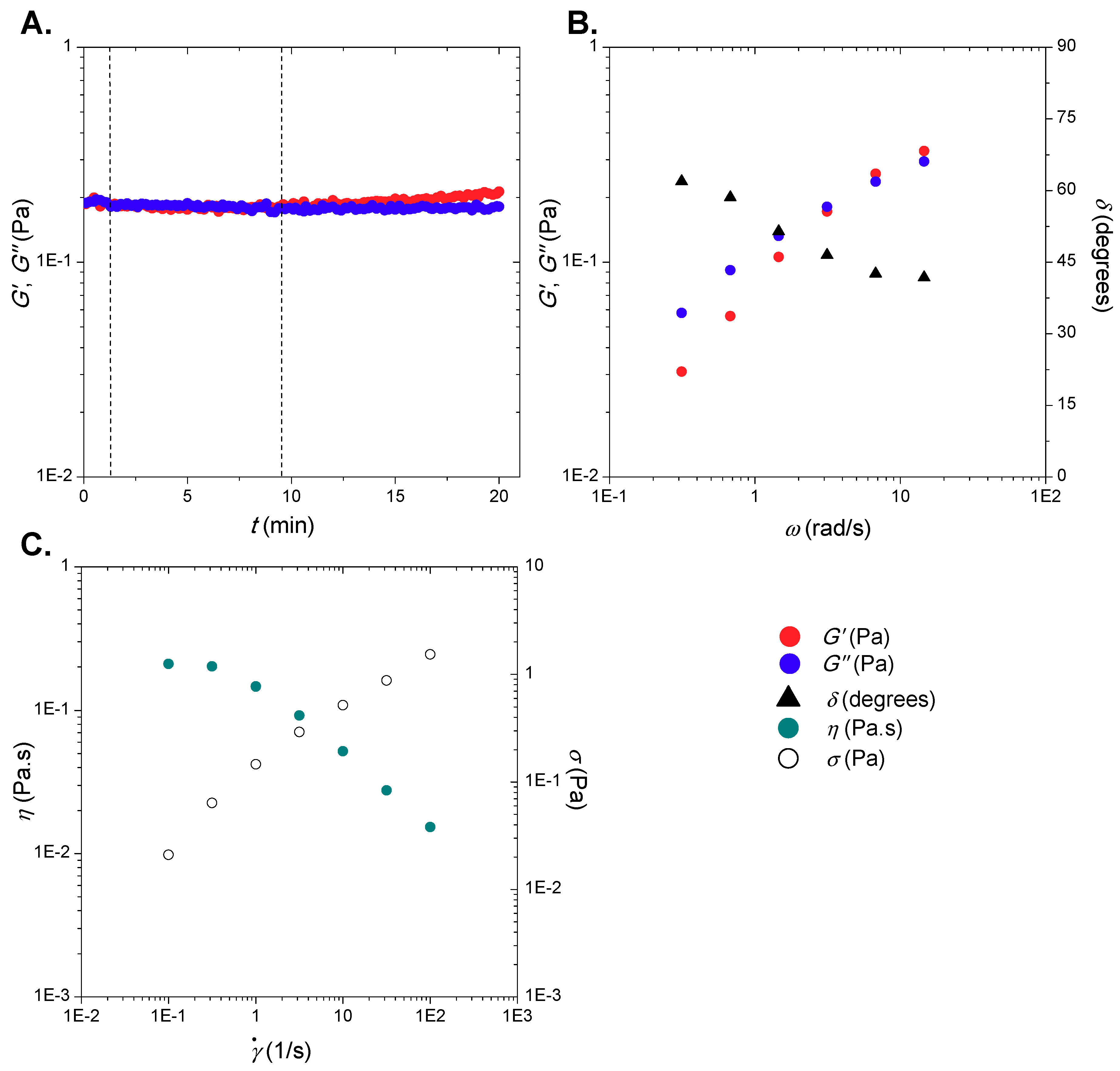
2.3. Rheological Studies
2.4. ELISA Testing
3. Results
3.1. Rheological Studies of Model HA Solutions and of SF Samples
3.2. HA Concentration in Healthy and Pathological Equine SF Samples
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample No | Breed | Joint | Sex | CHA,rheological (mg/mL) | CHA,biochemical9 (mg/mL) | C.F.10 |
|---|---|---|---|---|---|---|
| 1 | TB 1 | Carpus | Female | 3.0 3, 2.9 4, 2.9 5, 2.9 6, 3.2 7, 2.9 8 | 0.71 | 4.2 |
| 2 | Gelding | 4.1 4 | 1.23 | 3.3 | ||
| 3 | Gelding | 4.1 3, 4.2 5, 4.2 7 | 0.50 | 8.3 | ||
| 4 | Gelding | 4.3 3, 4.1 4, 4.2 5, 4.1 8 | 0.55 | 7.6 | ||
| 5 | Fetlock | Gelding | 2.4 1 | 0.30 | 8.0 | |
| 6 | Gelding | 4.6 4, 4.9 5, 4.4 6, 4.8 7, 4.5 8 | 0.57 | 8.1 | ||
| 7 | Gelding | 3.6 3, 3.6 5, 3.8 7 | 0.54 | 6.8 | ||
| 8 | WB 2 | Carpus | Gelding | 0.5 3, 0.6 7 | 0.55 | 1.0 |
| 9 | Gelding | 4.0 3, 3.9 5, 4.1 7 | 0.71 | 5.6 | ||
| 10 | Fetlock | Gelding | 3.8 3, 3.8 5, 3.9 7 | 0.67 | 5.7 | |
| 11 | Gelding | 5.3 3, 5.5 5 | 0.97 | 5.6 | ||
| 12 | - | 3.7 3, 4.1 5, 4.0 7 | 0.67 | 5.9 | ||
| 13 | Gelding | 4.0 5, 4.0 7 | 0.87 | 4.6 | ||
| 14 | Coffin | Gelding | 4.4 3, 4.4 5, 4.5 7 | 0.94 | 4.7 | |
| 15 | - | 2.7 3 | 0.37 | 7.3 | ||
| 16 | - | 1.4 3 | 0.90 | 1.6 |
| Sample No | Breed | Joint | Sex | CHA,rheological (mg/mL) | CHA,biochemical7 (mg/mL) | C.F.8 |
|---|---|---|---|---|---|---|
| 1 | TB | Carpus | Gelding | 3.2 1, 3.2 2, 3.1 3, 3.1 4, 3.0 6 | 0.55 | 5.3 |
| 2 | Gelding | 3.8 1, 3.9 3, 3.8 4, 4.0 5 | 0.37 | 10.5 | ||
| 3 | Gelding | 4.1 1, 4.4 2, 4.4 3, 4.5 4, 4.2 5, 4.0 6 | 0.62 | 6.9 | ||
| 4 | Female | 3.0 1, 2.7 3, 3.2 4, 2.9 6 | 0.23 | 12.8 | ||
| 5 | Fetlock | Gelding | 3.0 1, 2.7 2, 3.0 3, 3.2 4, 1.3 5, 1.9 6 | 0.24 | 12.4 | |
| 6 | Gelding | 3.0 1, 3.4 2, 2.9 3, 3.0 4, 1.4 5, 1.2 6 | 0.30 | 10.3 | ||
| 7 | Gelding | 2.2 1, 2.4 3, 2.5 5 | 0.32 | 7.4 | ||
