Abstract
Cardiovascular diseases remain the leading cause of morbidity and mortality worldwide, underscoring the need for continuous innovation in diagnostics and treatment. Mock circulation loops (MCLs) systems have recently emerged as new research platforms capable of replicating the hemodynamics of the human cardiovascular system. This review explores the expanding applications of MCLs to cardiovascular diseases beyond their traditional role in testing ventricular assist devices and heart failure management. We focus on their versatility in simulating various cardiovascular conditions, particularly arterial diseases such as atherosclerosis, stenosis, and aneurysms. This review traces the evolution of MCLs and their integration with computational simulations and real-time data acquisition systems. MCLs provide detailed insights into hemodynamic responses under diverse conditions, enhancing the precision and safety of cardiovascular interventions. This comprehensive review emphasizes the critical role of MCLs in advancing cardiovascular research, refining clinical interventions, and improving patient outcomes.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide, contributing significantly to the global health burden [1]. As of 2020, the crude prevalence of CVD was 607 million cases, reflecting a 29% increase since 2010 [2], according to the latest heart disease and stroke statistics in 2023. These diseases encompass a variety of conditions, such as myocardial infarction, heart failure, stroke, aneurysms, atherothrombosis, and peripheral artery diseases, which result from complex interactions between hemodynamic, genetic, environmental, and lifestyle factors [3,4,5,6,7]. Understanding the underlying mechanisms and developing effective treatments for CVDs are essential for improving patient outcomes and reducing healthcare costs.
To achieve this, a fundamental aspect of cardiovascular health and disease that must be studied is hemodynamics—the study of blood flow and the forces involved in circulation through the cardiovascular system [8]. Hemodynamic parameters, including blood pressure, endothelial stress, etc., are crucial for atherosclerotic lesion development and the progression of CVDs. Abnormal hemodynamic conditions can lead to pathologies such as atherosclerosis [9], aneurysms [10], and thrombosis [11], emphasizing the need for detailed hemodynamic assessment in both clinical and research settings. Meanwhile, understanding hemodynamics is essential for developing effective diagnostic tools and therapeutic strategies. It provides insights into the mechanical environment of the cardiovascular system, helping to predict disease progression and evaluate the efficacy of interventions. Advanced hemodynamic studies can guide personalized treatment plans, improving patient outcomes by tailoring therapies to individual physiological conditions.
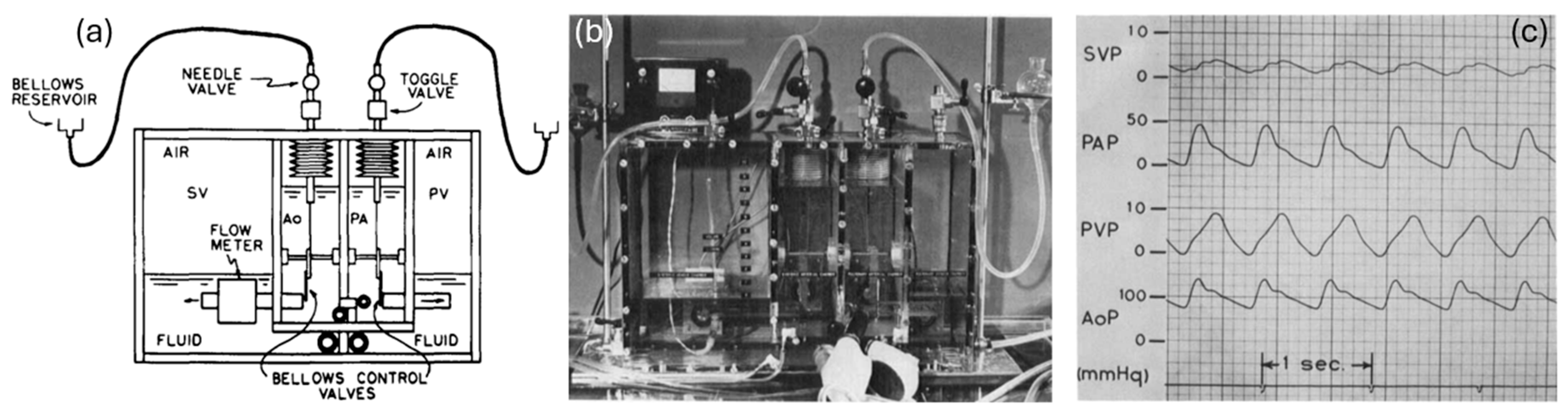
Mock circulation loops (MCLs) have emerged as novel research platforms to mimic the hemodynamics of the human cardiovascular system. Offering a controlled flow environment to simulate human circulatory conditions, MCLs replicate the hemodynamic environment of the cardiovascular system, allowing for researchers to study the effects of different pathological conditions and evaluate the performance of medical devices under realistic conditions. Figure 1 shows an early MCL prototype designed for artificial heart performance evaluation, simulating the human circulatory system [2]. The fluid starts in the systemic venous chamber and flows into the right ventricle. From there, it is pumped into the pulmonary arterial chamber. The fluid then passes through the pulmonary resistance valve into the pulmonary venous chamber. Next, it flows into the left ventricle, which pumps it into the systemic arterial chamber. Finally, the fluid returns to the systemic venous chamber by passing through the systemic resistance valve. This cycle replicates the continuous blood flow through the heart and vascular system.

Figure 1.
Early design of a hydraulic analog of an MCL [2]. (a) The schematics of a mock circulation system. (b) Experimental benchtop. (c) The pressure waveforms collected from an MCL.
The origin of MCLs traces back to the early understanding of the cardiovascular system, beginning with William Harvey’s foundational work “De Motu Cordis” in 1628, which described the circulatory system’s function [12]. In 1733, Stephen Hales’ first measurement of blood pressure further enhanced our understanding of vascular hemodynamics [13]. The late 19th century saw the development of the Windkessel model by Otto Frank, which explained the arterial system’s role in blood flow during the cardiac cycle and laid the groundwork for modern MCLs by simulating arterial compliance and resistance [14]. The 2000s were characterized by innovations in material science that improved the biocompatibility and durability of MCL components [15] along with the integration of real-time data systems for immediate simulation adjustments [16,17]. The 2010s ushered in the era of patient-specific simulations and the use of 3D printing, enabling highly personalized simulations for pre-surgical planning [18,19,20] and custom device testing [21,22]. These advancements have allowed for MCLs to provide more precise and tailored cardiovascular interventions. The 2020s are seeing further advances, with the integration of sophisticated computational models [23,24,25,26,27,28,29,30], enhancing the predictive power and clinical relevance of MCL simulations.
By replicating the pressures, flow rates, and resistance characteristic of human blood circulation, MCLs serve as critical tools in the development, testing, and validation of medical devices, such as ventricular assist devices (VADs) [21,31,32,33,34], pulsatile pumps [22,35,36], and artificial hearts [37,38]. The development of MCL technology began in the mid-20th century as a response to the growing need for more sophisticated testing methods in cardiovascular research. Initial models were simple, often only replicating single aspects of heart function [39]. As the complexity of cardiovascular devices increased, so too did the complexity of the MCLs. Over the decades, these systems have evolved from basic setups capable of simulating only the most fundamental heart dynamics to advanced systems that include adjustable compliance chambers, variable resistance units, and sophisticated control software capable of emulating various cardiac conditions and patient scenarios [40]. This evolution has been driven by advancements in materials science, fluid dynamics, and control systems technology, making today’s MCLs highly adaptable and capable of providing detailed feedback on device performance under a variety of simulated physiological conditions.
The clinical diagnosis of CVDs typically involves a combination of non-invasive and invasive techniques, each with its own advantages and limitations, but MCLs offer a valuable bridge between these methods. Non-invasive methods, such as echocardiography, magnetic resonance imaging (MRI), and computed tomography angiogram (CTA) scans, provide detailed anatomical and functional information about the heart and blood vessels [41]. These methods are valued for their safety, low cost, and ability to offer real-time insights into cardiovascular health. However, they may be limited by their availability, radiation exposure, and ability to comprehensively capture dynamic physiological changes [41]. In contrast, invasive methods, such as cardiac catheterization and angiography, offer direct measurements of hemodynamic parameters and detailed visualization of vascular structures [42]. These techniques are highly accurate and crucial for diagnosing conditions such as coronary artery diseases. Yet, they carry risks, including arterial injury, infection, bleeding, and radiation exposure, which can restrict their use, particularly in vulnerable patient populations [43]. MCLs provide a significant advantage by replicating patient-specific conditions and offering detailed hemodynamic data without the risks associated with invasive procedures, making them an invaluable tool for both research and clinical planning.
Medical treatments for CVDs, such as pharmacotherapy and surgical interventions, have significantly improved survival rates, and MCLs can further enhance the effectiveness and safety of these treatments [44,45]. Pharmacological agents, such as statins, beta-blockers, and antiplatelet drugs, effectively manage risk factors and have demonstrated benefits in disease progression [46,47]. Surgical and interventional procedures, including coronary artery bypass grafting [48] and percutaneous coronary intervention [49], are vital for treating advanced disease. However, they are associated with drawbacks, such as adverse side effects, the need for long-term medication adherence, and potential procedural complications [50]. The integration of MCLs in preclinical evaluations provides a controlled environment to compare specific drug effects on mechanical and hemodynamic properties in extracorporeal circuits [51]. This capability is particularly important in reducing adverse effects and improving patient-specific treatment plans, ultimately leading to better clinical outcomes.
While numerous valuable reviews exist on MCLs, they have predominantly focused on their established roles in cardiovascular medical device testing, including comprehensive overviews of MCL development for ventricular assist devices, mechanical heart valves, and extracorporeal circuits, with particular emphasis on flow control, hemocompatibility, and pump dynamics [52,53,54]. These reviews have provided valuable foundations in the field, particularly by establishing the role of MCLs in cardiac support technologies. While their primary focus has been on device performance and cardiovascular assistance, they have also opened avenues for expanding MCL applications. Some have touched upon in vitro vascular condition modeling [55], laying important groundwork that this review seeks to build upon by offering a more systematic perspective on patient-specific surgical planning and pathological flow simulation. This review, however, addresses a critical gap by shifting the perspective towards the direct clinical application of MCL technology. We will delve into the versatile capabilities of MCLs in simulating a wide spectrum of CVDs, moving significantly beyond heart failure models to focus specifically on arterial pathologies, such as aneurysms, arterial stenosis, and peripheral artery disease. The main goal and new contribution of this work are to organize and summarize how MCLs are now used. While MCLs are well-known for testing devices, we focus on how they help us really understand disease processes and, very importantly, help plan surgeries better and create personalized treatments in real hospitals. This focus on diseases and their real-world medical use shows a new way MCLs are being applied.
