Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
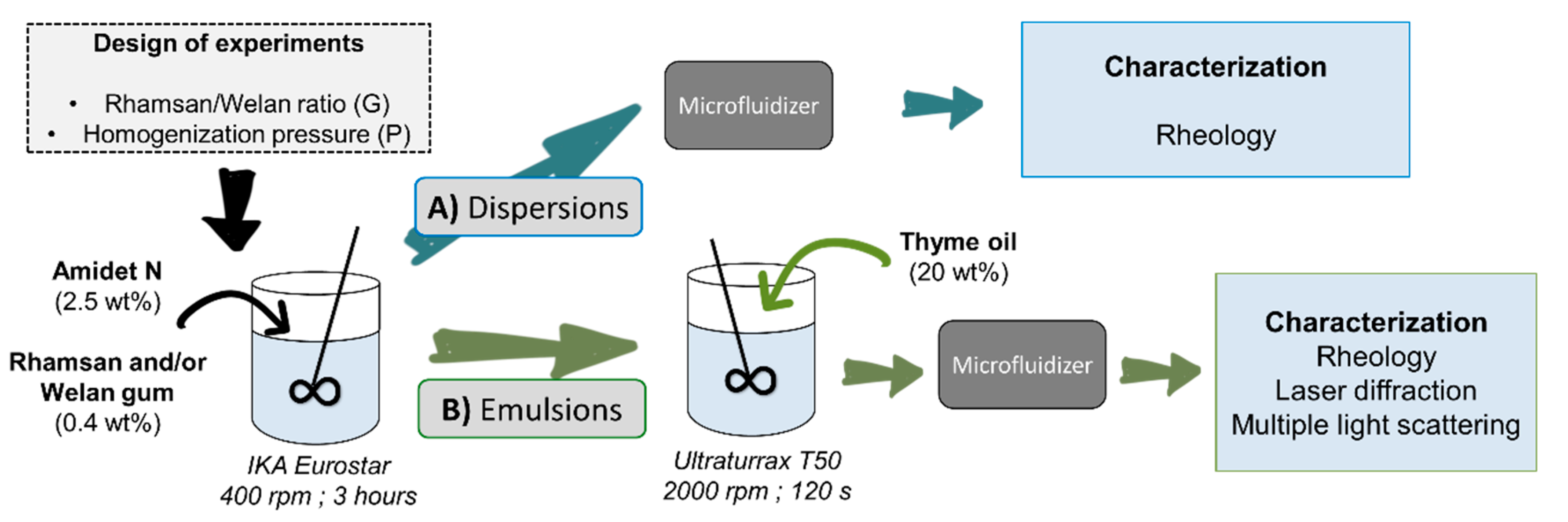
2.2. Preparation of Samples
2.3. Rheological Characterization
2.4. Droplet Size Distributions by Laser Diffraction Technique
2.5. Physical Stability of Emulsions
2.6. Statistical Analysis
3. Results and Discussion
3.1. Development of a Continuous Phase Containing Rhamsan and/or Welan Gums
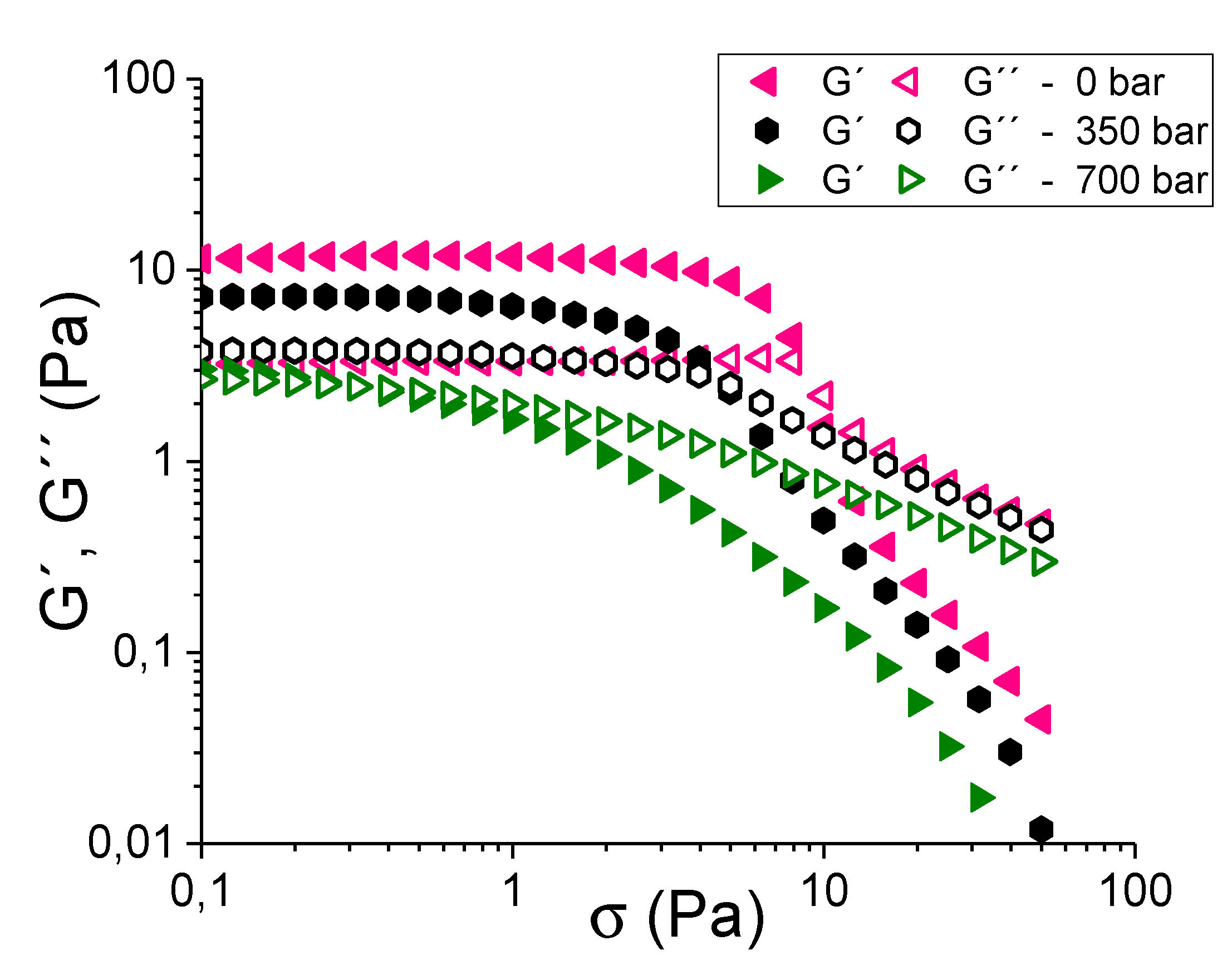
3.2. Development of Thyme Oil-in-Water Emulsions
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Robins, M.M.; Wilde, P.J. Colloids and emulsions. Science 2003, 1517–1524. [Google Scholar] [CrossRef]
- Pérez-Mosqueda, L.M.; Ramírez, P.; Trujillo-Cayado, L.A.; Santos, J.; Muñoz, J. Development of eco-friendly submicron emulsions stabilized by a bio-derived gum. Colloids Surf. B Biointerfaces 2014, 123, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Zhao, M.; McClements, D.J. Improving the stability of wheat protein-stabilized emulsions: Effect of pectin and xanthan gum addition. Food Hydrocoll. 2015, 43, 377–387. [Google Scholar] [CrossRef]
- Kang, K.S.; Pettitt, D.J. Xanthan, gellan, welan, and rhamsan. In Industrial Gums, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 341–397. [Google Scholar]
- Trujillo-Cayado, L.A.; Alfaro, M.C.; Muñoz, J.; Raymundo, A.; Sousa, I. Development and rheological properties of ecological emulsions formulated with a biosolvent and two microbial polysaccharides. Colloids Surf. B Biointerfaces 2016, 141, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Cayado, L.A.; Alfaro, M.C.; Raymundo, A.; Sousa, I.; Muñoz, J. Rheological behavior of aqueous dispersions containing blends of rhamsan and welan polysaccharides with an eco-friendly surfactant. Colloids Surf. B Biointerfaces 2016, 145, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Dong, S.H.; Li, S.; Chen, X.Y.; Wu, D.; Xu, H. Statistical experimental design optimization of rhamsan gum production by Sphingomonas sp. CGMCC 6833. J. Microbiol. 