Abstract
Biomass fluidized bed gasification technology has attracted significant attention due to its high efficiency and clean energy conversion capabilities. However, its industrial application has been limited by insufficient technological maturity. This paper systematically reviews the research progress on biomass fluidized bed gasification characteristics; compares the applicability of bubbling fluidized beds (BFBs), circulating fluidized beds (CFBs), and dual fluidized beds (DFBs); and highlights the comprehensive advantages of CFBs in large-scale production and tar control. The gas–solid flow characteristics within CFB reactors are highly complex, with factors such as fluidization velocity, gas–solid mixing homogeneity, gas residence time, and particle size distribution directly affecting syngas composition. However, experimental studies have predominantly focused on small-scale setups, failing to characterize the impact of flow dynamics on gasification reactions. Therefore, numerical simulation has become essential for in-depth exploration. Additionally, this study analyzes the influence of different gasification agents (air, oxygen-enriched, oxygen–steam, etc.) on syngas quality. The results demonstrate that oxygen–steam gasification eliminates nitrogen dilution, optimizes reaction kinetics, and significantly enhances syngas quality and hydrogen yield, providing favorable conditions for downstream processes such as green methanol synthesis. Based on the current research landscape, this paper employs numerical simulation to investigate oxygen–steam CFB gasification at a pilot scale (500 kg/h biomass throughput). The results reveal that under conditions of O2/H2O = 0.25 and 800 °C, the syngas H2 volume fraction reaches 43.7%, with a carbon conversion rate exceeding 90%. These findings provide theoretical support for the industrial application of oxygen–steam CFB gasification technology.
1. Introduction
As the global energy structure accelerates its transition to low-carbonization, biomass energy, being the only renewable carbon source that can be directly converted into high-quality liquid and gaseous fuels, is regarded as one of the core pillars to alleviate dependence on fossil energy and achieve carbon neutrality. According to the International Energy Agency (IEA), by 2050, the proportion of biomass energy in global energy consumption will increase to 20%, among which thermochemical gasification technology has become the focus of this field due to its high efficiency conversion potential. Compared with traditional combustion and anaerobic fermentation, biomass fluidized bed gasification converts complex organic matter into hydrogen-rich synthesis gas (H2/CO) through high-temperature partial oxidation, which not only realizes the closed loop of the carbon cycle but also provides low-carbon raw materials for downstream processes such as bio-hydrogen preparation and green methanol synthesis, which meets the dual needs of industrial decarbonization and energy diversification. However, although laboratory studies have confirmed its technical feasibility, the industrialization process of biomass fluidized bed gasification technology is still difficult. It mainly faces three challenges.
First, there is a lack of unified standards for the selection of reactor configuration. Although the bubbling bed (BFB) has a simple structure and low tar production, its low gas velocity operation characteristics limit the processing capacity and make it difficult to meet the needs of large-scale production; the dual-flow bed (DFB) improves fuel flexibility through the dual reaction zone design, but the complexity of the system leads to a surge in investment and operation and maintenance costs. In comparison, the circulating fluidized bed (CFB) has achieved a balance between large-scale production capacity and in situ tar cracking efficiency by virtue of its strong particle circulation at high gas velocity and built-in gas–solid separation function, but the high degree of non-uniformity of gas–solid flow in its furnace has not been fully analyzed, resulting in significant uncertainty in the regulation of syngas components. Secondly, the optimization path of the gasification medium needs to be clarified. The calorific value of the syngas in traditional air gasification is lower than 12 MJ/Nm3 due to the nitrogen dilution effect, while oxygen-enriched and oxygen–water vapor gasification can increase the calorific value to 15–20 MJ/Nm3 by eliminating nitrogen interference and strengthening endothermic reactions (such as tar reforming), while the hydrogen gas volume fraction is increased to more than 40%. However, the cost of oxygen medium and the risk of catalytic deactivation of bed materials (such as olivine) by water vapor at high temperature still need to be solved through process integration and material innovation. Finally, current research lacks a unified theoretical understanding of gas–solid flow mechanisms and their coupling with multi-step reactions in biomass CFB gasification processes.
In view of the above three challenges faced by biomass fluidized beds, this paper reviews the literature around the selection of reactor configuration, gas–solid flow characteristics inside the reactor, and the comparative analysis of the optimization path of gasification medium. On this basis, numerical simulations of coupled gas–solid flow, heat transfer, and multi-step gasification reactions were carried out for an oxygen–water vapor CFB gasifier with a biomass processing capacity of 500 kg/h. By systematically comparing the performance boundaries of different fluidized bed configurations, the core advantages of CFB in large-scale gasification are revealed; by comparing and analyzing the optimization path of gasification medium, the key role of oxygen–water vapor medium in eliminating nitrogen dilution and strengthening water–gas shift reaction is clarified. Through numerical simulation, the regulation mechanism of fluidization velocity, particle residence time, and O2/H2O ratio on syngas composition and carbon conversion rate in the CFB gasification process is characterized. The research results show that under the gasification conditions of O2/H2O = 0.25 and 800 °C, the hydrogen gas volume fraction in the synthesis gas can reach 43.7% and the carbon conversion rate exceeds 90%. This article provides theoretical support for the design of industrial-grade gasifiers.
2. Biomass Gasification Reactor Configurations
The core of biomass gasification technology is the gasification reactor, and its configuration design directly affects the gasification efficiency, synthesis gas quality, and engineering economy. At present, the mainstream gasification furnace types include fixed beds and fluidized beds. The two types of reactors show significant differences in structural characteristics and process adaptability.
2.1. Fixed Bed Gasifier
According to the contact mode between biomass and gasifier, fixed bed gasifiers can be divided into three types: updraft type, downdraft type, and horizontal suction type [1], as shown in Figure 1a–c. The fixed bed has a simple structure and low investment cost and is suitable for small-scale distributed energy supply.
For this reason, domestic and foreign scholars have carried out relevant research. Ismail TM et al. [2] showed that the increase in ER helps to obtain higher gasification efficiency. In addition, the tar content and carbon dioxide content will be significantly reduced. Cerone et al. [3], in a pilot-scale up-suction fixed-bed gasifier, the gasification study was carried out using almond shells as feedstock, and the syngas obtained was dominated by N2 (43.7%), CO (30.8%), and H2 (14.3%), whereas the use of air–steam gasification resulted in an increase in the concentration of H2 to 20% and the concentration of CO2 from 8.4% to 11.6%. The air gasification CGE was 67%, which was elevated to 72.5% with the addition of steam. The tar content was 70 g/Nm3 for air gasification and increased to 78–80 g/Nm3 for steam gasification, and the calorific value was 6.20 MJ/Nm3 for air gasification and increased to 6.43 MJ/Nm3 for steam gasification.
The downdraft fixed bed gasifier is introduced from the top or middle, and the airflow flows downward and is discharged from the bottom. The tar flows through the high-temperature oxidation zone for cracking and has a low content. Singh A. et al. [4] investigated the syngas generation in a laboratory-scale downdraft gasifier of mixed agro-residue pellets, utilizing a mixture of oxygen and steam as the gasifying agent. They found that by using an equivalence ratio of 0.21 and a steam to biomass ratio around 2.15, the concentration of hydrogen in the output syngas was very high (42.2 ± 0.9 vol%), with a lower heating value of 8.3 ± 0.2 MJ/Nm3 and a cold gas efficiency of 78.9%. However, there are limitations in the fuel adaptability of the downdraft fixed bed, and it was found that biomass particles are not completely suitable for downdraft fixed bed gasification. Zhang et al. [5] numerically investigated the biomass oxy-gasification in a downdraft gasifier with an integrated H2-reforming module in the Aspen Plus environment. Their research activity demonstrated that the water vapor addition at the inlet of the gasifier, using a correct ER, operating conditions, and gasification agent temperature, leads to a higher H2 content in the produced syngas, thus improving the efficiency of the downstream reforming section.
In the horizontal suction gasifier, biomass raw materials are added from the top of the gasifier, the gasifier enters the furnace from a certain height of the furnace body, the ash falls into the ash chamber at the bottom of the grate, and the gas flows horizontally. A high-temperature combustion gasification zone is formed around the air inlet of this gasifier, and pyrolysis and drying zones are formed in the upper part of the vessel. The ash is removed at the bottom, and the gas leaving the unit has a temperature of 800–900 °C. Therefore, the furnace is only suitable for fuels containing tar and ash content not exceeding 5%, such as anthracite, coke, and charcoal, which limits its wide application [6].
In summary, the application of fixed bed gasification in the field of industrial alcohol production is restricted in terms of carbon conversion rate, biomass tar content, fuel adaptability, and especially scale expansion. Therefore, in recent years, the research on fixed bed gasifiers has been relatively reduced, and researchers have shifted their focus to fluidized bed gasifiers.
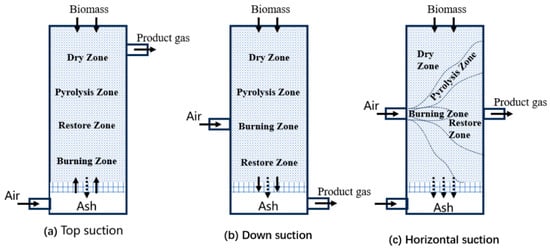
Figure 1.
Fixed bed gasifiers: (a) Top suction; (b) Down suction; (c) Horizontal suction [7].
Figure 1.
Fixed bed gasifiers: (a) Top suction; (b) Down suction; (c) Horizontal suction [7].
2.2. Fluidized Bed Gasifiers
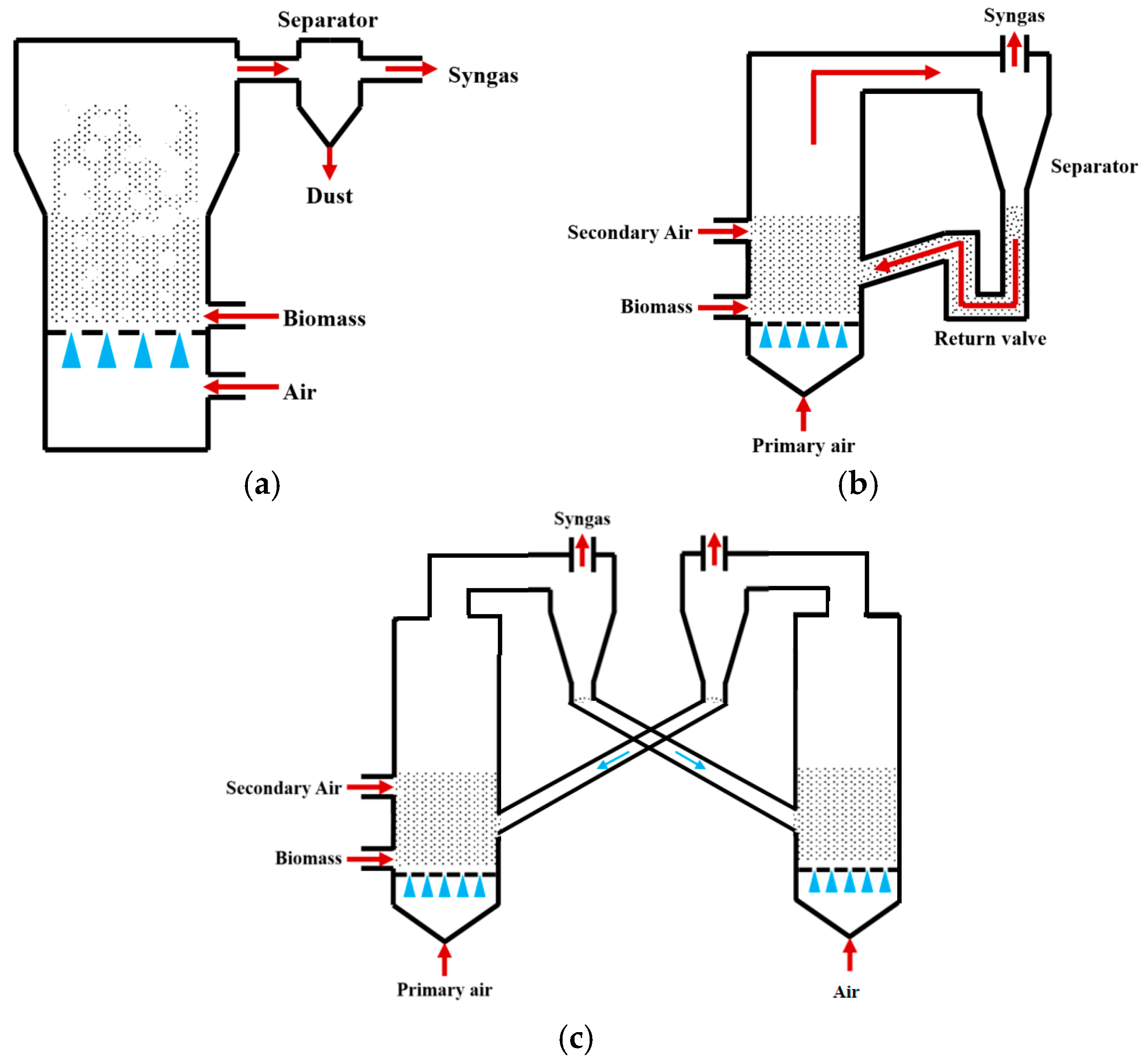
Fluidized bed gasifiers achieve efficient gas–solid heat transfer through fluidization of inert bed materials (e.g., quartz sand), offering advantages such as broad fuel adaptability (capable of processing powdered or granular biomass), uniform operating temperatures (800–1000 °C), and high carbon conversion rates (>85%) [8]. They are primarily classified into three types: bubbling fluidized beds (BFBs), circulating fluidized beds (CFBs), and dual fluidized beds (DFBs), as shown in Figure 2a–c.