2. Key Components of an MCL
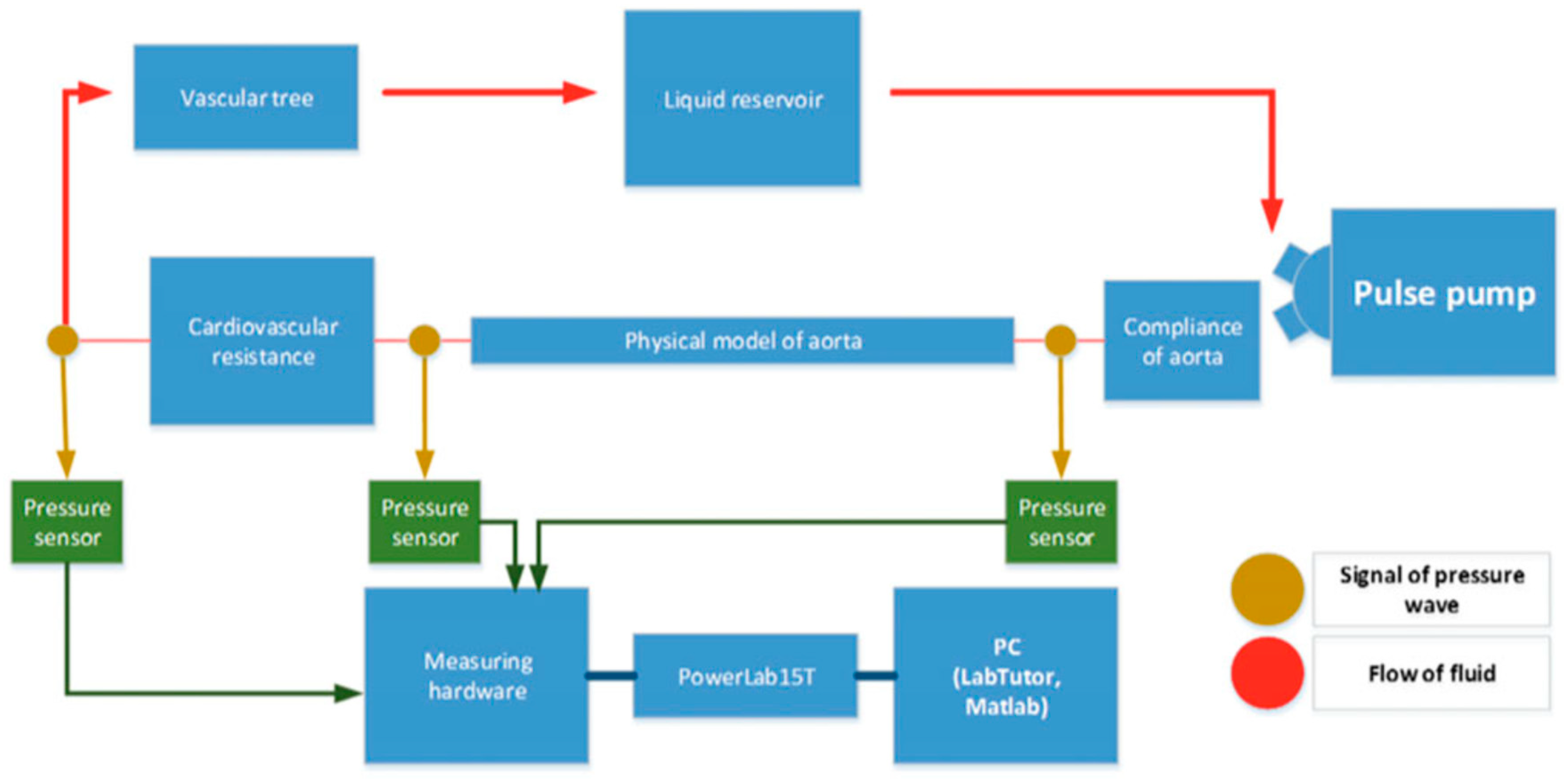
An MCL is composed of several key components that collectively simulate the cardiovascular system’s dynamics. Figure 2 provides a schematic overview of a typical MCL setup, illustrating the arrangement and function of these key components. At the core of an MCL is a programmable pump that mimics the heart’s pumping action, which can be adjusted to vary the heart rate and stroke volume according to the simulation requirements. Compliance chambers are used to simulate the elasticity of arterial walls, absorbing the pulse waves generated by the pump. Resistance elements replicate the peripheral resistance of the vascular system and can be adjusted to model different levels of vascular constriction. A venous reservoir is often included at the end of the loop to collect and recirculate the fluid, ensuring volume stability and enabling continuous closed-loop operation. Together, these components allow for MCLs to model heart and vascular functions across a range of physiological and pathological conditions. The integration of these components—programmable pumps, compliance chambers, and resistance elements—enables MCLs to accurately simulate the complex dynamics of the cardiovascular system. By providing a highly controllable and adaptable platform, MCLs facilitate detailed studies of hemodynamic responses under various physiological and pathological conditions. This capability is essential for advancing our understanding of cardiovascular function and for developing and testing new medical devices and treatments. As technology continues to evolve, the precision and applicability of MCLs are expected to further enhance their role in cardiovascular research, ultimately leading to improved diagnostic and therapeutic strategies for a wide range of CVDs.
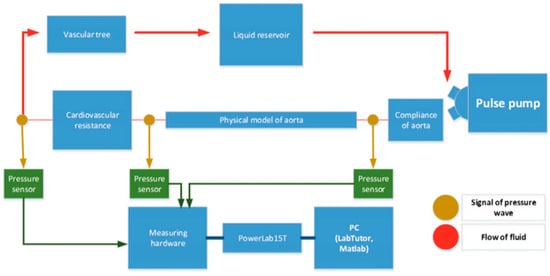
Figure 2.
Simplified schematic of a closed-loop mock circulation system representing the cardiovascular system [56]. A pulsatile pump generates flow that passes through the first compliance chamber, a simulated aortic segment, and the second compliance chamber before returning to the reservoir.
2.1. Pulsatile Pump
At the heart of every MCL is the programmable pump, which serves as the heart substitute. This pump is specifically designed to mimic the heart’s pumping action and is capable of generating pulsatile or continuous blood flow depending on the study requirements. It can be finely tuned to vary the heart rate and stroke volume, allowing for researchers to simulate different cardiac conditions from rest to stress. The programmability of the pump enables the replication of various cardiac outputs that would be seen in different patient populations from infants with low cardiac output to adults with high output due to physiological stress or disease.
Various pumps are employed to simulate cardiovascular dynamics, each offering unique advantages. Diaphragm and piston pumps are commonly used for their ability to generate precise, pulsatile flow patterns that mimic the heart’s contractions, making them ideal for ventricular simulations. Peristaltic pumps are valued for handling delicate fluids without contamination, using a series of rollers to push fluid through a flexible tube, which is essential for maintaining a sterile environment [57,58]. Centrifugal pumps provide a continuous, smooth flow, suitable for scenarios requiring steady, non-pulsatile simulations, while gear and rotary vane pumps offer high precision and repeatability, capable of handling high pressures and moderate flow rates, respectively [34,59]. Herreros reported that the centrifugal pump, when exclusively controlled by a pulsatile console, can provide pulsatile flow but concluded that its pulsatility is approximately 25% of that created by a truly pulsatile pump [60]. These diverse pumps enable MCLs to replicate a wide range of physiological conditions, enhancing the accuracy and applicability of cardiovascular research.
Beyond direct pumping methods, ventricular simulation in MCLs can also be achieved through indirect compression using flexible chambers. In this approach, a compliant sac or bladder serving as a ventricle analog is rhythmically compressed by external driving mechanisms, such as pneumatic pistons, hydraulic actuators, or servo-controlled mechanical pusher plates [61,62]. This technique offers a more physiologically accurate replication of ventricular deformation and diastolic filling, making it particularly suitable for applications requiring biomimetic cardiac motion [63,64]. Pumps in MCL systems can be connected to computer systems that control their operation, ensuring that the desired heart rates, pressures, and flow rates are accurately maintained throughout the experiments. These pumps can also simulate heart rate variabilities or arrhythmic conditions, providing a comprehensive platform for cardiovascular research and device testing.
2.2. Compliance Chambers
Compliance chambers in an MCL play a crucial role in simulating the elasticity of arterial walls. These chambers are designed to absorb and dampen the pulse waves generated by the pump, mimicking the natural compliance of human arteries. By doing so, they help to create a more realistic simulation of the arterial pulse pressure waveforms seen in vivo. The material and design of these chambers are chosen to replicate the capacitive properties of the vascular system, which are essential for understanding how blood pressure and flow interact in real biological systems. Adjustments to the compliance chambers can be made to model different levels of arterial stiffness, which is particularly useful in studies focused on aging or CVDs like arteriosclerosis. These chambers are integral in studying how changes in arterial compliance affect overall cardiovascular function and the performance of cardiovascular devices such as stents and grafts.
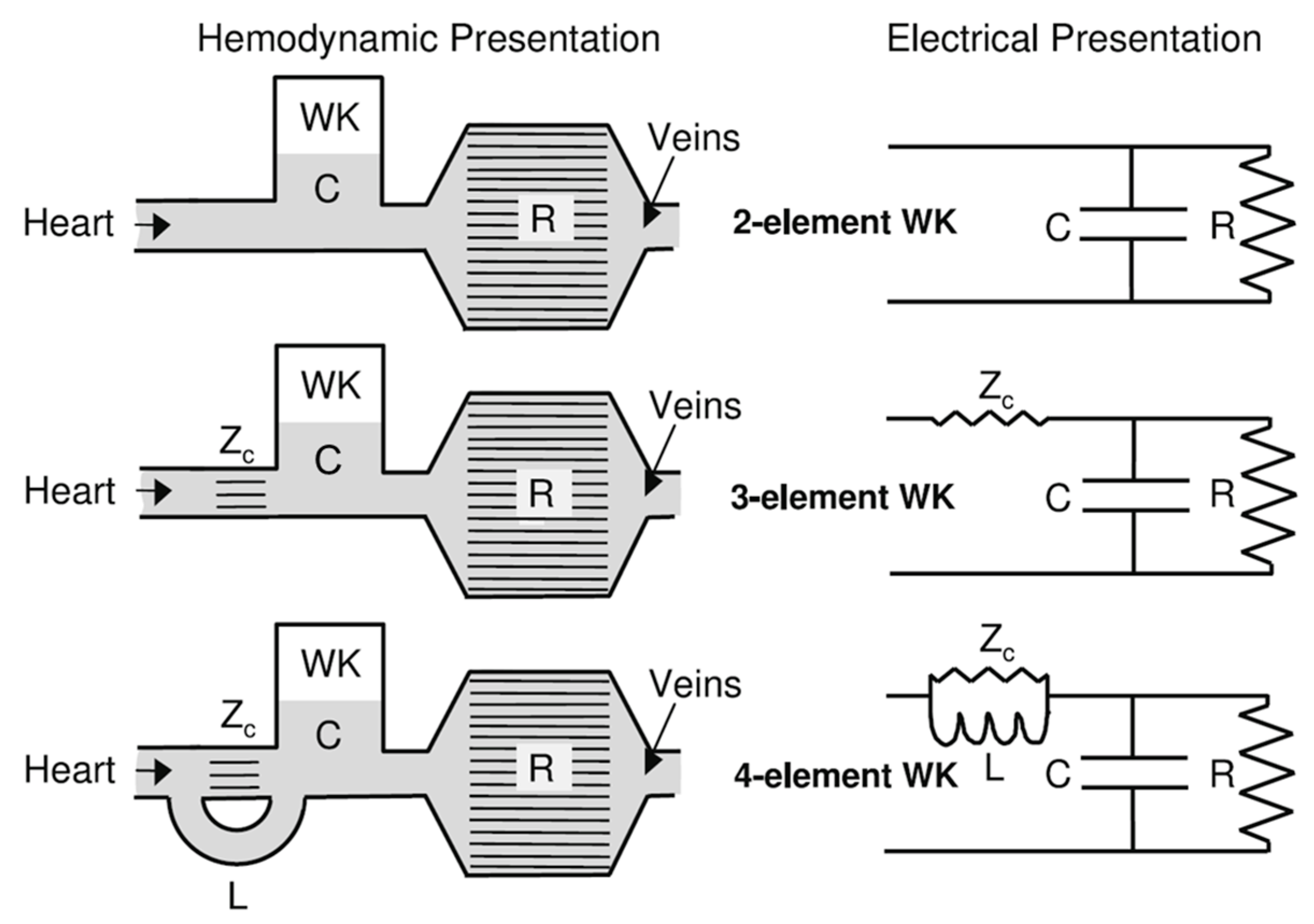
One of the seminal models in hemodynamics is the Windkessel model, developed in the late 19th century [39,65]. This model was initially proposed to describe the aortic pressure curve and how blood flows through the elastic arteries. The term “Windkessel” translates to “air chamber” in German, reflecting the model’s conception of arteries acting as reservoirs that maintain blood flow during diastole, much like air in a pressurized chamber. The basic Windkessel model treats the arterial system as a simple compliance chamber connected to a resistance, as shown in Figure 3, where compliance represents the elasticity of the arterial walls and resistance represents the arterioles’ control of blood flow [66,67].