2015, 53, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Imai, N.; Doi, Y.; Sano, M.; Tamano, S.; Omoto, T.; Asai, I.; Yasuhara, K.; Hayashi, S. Ninety-day oral toxicity study of rhamsan gum, a natural food thickener produced from Sphingomonas ATCC 31961, in Crl:CD(SD)IGS rats. J. Toxicol. Sci. 2010, 35, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, G.R. Gellan gum. In Food Gels; Springer: New York, NY, USA, 1990; pp. 201–232. [Google Scholar]
- Lee, L.; Norton, I.T. Comparing droplet breakup for a high-pressure valve homogeniser and a microfluidizer for the potential production of food-grade nanoemulsions. J. Food Eng. 2013, 114, 158–163. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Optimization of nano-emulsions production by microfluidization. Eur. Food Res. Technol. 2007, 225, 733–741. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Calero, N.; Alfaro, M.C.; Muñoz, J. Development of eco-friendly emulsions produced by microfluidization technique. J. Ind. Eng. Chem. 2016, 36, 90–95. [Google Scholar] [CrossRef]
- Lagoueyte, N.; Paquin, P. Effects of microfluidization on the functional properties of xanthan gum. Food Hydrocoll. 1998, 12, 365–371. [Google Scholar] [CrossRef]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Baratta, M.T.; Deans, S.G.; Dorman, H.J.D. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000, 66, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Hayakawa, M.; Goto, Y.; Masuo, Y.; Koike, K. Anxiolytic-like effects of essential oil from Thymus vulgaris was increased during stress. Flavour Fragr. J. 2018, 33, 191–195. [Google Scholar] [CrossRef]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzięcioł, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The effect of fennel essential oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Ryu, V.; McClements, D.J.; Corradini, M.G.; McLandsborough, L. Effect of ripening inhibitor type on formation, stability, and antimicrobial activity of thyme oil nanoemulsion. Food