| 8 | Gelding | 3.9 1, 4.1 2, 3.8 3, 3.8 4, 3.8 5, 3.7 6 | 0.50 | 7.7 | ||
| 9 | Gelding | 1.2 2, 0.9 4, 1.2 6 | 0.26 | 4.2 | ||
| 10 | WB | Fetlock | Female | 3.7 1, 3.5 2, 3.5 3, 3.7 5 | 0.41 | 8.8 |
| 11 | Female | 3.7 1, 3.5 2, 3.7 3, 3.7 4, 3.9 5, 3.6 6 | 0.61 | 6.0 | ||
| 12 | Female | 4.7 3, 4.8 5, 4.7 7 | 0.73 | 6.5 | ||
| 13 | Gelding | 0.8 1, 1.0 3, 0.9 5 | 0.27 | 3.3 | ||
| 14 | Coffin | Gelding | 2.6 1 | 0.37 | 7.0 | |
| 15 | Gelding | 0.6 1, 0.7 3, 0.7 5 | 0.11 | 6.1 | ||
| 16 | Gelding | 0.8 1, 1.0 5 | 0.24 | 3.8 | ||
| 17 | Female | 1.8 1 | 0.10 | 18.0 | ||
| 18 | Female | 1.6 1 | 0.32 | 5.0 | ||
| 19 | Gelding | 0.9 1 | 0.20 | 4.5 |
Appendix B
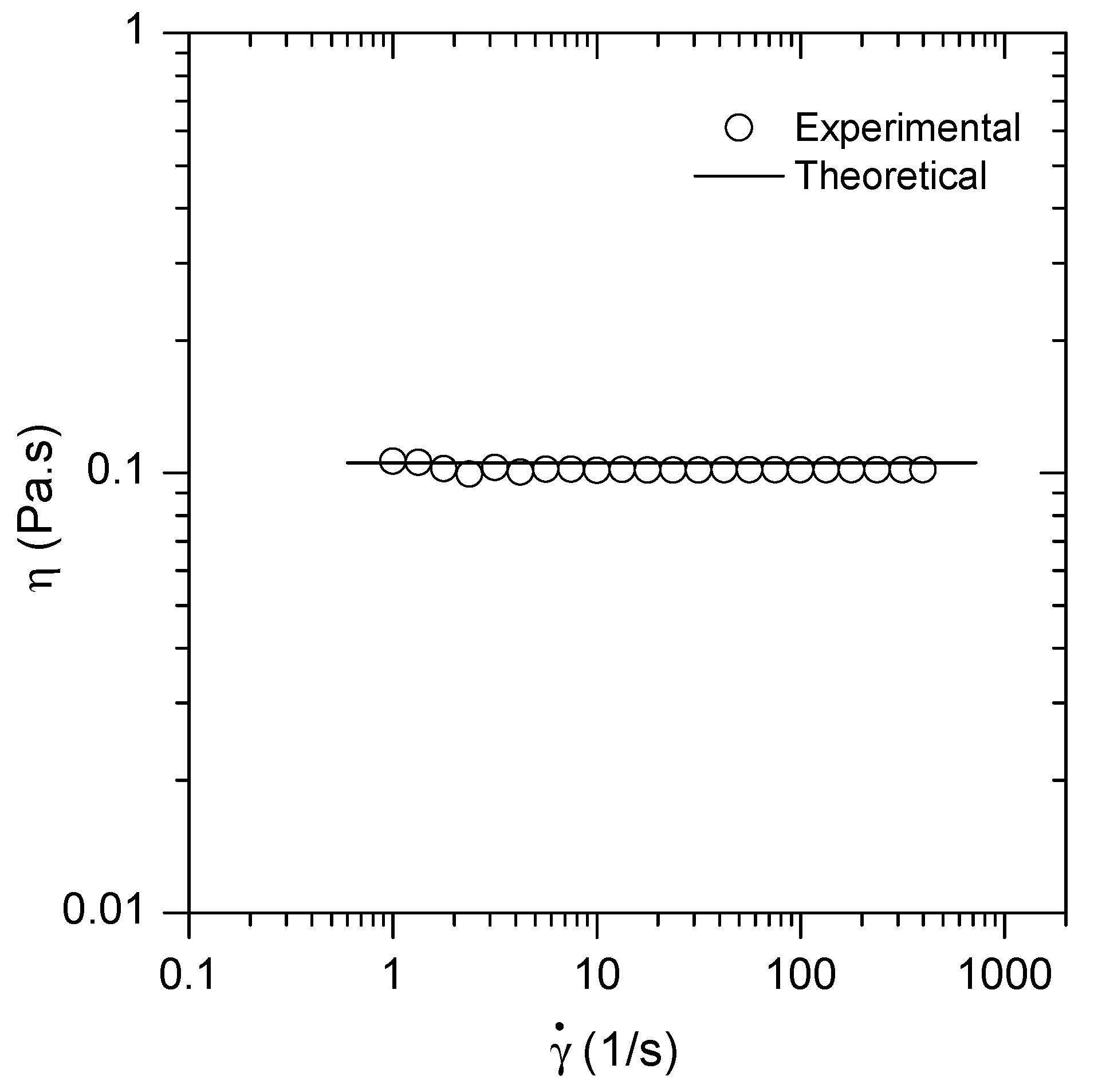
References
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Mathieu, P.; Conrozier, T.; Vignon, E.; Rozand, Y.; Rinaudo, M. Rheologic behavior of osteoarthritic synovial fluid after addition of hyaluronic acid: A pilot study. Clin. Orthop. Relat. Res. 2009, 467, 3002–3009. [Google Scholar] [CrossRef]
- Altman, R.D.; Abramson, S.; Bruyere, O.; Clegg, D.; Herrero-Beaumont, G.; Maheu, E.; Moskowitz, R.; Pavelka, K.; Reginster, J.Y. Commentary: Osteoarthritis of the knee and glucosamine. Osteoarthr. Cartil. 2006, 14, 963–966. [Google Scholar] [CrossRef][Green Version]
- Bagga, H.; Burkhardt, D.; Sambrook, P.; March, L. Longterm effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J. Rheumatol. 2006, 33, 946–950. [Google Scholar]
- Lee, A.S.; Ellman, M.B.; Yan, D.; Kroin, J.S.; Cole, B.J.; van Wijnen, A.J.; Im, H.J. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 2013, 527, 440–447. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Cohen, I.; Bonassar, L.J. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS ONE 2015, 10, e0143415. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef]