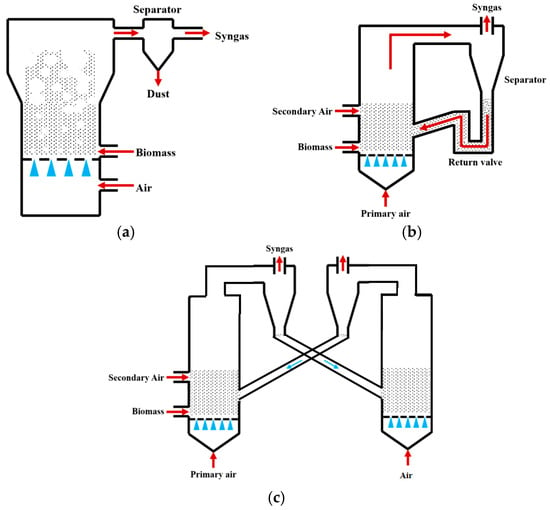
Figure 2.
Fluidized bed gasifiers: (a) BFBgasifiers; (b) CFBgasifiers; (c) DFBgasifiers [7].
2.2.1. Bubbling Fluidized Bed (BFB) Gasifiers
In BFB gasifiers, the gasification agent enters from the bottom air distributor plate, inducing low-velocity fluidization (typically 0.5–3 m/s). Particles undergo bubbling and mixing within the bed layer, enabling intense gas–solid contact and uniform temperatures (800–900 °C). The high bed material concentration makes BFBs suitable for gasifying high-ash biomass. Kulkarni et al. [9] investigated a BFB gasifier using pinewood as feedstock. The results showed that torrefied pinewood at 935 °C and ER = 0.25 produced syngas with H2: 6.91%, CO: 11.78% (H2/CO ≈ 0.59), achieving 79% carbon conversion at 800 °C. The highest heating value (6.29 MJ/Nm3) occurred at 790 °C and gradually decreased with rising temperature. Cold gas efficiency reached 52.12% at 935 °C. Sik Hwang et al. [10] investigated the air gasification process of wood pellets in a BFB gasifier by CFD-DEM modeling, focusing on the effects of ER and fluidization number (FN) on the gasification performance, which was about 17% for CO, 10% for H2, 3% for CH4, and 17% for CO2 under the conditions of ER = 0.27 and FN = 3.2, an LHV of about 5 MJ/m3, and a cold gas efficiency (CGE) of 74.82%. Nguyen et al. [11] experimentally investigated the steam gasification of baked wood chips in a bubbling fluidized bed with 48.41% H2, 23.92% CO, 20.95% CO2, and 4.32% CH4 at SBR = 1.2, and when the ER was increased to 0.22, the H2 was decreased to 40.96% and the CO2 was increased to 24.8%, and the steam gasification carbon conversion efficiency (CCE) was 75.01% at steam gasification. The calorific value (LHV) was up to 10.52 MJ/m3. Thakkar et al. [12] gasified rice husk using catalytic bed materials. At ER = 0.3, baseline syngas (without catalyst) contained H2 (2.7%) and CO (15.2%) with H2/CO ≈ 0.18. With dolomite and olivine additives, H2 increased by 1.08% and 0.95%, CO by 2.1% and 1.8%, H2/CO by 0.04% and 0.03%, and carbon conversion improved by 5% and 4%, respectively. Compared with fixed beds, BFBs exhibit superior mass transfer efficiency, enhanced tar control, broader feedstock adaptability, and scalability potential. However, challenges persist, including short gas residence time leading to low H2/CO ratios and scale-up limitations imposed by fluidization velocity constraints.
2.2.2. Circulating Fluidized Bed (CFB) Gasifiers
CFB employs high-velocity gas flow (typically 3–8 m/s) to achieve complete fluidization of particles and gas. Particles are separated via cyclones and recirculated to the bed, enhancing mass transfer and secondary tar cracking. This extends the gas residence time to 10–15 s, significantly improving tar cracking efficiency (tar content <1 g/Nm3) and enabling dynamic H2/CO ratio adjustment. Gao Zhongming et al. [13] reported syngas compositions from wood chips at ER = 0.20 and 750 °C: H2 = 5.1% and CO = 18.2% (H2/CO = 0.28), with a heating value of 5 800 kJ/m3. For rice husk, corn stalk, and rice straw feedstocks, H2 decreased by 0.6%, 1.2%, and 0.9%, CO by 0.7%, 1.4%, and 1.9%, and H2/CO by 0.02, 0.05, and 0.02, respectively, indicating wood chips as the optimal feedstock for hydrogen-rich syngas with superior heating value. Ramzan et al. [14] developed a CFB coal gasification model using Aspen Plus, showing that elevated gasification agent temperatures (>850 °C) increased CO (35%) and H2 (30%) concentrations while reducing CO2 (15%), N2, and CH4, achieving an LHV of ~10 MJ/Nm3. Liu et al. [15] simulated the gasification of corn stover in a 20 MW circulating fluidized bed (CFB) based on the CPFD method to analyze the effect of steam (S/B) and carbon dioxide (C/B) as gasification media on syngas components and tar. Under air conditions, the content of H2 was 8.53%, CO was 16.43%, CH4 was 5.14%, CO2 was 18.17%, steam increase H2 significantly elevated to 20%, and CO and CH4 decreased; CO2 increased to 22%. There was an increase in CO2, CO elevated to ~18%, and H2 and CH4 decreased slightly. tar mass fraction decreased by ~50% at S/B = 0.6, and CO2 addition (C/B = 0.6) slightly decreased the tar (~10%). Wu et al. [16] studied cattle manure gasification, reporting H2 = 10–14% (peaking at S/B = 0.8), CO = 1.86–4%, CH4 = 2–8%, H2/CO = 3.5–7.5, LHV = 2.0–4.7 MJ/m3, and tar = 0.846 g/m3, with low heating values being attributed to high ash content.
Compared with BFBs, CFBs excel in tar treatment through cyclic particle-catalyzed cracking and prolonged high-temperature exposure, whereas BFBs rely on localized high-temperature zones. CFBs feature particle recirculation via cyclones, intense gas–solid mixing, and expanded contact areas, while BFBs lack particle recycling and are confined to bubble phase contact, resulting in inferior mass transfer efficiency. CFBs operate at higher fluidization velocities, which facilitates reactor scaling. This feature makes them more suitable for large-scale industrial biomass gasification.
2.2.3. Dual Fluidized Bed (DFB) Gasifiers