Chem. 2018, 245, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Calero, N.; Trujillo-Cayado, L.A.; Muñoz, J. Development and characterisation of a continuous phase based on a fumed silica and a green surfactant with emulsion applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 351–357. [Google Scholar] [CrossRef]
- Zasypkin, D.V.; Braudo, E.E.; Tolstoguzov, V.B. Multicomponent biopolymer gels. Food Hydrocoll. 1997, 11, 159–170. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Woods, J.R.; Harding, S.E.; Hill, S.E.; Mitchell, J.R. Biopolymer Mixtures. Bioseparation 1998, 7, 64. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo, L.A.; Calero, N.; Alfaro, M.C.; Munoz, J. Physical Characterization of a Commercial Suspoemulsion as a Reference for the Development of Suspoemulsions. Chem. Eng. Technol. 2013, 36, 1883–1890. [Google Scholar] [CrossRef]
- Santos, J.; Calero, N.; Trujillo-Cayado, L.A.; Garcia, M.C.; Muñoz, J. Assessing differences between Ostwald ripening and coalescence by rheology, laser diffraction and multiple light scattering. Colloids Surf. B Biointerfaces 2017, 159, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Cayado, L.A.; Alfaro, M.C.; Muñoz, J. Effects of ethoxylated fatty acid alkanolamide concentration and processing on D-limonene emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 198–203. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Santos, J.; Ramírez, P.; Alfaro, M.C.; Muñoz, J. Strategy for the development and characterization of environmental friendly emulsions by microfluidization technique. J. Clean. Prod. 2018, 178, 723–730. [Google Scholar] [CrossRef]
- Martin-Piñero, M.J.; Ramirez, P.; Muñoz, J.; Alfaro, M.C. Development of rosemary essential oil nanoemulsions using a wheat biomass-derived surfactant. Colloids Surf. B Biointerfaces 2019, 173, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Kwon, Y.-J. Characterization of β-carotene nanoemulsions prepared by microfluidization technique. Food Sci. Biotechnol. 2014, 23, 107–113. [Google Scholar] [CrossRef]