- Majd, S.E.; Kuijer, R.; Kowitsch, A.; Groth, T.; Schmidt, T.A.; Sharma, P.K. Both hyaluronan and collagen type ii keep proteoglycan 4 (lubricin) at the cartilage surface in a condition that provides low friction during boundary lubrication. Langmuir ACS J. Surf. Colloids 2014, 30, 14566–14572. [Google Scholar] [CrossRef]
- Khabarov, V.N.; Boykov, P.Y.; Selyanin, M.A. Hyaluronic Acid: Preparation, Properties, Application in Biology and Medicine; John Wiley & Sons, Ltd.: Chichester, West Sussex, UK, 2015. [Google Scholar]
- Matteini, P.; Dei, L.; Carretti, E.; Volpi, N.; Goti, A.; Pini, R. Structural behavior of highly concentrated hyaluronan. Biomacromolecules 2009, 10, 1516–1522. [Google Scholar] [CrossRef]
- Kung, M.; Markantonis, J.; Nelson, S.; Campbell, P. The synovial lining and synovial fluid properties after joint arthroplasty. Lubricants 2015, 3, 394–412. [Google Scholar] [CrossRef]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Blewis, M.E.; Nugent-Derfus, G.E.; Schmidt, T.A.; Schumacher, B.L.; Sah, R.L. A model of synovial fluid lubricant composition in normal and injured joints. Eur. Cells Mater. 2007, 13, 26–39. [Google Scholar] [CrossRef]
- Steel, C.M. Equine synovial fluid analysis. Vet. Clin. N. Am. Equine Pract. 2008, 24, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Mayol, L.; Schiavinato, A.; Ambrosio, L. Effect of hyaluronic acid amide derivative on equine synovial fluid viscoelasticity. J. Biomed. Mater. Res. Part A 2010, 92, 1162–1170. [Google Scholar] [CrossRef]
- Li, Z.; Yao, S.; Alini, M.; Grad, S. Different response of articular chondrocyte subpopulations to surface motion. Osteoarthr. Cartil. 2007, 15, 1034–1041. [Google Scholar] [CrossRef]
- Williams, V.S. Intraarticular hyaluronic acid supplementation in the horse: The role of molecular weight. J. Equine Vet. Sci. 2007, 27, 298–303. [Google Scholar] [CrossRef]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. Suppl. 1993, 39, 3–9. [Google Scholar]