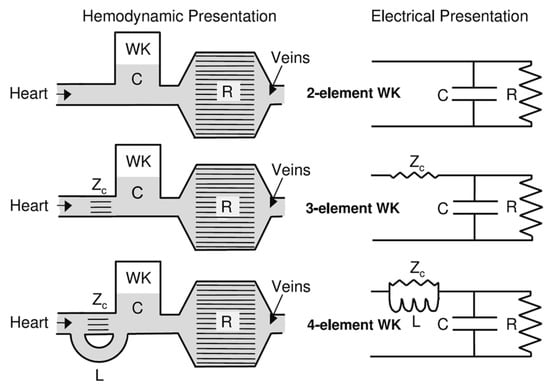
Figure 3.
The two-element Windkessel, the three-element Windkessel, and the four-element Windkessel presented in hydraulic and electrical form [66] (WK—Windkessel Model, C—compliance, R—resistance, Zc—impedance, L—inductance).
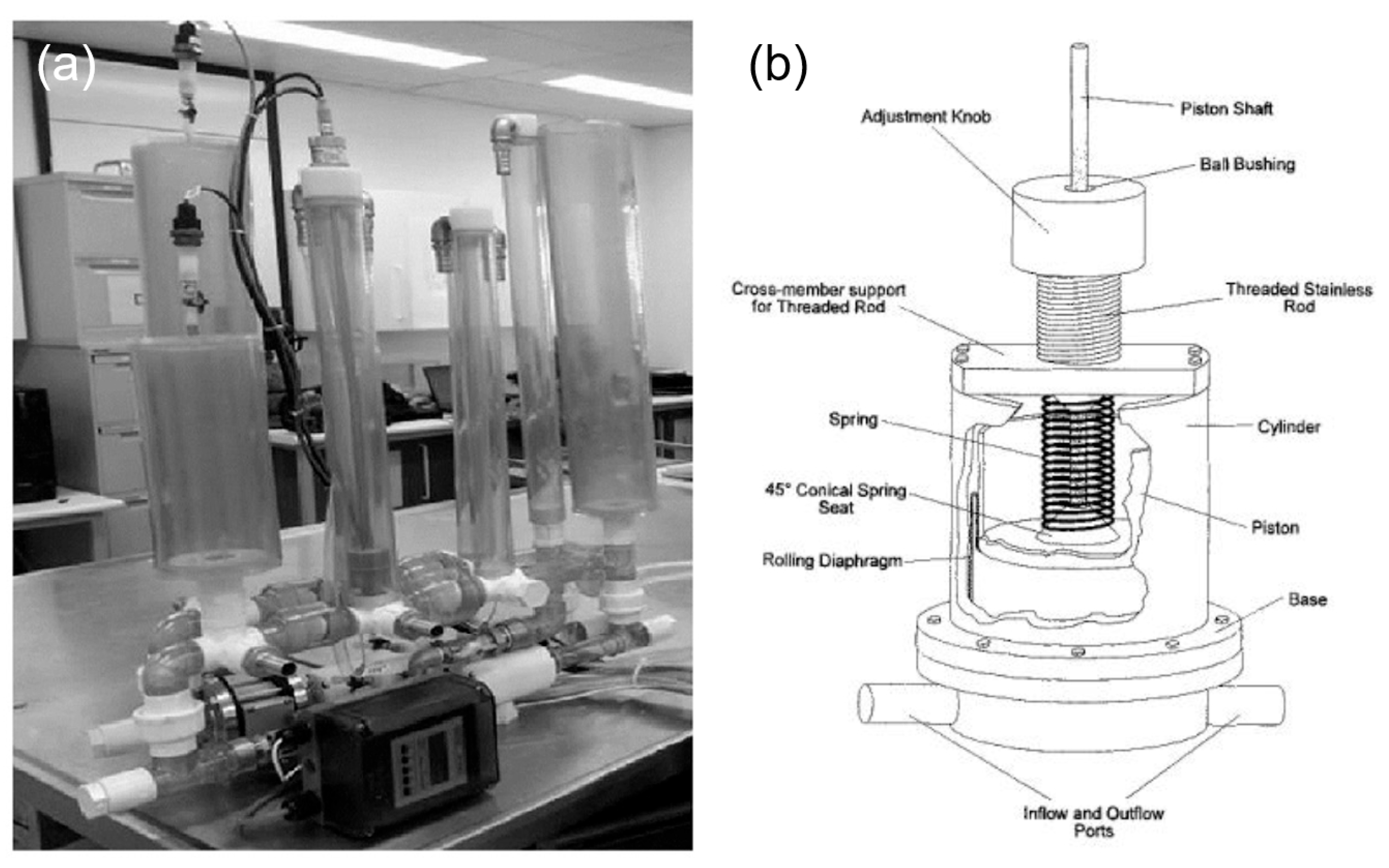
Various types of compliance chambers are utilized to simulate the elasticity of arterial walls, each offering different features. Static compliance chambers, which can be adjusted by varying the air or water volume within them, are used for consistent simulations where simple adjustments to compliance are sufficient [68]. Adjustable compliance chambers incorporate elements like springs or membranes that can be fine-tuned to replicate different levels of arterial stiffness in real-time (Figure 4), making them ideal for studies focused on aging or diseases like arteriosclerosis [69]. These advanced chambers allow for dynamic adjustments during simulations, providing a versatile and realistic platform for accurately modeling a wide range of physiological and pathological conditions in cardiovascular research.
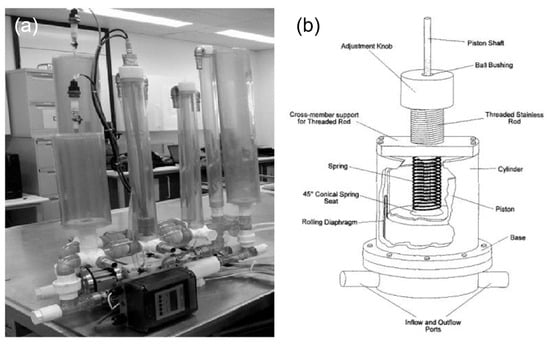
Figure 4.
Compliance chambers applied in MCLs. (a) Static compliance chambers [68]. (b) Variable stiffness spring compliance chamber.
2.3. Resistance Valves/Resistors
Resistance elements in an MCL are critical components used to simulate the peripheral vascular resistance found in human circulation. Their primary function is to replicate the hemodynamic load imposed on the heart by the systemic vasculature, commonly referred to as systemic vascular resistance (SVR) [66,70,71]. These resistance elements work by restricting the flow path of the circulating fluid, thereby creating a pressure drop analogous to that which occurs across capillary beds and arterioles in vivo. The resistance is typically generated by narrowing the fluid channel, forcing the fluid through longer or thinner pathways, or through geometrical constrictions that mimic arteriolar tone [72,73]. The ability to adjust resistance is crucial for simulating SVR, a key determinant of cardiac afterload [74].
To achieve this, MCLs incorporate various types of resistance components tailored for different control needs. For instance, manual clamp-type resistors, such as Hoffman clips, allow for coarse adjustment of resistance by manually altering tubing diameter using an external clamp [20,75]. This method is fast and easy to implement but lacks high precision. For finer control, adjustable resistance valves are employed, such as rotary or diaphragm valves, which allow for researchers to gradually tune resistance with higher precision. These are useful when replicating subtle changes in vascular tone or studying graded vascular responses under pharmacological or device interventions [40,70,76]. Additionally, needle gauges or micrometer-controlled valves provide high-resolution control of flow resistance and are particularly beneficial in studies requiring exact replication of peripheral resistance or when simulating small arterial branches [73].
In certain setups, resistance is not only modulated to mimic normal physiology but is also dynamically adjusted to simulate pathological conditions. For example, researchers may increase resistance to mimic hypertensive vascular beds or decrease it to simulate arteriolar dilation, thereby analyzing cardiac responses under altered afterload conditions [38]. This is especially relevant when testing mechanical circulatory support devices, such as VADs [77,78,79] or total artificial hearts [38], to observe their performance under different afterload profiles.
3. Applications in Surgical Planning
MCLs have played a critical role in the field of surgical planning by providing a controlled and replicable environment to simulate various cardiovascular conditions and interventions. The application of MCLs in surgical planning can be categorized into three main areas: interventional procedure optimization, preclinical and surgery evaluations, and patient-specific surgical planning.