DFB adopts a dual reactor structure to decouple the gasification and combustion processes. Water vapor or oxygen is introduced into the gasifier for gasification reaction, and the burner provides heat for gasification through the combustion of residual carbon. This design avoids the reflux of synthesis gas and can increase the calorific value to 12–15 MJ/m3. At the same time, the high-temperature combustion zone (>1000 °C) can achieve near-zero tar emissions [17]. Karl et al. [18] studied pine sawdust under the conditions of steam/biomass ratio of 0.6 and gasification temperature of 850 °C. The H2 volume fraction in the synthesis gas reached 45.2%, and the tar content was 0.05 g/Nm3. Nyoman [19] et al. used rice husk as raw material to study the effect of temperature on gasification reaction in DFB. The results showed that at 700 °C, the CO content in the synthesis gas was 15.79%, the H2 content was 4.13%, the CH4 content was 1.10%, and the H2/CO ratio was 0.26. When the gasification temperature increases from 600 °C to 700 °C, the gasification efficiency increases from 40.95% to 43.77%. Wilk et al. [20] studied the CO content of waste wood gasification syngas, which was 22%, and H2 content was 12.3%, LHV was 13.9 MJ/Nm3, and the H2/CO ratio was 0.56. The H2 content of bark was the highest with 18.5%, LHV was 12.5 MJ/Nm3, and the H2/CO ratio was 1.48.
Despite advantages in gasification efficiency and tar reduction, DFBs face challenges including complex system architecture, difficulty in bed material circulation control, and ash contamination from thermal carrier abrasion, which compromise long-term operational stability.
2.3. Comparative Analysis and Conclusions
Based on a comprehensive literature analysis, the key performance parameters of the three reactor configurations are summarized in Table 1. CFB demonstrates superior overall performance in scaled production, tar control, and syngas quality, particularly suited for oxygen–steam gasification processes. Its high gas velocity operation enhances mass transfer while suppressing ash agglomeration, and the introduction of circulating bed materials further promotes steam reforming reactions (H2 yield +20%) [21].

Table 1.
Comparison of key performance parameters for biomass gasifier configurations.
3. Gas–Solid Flow Characteristics of Biomass Fluidized Bed
CFB is an ideal reactor for biomass gasification due to its high gas velocity and strong particle circulation characteristics. However, its complex gas–solid flow characteristics (fluidizing gas velocity, gas–solid mixing uniformity, and particle residence time) significantly affect the composition of syngas and gasification efficiency. Therefore, optimizing the above flow parameters is the key to improving the gasification performance of CFB. It is necessary to achieve gas–solid synergy through multi-scale regulation. Many scholars at home and abroad have conducted research on this.
3.1. Fluidizing Gas Velocity
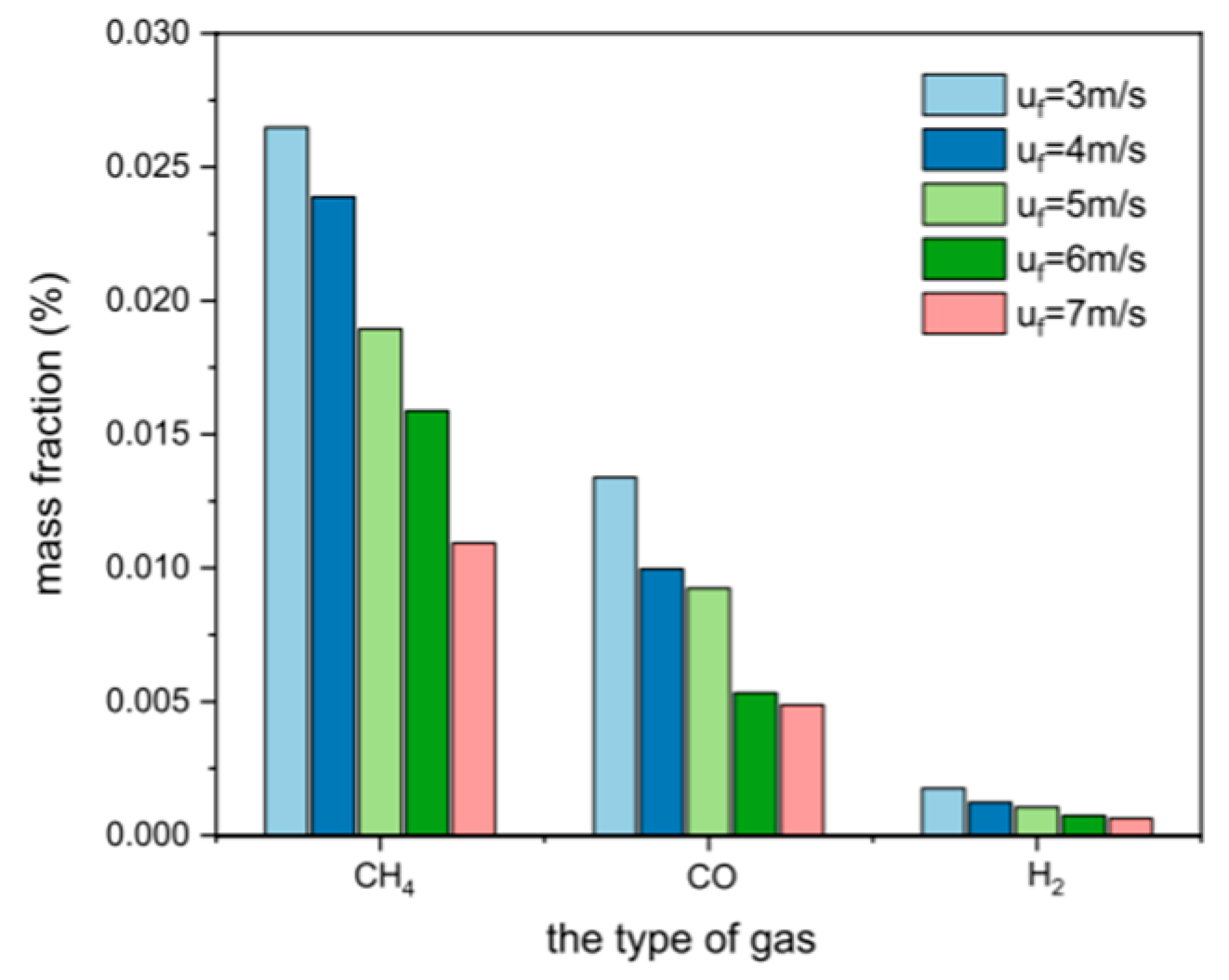
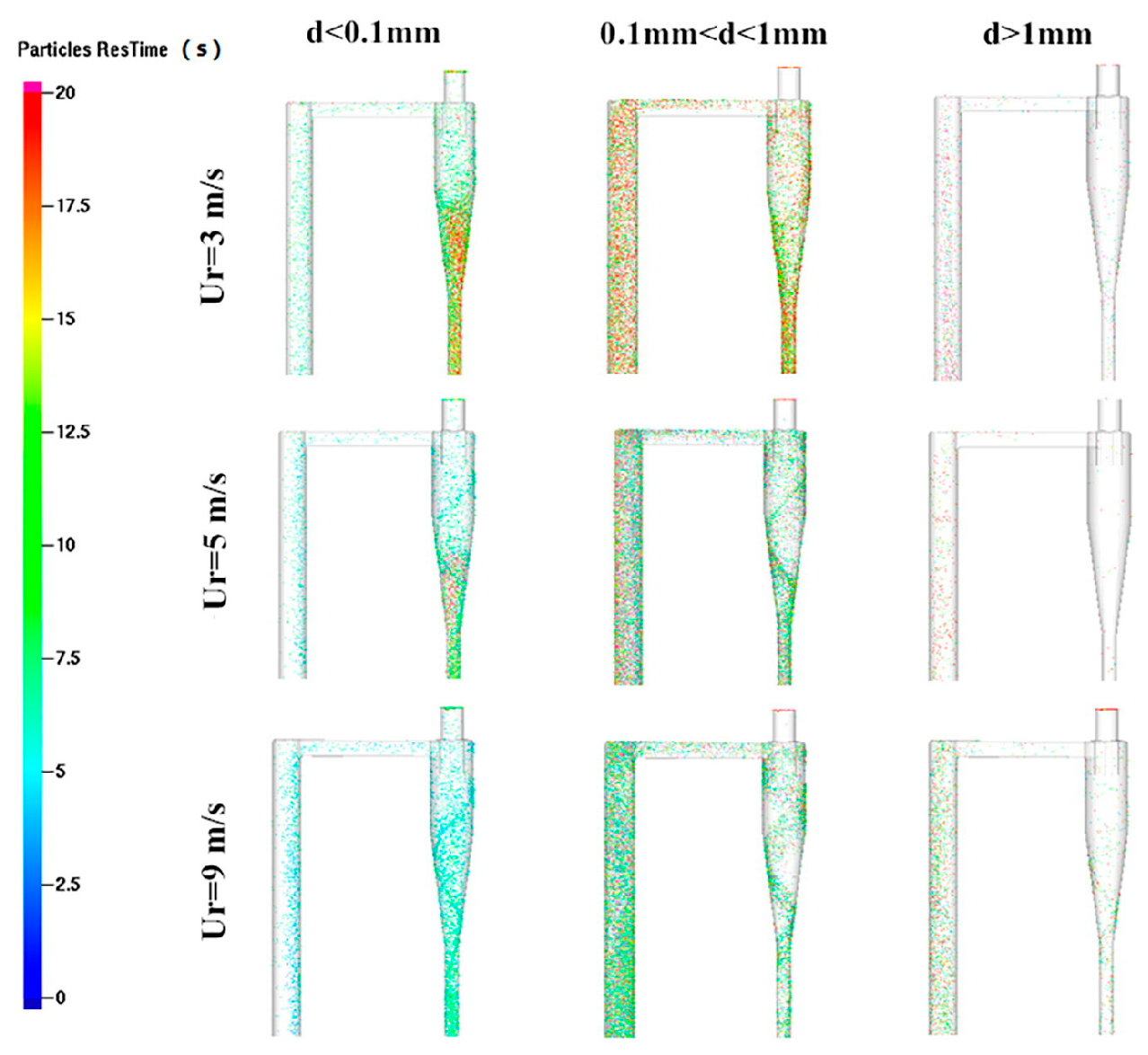
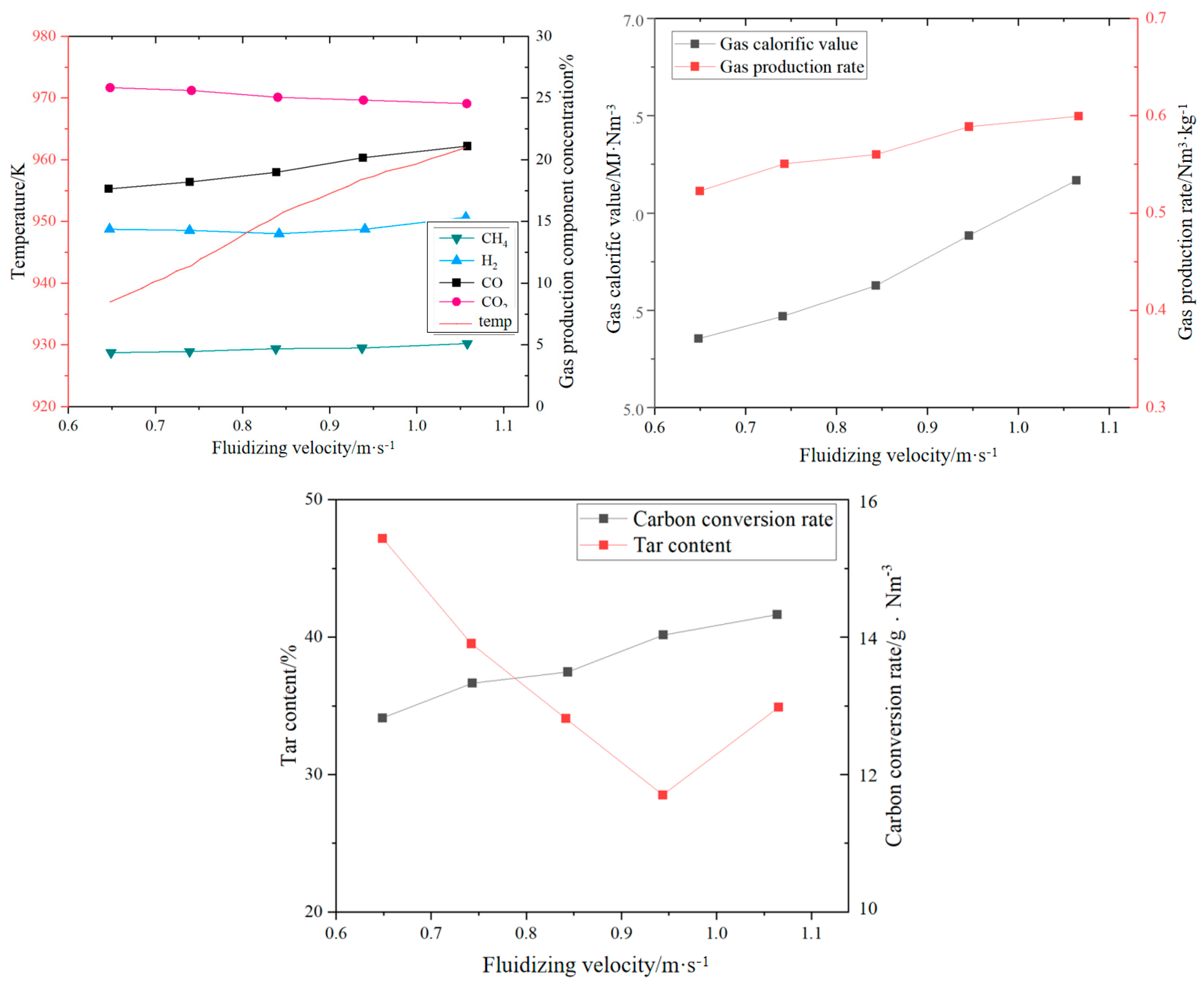
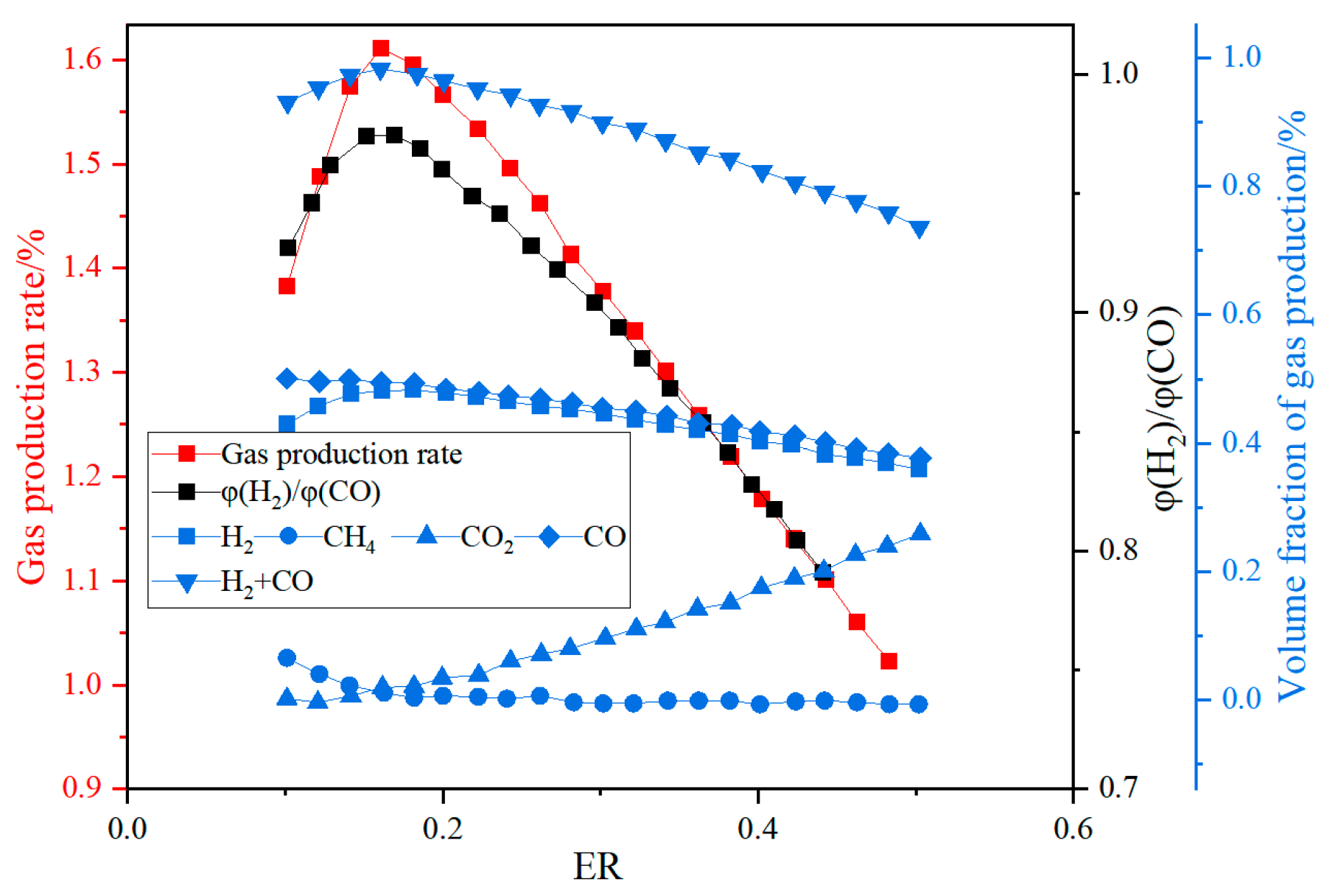
The fluidizing gas velocity directly affects the particle entrainment rate and gas–solid contact strength: too low of a gas velocity can easily lead to bed collapse, prolonged fuel residence time, and increased tar production; too high of a gas velocity will aggravate particle segregation, shorten gas residence time, and reduce the generation efficiency of CO and H2. Wang et al. [22] established a numerical model of CFB air–water vapor mixture using computational particle fluid dynamics. The calculation shows that with the increase in fluidizing gas velocity, the mass fraction of combustible components in the syngas gradually decreases (see Figure 3). Zhao et al. [23] used the CFD-DEM method to simulate the gasification of glucose in a supercritical water fluidized bed reactor (SCWFBR), indicating that high wall temperature, low flow rate, and high initial bed height are conducive to gasification. However, at flow rates below the minimum fluidization velocity, it was found that the H2 yield was low and the gasification efficiency (GE) was high. Liu et al. [24] studied the effect of fluidization velocity on gasification characteristics during CFB gasification. The study showed that small particles (≤0.1 mm) can only be well fluidized when the fluidization velocity is 3 m/s, and large particles (≤1 mm) will stay in the riser for a long time when the fluidization velocity is 3 m/s; particles of 0.1–1 mm will stay in the riser for a relatively long time when the fluidization velocity is 5 m/s. All particles leave the riser quickly at a fluidization velocity of 9 m/s. A good fluidization state can be maintained at a fluidization velocity of 5 m/s (see Figure 4). Lu Jie et al. [25] studied the gasification characteristics of biomass under low equivalence ratio conditions using a BFB device (see Figure 5). When the fluidization velocity increases, the CO concentration increases, the CO2 concentration decreases, the H2 concentration increases slightly, and the gas production rate and carbon conversion rate gradually increase. Although the study shows that the higher the fluidization velocity, the higher the gas production rate and the greater the effective gas ratio, it did not study the change trend after the velocity exceeds 1.1 m/s, which has certain limitations.
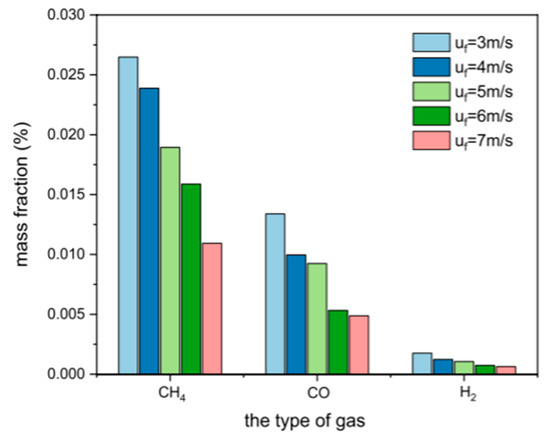
Figure 3.
Effect of fluidization velocity on syngas composition [22].