| Sample | X1 | X2 | Rhamsan–Welan Ratio | Pressure (bar) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0.3 | 100 |
| 2 | 1 | −1 | 0.7 | 100 |
| 3 | −1 | 1 | 0.3 | 600 |
| 4 | 1 | 1 | 0.7 | 600 |
| 5 | 0 | 0 | 0.5 | 350 |
| 6 | 0 | 0 | 0.5 | 350 |
| 7 | 0 | 0 | 0.5 | 350 |
| 8 | −1.414 | 0 | 0 | 350 |
| 9 | −1 | 0 | 0.3 | 350 |
| 10 | 1 | 0 | 0.7 | 350 |
| 11 | 1.414 | 0 | 1 | 350 |
| 12 | 0 | −1.414 | 0.5 | 0 |
| 13 | 0 | −1 | 0.5 | 100 |
| 14 | 0 | 1 | 0.5 | 600 |
| 15 | 0 | 1.414 | 0.5 | 700 |
| Sample | Rhamsan–Welan Ratio | Pressure (bar) | τc (Pa) |
|---|---|---|---|
| 1 | 0.3 | 100 | 1.45 ± 0.09 |
| 2 | 0.7 | 100 | 2.80 ± 0.14 |
| 3 | 0.3 | 600 | 0.13 ± 0.01 |
| 4 | 0.7 | 600 | 0.15 ± 0.01 |
| 5 | 0.5 | 350 | 0.83 ± 0.05 |
| 6 | 0.5 | 350 | 0.81 ± 0.06 |
| 7 | 0.5 | 350 | 0.84 ± 0.05 |
| 8 | 0 | 350 | 0.47 ± 0.02 |
| 9 | 0.3 | 350 | 0.79 ± 0.04 |
| 10 | 0.7 | 350 | 1.06 ± 0.08 |
| 11 | 1 | 350 | 1.24 ± 0.08 |
| 12 | 0.5 | 0 | 3.28 ± 0.19 |
| 13 | 0.5 | 100 | 2.27 ± 0.12 |
| 14 | 0.5 | 600 | 0.29 ± 0.02 |
| 15 | 0.5 | 700 | 0.11 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trujillo-Cayado, L.A.; Santos, J.; Calero, N.; Alfaro, M.d.C.; Muñoz, J. Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil. Fluids 2019, 4, 29. https://doi.org/10.3390/fluids4010029
Trujillo-Cayado LA, Santos J, Calero N, Alfaro MdC, Muñoz J. Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil. Fluids. 2019; 4(1):29. https://doi.org/10.3390/fluids4010029
Chicago/Turabian StyleTrujillo-Cayado, Luis A., Jenifer Santos, Nuria Calero, Maria del Carmen Alfaro, and José Muñoz. 2019. "Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil" Fluids 4, no. 1: 29. https://doi.org/10.3390/fluids4010029
APA StyleTrujillo-Cayado, L. A., Santos, J., Calero, N., Alfaro, M. d. C., & Muñoz, J. (2019). Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil. Fluids, 4(1), 29. https://doi.org/10.3390/fluids4010029