3.1. Interventional Procedure Optimization
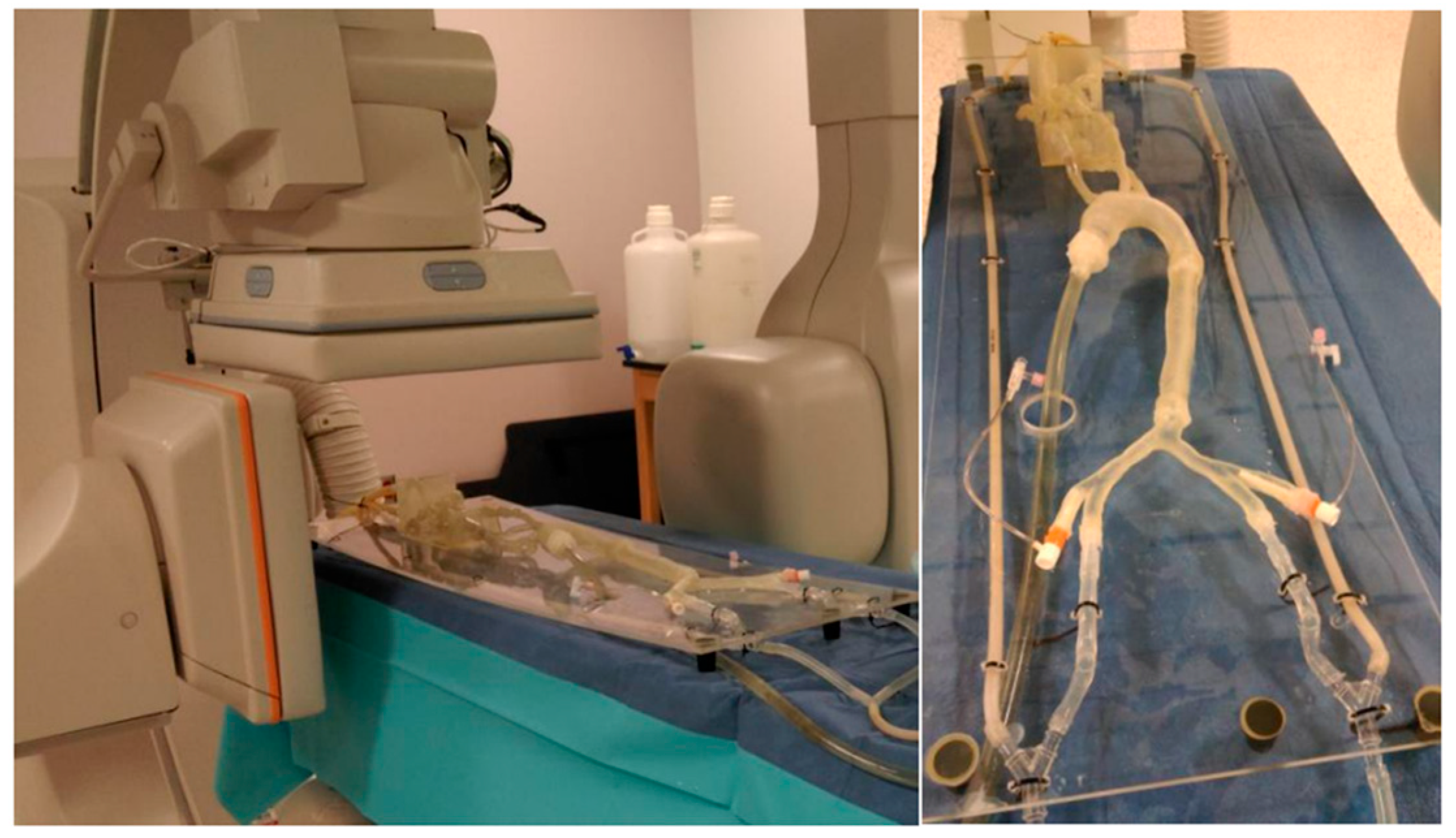
Surgical and interventional procedures, including coronary artery bypass grafting and percutaneous coronary intervention, remain critical for managing advanced cardiovascular disease. However, they are often accompanied by complications such as bleeding, infection, restenosis, and the long-term reliance on antiplatelet medications [50]. MCLs provide a realistic platform for testing and optimizing interventional procedures. These procedures include angioplasty, stenting, and valve replacement surgeries. By simulating patient-specific conditions, MCLs enable surgeons to evaluate the efficacy and safety of different interventional techniques before applying them in clinical practice [80]. As shown in Figure 5, utilizing 3D phantoms in MCL is effective in evaluating the performance of distal access catheters by measuring the required force applied to the tissues during neuroendovascular procedures [19]. MCLs can study the forces on the artery, aneurysm wall, and the stent when stent grafts are used to treat aneurysmal disease.
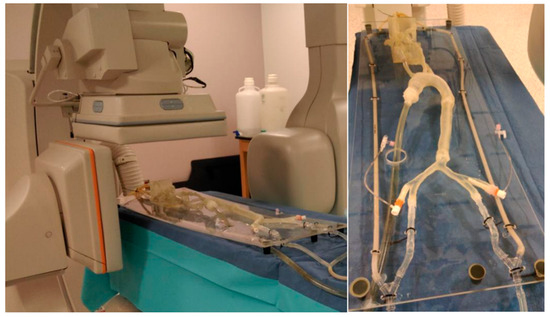
Figure 5.
Several printed phantoms are put together to better simulate the traversal of the catheter from the entry point in the femoral artery to the treatment site [19].
Meanwhile, 3D printing enables the creation of highly precise benchtop experiments where pressure sensors can be integrated into vascular phantoms, allowing for the study of hemodynamic changes resulting from various therapies, such as the treatment of intracranial aneurysms with flow diverters [20]. Agrafiotis et al. [81] demonstrate the use of MCL to assess the biomechanical impact of interventional surgeries, such as stent placement, on aortic tissues. By simulating physiological conditions and performing extended perfusion, the research showed that stent placement significantly stiffens the aorta, reduces its flexibility, and causes structural changes in the tissue. By evaluating these impacts under simulated physiological conditions, MCLs provide critical data that can guide enhancements in stent technology, ultimately aiming to reduce adverse effects and improve patient outcomes.
3.2. Preclinical and Surgery Evaluations
MCLs play a crucial role in preclinical evaluations of new surgical techniques. They offer a controlled environment to test innovations such as novel assessment methods, including patient-specific angiographic phantoms developed for evaluating endovascular image-guided interventional devices and assessing imaging performance under realistic hemodynamic conditions [82]. In addition, new interventional techniques have been studied using MCLs, such as flow-modifying strategies tested in patient-specific aneurysm phantoms with optical flow visualization systems [83] and the integration of 3D-printed vascular models into treatment planning workflows for image-guided neurovascular interventions [19]. These applications demonstrate the value of MCLs in refining both diagnostic and therapeutic technologies before clinical implementation. Graft materials [84] are evaluated in MCLs to assess mechanical performance, durability, and hemodynamic compatibility, while bypass/stenting strategies [18] are tested to optimize placement techniques and improve flow restoration in occluded or narrowed vessels.
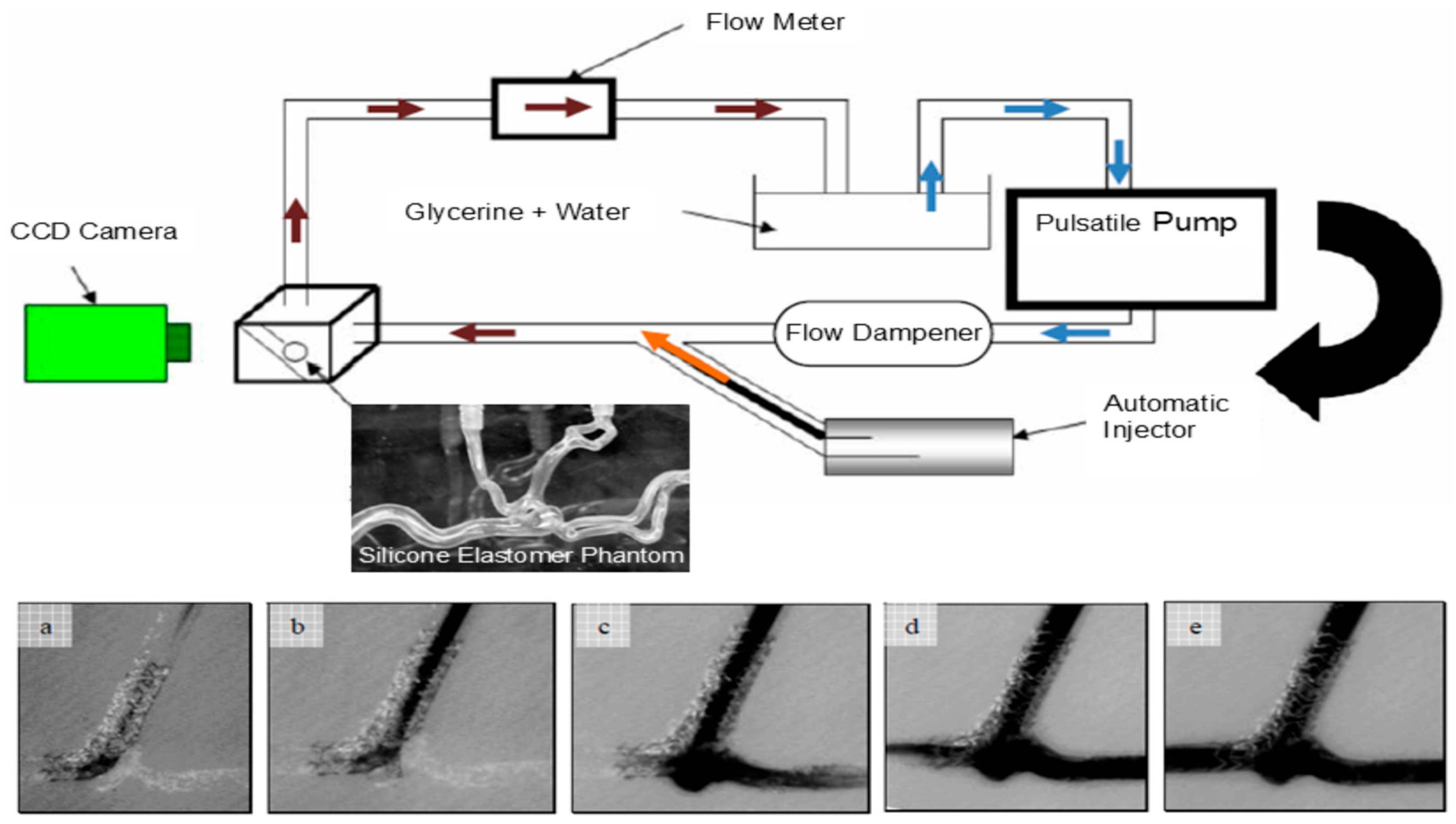
These applications demonstrate the value of MCLs in refining both diagnostic and therapeutic technologies before clinical implementation. The correlation between angiographic parametric imaging features and the severity of carotid artery disease was investigated by 3D-printed patient-specific neurovascular phantoms [30]. MCLs using 3D-printed vascular phantoms offer a standardized and patient-specific experimental platform for device testing and physician training (as shown in Figure 6), improving the safety and reliability of endovascular image-guided interventions by allowing for pre-procedural simulations to optimize treatment plans, reduce procedural duration and complications, and enhance overall outcomes [83].
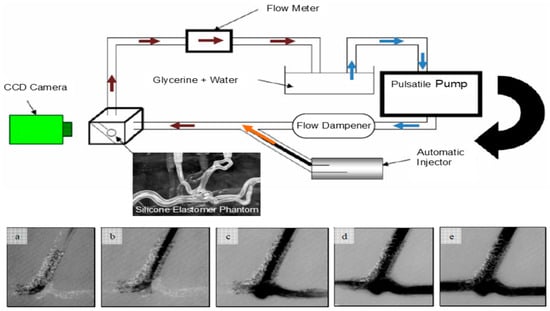
Figure 6.