- Balazs, E.A. Analgesic effect of elastoviscous hyaluronan solutions and the treatment of arthritic pain. Cells Tissues Organs 2003, 174, 49–62. [Google Scholar] [CrossRef]
- Guidolin, D.; Franceschi, F. Viscosupplementation with high molecular weight native hyaluronan. Focus on a 1500–2000 kda fraction (hyalubrix(r)). Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3326–3338. [Google Scholar]
- Schiraldi, C.; Stellavato, A.; de Novellis, F.; La Gatta, A.; De Rosa, M. Hyaluronan viscosupplementation: State of the art and insight into the novel cooperative hybrid complexes based on high and low molecular weight HA of potential interest in osteoarthritis treatment. Clin. Cases Miner. Bone Metab. 2016, 13, 36–37. [Google Scholar] [CrossRef]
- Moyer, W.; Schumacher, J.; Schumacher, J. A Guide to Equine Joint Injection and Regional Anesthesia; Veterinary Learning Systems: Yardley, PA, USA, 2007. [Google Scholar]
- Tyrnenopoulou, P.C.; Rizos, E.; Kritsepi-Konstantinou, M.; Papadopoulou, P.L.; Patsikas, M.N.; Papazoglou, L.G.; Aggeli, A.; Diakakis, N.E. Quantitative characterization of viscoelastic properties of normal equine synovial fluid from Thoroughbred and Warmblood horses. Am. J. Vet. Res. 2018, 80, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Verwilghen, D.; Busoni, V.; Gangl, M.; Franck, T.; Lejeune, J.P.; Vanderheyden, L.; Detilleux, J.; Grulke, S.; Deberg, M.; Henrotin, Y.; et al. Relationship between biochemical markers and radiographic scores in the evaluation of the osteoarticular status of warmblood stallions. Res. Vet. Sci. 2009, 87, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Itenov, T.S.; Kirkby, N.S.; Bestle, M.H.; Nilsson, A.C.; Erlandsen, E.J.; Peters, L.; Jensen, J.U. Hyaluronic Acid Assays: Turbidimetric or Enzyme-Based Immune Assay? A Method Comparison Study. J. Clin. Lab. Anal. 2016, 30, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V.; Chhabra, A.; Kumar, A.; Soni, S.; Singh, S.; Aggarwal, Y. Correlation of serum hyaluronic acid with clinical and radiological severity in primary knee osteoarthritis. Int. J. Orthop. Sci. 2018, 4, 420–425. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Bonovolias, I.; Roth, M.; Tamm, M.; Schumann, D.; Baty, F.; Louis, R.; Milenkovic, B.; Boersma, W.; Stieltjes, B.; et al. Serum levels of hyaluronic acid are associated with COPD severity and predict survival. Eur. Respir. J. 2019, 53, 1801183. [Google Scholar] [CrossRef] [PubMed]
- Jolly, A.; Mascaró, M.; Ingratta, G.G.; Pibuel, M.; Hajos, S.E.; Mundo, S.L. Evaluation of Hyaluronic Acid in Cattle: Physiological Variations Related to Age, Periparturition and in Clinical Cases of Paratuberculosis. J. Vet. Sci. Technol. 2016, 7, 342–346. [Google Scholar] [CrossRef]
- Peng, C.; Wallwiener, M.; Rudolph, A.; Ćuk, K.; Eilber, U.; Celik, M.; Modugno, C.; Trumpp, A.; Heil, J.; Marm, F.; et al. Plasma hyaluronic acid level as a prognostic and monitoring marker of metastatic breast cancer. Int. J. Cancer 2016, 138, 2499–2509. [Google Scholar] [CrossRef]
- Milas, M.; Rinaudo, M. Characterization and properties of hyaluronic acid (hyaluronan). In Polysaccharides; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Milas, M.; Rinaudo, M.; Roure, I.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Comparative rheological behavior of hyaluronan from bacterial and animal sources with cross-linked hyaluronan (hylan) in aqueous solution. Biopolymers 2001, 59, 191–204. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, F.; Luan, T.; Zhang, Z.; Zhang, H. Rheological studies of hyaluronan solutions based on the scaling law and constitutive models. Polymer 2014, 55, 295–301. [Google Scholar] [CrossRef]