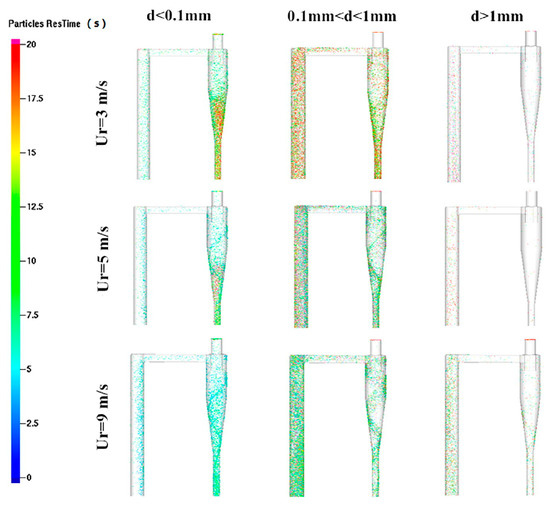
Figure 4.
Residence time of particles of different sizes at different fluidization gas velocities in the area near the top of the CFB [24].
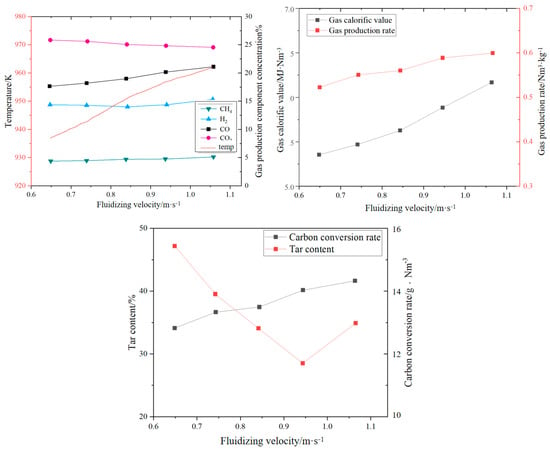
Figure 5.
Impact of fluidization velocity on syngas components [25].
In summary, the selection of fluidizing gas velocity is closely related to the characteristics of biomass particles (such as particle size and density) and should be selected in combination with particle size, initial bed height, and other conditions. Excessive fluidizing gas may dilute the synthesis gas, reduce the concentration of effective components (H2, CO), and increase dust entrainment. If the fluidizing gas velocity is too low, a fixed bed may form in a local area of the bed, resulting in incomplete pyrolysis of biomass. Therefore, the fluidizing gas velocity is reasonably set, and numerical simulation provides an effective means for selecting the optimal working condition.
3.2. Gas–Solid Mixing Uniformity
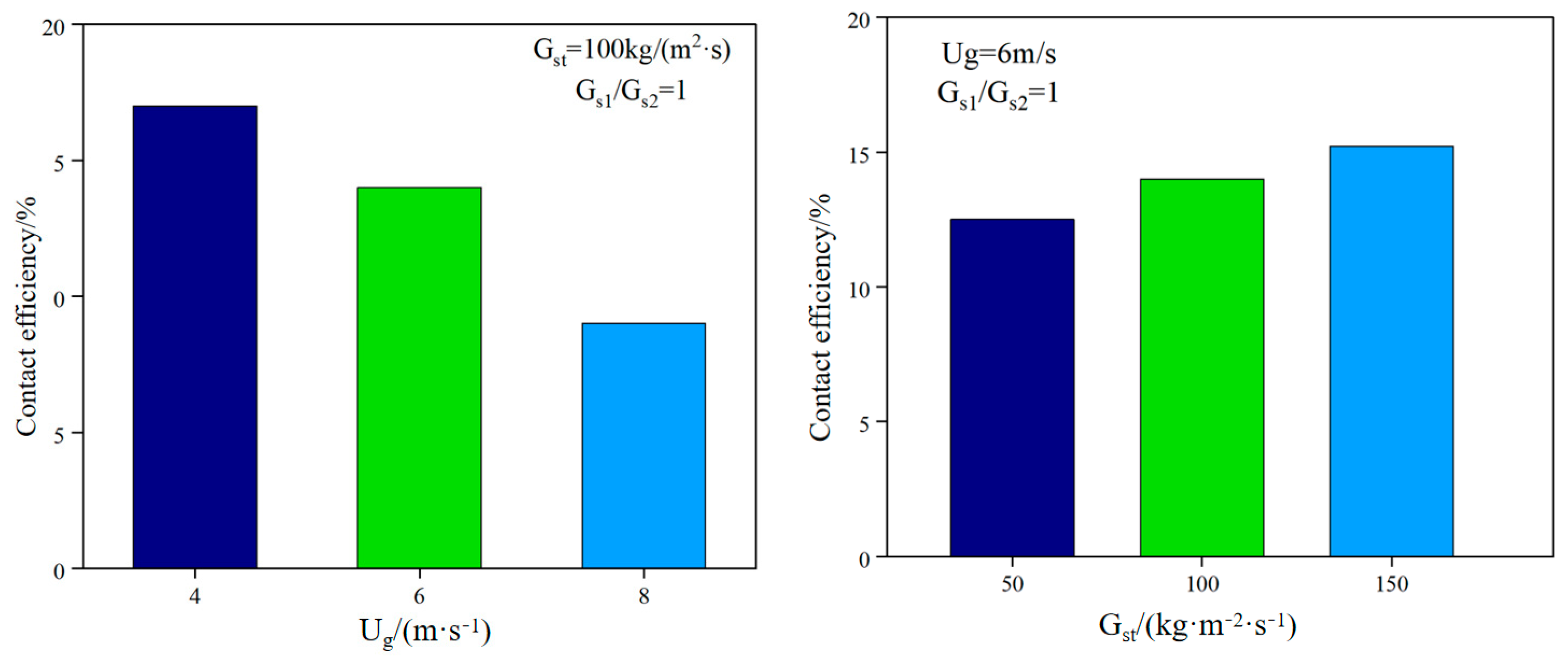
The uniformity of gas–solid mixing determines the renewal rate of the reaction interface. Non-uniform mixing (such as local particle agglomeration or bubble short-circuiting) will lead to an imbalance in the distribution of fuel pyrolysis products. CH4 and tar will be generated in some areas due to lack of oxygen, reducing the proportion of effective components (H2 + CO) in the synthesis gas. To this end, Han Chaoyi et al. [26] studied the gas–solid flow characteristics on a new DFB cold device. The study showed that reducing the superficial gas velocity (from 8 m/s to 4 m/s) can significantly improve the contact efficiency (from 9.0% to 16.5%) because the gas–solid contact time is prolonged by reducing the gas velocity. Increasing the particle circulation rate (from 50 kg/(m2·s) to 150 kg/(m2·s)) can improve the contact efficiency (from 12.3% to 15.8%) because the increase in particle concentration enhances the reaction intensity (see Figure 6). Fang Mingming et al. [27] conducted a systematic study on the dense gas–solid two-phase flow characteristics in ICFB and CFB. Increasing the fluidizing gas velocity can significantly increase the particle circulation flux, but there is a saturation effect; the three-dimensional flow characteristics of CFB were compared with the circular and square riser cross-sections, and it was found that the radial inhomogeneity of the gas–solid flow in the square riser was more significant, and the particles were more violently mixed near the wall. In Wan et al. [28], the advantages of the CFD-DEM model in revealing local mixing characteristics are summarized, the universality of the three-zone division of the fluidized bed is clarified, and the effects of gas velocity, density ratio and size ratio on mixing/separation are quantified. The study shows that under partial mixing conditions, the fluidized bed is divided into a complete separation zone, a transition zone, and a stable mixing zone along the height. Increasing the gas velocity can accelerate mixing and expand the stable mixing zone. Increasing the density ratio and size ratio will aggravate separation and reduce mixing efficiency. The initial particle arrangement affects the mixing dynamic process but does not change the final mixing degree.
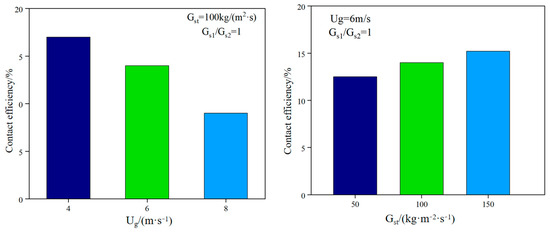
Figure 6.
Effect of operating conditions on gas–solid contact efficiency [27].
From the above analysis, it can be seen that increasing the particle circulation rate can significantly enhance the reaction intensity, and uniform mixing promotes full contact between the gasification medium and the biomass particles, accelerating the release of volatiles and tar cracking. However, excessively uniform mixing may cause the oxygen to be distributed too widely, resulting in the oxidation of CO and H2, reducing the calorific value of the syngas. The mixing index of CFB is usually 40~60% higher than that of BFB, corresponding to a 15%~20% increase in carbon conversion rate.
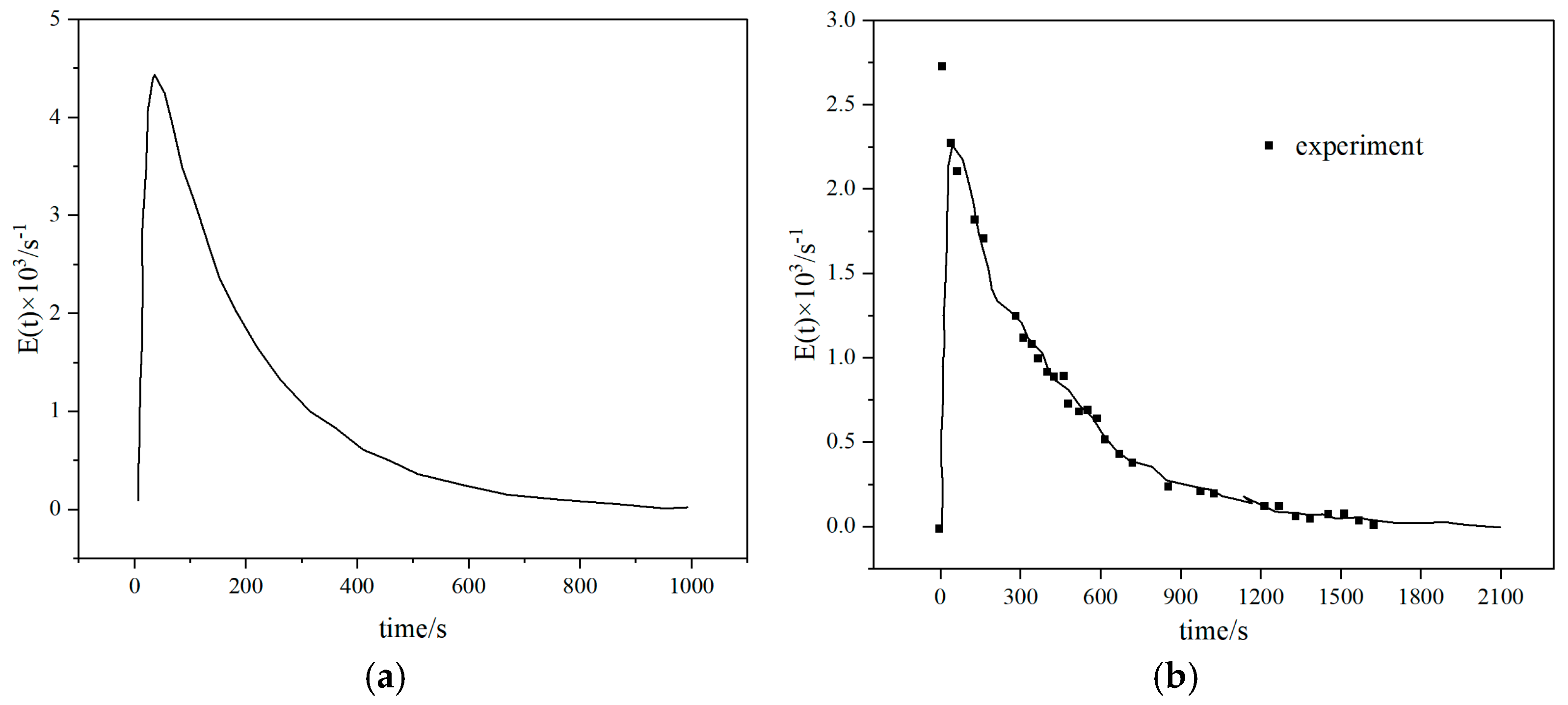
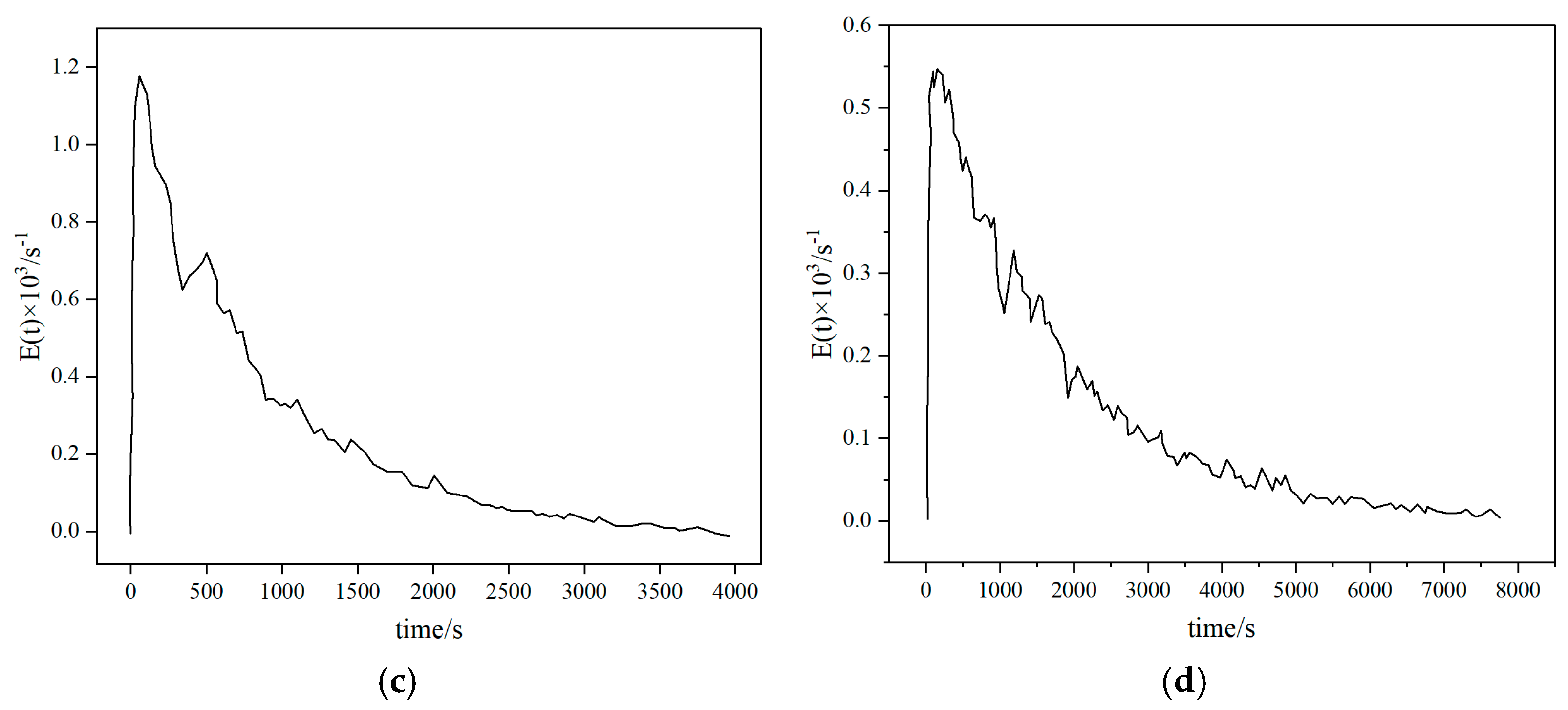
3.3. Particle Residence Time
Particle residence time is closely related to carbon conversion rate and ash residue. Too short of a residence time will prevent biomass coke from fully participating in the gasification reaction, resulting in a decrease in carbon conversion rate, while too long of a residence time may cause ash melting and slagging, destroying fluidization stability. Therefore, optimizing the above flow parameters is the key to improving CFB gasification performance, and multi-scale regulation is required to achieve gas–solid synergy. Yang Hairui et al. [29] first proposed the concept of particle residence time (RTD) for the material balance problem in a circulating fluidized bed (CFB) boiler. The study showed that large particles (such as >200 μm) have a shorter residence time because they escape through the slag outlet first; medium-sized particles (100–200 μm) have the longest residence time because they participate in the circulation; and fine particles (<50 μm) are easily carried by the airflow and escape and have the shortest residence time. An increase in gas velocity will increase the fly ash carrying capacity, resulting in a decrease in the residence time of fine particles, but the residence time of large particles will be extended due to a decrease