Experimental schematics for an MCL incorporating high-speed cameras. The bottom images (a–e) show the initial inflow of optical dye into an aneurysm phantom treated with stenting [83].
3.3. Patient-Specific Surgical Planning
By incorporating patient-specific data, MCLs help surgeons design tailored surgical approaches, improving precision and reducing intraoperative surprises. Advanced imaging technologies play a crucial role in the evaluation of test samples within MCL setups. Techniques such as MRI, CTA scans, and ultrasound are employed to visualize how a device interacts with blood flow within the loop. These imaging tools provide real-time, detailed visualizations of the device in operation, offering insights into the flow dynamics and mechanical interactions at play. Imaging technology also allows for the assessment of wear and tear on the devices over time, which is crucial for understanding the longevity and durability of implants [18,19,20,83]. By visualizing how a device operates within the MCL, researchers can make precise adjustments to improve design and functionality, ensuring that the device will perform as intended in the human body [30].
Three-dimensional printing technology has revolutionized the fabrication of test samples for MCL setups by enabling the creation of complex, patient-specific cardiovascular structures [15,18,19,20,21,29,30,85,86,87,88,89]. Using data from patient imaging studies, such as CTA scans or MRIs, researchers can produce anatomically accurate models of a patient’s heart or blood vessels. These models are then used as test samples in MCLs to evaluate how a device will perform in a specific anatomical context. The use of 3D-printed models is particularly advantageous when testing devices for congenital heart diseases or other unique anatomical challenges. It allows for the customization of devices to fit precise anatomical needs, significantly improving the relevance and accuracy of the testing process. For example, a custom-fit vascular graft can be 3D printed to match the exact dimensions and curvature of a patient’s blood vessels, ensuring that the graft integrates seamlessly when deployed in actual surgical procedures.
Furthermore, patient-specific MCL studies are invaluable for customizing medical devices, such as tailor-made stents or grafts that need to fit individual anatomical requirements. By testing these devices in an MCL configured according to patient-specific data, manufacturers can ensure a higher degree of safety and effectiveness, ultimately leading to better patient outcomes.
4. MCLs in Cardiovascular Disease Studies
MCLs are essential tools in cardiovascular disease research due to their ability to provide highly adaptable and precise simulations of the human circulatory system. These systems are crucial for accurately replicating the diverse and complex conditions found in cardiovascular medicine, allowing for researchers to study disease mechanisms and evaluate therapeutic interventions in a controlled environment. MCLs can be customized in terms of size, functional components, and the complexity of the physiological conditions they replicate. For instance, studies of arterial stenosis require MCLs to incorporate variable resistance elements that simulate the narrowing of vessels and recreate steep pressure gradients and disturbed flow patterns [40]. In aneurysm-related MCL studies, while rupture risk remains an important concern, increasing attention is directed toward optimizing endovascular treatment strategies, particularly stent and graft interventions. Researchers often focus on how these devices alter local hemodynamics, such as flow diversion, vortex suppression, and pressure redistribution [18,83,84]. For peripheral artery disease, MCLs must replicate reduced flow and pressure in distal regions, often involving long vascular paths with adjustable compliance and resistance to reflect segmental occlusions [63]. When investigating venous insufficiency or valve-related pathologies, the inclusion of one-way valves and reversed flow conditions becomes necessary. This adaptability is vital for investigating specific CVDs, as it enables the fine-tuning of flow and pressure parameters to mimic precise pathological conditions. By offering a robust platform for various cardiovascular investigations, MCLs facilitate the study of disease progression, the impact of interventions, and the development of new treatments, ultimately leading to improved patient outcomes and advancements in cardiovascular medicine.
4.1. Stenosis
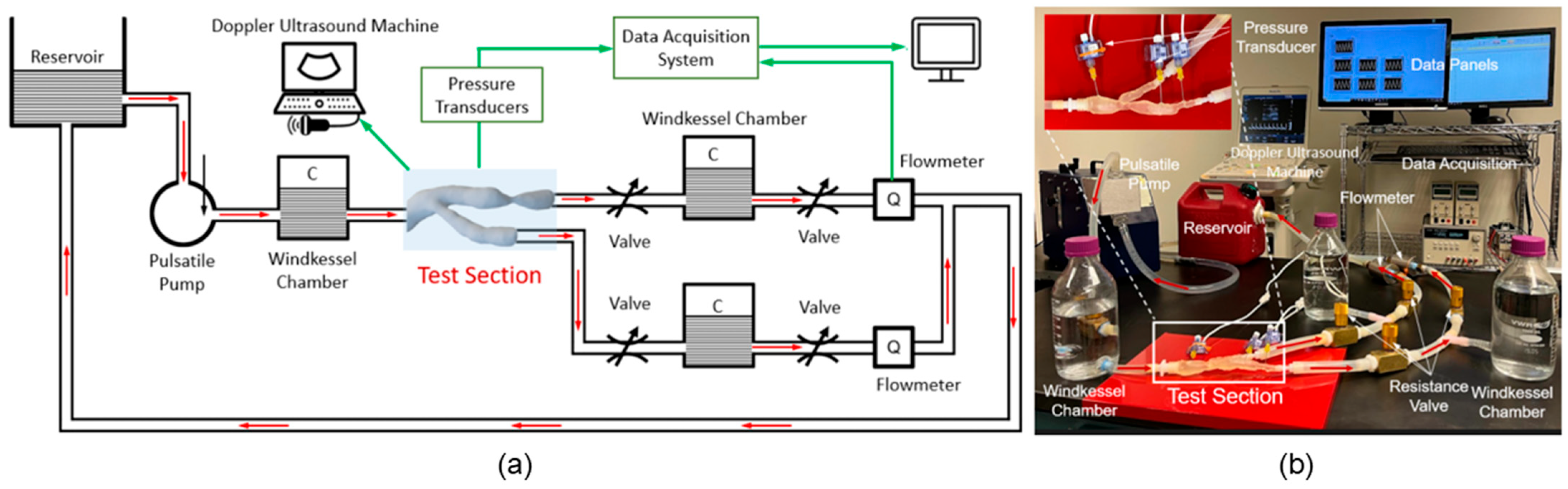
Arterial stenosis involves the narrowing of arteries, such as iliac [40], renal [90], and coronary [91], due to plaque buildup or other pathological changes, significantly altering hemodynamics [92]. MCLs are equipped to simulate these conditions using adjustable resistance elements that precisely mimic arterial narrowing. This setup enables researchers to study the effects of stenosis on blood flow dynamics, pressure gradients, and the overall cardiovascular health impact. MCLs provide a controlled environment to evaluate interventions like balloon angioplasty and stenting, assessing their effectiveness in restoring normal flow and preventing restenosis. Malone et al. examined how stents deploy within narrowed arteries and their subsequent impact on blood flow and arterial pressure [72] via MCLs, which helps in optimizing therapeutic approaches. Hong et al. [40] demonstrated the use of an MCL (as shown in Figure 7) to characterize in vitro hemodynamics in human systemic arteries with stenosis. By adjusting the diameter reduction of the 3D model to simulate arterial narrowing, they were able to study the impact on blood flow and pressure, providing valuable insights into the efficacy of interventions like balloon angioplasty or stent placement.
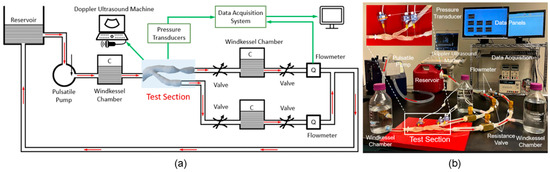
Figure 7.
Life-sized MCL [40]: (a) schematic diagram and (b) benchtop setup to mimic the blood flow in a 3D-printed silicone arterial system for in vitro measurement of pressure and velocity waveforms.
4.2. Aneurysm
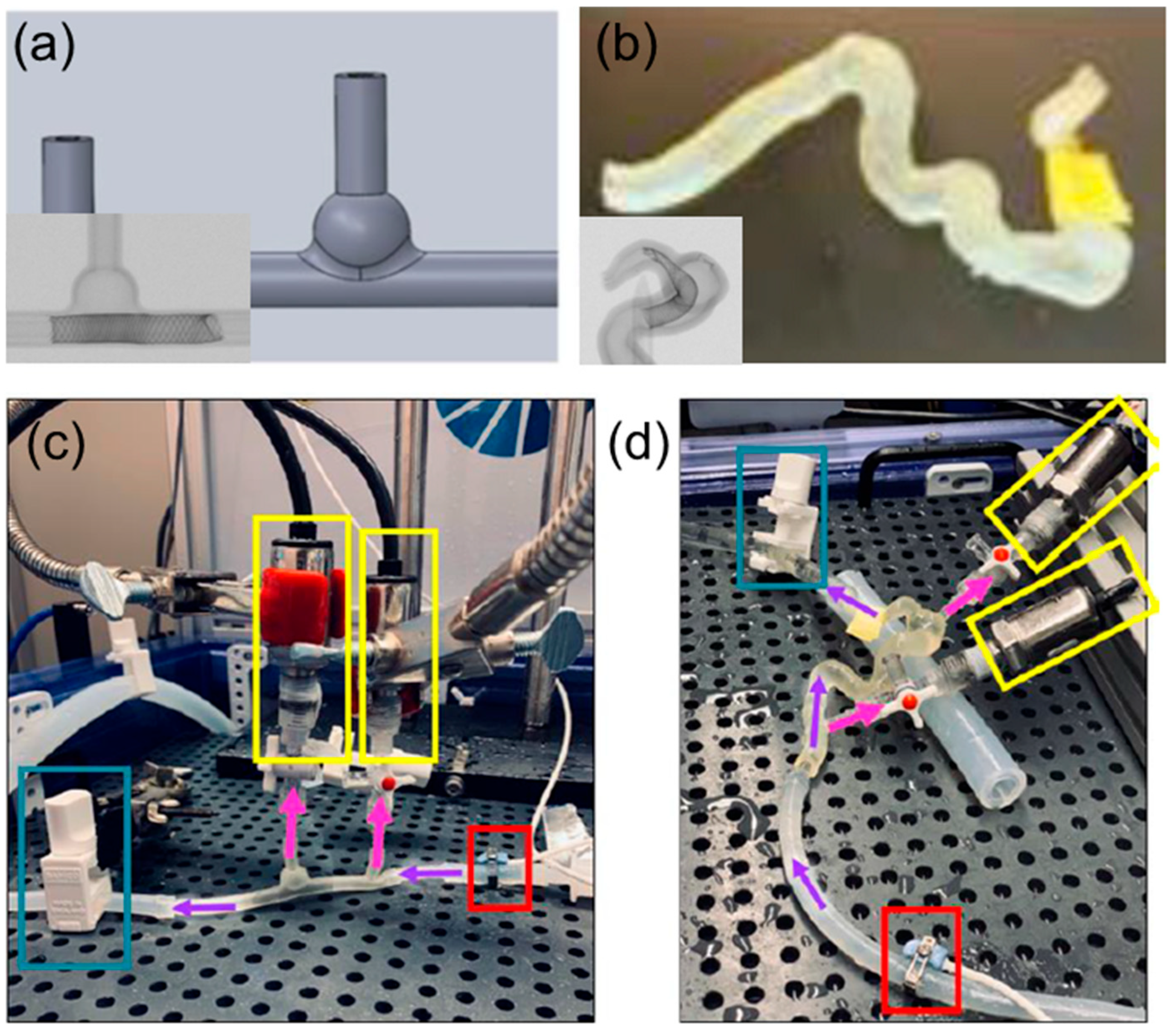
Arterial aneurysms involve abnormal bulging of the artery wall, posing significant rupture risks with serious complications. MCLs can be adapted to model the biomechanical properties of arterial walls with aneurysms, allowing for simulation under various blood pressure conditions. This capability is crucial for understanding stress distribution across the aneurysmal wall and for developing risk assessments and treatment strategies. The simulation of aneurysm dynamics in MCLs includes testing surgical stenting repairs and devices designed to reinforce the arterial wall, providing insights into the effectiveness of these treatments in preventing rupture and enhancing patient safety [10,18,20]. Lieber et al. used MCLs to study the alteration of hemodynamics in aneurysm models by stenting [10]. Their research highlighted the influence of stent porosity on flow patterns and provided critical insights into optimizing stent designs to prevent aneurysm rupture. Meess et al. [18] created a 3D-printed abdominal aortic aneurysm phantom for image-guided surgical planning with a patient-specific fenestrated endovascular graft system, providing insights into the effectiveness of these treatments in preventing rupture and enhancing patient safety. Allman et al. utilized 3D-printed intracranial aneurysm phantoms in MCLs to test the effect of a flow diverter’s geometry on hemodynamics. They compared idealized models with patient-specific models, as shown in Figure 8, and found that undersizing flow diverters increased hydraulic resistance and pressure differences, potentially leading to better occlusion rates. This study highlights the importance of precise sizing in flow diverter placement and aids in developing better treatment strategies for aneurysms [20].