- Krause, W.E.; Bellomo, E.G.; Colby, R.H. Rheology of sodium hyaluronate under physiological conditions. Biomacromolecules 2001, 2, 65–69. [Google Scholar] [CrossRef]
- Braithwaite, G.J.; Daley, M.J.; Toledo-Velasquez, D. Rheological and molecular weight comparisons of approved hyaluronic acid products—Preliminary standards for establishing class iii medical device equivalence. J. Biomater. Sci. Polym. Ed. 2016, 27, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Milas, M.; Rinaudo, M.; Roure, I.; Al-Assaf, S.; Phillips, G.; Phillips, G.; Williams, P.A. Rheological behaviour of hyaluronan, healon and hylan in aqueous solutions. In Hyaluronan; Elsevier: Amsterdam, The Netherlands, 2002; pp. 181–193. [Google Scholar]
- Meyer, F.; Lohmann, D.; Kulicke, W.M. Determination of the viscoelastic behavior of sodium hyaluronate in phosphate buffered saline with rheo-mechanical and rheo-optical methods. J. Rheol. 2009, 53, 799–818. [Google Scholar] [CrossRef]
- Fouissac, E.; Milas, M.; Rinaudo, M. Shear-rate, concentration, molecular weight, and temperature viscosity dependences of hyaluronate, a wormlike polyelectrolyte. Macromolecules 1993, 26, 6945–6951. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Meyer, F.; Bingöl, A.Ö.; Lohmann, D. Visco-elastic properties of sodium hyaluronate solutions. AIP Conf. Proc. 2008, 1027, 585–587. [Google Scholar]
- Teraoka, I. Polymer Solutions: An Introduction to Physical Properties; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Pires, A.M.B.; Santana, M.H.A. Rheological aspects of microbial hyaluronic acid. J. Appl. Polym. Sci. 2011, 122, 126–133. [Google Scholar] [CrossRef]
- Fam, H.; Bryant, J.T.; Kontopoulou, M. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar]