in the amount of slag discharge. Lan Bin et al. [30] conducted a study on industrial fluidized beds and found that the mean residence time (MRT) of particles is linearly positively correlated with the height of the fluidized bed. (See Figure 7) The MRT of a larger size fluidized bed can be predicted by a linear relationship. The MRT of coarse particles (640 μm) is significantly higher than that of fine particles (251 μm), and the difference increases with the increase in bed height (1.3 times for a 0.07 m bed and 1.4 times for a 0.63 m bed), indicating that bed height can regulate the heterogeneity of particle residence time. Gao Wei et al. [31] conducted a study on the BFB reactor in the DFB and found that as the material flow rate increased, particle mixing weakened and the MRT shortened. As the bed height increased, particle mixing increased and the MRT prolonged. As the particle size increased, the RTD peak increased and the mixing efficiency decreased. However, the experiment was based on a cold state model and did not consider the effects of chemical reactions and thermal effects. Basu et al. [32] showed that the residence time of high-volatile biomass should be short (1–3 s) because it releases volatiles quickly, and too long a residence time can easily lead to secondary reactions of effective gases. Low-volatile biomass needs to be extended to 3–5 s to ensure that fixed carbon is fully gasified. Huang et al. [33] showed that the residence time of a fixed bed is longer (10–30 min), but the tar content is high (>100 mg/Nm3), which requires subsequent purification. The residence time of a fluidized bed is shorter (2–10 s), the tar content is low (<50 mg/Nm3), and it is suitable for continuous production; high H2 synthesis gas requires a medium residence time (3–5 s) to promote the water–gas reaction; high-calorific value fuel gas requires a shorter residence time (1–2 s) to retain CH4.
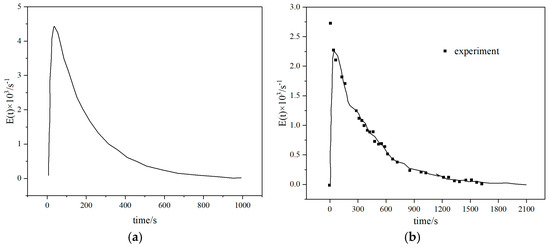
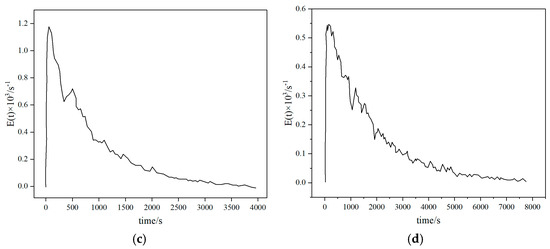
Figure 7.
Residence time distribution of particles in fluidized beds with different scales: (a) Lb = 0.07 m (b) Lb = 0.15 (c) Lb = 0.31 m (d) Lb = 0.63 m [30].
In summary, the particle residence time is a key parameter in the fluidized bed gasification process, which is synergistically affected by particle size, fluidized bed size (such as reactor diameter and height), bed material stacking height, and biomass raw material characteristics (such as density, shape, and volatile content). Therefore, optimizing the residence time requires multi-scale regulation based on raw material characteristics and reactor design parameters.
3.4. Analysis and Conclusions
Based on the above investigation, it can be seen that the fluidization gas velocity of 5 m/s can maintain a good fluidization state. Strictly controlling the particle residence time can ensure the reaction depth and ash control, reduce the unreacted carbon residue, and promote the secondary cracking of tar at high temperature.
4. Biomass Gasification Medium
The gasification medium is a key factor in regulating the thermochemical conversion path of biomass. Its type and ratio directly affect the distribution of gasification products, tar generation characteristics, and system energy efficiency. According to the composition of the medium, gasification technology can be divided into a single medium (oxygen, air, water vapor, etc.) and combined medium, and their reaction mechanism and economic efficiency are significantly different.
4.1. Single Gasification Medium
4.1.1. Air
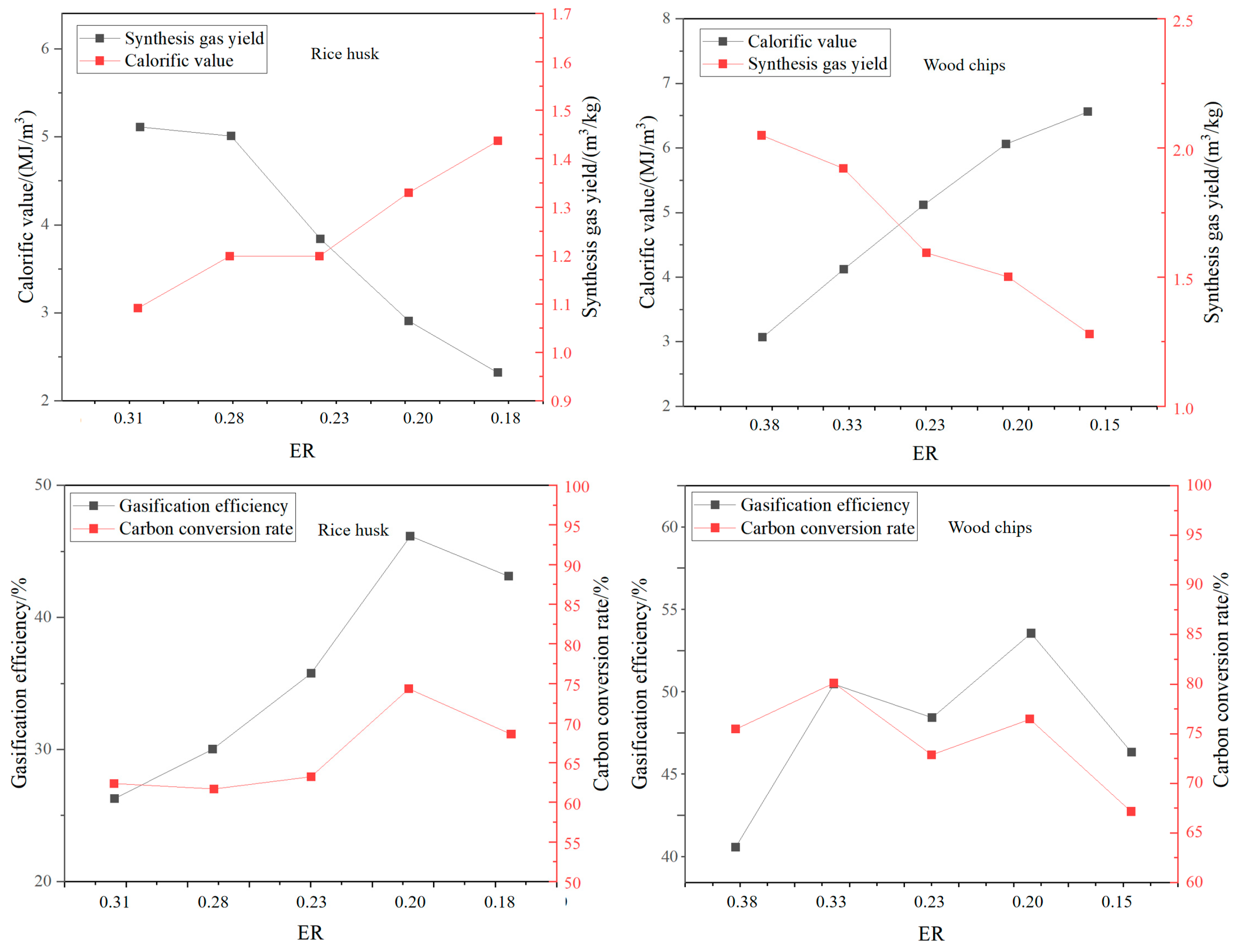
As the cheapest gasification medium, air releases heat through partial oxidation to maintain the gasification reaction, but nitrogen dilution leads to low calorific value of syngas (4–6 MJ/Nm3) and high tar content (10–20 g/Nm3) [21]. Li Jianghao et al. [34] conducted a study on a pilot-scale fluidized bed. The results showed that sawdust and rice husks reached the best gasification conditions when the ER was about 0.2, with calorific values of 5.39 MJ/m3 and 6.04 MJ/m3, respectively, and gasification efficiencies of 46.15% and 51.91%, respectively (see Figure 8). It can be seen that the gasification efficiency of air gasification is relatively low. Air gasification is suitable for small-scale distributed power generation, but it requires a complex purification system.
Figure 8.
Gasification performance indices under different equivalence ratios (ERs) [34].
4.1.2. Oxygen (O2)
Oxygen gasification eliminates nitrogen dilution, thereby increasing the syngas calorific value to 10–12 MJ/Nm3 while reducing tar content to <5 g/Nm3 [35]. Li Bin et al. [36] used Aspen Plus® software to establish a model for biomass oxygen gasification to produce synthesis gas and conducted an in-depth analysis of various influencing factors. The results showed that with the increase in the O2 equivalent ratio, the volume fraction and yield of the synthesis gas first increased and then decreased (see Figure 9). When the oxygen equivalent ratio was 0.16, the volume fraction and yield of the synthesis gas reached the maximum value, with a volume fraction of 97.63% and a yield of 1.61 m3/kg. When S/B = 0.75, H2/CO = 2.03, which is suitable for methanol synthesis.
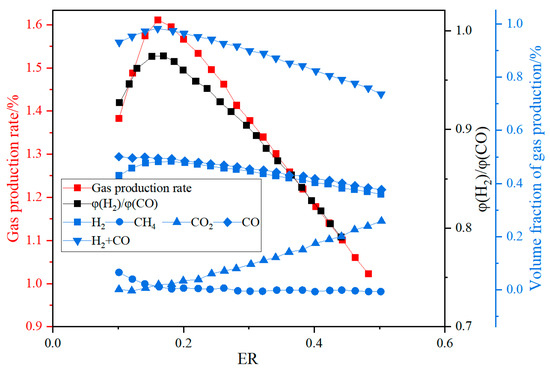
Figure 9.
Variation of gas composition, syngas yield, and p(H2)/p(CO) ratio with oxygen equivalence ratio [36].