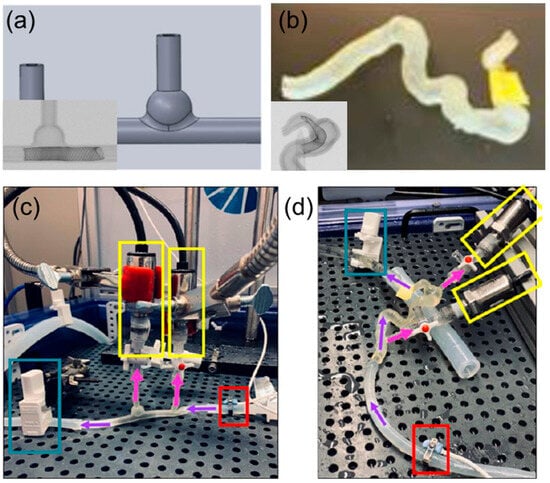
Figure 8.
Examples of test models and experimental setup of an MCL. (a) Ideal intracranial aneurysm model and micro-CT scan containing a flow director. (b) Patient-specific intracranial aneurysm model and micro-CT scan containing a flow director. (c) Experimental setup for idealized model and (d) patient-specific model. Purple arrows indicate the direction of flow. Pink arrows indicate the direction of flow to the pressure sensors (yellow box). The red box highlights the ultrasound sensor that keeps track of the incoming flow rate in mL/min. The blue box highlights a clamp to simulate the pressure seen in the intracranial vasculature [20].
4.3. Peripheral Artery Disease
Peripheral artery disease (PAD) is characterized by the narrowing or blockage of peripheral arteries, often leading to significant blood flow reduction and associated complications. MCLs configured for PAD research can simulate the conditions of peripheral arterial narrowing by adjusting resistance elements to mimic different levels of vascular constriction. This setup allows for researchers to study the impact of PAD on blood flow and tissue perfusion, providing valuable insights into disease progression. MCLs are instrumental in evaluating the efficacy of various therapeutic interventions, such as bypass surgery and angioplasty.
By simulating the hemodynamic environment of PAD, MCLs enable the testing and optimization of treatments aimed at restoring blood flow and improving patient outcomes. Hirsch et al. [11] provided comprehensive practice guidelines for managing PAD, emphasizing the importance of accurate simulation and testing of interventions in MCLs to optimize patient outcomes. Their guidelines underscore the critical role of MCLs in developing effective treatments for PAD. Mukherjee et al. [46] discussed the considerations in the risks, diagnosis, and treatment of PAD, highlighting how MCLs can simulate different levels of arterial constriction to study the disease’s impact and evaluate potential therapeutic interventions.
5. Challenges and Limitations
MCLs have become invaluable tools in studying surgical planning and CVDs, yet they face several challenges and limitations, as highlighted in various published studies. One significant challenge is the difficulty in accurately replicating the complex physiological conditions of the human cardiovascular system [52]. While MCLs can simulate basic hemodynamic parameters, they often fall short in reproducing the intricate interactions between blood flow, vessel elasticity, and cellular responses [86]. Additionally, MCLs are limited in their ability to mimic long-term biological responses and disease progression, critical for understanding chronic conditions and the effectiveness of long-term treatments. Creating patient-specific models, though beneficial for personalized medicine, is both time-consuming and costly, requiring advanced imaging and 3D printing technologies [15].
Moreover, the successful implementation of MCLs in surgical planning demands a high level of technical expertise and sophisticated equipment [87]. The need for precise calibration and maintenance of the MCL to ensure realistic and reliable simulations can be a barrier, particularly in resource-limited settings. The integration of advanced computational models and real-time data acquisition systems adds further complexity and expense. Additionally, while MCLs provide valuable insights into the mechanical performance of devices and procedural techniques, they cannot fully replicate the biological environment, such as the immune response, healing processes, and potential complications like infections [93]. Consequently, the use of complementary animal models or clinical trials remains necessary to validate findings from MCL studies [17], extending the overall time and cost of research.
6. Summary
MCLs have become increasingly important in cardiovascular research. They provide a reliable way to simulate different cardiovascular conditions and test various interventions. Researchers typically use MCLs in three main areas of surgical planning: optimizing interventional procedures, evaluating surgical techniques, and tailoring surgical plans to individual patients. By creating patient-specific conditions, MCLs allow for more accurate testing and fine-tuning of procedures like stenting and angioplasty. This leads to better clinical outcomes overall. They also play a crucial role in the early evaluation of new surgical techniques and devices, ensuring they are safe and effective before being used in real-world applications.
On the disease side of things, MCLs enable researchers to closely study specific conditions such as arterial stenosis, aneurysms, etc. By mimicking how these diseases progress and how interventions might work, MCLs provide valuable insights into the effectiveness of treatments, such as stent placements for aneurysms and bypass surgeries for PAD. Plus, with advancements in 3D printing technology, MCL studies can incorporate patient-specific models that enhance the accuracy and relevance of testing cardiovascular devices.
That said, MCLs do have their drawbacks. They often struggle to fully replicate long-term biological responses and chronic conditions. The need for advanced equipment and technical know-how, along with the complexity of human physiology, presents some hurdles. Despite these challenges, MCLs are indispensable tools in cardiovascular research, driving the development of new treatments and interventions that can improve patient outcomes.
Author Contributions
Concept/design, A.P.S., H.Y., J.C., and W.H.; Data analysis/interpretation, W.H. and V.T.; Drafting article, W.H.; Critical revision of article, H.Y. and A.P.S.; Approval of article, H.Y.; Data collection, A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The second author was supported by the Indiana University Medical Student Program for Research and Scholarship (IMPRS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 3D | 3-Dimensional |
| CTA | Computed Tomography Angiogram |
| CVDs | Cardiovascular Diseases |
| MCLs | Mock Circulation Loops |
| micro-CT | Micro-Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PAD | Peripheral Artery Disease |
| SVR | Systemic Vascular Resistance |
| VADs | Ventricular Assist Devices |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar]
- Donovan, F. Design of a hydraulic analog of the circulatory system for evaluating artificial hearts. Biomater. Med. Devices Artif. Organs 1975, 3, 439–449. [Google Scholar] [CrossRef]
- Elefteriades, J.A. Thoracic aortic aneurysm: Reading the enemy’s playbook. Curr. Probl. Cardiol. 2008, 33, 203–277. [Google Scholar] [CrossRef]
- Marsh, J.D.; Keyrouz, S.G. Stroke prevention and treatment. J. Am. Coll. Cardiol. 2010, 56, 683–691. [Google Scholar] [CrossRef]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Kullo, I.J.; Rooke, T.W. Peripheral artery disease. N. Engl. J. Med. 2016, 374, 861–871. [Google Scholar] [CrossRef]
- Fuster, V.; Moreno, P.R.; Fayad, Z.A.; Corti, R.; Badimon, J.J. Atherothrombosis and high-risk plaque: Part I: Evolving concepts. J. Am. Coll. Cardiol. 2005, 46, 937–954. [Google Scholar] [CrossRef]
- Secomb, T.W. Hemodynamics. Compr. Physiol. 2016, 6, 975. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Lieber, B.B.; Stancampiano, A.P.; Wakhloo, A.K. Alteration of hemodynamics in aneurysm models by stenting: Influence of stent porosity. Ann. Biomed. Eng. 1997, 25, 460–469. [Google Scholar] [CrossRef]
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery,* Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar]
- Harvey, W.; Willis, R. The Works of William Harvey; Sydenham Society: London, UK, 1847; Volume 11. [Google Scholar]