- Vincent, H.K.; Percival, S.S.; Conrad, B.P.; Seay, A.N.; Montero, C.; Vincent, K.R. Hyaluronic acid (HA) viscosupplementation on synovial fluid inflammation in knee osteoarthritis: A pilot study. Open Orthop. J. 2013, 7, 378–384. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E.; Laurent, U.B.; Engström-Laurent, A. Hyaluronan in inflammatory joint disease. Acta Orthop. Scand. 1995, 266, 116–120. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; Boger, D.V.; Fraser, J.R.E. The dynamic and steady shear properties of synovial fluid and of the components making up synovial fluid. Rheol. Acta 1984, 23, 311–321. [Google Scholar] [CrossRef]

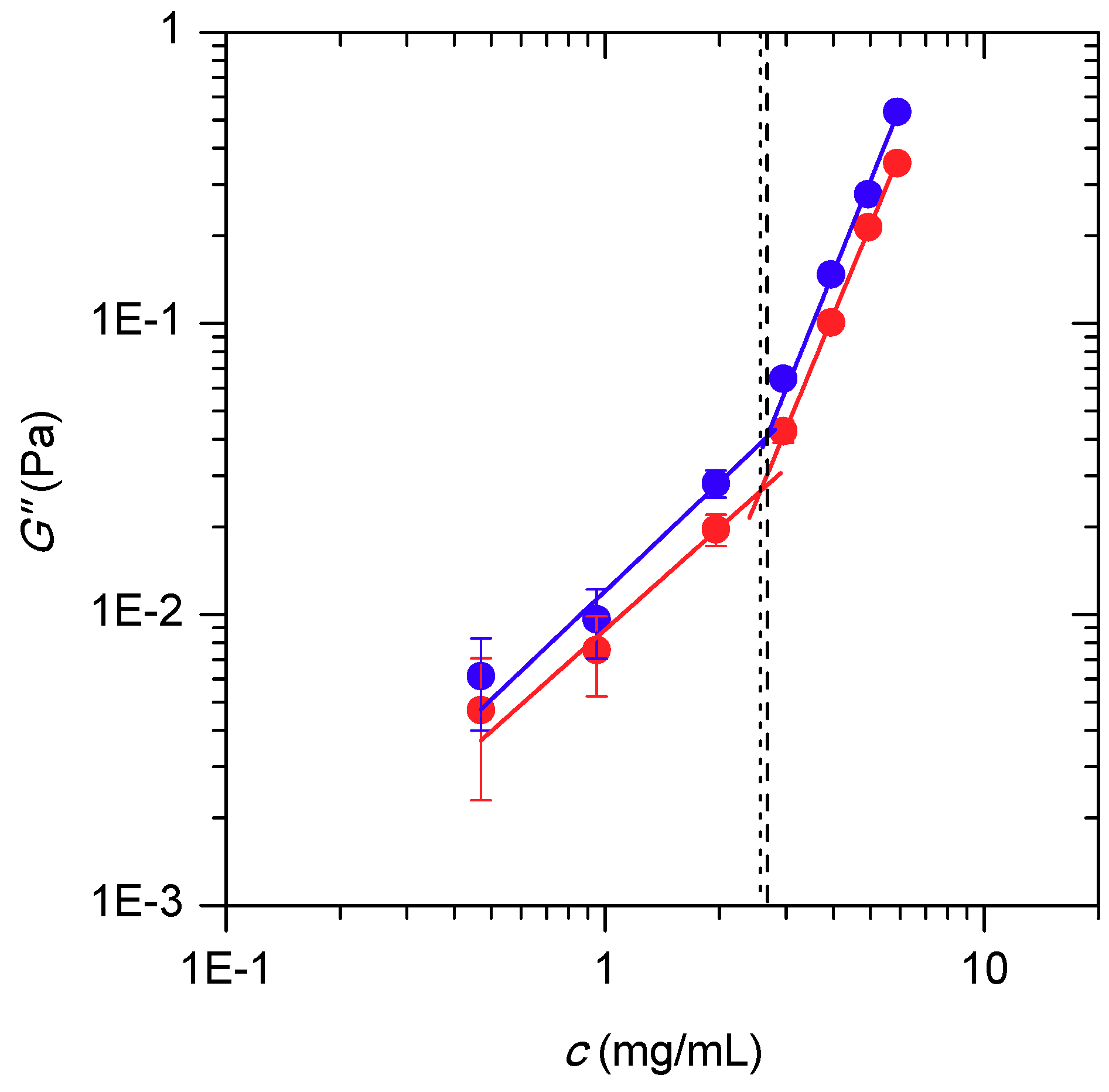
| Regime | T/°C | R2 | A | Δa | b | Δb | G″* (Pa) |
|---|---|---|---|---|---|---|---|
| Dilute | 37.5 | 0.97176 | 8.88 × 10−3 | 0.98 × 10−3 | 1.16 | 0.18 | 2.65 × 10−2 |
| Semidilute | 0.99716 | 1.37 × 10−3 | 0.20 × 10−3 | 3.15 | 0.09 | ||
| Dilute | 25 | 0.95182 | 1.21 × 10−2 | 1.75 × 10−3 | 1.24 | 0.24 | 4.05 × 10−2 |
| Semidilute | 0.99172 | 1.73 × 10−3 | 0.51 × 10−3 | 3.22 | 0.18 |
| Regime | T/°C | R2 | A | Δa | b | Δb | G″* (Pa) |
|---|---|---|---|---|---|---|---|
| Dilute | 37.5 | - | 7.59 × 10−3 | - | 1.35 | - | 2.25 × 10−2 |
| Semidilute | 0.99675 | 2.52 × 10−3 | 0.51 × 10−3 | 2.71 | 0.12 | ||
| Dilute | 25 | - | 1.28 × 10−2 | - | 1.13 | - | 3.80 × 10−2 |
| Semidilute | 0.99608 | 1.87 × 10−3 | 0.51 × 10−3 | 3.13 | 0.16 |
| Regime | T/°C | R2 | A | Δa | b | Δb | η* (Pa·s) |
|---|---|---|---|---|---|---|---|
| Dilute | 37.5 | 0.99324 | 2.37 × 10−3 | 0.14 × 10−3 | 1.18 | 0.09 | 7.80 × 10−3 |
| Semidilute | 0.99810 | 0.26 × 10−3 | 0.56 × 10−4 | 3.40 | 0.13 | ||
| Dilute | 25 | 0.99182 | 3.66 × 10−3 | 0.26 × 10−3 | 1.28 | 0.11 | 1.28 × 10−2 |
| Semidilute | 0.99731 | 0.66 × 10−3 | 0.15 × 10−3 | 3.07 | 0.13 |
| Breed | CHA,rheological (mg/mL) | CHA,biochemical (mg/mL) 3 | C.F.4 |
|---|---|---|---|
| TB 1 | 2.4 | 0.30 | 8.0 |
| 4.6 | 0.57 | 8.1 | |
| 3.7 | 0.54 | 6.8 | |
| WB 2 | 3.8 | 0.67 | 5.7 |
| 5.4 | 0.97 | 5.6 | |
| 3.9 | 0.67 | 5.9 | |
| 4.0 | 0.87 | 4.6 |