4.1.3. Water Vapor (H2O)
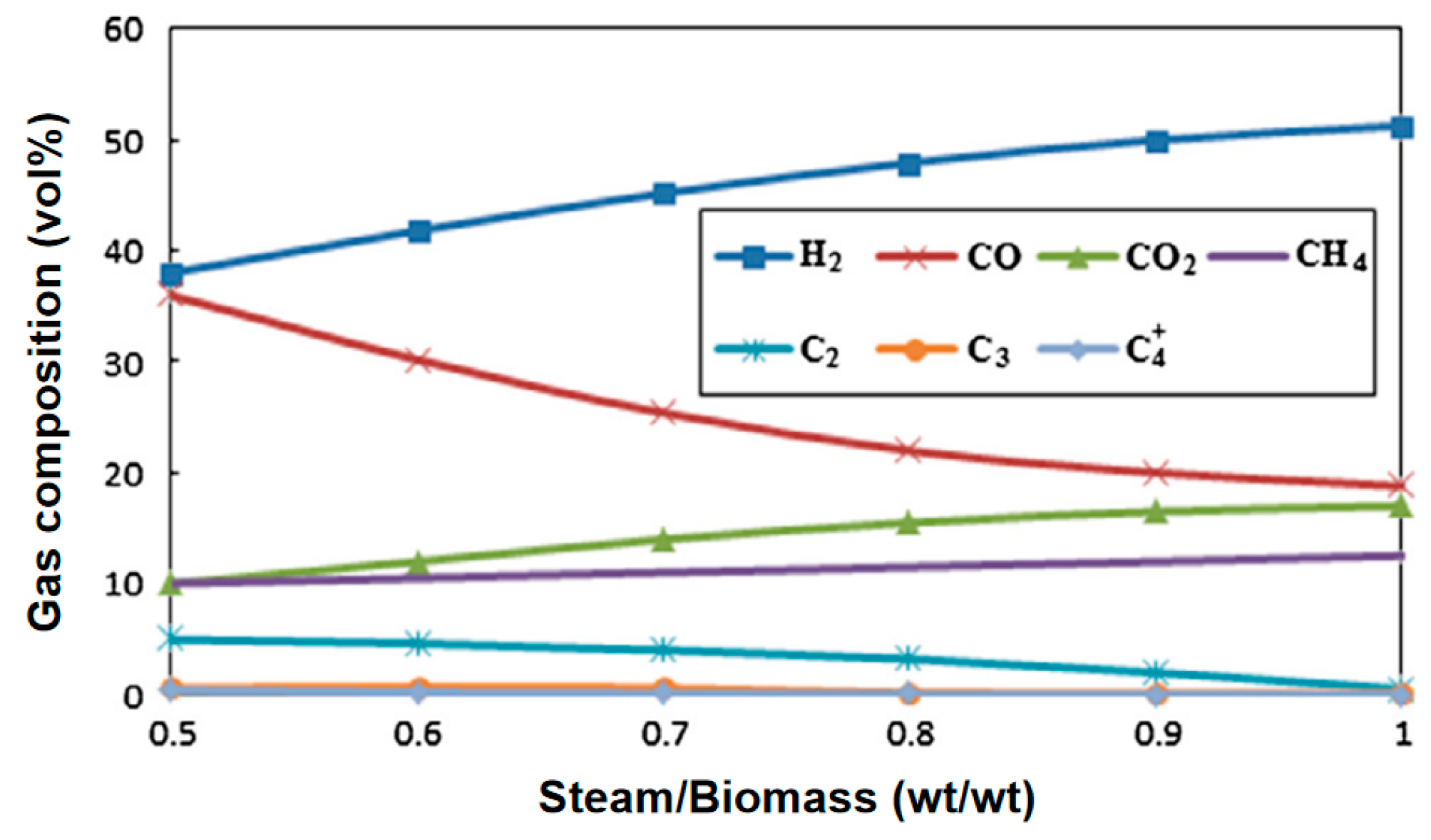
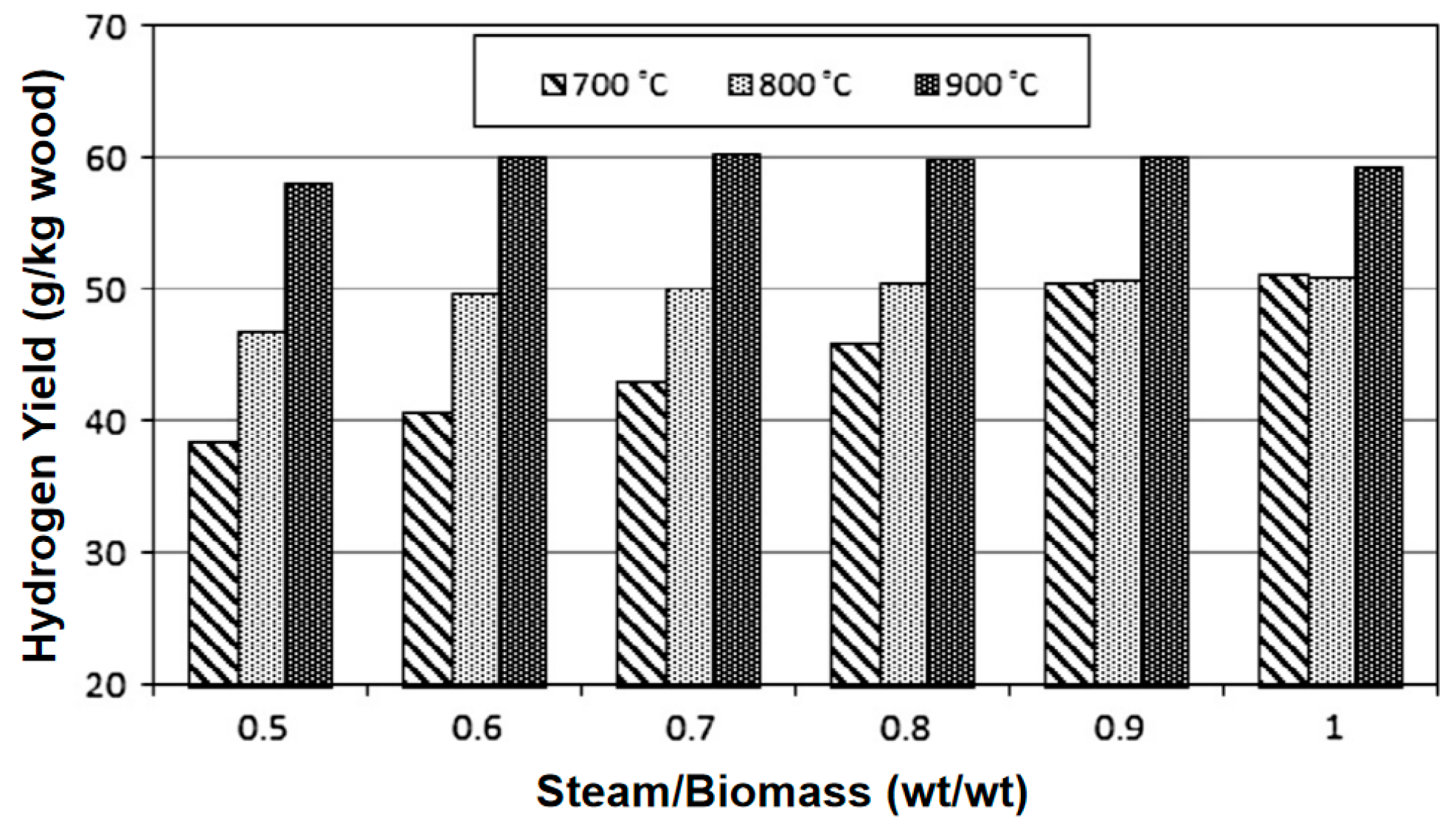
Water vapor gasification significantly increases the H2 yield (volume fraction can reach 40–50%) through an endothermic reforming reaction and inhibits tar formation. Kurkela et al. [37] conducted gasification activities of seven biomasses at 900 °C using a high-temperature steam atmosphere. The contents of H2, CO, CH4, and CO2 in the product gas were 50.77%, 21.39%, 5.51%, and 21.39%, respectively. It can be seen that the H2 content in the synthesis gas under a steam atmosphere can reach more than 50%. Phuhiran C et al. [38] conducted an experimental study on a fixed bed reactor using eucalyptus trunk branches as gasification raw materials and water vapor as a gasification agent. The synthesis gas obtained contained 46.68 mol% H2, 37.71 mol% CO, and an H2/CO ratio of 1.24. The tar content was high in the absence of a catalyst. Fremaux et al. [39] built a pilot-scale fluidized bed reactor. The study showed that as the experimental time increased, the H2 production also increased. As the steam/biomass ratio increased, CO decreased and the H2 concentration increased. The increase in reactor temperature will lead to a significant increase in H2. When S/B is 0.8, the proportion of hydrogen can reach 46.3%, CO accounts for 22.4%, and H2/CO is 2.07, which meets the requirements for alcohol production (see Figure 10 and Figure 11). Loha et al. [40] studied the generation of hydrogen-rich syngas by steam gasification of the rice husk bubbling fluidized bed. The study showed that under the conditions of temperature 770 °C and S/B = 1.0, the volume fraction of H2 in the syngas is 57.98%, CO is 23.79%, H2/CO is 2.44, and LHV is 10.8 MJ/Nm3.
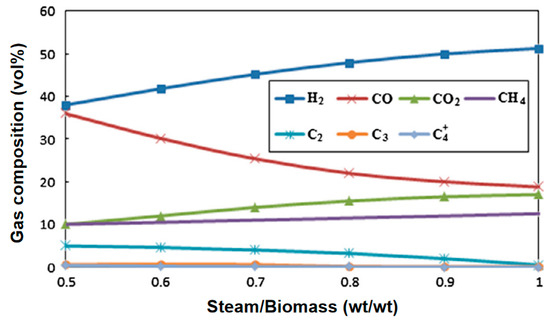
Figure 10.
Effect of steam-to-biomass ratio on gas composition [39].
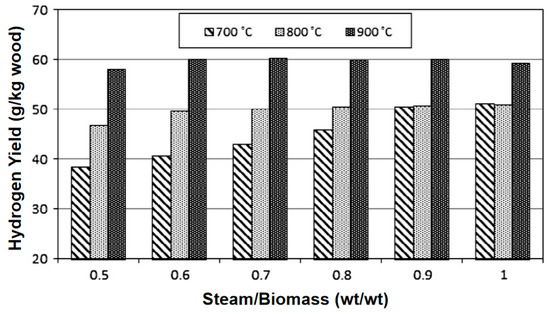
Figure 11.
Effect of temperature on hydrogen yield [39].
Compared with traditional air gasification, the H2 content in the syngas is significantly increased and the calorific value of the syngas is increased, but steam injection requires additional heat input (usually relying on external combustion or electric heating), resulting in reduced system energy efficiency.
4.1.4. Carbon Dioxide (CO2)
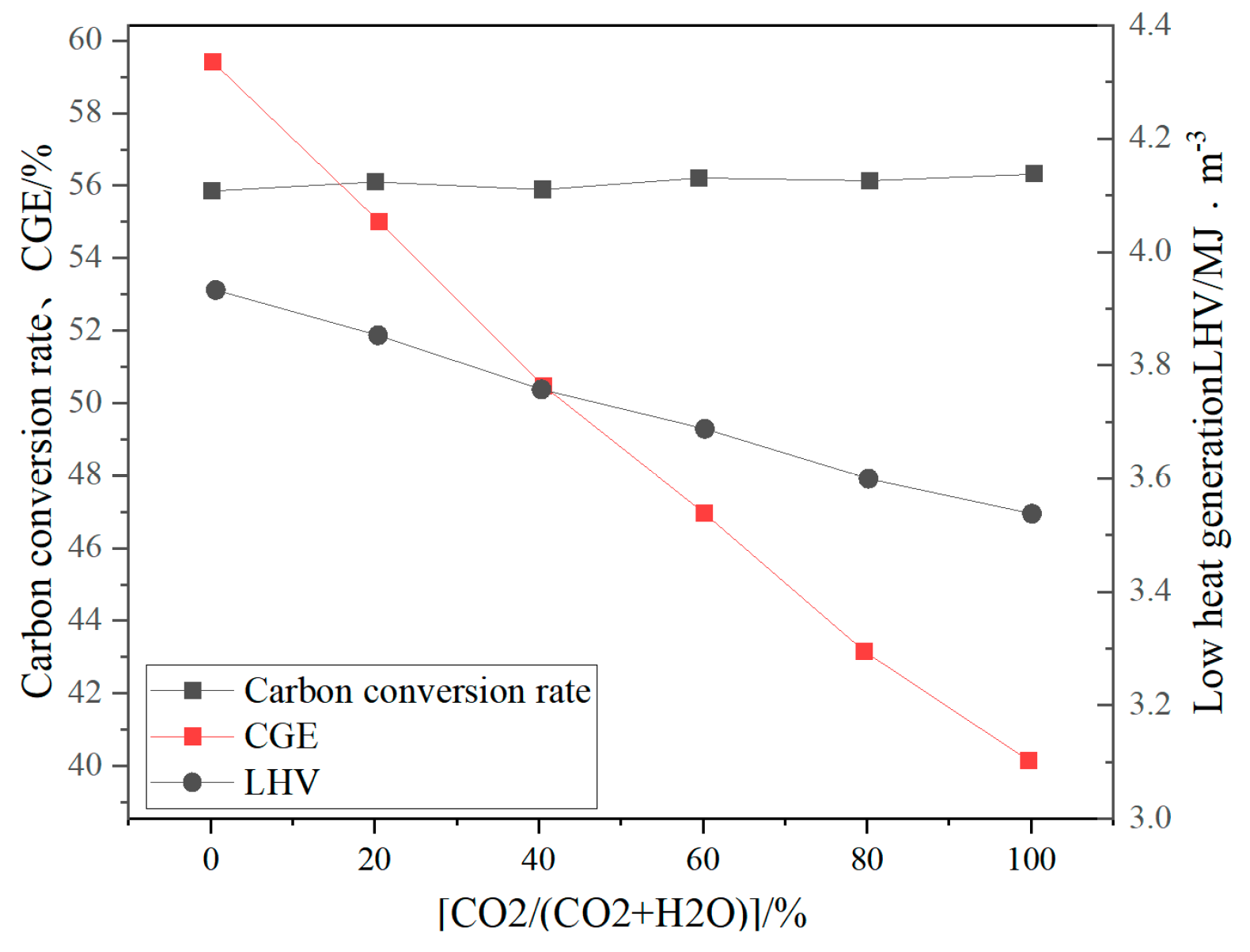
CO2 as a gasification medium can promote the Boudouard reaction (C + CO2 → 2CO) and increase the proportion of CO in the synthesis gas (volume fraction up to 35–45%) and is suitable for synthesizing methanol or Fischer–Tropsch fuels [41]. Pandey B et al. [42] experimentally analyzed the effects of different concentrations of CO2 and O2 in the gasifier on the gasification performance of the gasifier. The results showed that when 30 vol% CO2 was introduced, CO accounted for 42.37%, H2 accounted for 16.79%, CO2 accounted for 37.82%, and CH4 accounted for 3%. The LHV was 4.39 MJ/Nm3, the CO2 conversion rate was 62–69%, and the H2/CO ratio was 0.4, with a relatively low proportion of H2. Zhu et al. [43] studied the gasification process of biomass using CO2 as a gasifier in a 0.3 MWth CFB gasifier. Studies have shown that CO accounts for about 27% of the syngas by volume, H2 accounts for about 19% by volume, the H2/CO ratio is 1.42, the calorific value is 3.87 MJ/Nm3, and the cold gas efficiency (CGE) is 73%. Ren Xixi et al. [44] established a three-dimensional model of a BFB gasifier based on the CPFD method, using CO2 to replace part or all of the water vapor as a gasifier. Studies have shown that the increase in CO2 content reduces the gasification efficiency and the low calorific value of the gasified syngas, while slightly increasing the carbon conversion rate (see Figure 12). When the gasifier contains 60% CO2, CO accounts for 13.21% of the syngas, H2 accounts for 11.83%, CH4 accounts for 2.80%, and CO2 accounts for 17.77%. The H2/CO ratio is 0.64, and the lower calorific value is only 3.94 MJ/m3. It can be found that regardless of whether it is a fixed bed, BFB, or CFB, the proportion of H2 in the synthesis gas gasified by CO2 is small, and the overall calorific value of the synthesis gas is low, which is not suitable for the production of hydrogen-rich synthesis gas.