- Booth, J. A short history of blood pressure measurement. Proc. R. Soc. Med. 1977, 70, 793–799. [Google Scholar] [CrossRef]
- Frank, O. The basic shape of the arterial pulse. First treatise: Mathematical analysis. J. Mol. Cell. Cardiol. 1990, 22, 255–277. [Google Scholar] [CrossRef]
- Ionita, C.N.; Mokin, M.; Varble, N.; Bednarek, D.R.; Xiang, J.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I.; Meng, H.; Rudin, S. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. In Proceedings of the Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging, San Diego, CA, USA, 16–18 February 2014. [Google Scholar]
- Colacino, F.M.; Moscato, F.; Piedimonte, F.; Danieli, G.; Nicosia, S.; Arabia, M. A Modified Elastance Model to Control Mock Ventricles in Real-Time: Numerical and Experimental Validation. ASAIO J. 2008, 54, 563–573. [Google Scholar] [CrossRef]
- Gregory, S.D.; Greatrex, N.; Timms, D.; Gaddum, N.; Pearcy, M.J.; Fraser, J.F. Simulation and enhancement of a cardiovascular device test rig. J. Simul. 2010, 4, 34–41. [Google Scholar] [CrossRef]
- Meess, K.M.; Izzo, R.L.; Dryjski, M.L.; Curl, R.E.; Harris, L.M.; Springer, M.; Siddiqui, A.H.; Rudin, S.; Ionita, C.N. 3D printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific fenestrated endovascular graft system. In Proceedings of the Medical Imaging 2017: Imaging Informatics for Healthcare, Research, and Applications, Orlando, FL, USA, 15–16 February 2017. [Google Scholar]
- Russ, M.; O’Hara, R.; Nagesh, S.S.; Mokin, M.; Jimenez, C.; Siddiqui, A.; Bednarek, D.; Rudin, S.; Ionita, C. Treatment planning for image-guided neuro-vascular interventions using patient-specific 3D printed phantoms. In Proceedings of the Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging, Orlando, Fl, USA, 24–26 February 2015. [Google Scholar]
- Allman, A.B.; Bhurwani, M.M.S.; Senko, J.L.; Rava, R.A.; Podgorsak, A.R.; Rudin, S.; Ionita, C.N. Use of 3D printed intracranial aneurysm phantoms to test the effect of flow diverters geometry on hemodynamics. In Proceedings of the Medical Imaging 2020: Imaging Informatics for Healthcare, Research, and Applications, Houston, TX, USA, 16–20 February 2020. [Google Scholar]
- Thaker, R.; Araujo-Gutierrez, R.; Marcos-Abdala, H.G.; Agrawal, T.; Fida, N.; Kassi, M. Innovative modeling techniques and 3D printing in patients with left ventricular assist devices: A bridge from bench to clinical practice. J. Clin. Med. 2019, 8, 635. [Google Scholar] [CrossRef]
- Chaudhury, R.A.; Atlasman, V.; Pathangey, G.; Pracht, N.; Adrian, R.J.; Frakes, D.H. A high performance pulsatile pump for aortic flow experiments in 3-dimensional models. Cardiovasc. Eng. Technol. 2016, 7, 148–158. [Google Scholar] [CrossRef]
- Yu, H.; Khan, M.; Wu, H.; Du, X.; Chen, R.; Rollins, D.; Fang, X.; Long, J.; Xu, C.; Sawchuk, A. A new noninvasive and patient-specific hemodynamic index for assessing the severity of renal arterial stenosis. Int. J. Numer. Methods Biomed. Eng 2022, 38, e3611. [Google Scholar] [CrossRef]
- Yu, H.; Khan, M.; Wu, H.; Zhang, C.; Du, X.; Chen, R.; Fang, X.; Long, J.; Sawchuk, A.P. Inlet and outlet boundary conditions and uncertainty quantification in volumetric lattice boltzmann method for image-based computational hemodynamics. Fluids 2022, 7, 30. [Google Scholar] [CrossRef]
- Zhang, X.; Gomez-Paz, J.; Chen, X.; McDonough, J.M.; Islam, M.M.; Andreopoulos, Y.; Zhu, L.; Yu, H. Volumetric lattice Boltzmann method for wall stresses of image-based pulsatile flows. Sci. Rep. 2022, 12, 1697. [Google Scholar] [CrossRef]
- Yu, H. Non-Invasive Functional Assessment Technique for Determining Hemodynamic Severity of an Arterial Stenosis. U.S. Patent 11,538,153, 27 December 2022. [Google Scholar]
- An, S.; Yu, H.; Islam, M.M.; Zhang, X.; Zhan, Y.; Olivieri, J.J.; Ambati, J.; Yao, J.; Gelfand, B.D. Effects of donor-specific microvascular anatomy on hemodynamic perfusion in human choriocapillaris. Sci. Rep. 2023, 13, 22666. [Google Scholar] [CrossRef]
- Shang, B.; Chen, R.; Yan, W.; Yu, H.W. GPU accelerated volumetric lattice Boltzmann model for image-based hemodynamics in portal hypertension. Comput. Fluids 2023, 266, 106038. [Google Scholar] [CrossRef]
- Paccione, E.; Ionita, C.N. Challenges in hemodynamics assessment in complex neurovascular geometries using computational fluid dynamics and benchtop flow simulation in 3D printed patient-specific phantoms. In Proceedings of the Medical Imaging 2021: Biomedical Applications in Molecular, Structural, and Functional Imaging, Meloneras, Spain, 15–19 February 2021. [Google Scholar]
- Shepard, L.M.; Sommer, K.N.; Paccione, E.; Mokin, M.; Siddiqui, A.H.; Snyder, K.V.; Levy, E.I.; Davies, J.M.; Rudin, S.; Ionita, C.N. Use of 3D-printed patient-specific neurovascular phantoms to investigate the correlation between disease severity and quantitative angiography results. In Proceedings of the Medical Imaging 2020: Imaging Informatics for Healthcare, Research, and Applications, Houston, TX, USA, 15–20 February 2020. [Google Scholar]
- Pantalos, G.M.; Koenig, S.C.; Gillars, K.J.; Giridharan, G.A.; Ewert, D.L. Characterization of an adult mock circulation for testing cardiac support devices. ASAIO J. 2004, 50, 37–46. [Google Scholar] [CrossRef]
- Timms, D.; Hayne, M.; McNeil, K.; Galbraith, A. A complete mock circulation loop for the evaluation of left, right, and biventricular assist devices. Artif. Organs 2005, 29, 564–572. [Google Scholar] [CrossRef]
- Timms, D.L.; Gregory, S.D.; Greatrex, N.A.; Pearcy, M.J.; Fraser, J.F.; Steinseifer, U. A compact mock circulation loop for the in vitro testing of cardiovascular devices. Artif. Organs 2011, 35, 384–391. [Google Scholar] [CrossRef]
- Sénage, T.; Février, D.; Michel, M.; Pichot, E.; Duveau, D.; Tsui, S.; Trochu, J.N.; Roussel, J.C. A mock circulatory system to assess the performance of continuous-flow left ventricular assist devices (LVADs): Does axial flow unload better than centrifugal LVAD? ASAIO J. 2014, 60, 140–147. [Google Scholar] [CrossRef]
- Kolyva, C.; Biglino, G.; Pepper, J.R.; Khir, A.W. A mock circulatory system with physiological distribution of terminal resistance and compliance: Application for testing the intra-aortic balloon pump. Artif. Organs 2012, 36, E62–E70. [Google Scholar] [CrossRef]
- Najjari, M.R.; Plesniak, M.W. PID controller design to generate pulsatile flow rate for in vitro experimental studies of physiological flows. Biomed. Eng. Lett. 2017, 7, 339–344. [Google Scholar] [CrossRef]
- Verdonck, P.; Kleven, A.; Verhoeven, R.; Angelsen, B.; Vandenbogaerde, J. Computer-controlled in vitro model of the human left heart. Med. Biol. Eng. Comput. 1992, 30, 656–659. [Google Scholar] [CrossRef]
- Nestler, F.; Bradley, A.P.; Wilson, S.J.; Timms, D.L.; Frazier, O.H.; Cohn, W.E. A hybrid mock circulation loop for a total artificial heart. Artif. Organs 2014, 38, 775–782. [Google Scholar] [CrossRef]
- Westerhof, N.; Elzinga, G.; Sipkema, P. An artificial arterial system for pumping hearts. J. Appl. Physiol. 1971, 31, 776–781. [Google Scholar] [CrossRef]
- Hong, W.; Yu, H.; Chen, J.; Talamantes, J.; Rollins, D.M.; Fang, X.; Long, J.; Xu, C.; Sawchuk, A.P. A mock circulation loop to characterize in vitro hemodynamics in human systemic arteries with stenosis. Fluids 2023, 8, 198. [Google Scholar] [CrossRef]
- Scholz, A.M.; Bünger, L.; Kongsro, J.; Baulain, U.; Mitchell, A.D. Non-invasive methods for the determination of body and carcass composition in livestock: Dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: Invited review. Animal 2015, 9, 1250–1264. [Google Scholar] [CrossRef]
- Grossman, W. Cardiac Catheterization and Angiography, 3rd ed.; OSTI: Oak Ridge, TN, USA, 1986.