| Breed | CHA,rheological (mg/mL) | CHA,biochemical (mg/mL) | C.F. |
|---|---|---|---|
| TB | 2.5 | 0.24 | 10.4 |
| 2.5 | 0.30 | 8.3 | |
| 2.4 | 0.32 | 7.5 | |
| 3.9 | 0.50 | 7.8 | |
| 1.1 | 0.26 | 4.2 | |
| WB | 3.6 | 0.41 | 8.8 |
| 3.7 | 0.61 | 6.1 | |
| 4.7 | 0.73 | 6.4 | |
| 0.9 | 0.27 | 3.3 |
| Healthy Joints | Osteoarthritic Joints | ||
|---|---|---|---|
| Fetlock | Thoroughbred C.F. = 7.5 ± 0.7, n = 3 | Fetlock | Thoroughbred C.F.= 7.3 ± 3.1, n = 5 |
| Warmblood C.F. = 5.3 ± 0.7, n = 4 | Warmblood C.F. = 6.1 ± 2.8, n = 4 | ||
| Carpus | Thoroughbred C.F.= 5.8 ± 2.5, n = 4 | Carpus | Thoroughbred C.F. = 9.1 ± 3.8, n = 4 |
| Warmblood C.F. = 3.3 ± 2.3, n = 2 | Warmblood - | ||
| Coffin | Warmblood C.F. = 4.5 ± 2.9, n = 3 | Coffin | Warmblood C.F. = 10.9 ± 7.1, n = 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matisioudis, N.; Rizos, E.; Tyrnenopoulou, P.; Papazoglou, L.; Diakakis, N.; Aggeli, A. Comparative Studies of Hyaluronic Acid Concentration in Normal and Osteoarthritic Equine Joints. Fluids 2019, 4, 193. https://doi.org/10.3390/fluids4040193
Matisioudis N, Rizos E, Tyrnenopoulou P, Papazoglou L, Diakakis N, Aggeli A. Comparative Studies of Hyaluronic Acid Concentration in Normal and Osteoarthritic Equine Joints. Fluids. 2019; 4(4):193. https://doi.org/10.3390/fluids4040193
Chicago/Turabian StyleMatisioudis, Nikolaos, Eleftherios Rizos, Panagiota Tyrnenopoulou, Lysimachos Papazoglou, Nikolaos Diakakis, and Amalia Aggeli. 2019. "Comparative Studies of Hyaluronic Acid Concentration in Normal and Osteoarthritic Equine Joints" Fluids 4, no. 4: 193. https://doi.org/10.3390/fluids4040193
APA StyleMatisioudis, N., Rizos, E., Tyrnenopoulou, P., Papazoglou, L., Diakakis, N., & Aggeli, A. (2019). Comparative Studies of Hyaluronic Acid Concentration in Normal and Osteoarthritic Equine Joints. Fluids, 4(4), 193. https://doi.org/10.3390/fluids4040193