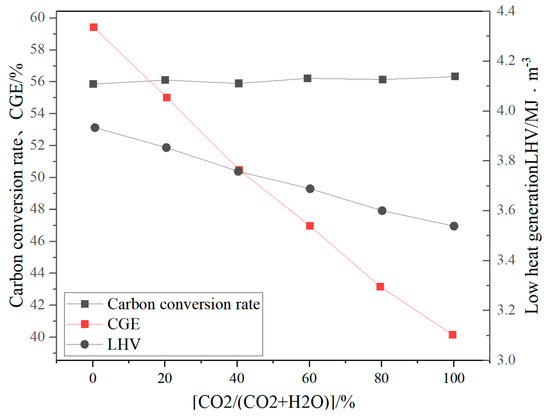
Figure 12.
Effects of different CO2 replacement ratios on LHV, CGE, and carbon conversion rate [44].
4.2. Combined Gasification
4.2.1. Air/H2O
Air/H2O gasification dilutes the oxygen concentration in the oxidation zone through steam, inhibits combustion heat release, alleviates the ash slagging problem, and increases the H2 yield. Sharma et al. [45] used air/H2O as a gasifier and simulated the fluidized bed biomass gasification process by numerical simulation. The syngas contained 12–18% H2, 14–20% CO, 10–15% CO2, and 1–3% CH4. The H2/CO ratio was as high as 1.06, and the calorific value was 4.01 MJ/Nm3. The air/H2O gasification method combines the advantages of air gasification and steam gasification, improves the H2 yield, and solves the problem of external heat source of steam gasification. However, the calorific value of the syngas is still limited by nitrogen (6–8 MJ/Nm3), and the tar content is relatively high (3–5 g/Nm3), which is only suitable for gasification of low-quality fuels.
4.2.2. Oxygen-Enriched Water Vapor (Air-O2/H2O)
The combination of O2 and H2O can synergistically optimize the gasification efficiency: O2 provides reaction heat and H2O promotes the water–gas shift reaction and realizes the dynamic regulation of the H2/CO ratio. Oxygen-enriched water vapor gasification is actually evolved on the basis of air–water vapor gasification, which increases the oxygen content in the air and reduces the effect of N2 dilution. At the same time, high temperature is conducive to the cracking of tar, accelerates the reaction rate, and thus increases the carbon conversion rate. Therefore, many scholars have conducted research on this. Erkiaga et al. [46] used O2/H2O mixed gasification of pine sawdust in a circulating fluidized bed. The H2 yield was increased by 28% compared with oxygen gasification, and the tar content was reduced to 1.2 g/Nm3. Ye Renwen et al. [47] used a self-heating BFB to conduct oxygen-enriched water vapor directional gasification experiments on sawdust. The results show that under the optimal working conditions, H2 accounts for 21.40%, CO accounts for 19.00%, CO2 accounts for 32.50%, CH4 accounts for 4.40%, and H2/CO accounts for 1.13. Frigo et al. [48] experimentally explored the performance of wood chip particles in oxygen–steam gasification and the efficiency of the subsequent syngas-to-hydrogen system using a downdraft gasifier, with a syngas fraction of 40–45% H2, 25–32% CO2, 20–28% CO, 4.5–5% CH4, and a tar content of only 0.1–0.15 g/Nm3; an LHV of 9.0–10.2 MJ/Nm3, significantly higher than the traditional air gasification (5.3–6.0 MJ/Nm3); and a CGE of 78–83%, indicating excellent gasification effect.
Although oxygen-enriched steam gasification increases the H2 content in the syngas to a certain extent, it still has the problem of reduced calorific value due to the dilution of the syngas by N2 in the oxygen-enriched air.
4.2.3. Oxygen–Water Vapor (O2/H2O)
In order to eliminate the influence of N2 on the gasification process, oxygen–water vapor gasification is used on the basis of oxygen-rich water vapor gasification. Oxygen–water vapor gasification combines the characteristics of oxygen gasification and water vapor gasification, realizing self-heating and reducing oxygen consumption. It can also produce fuel gas rich in H2 and CO, further reducing the tar content and improving the calorific value of the synthesis gas. Xu Wei et al. [49] used bamboo chips as raw materials and oxygen–water vapor as mixed gasification agents to conduct gasification experiments in a fixed bed gasification reactor. The results showed that the H2 volume fraction in the prepared fuel gas was 32.04%, CO was 26.34%, the gas production rate was 1.40 L/g, the H2/CO ratio was 1.22 at the optimal equivalence ratio of 0.3, the calorific value was 11.37 MJ/m3, and the synthesis gas components under different oxygen dosage ratios were as seen in Table 2. Frigo et al. [50] experimentally explored the performance of wood chip particles in oxygen–steam gasification and the efficiency of the subsequent syngas-to-hydrogen system using a downdraft gasifier, with a syngas fraction of 40–45% H2, 25–32% CO2, 20–28% CO, 4.5–5% CH4, and a tar content of only 0.1–0.15 g/Nm3; an LHV of 9.0–10.2 MJ/Nm3, significantly higher than the traditional air gasification (5.3–6.0 MJ/Nm3); and a CGE of 78–83%, indicating excellent gasification effect. Ruoppolo et al. [51] experimentally studied the gasification of wood and olive shell/coal particles using oxygen and steam as gasification media in a pilot-scale BFB reactor. The study showed that compared with the air–steam gasification device, the carbon conversion rate in the gasification process was the highest, and the N2 content in the oxygen-enriched gasification process was limited. At the same time, the tar content and CO2 level were also higher.

Table 2.
Effect of oxygen dosage ratio on oxygen–steam gasification of bamboo chips [49].
4.3. Performance Comparison and Optimization Direction
The comparative study in Table 3 reveals that oxygen–Steam gasification significantly increases the H2 content and calorific value in the syngas, effectively suppresses tar and pollutants, optimizes carbon conversion efficiency and energy efficiency, and has industrial advantages in bio-hydrogen and chemical production (such as synthetic ammonia, methanol); carbon-neutral energy systems; and difficult-to-treat waste resource utilization (municipal sludge, agricultural residues).

Table 3.
Effect of gasification medium on syngas characteristics and system energy efficiency.
5. Conclusions
This paper systematically reviews the research progress of biomass fluidized bed gasification technology, studies and analyzes the scale advantages and gas–solid flow control difficulties of CFB, conducts pilot-scale numerical simulations for oxygen–steam gasification media, and explores the influence of operating parameters (O2/H2O ratio, temperature) on syngas components and carbon conversion rate, providing theoretical support for downstream processes such as bio-hydrogen preparation and green alcohol synthesis. Oxygen–Steam combined gasification performs best in syngas quality (H2/CO ratio > 1.5), tar control (<1 g/Nm3), and carbon conversion rate (>90%) and is particularly suitable for high temperature and high gas velocity conditions of circulating fluidized beds.
6. Numerical Simulation Analysis of Pilot-Scale Biomass Fluidized Bed Gasification
Based on the above discussion, this paper uses the MP-PIC method to carry out pilot-scale (500 kg/h) material fluidized bed gasification numerical simulation.
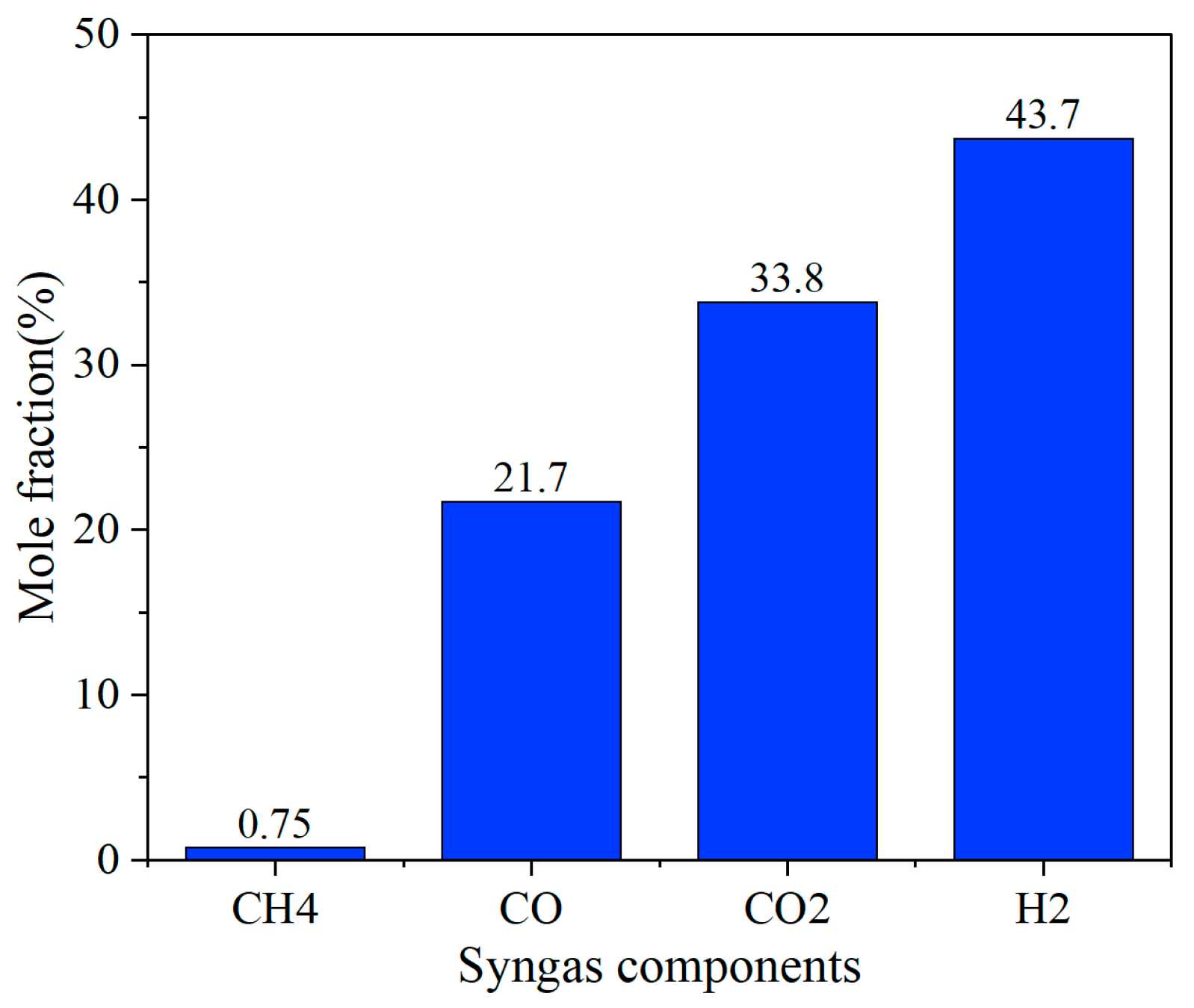
Under the conditions of a furnace temperature of 800 °C, fluidizing gas velocity of 4.5 m/s, equivalence ratio of 0.22, and O2/H2O = 0.25, the composition of the syngas was obtained, as shown in Figure 13.