- Kennedy, J.W.; Baxley, W.A.; Bunnel, I.L.; Gensini, G.G.; Messer, J.V.; Mudd, J.G.; Noto, T.J.; Paulin, S.; Pichard, A.D.; Sheldon, W.C. Mortality related to cardiac catheterization and angiography. Catheter. Cardiovasc. Diagn. 1982, 8, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Mutrie, C.; Kasirajan, K.; Milner, R.; Chen, E.P.; Veeraswamy, R.K.; Dodson, T.F.; Salam, A.A. Endovascular repair for diverse pathologies of the thoracic aorta: An initial decade of experience. J. Am. Coll. Surg. 2009, 208, 802–816. [Google Scholar] [CrossRef]
- Booher, A.M.; Isselbacher, E.M.; Nienaber, C.A.; Trimarchi, S.; Evangelista, A.; Montgomery, D.G.; Froehlich, J.B.; Ehrlich, M.P.; Oh, J.K.; Januzzi, J.L. The IRAD classification system for characterizing survival after aortic dissection. Am. J. Med. 2013, 126, 730.e19–730.e24. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Cho, L. Peripheral arterial disease: Considerations in risks, diagnosis, and treatment. J. Natl. Med. Assoc. 2009, 101, 999–1008. [Google Scholar] [CrossRef]
- Dey, S.; Mukherjee, D. Clinical perspectives on the role of anti-platelet and statin therapy in patients with vascular diseases. Curr. Vasc. Pharmacol. 2003, 1, 329–333. [Google Scholar] [CrossRef]
- Reicher, B.; Poston, R.S.; Mehra, M.R.; Joshi, A.; Odonkor, P.; Kon, Z.; Reyes, P.A.; Zimrin, D.A. Simultaneous “hybrid” percutaneous coronary intervention and minimally invasive surgical bypass grafting: Feasibility, safety, and clinical outcomes. Am. Heart J. 2008, 155, 661–667. [Google Scholar] [CrossRef]
- Agostoni, P.; Biondi-Zoccai, G.G.; De Benedictis, M.L.; Rigattieri, S.; Turri, M.; Anselmi, M.; Vassanelli, C.; Zardini, P.; Louvard, Y.; Hamon, M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: Systematic overview and meta-analysis of randomized trials. J. Am. Coll. Cardiol. 2004, 44, 349–356. [Google Scholar] [CrossRef]
- Werner, J.; Feuerbach, S.; Uhl, W.; Büchler, M. Management of acute pancreatitis: From surgery to interventional intensive care. Gut 2005, 54, 426–436. [Google Scholar] [CrossRef]
- Bleilevens, C.; Hill, A.; Grzanna, T.; Fechter, T.; Bohnen, M.; Weber, H.-J.; Beckers, C.; Borosch, S.; Zayat, R.; Benstoem, C. In vitro head-to-head comparison of anticoagulation properties of two heparin brands in a human blood miniature mock loop. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Cappon, F.; Wu, T.; Papaioannou, T.; Du, X.; Hsu, P.-L.; Khir, A.W. Mock circulatory loops used for testing cardiac assist devices: A review of computational and experimental models. Int. J. Artif. Organs 2021, 44, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Khudzari, A.; Kadir, M.; Osman, K.; Hudzari, A. Mock circulatory loop for cardiovascular assist device testing. In Cardiovascular Engineering: Technological Advancements, Reviews, and Applications; Spinger: Berlin/Heidelberg, Germany, 2019; pp. 177–200. [Google Scholar]
- Shi, Y.; Yang, H. Mock circulatory test rigs for the in vitro testing of artificial cardiovascular organs. J. Med. Eng. Technol. 2019, 43, 223–234. [Google Scholar] [CrossRef]
- Xu, K.-W.; Gao, Q.; Wan, M.; Zhang, K. Mock circulatory loop applications for testing cardiovascular assist devices and in vitro studies. Front. Physiol. 2023, 14, 1175919. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Noury, N.; Cerny, M. Proposal of Physical Model of Cardiovascular System; Improvement of Mock Circulatory Loop. In World Congress on Medical Physics and Biomedical Engineering 2018, Prague, Czech Republic, 3–8 June 2018; Springer Nature Singapore: Singapore, 2019. [Google Scholar]
- Spurlock, D.J.; Raney, D.N.; Fracz, E.M.; Mazur, D.E.; Bartlet, R.; Haft, J.W. In vitro testing of a novel blood pump designed for temporary extracorporeal support. Asaio J. 2012, 58, 109–114. [Google Scholar] [CrossRef][Green Version]
- Mazur, D.E.; Osterholzer, K.R.; Toomasian, J.M.; Merz, S.I. A novel, low cost, disposable, pediatric pulsatile rotary ventricular pump for cardiac surgery that provides a physiological flow pattern. ASAIO J. 2008, 54, 523–528. [Google Scholar] [CrossRef]
- Timms, D.; Hayne, M.; Tan, A.; Pearcy, M. Evaluation of left ventricular assist device performance and hydraulic force in a complete mock circulation loop. Artif. Organs 2005, 29, 573–580. [Google Scholar] [CrossRef]
- Herreros, J.; Berjano, E.J.; Sales-Nebot, L.; Más, P.; Calvo, I.; Mastrobuoni, S.; Mercé, S. A new method of providing pulsatile flow in a centrifugal pump: Assessment of pulsatility using a mock circulatory system. Artif. Organs 2008, 32, 490–494. [Google Scholar] [CrossRef]
- Timms, D. A review of clinical ventricular assist devices. Med. Eng. Phys. 2011, 33, 1041–1047. [Google Scholar] [CrossRef]
- Gregory, S.D. Simulation and Development of a Mock Circulation Loop with Variable Compliance. Ph.D. Thesis, Queensland University of Technology, Brisbane, Australia, 2009. [Google Scholar]
- Wang, Y.; Smith, P.; Wheeler, J.; John, M.; Broda, C.; Frazier, O.; Taylor, D.; Sampaio, L. A 4-chamber model heart with 3d printed silicone aorta and peripheral arteries to simulate the human cardiovascular system in a mock circulatory loop. J. Heart Lung Transplant. 2019, 38, S342. [Google Scholar] [CrossRef]
- Lee, K.B.; Sun, K.; Kim, H.W.; Chang, J.K.; Park, C.Y.; Chung, C.; Kim, J.K.; Kim, J.; Kim, H.M. Effective reduction of blood cell damage by using a compliance chamber in a pulsatile extracorporeal life support system. ASAIO J. 2000, 46, 233. [Google Scholar] [CrossRef]
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Lankhaar, J.-W.; Westerhof, B.E. The arterial windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef]
- Catanho, M.; Sinha, M.; Vijayan, V. Model of Aortic Blood Flow Using the Windkessel Effect; University of California of San Diago: San Diago, CA, USA, 2012. [Google Scholar]
- Mansouri, M.; Gregory, S.D.; Salamonsen, R.F.; Lovell, N.H.; Stevens, M.C.; Pauls, J.P.; Akmeliawati, R.; Lim, E. Preload-based Starling-like control of rotary blood pumps: An in-vitro evaluation. PLoS ONE 2017, 12, e0172393. [Google Scholar] [CrossRef]
- Taylor, C.E.; Miller, G.E. Mock circulatory loop compliance chamber employing a novel real-time control process. J. Med. Devices 2012, 6, 045003. [Google Scholar] [CrossRef]
- Gregory, S.D.; Pauls, J.P.; Wu, E.L.; Stephens, A.; Steinseifer, U.; Tansley, G.; Fraser, J.F. An advanced mock circulation loop for in vitro cardiovascular device evaluation. Artif. Organs 2020, 44, E238–E250. [Google Scholar] [CrossRef]
- de Jong, M.M.; Parise, O.; Matteucci, F.; Rutten, M.; Devos, M.; Romano, M.; Micali, L.R.; Parise, G.; Maessen, J.G.; Gelsomino, S. Aortic flow below and visceral circulation during aortic counterpulsation: Evaluation of an in vitro model. Perfusion 2020, 37, 69–77. [Google Scholar] [CrossRef]
- Malone, A.; Chari, D.; Cournane, S.; Naydenova, I.; Fagan, A.; Browne, J. Investigation of the assessment of low degree (<50%) renal artery stenosis based on velocity flow profile analysis using Doppler ultrasound: An in-vitro study. Phys. Medica 2019, 65, 209–218. [Google Scholar]
- Santamore, W.P.; Kent, R.L.; Carey, R.A.; Bove, A.A. Synergistic effects of pressure, distal resistance, and vasoconstriction on stenosis. Am. J. Physiol.-Heart Circ. Physiol. 1982, 243, H236–H242. [Google Scholar] [CrossRef]
- Lankhaar, J.-W.; Westerhof, N.; Faes, T.J.; Marques, K.M.; Marcus, J.T.; Postmus, P.E.; Vonk-Noordegraaf, A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2006, 291, H1731–H1737. [Google Scholar] [CrossRef]
- Rezaienia, M.; Paul, G.; Avital, E.; Mozafari, S.; Rothman, M.; Korakianitis, T. In-vitro investigation of the hemodynamic responses of the cerebral, coronary and renal circulations with a rotary blood pump installed in the descending aorta. Med. Eng. Phys. 2017, 40, 2–10. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Chen, D. A mock circulation loop for in vitro haemodynamic evaluation of aorta. J. Phys. Conf. Ser. 2020, 1600, 012066. [Google Scholar] [CrossRef]
- Leopaldi, A.; Vismara, R.; Van Tuijl, S.; Redaelli, A.; van de Vosse, F.; Fiore, G.B.; Rutten, M. A novel passive left heart platform for device testing and research. Med. Eng. Phys. 2015, 37, 361–366. [Google Scholar] [CrossRef]
- Biglino, G.; Verschueren, P.; Zegels, R.; Taylor, A.M.; Schievano, S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. J. Cardiovasc. Magn. Reson. 2013, 15, 2. [Google Scholar] [CrossRef]
- Ochsner, G.; Amacher, R.; Amstutz, A.; Plass, A.; Daners, M.S.; Tevaearai, H.; Vandenberghe, S.; Wilhelm, M.J.; Guzzella, L. A novel interface for hybrid mock circulations to evaluate ventricular assist devices. IEEE Trans. Biomed. Eng. 2012, 60, 507–516. [Google Scholar] [CrossRef]
- Mokin, M.; Waqas, M.; Nagesh, S.V.S.; Karkhanis, N.V.; Levy, E.I.; Ionita, C.N.; Siddiqui, A.H. Assessment of distal access catheter performance during neuroendovascular procedures: Measuring force in three-dimensional patient specific phantoms. J. Neurointerventional Surg. 2019, 11, 619–622. [Google Scholar] [CrossRef]
- Agrafiotis, E.; Mayer, C.; Grabenwöger, M.; Zimpfer, D.; Regitnig, P.; Mächler, H.; Holzapfel, G.A. Global and local stiffening of ex vivo-perfused stented human thoracic aortas: A mock circulation study. Acta Biomater. 2023, 161, 170–183. [Google Scholar] [CrossRef]
- Sherman, J.; Rangwalla, H.; Dohatcu, A.; Minsuok, K.; Ionita, C.; Rudin, S. SU-FF-I-127: Patient Specific Angiography Phantoms for Investigating New Endovascular Image-Guided Interventional (EIGI) Devices. Med. Phys. 2007, 34, 2367. [Google Scholar] [CrossRef]
- Sherman, J.; Rangwala, H.S.; Ionita, C.N.; Dohatcu, A.; Lee, J.; Bednarek, D.R.; Hoffmann, K.R.; Rudin, S. Investigation of new flow modifying endovascular image-guided interventional (EIGI) techniques in patient-specific aneurysm phantoms (PSAPs) using optical imaging. In Proceedings of the Medical Imaging 2008: Visualization, Image-Guided Procedures, and Modeling, San Diego, CA, USA, 16–21 February 2008. [Google Scholar]
- Rangwala, H.S.; Ionita, C.N.; Rudin, S.; Baier, R.E. Partially polyurethane-covered stent for cerebral aneurysm treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 89, 415–429. [Google Scholar] [CrossRef]
- Cloonan, A.J.; Shahmirzadi, D.; Li, R.X.; Doyle, B.J.; Konofagou, E.E.; McGloughlin, T.M. 3D-printed tissue-mimicking phantoms for medical imaging and computational validation applications. 3D Print. Addit. Manuf. 2014, 1, 14–23. [Google Scholar] [CrossRef]
- Jang, J.; Yi, H.-G.; Cho, D.-W. 3D printed tissue models: Present and future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D printing in medicine for preoperative surgical planning: A review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Sommer, K.N.; Bhurwani, M.M.S.; Tutino, V.; Siddiqui, A.; Davies, J.; Snyder, K.; Levy, E.; Mokin, M.; Ionita, C.N. Use of patient specific 3D printed neurovascular phantoms to simulate mechanical thrombectomy. 3D Print. Med. 2021, 7, 32. [Google Scholar] [CrossRef]
- Tan, K.; Van Beek, E.; Brown, P.; Van Delden, O.; Tijssen, J.; Ramsay, L. Magnetic resonance angiography for the diagnosis of renal artery stenosis: A meta-analysis. Clin. Radiol. 2002, 57, 617–624. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.-B.; Andersen, B.K.; Holck, E.N.; Fox Maule, C.; Johansen, J.K.; Andreasen, L.N. Evaluation of coronary artery stenosis by quantitative flow ratio during invasive coronary angiography: The WIFI II study (wire-free functional imaging II). Circ. Cardiovasc. Imaging 2018, 11, e007107. [Google Scholar] [CrossRef]
- Holmstedt, C.A.; Turan, T.N.; Chimowitz, M.I. Atherosclerotic intracranial arterial stenosis: Risk factors, diagnosis, and treatment. Lancet Neurol. 2013, 12, 1106–1114. [Google Scholar] [CrossRef]
- Agrafiotis, E.; Zimpfer, D.; Mächler, H.; Holzapfel, G.A. Review of Systemic Mock Circulation Loops for Evaluation of Implantable Cardiovascular Devices and Biological Tissues. J. Endovasc. Ther. 2024, 15266028241235876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).