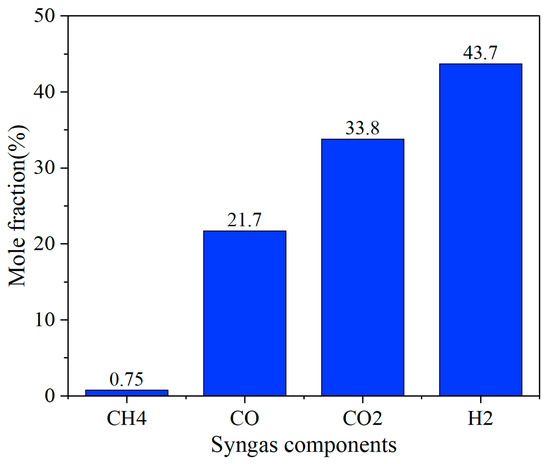
Figure 13.
Syngas mole fraction.
The numerical simulation results show that the syngas components show significant hydrogen-rich characteristics, among which H2 has the highest volume fraction, reaching 43.7%, followed by CO and CO2, which are 21.7% and 33.8%, respectively, while CH4 accounts for only 0.75%, H2/CO = 1.29, which is much closer to that required to make alcohol. This component distribution shows that under the optimized working conditions, the steam gasification reaction (C + H2O → H2 + CO) and the water–gas shift reaction (CO + H2O → CO2 + H2) are fully enhanced, resulting in a significant increase in the proportion of H2. At the same time, the effective conduct of the CH4 reforming reaction (CH4 + H2O → CO + 3H2) further inhibits the formation of methane. The high proportion of CO2 may be related to the high oxygen content of the biomass raw material and the excessive supply of water vapor in the gasification medium. The directional conversion of carbon elements can be achieved by adjusting the O2/H2O ratio in the future.
In summary, the CPFD numerical simulation based on the pilot scale (500 kg/h) verified the gain effect of high temperature (800 °C) and O2/H2O ratio (0.25) on the quality of syngas, providing a theoretical basis for industrial reactor design and process parameter optimization. Future work will further explore and optimize the gasification process under various reaction conditions (temperature, fluidizing gas velocity, equivalence ratio, oxygen–water vapor ratio, etc.), and promote the leapfrog development of biomass gasification technology from laboratory verification to industrial implementation.
Author Contributions
Writing—original draft preparation, methodology, validation, data curation: L.W.; methodology, formal analysis: T.Z.; methodology: B.H.; investigation, formal analysis, writing—review and editing: H.Y. formal analysis, writing—review and editing: M.Z.; investigation: N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Province Science and Technology Development Plan Project, grant number 20240304088SF, and supported by the Huaneng Group Science and Technology Research Project (HNKJ23-H71).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
Author Bo Hou was employed by the company Guoneng Longyuan Environmental Protection Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BFBs | Bubbling fluidized beds |
| CFBs | Circulating fluidized beds |
| DFBs | Dual fluidized beds |
| LHV | Lower heating value |
| ER | Equivalence ratio |
| MP-PIC | Multiphase particle-in-cell |
| CPFD | Computational Particle Fluid Dynamics |
References
- Niu, Y. Simulation Study on Flow and Heat-Mass Transfer Characteristics of Downdraft Biomass Gasification. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2023. Volume 105. [Google Scholar]
- Ismail, T.M.; El-Salam, M.A. Parametric studies on biomass gasification process on updraft gasifier high temperature air gasification. Appl. Therm. Eng. 2017, 112, 1460–1473. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Baleta, J.; Cerinski, D.; Skvorčinskienė, R. Experimental investigation of syngas composition variation along updraft fixed bed gasifier. Energy Convers. Manag. 2020, 221, 113116. [Google Scholar] [CrossRef]
- Singh, A.; Shivapuji, A.M.; Dasappa, S. Hydrogen production through agro-residue gasification and adsorptive separation. Appl. Therm. Eng. 2023, 234. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, A.; Li, Z.; Zhang, H.; Xiong, Y.; Xiao, R.; Hu, Z.; Wang, X. Numerical simulation analysis of biomass gasification and rich-H2 production process in a downdraft gasifier. J. Energy Inst. 2024, 114, 101596. [Google Scholar] [CrossRef]
- Min, P.; Dapeng, B.; Ruyi, S.; Liu, S. Research status and future challenges of biomass gasifiers in China. Energy Chem. Ind. 2020, 41, 1–6. [Google Scholar]
- Liu, N.; Liu, Z.; Wang, Y.; Zhou, T.; Zhang, M.; Yang, H. Clean and Efficient Thermochemical Conversion Technologies for Biomass in Green Methanol Production. Biomass 2025, 5, 13. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Y. Development of wet purification system for straw pyrolysis gasification. Trans. CSAM 2000, 31, 48–49. [Google Scholar]
- Kulkarni, A.; Baker, R.; Abdoulmomine, N.; Adhikari, S.; Bhavnani, S. Experimental study of torrefied pine as a gasification fuel using a bubbling fluidized bed gasifier. Renew. Energy 2016, 93, 460–468. [Google Scholar] [CrossRef]
- Hwang, I.S.; Sohn, J.; Lee, U.D.; Hwang, J. CFD-DEM simulation of air-blown gasification of biomass in a bubbling fluidized bed gasifier: Effects of equivalence ratio and fluidization number. Energy 2021, 219, 119533. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; May, J.; Peters, J.; Epple, B. Experimental study on steam gasification of torrefied woodchips in a bubbling fluidized bed reactor. Energy 2020, 202, 117744. [Google Scholar] [CrossRef]
- Thakkar, M.; Makwana, J.; Mohanty, P.; Shah, M.; Singh, V. In bed catalytic tar reduction in the autothermal fluidized bed gasification of rice husk: Extraction of silica, energy and cost analysis. Ind. Crop. Prod. 2016, 87, 324–332. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, D.; Chen, Y.; Liu, S.; Wang, Q. Experimental study on air gasification characteristics of agricultural and forestry wastes in circulating fluidized bed. Power Gener. Technol. 2024, 45, 535–544. [Google Scholar]
- Ramzan, N.; Athar, M.; Begum, S.; Ahmad, S.W.; Naveed, S. Simulation of circulating fluidized bed gasification for characteristic study of pakistani coal. Pol. J. Chem. Technol. 2015, 17, 66–78. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Huang, Y.; Zhou, M.; Jia, C.; Gu, Z.; Sun, B.; Wang, Q. CPFD simulations of corn stalk gasification in a circulating fluidized bed. Chem. Eng. Res. Des. 2024, 205, 246–256. [Google Scholar] [CrossRef]
- Wu, H.; Hanna, A.M.; Jones, D.D. Fluidized-bed gasification of dairy manure by Box–Behnken design. Waste Manag. Res. 2012, 30, 506–511. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.M.; Molina, G. Steam gasification of coal at low−medium (600−800 °C) temperature with simultaneous CO2 capture in fluidized bed at atmospheric pressure: The effect of inorganic species. 1. Literature review and comments. Ind. Eng. Chem. Res. 2007, 46, 6831–6839. [Google Scholar] [CrossRef]
- Karl, J.; Pröll, T. Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar] [CrossRef]
- Winaya, I.N.S.; Wirawan, I.K.G.; Darma, I.W.A.; Lokantara, I.P.; Hartati, R.S. An increase in bed temperature on gasification of dual reactor fluidized bed. E3S Web Conf. 2018, 67, 02059. [Google Scholar] [CrossRef]
- Wilk, V.; Kitzler, H.; Koppatz, S.; Pfeifer, C.; Hofbauer, H. Gasification of waste wood and bark in a dual fluidized bed steam gasifier. Biomass- Convers. Biorefinery 2011, 1, 91–97. [Google Scholar]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-rich gas production by steam gasification of hydrochar derived from sewage sludge. Int. J. Hydrog Energy 2020, 45, 1997–2006. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Dong, H.; Pan, Y.; Chen, H. Numerical simulation of gasification characteristics in a circulating fluidized bed biomass gasifiers. Biomass- Convers. Biorefinery 2025, 15, 6269–6286. [Google Scholar]
- Zhao, L.; Lu, Y. Hydrogen production by biomass gasification in a super critical water fluidized bed reactor: A CFD-DEM study. J. Supercrit. Fluids 2018, 131, 26–36. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Wang, Q. Simulation of gas–solid flow characteristics in a circulating fluidized bed based on a computational particle fluid dynamics model. Powder Technol. 2017, 321, 132–142. [Google Scholar] [CrossRef]
- Lu, J.; Jin, B. Three-dimensional numerical simulation of biomass fluidized bed gasification. Acta Energiae Solaris Sin. 2018, 39, 2863–2868. [Google Scholar]
- Chang, J.; Zhang, K.; Zhu, W.; Yang, Y. Gas-solid flow characteristics and contact efficiency in dual circulating fluidized bed riser. Pet. Process. Petrochem. 2016, 47, 16–24. [Google Scholar]
- Fang, M. DEM-LES Simulation of Dense Gas-Solid Two-Phase Flow in Circulating Fluidized Bed. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. [Google Scholar]
- Wan, Z.; Lu, Y. Numerical simulation of local and global mixing/segregation characteristics in a gas–solid fluidized bed. Chin. J. Chem. Eng. 2022, 44, 72–86. [Google Scholar] [CrossRef]
- Yang, H.; Wirsum, M.; Lü, J.; Yang, H. Modeling study on particle residence time in CFB boilers. J. Eng. Therm. Energy Power 2003, 143–146. [Google Scholar]
- Lan, B.; Xu, J.; Liu, Z.; Wang, J. Scale-up simulation study on particle residence time distribution characteristics in continuous dense-phase fluidized bed. CIESC J. 2021, 72, 521–533. [Google Scholar]
- Gao, W.; Zhang, J.; Wang, Y.; Huang, B.; Xu, G. Particle residence time distribution in continuous feeding/discharging bubbling fluidized bed. Chin. J. Process Eng. 2012, 12, 9–13. [Google Scholar]
- Basu, P. Combustion and Gasification in Fluidized Beds; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Huang, Y.; Wang, Y.; Zhang, C.; Xu, G. Optimization of gas residence time for high-calorific syngas production in a dual-bed gasifier. Appl. Energy 2015, 250, 1480–1489. [Google Scholar]
- Li, J.; Zhang, S.; Shao, J.; Yang, H.; Chen, H. Pilot-scale experimental study on typical biomass fluidized bed gasification. Energy Environ. 2020, 42–45. [Google Scholar] [CrossRef]
- Li, W.; Wu, S.; Wu, Y.; Huang, S.; Gao, J. Gasification characteristics of biomass at a high-temperature steam atmosphere. Fuel Process. Technol. 2019, 194, 106090. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Yang, H.; Wang, X.; Dai, Z. Simulation study on biomass oxygen gasification for syngas production based on ASPEN PLUS platform. J. Combust. Sci. Technol. 2011, 17, 432–436. [Google Scholar]
- Kurkela, E.; Kurkela, M. Hiltunen Pilot-scale development of pressurized air-blown fluidized-bed gasification for biomass. Energy Fuels 2013, 27, 6713–6722. [Google Scholar]
- Phuiran, C.; Takarada, T.; Chaiklangmuang, S. Hydrogen-rich gas from catalytic steam gasification of eucalyptus using nickel-loaded Thai brown coal char catalyst. Int. J. Hydrog Energy 2014, 39, 3649–3656. [Google Scholar] [CrossRef]
- Fremaux, S.; Beheshti, S.-M.; Ghassemi, H.; Shahsavan-Markadeh, R. An experimental study on hydrogen-rich gas production via steam gasification of biomass in a research-scale fluidized bed. Energy Convers. Manag. 2015, 91, 427–432. [Google Scholar] [CrossRef]
- Loha, C.; Chattopadhyay, H.; Chatterjee, P.K. Thermodynamic analysis of hydrogen rich synthetic gas generation from fluidized bed gasification of rice husk. Energy 2011, 36, 4063–4071. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Pandey, B.; Sheth, P.N.; Prajapati, Y.K. Air-CO2 and oxygen-enriched air-CO2 biomass gasification in an autothermal downdraft gasifier: Experimental studies. Energy Convers. Manag. 2022, 270, 116216. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Ocone, R.; Wang, H. MP-PIC simulation on CO2 gasification of biomass in a pilot plant circulating fluidized bed gasifier. Fuel 2023, 332, 125992. [Google Scholar] [CrossRef]
- Ren, X.; Yang, H.; Chen, Q.; Zhao, C.; Zhang, S.; Chen, H. Numerical simulation of CO2 biomass gasification. Acta Energiae Solaris Sinica 2021, 42, 1–9. [Google Scholar]
- Sharma, S.; Sheth, P.N. Air-steam biomass gasification: Experiments, modeling and simulation. Energy Convers. Manag. 2016, 110, 307–318. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M. Syngas from steam-oxygen fluidized bed gasification of MSW: Effect of temperature and oxygen concentration. Fuel Process. Technol. 2015, 133, 202–208. [Google Scholar]
- Ye, R.; Liu, J.; Li, X.; Zheng, L.; Li, M.; Wang, G.; Zhang, D. Experimental study on oxygen-steam oriented gasification in biomass bubbling fluidized bed. Acta Energiae Solaris Sin. 2016, 37, 1636–1642. [Google Scholar]
- Frigo, S.; Flori, G.; Barontini, F.; Gabbrielli, R.; Sica, P. Experimental and numerical performance assessment of green-hydrogen production from biomass oxy-steam gasification. Int. J. Hydrogen Energy 2024, 71, 785–796. [Google Scholar] [CrossRef]
- Xu, W.; Ren, J.; Ying, H.; Sun, Y.; Xu, Y. Hydrogen-rich gas production from bamboo chips via oxygen-steam gasification. Biomass Chem. Eng. 2023, 57, 44–50. [Google Scholar]
- Su, D.; Liu, H.; Zhou, Z.; Yin, X.; Wu, C. Experimental study on oxygen-steam gasification of biomass in a fluidized bed. J. Fuel Chem. Technol. 2012, 40, 309–314. [Google Scholar]
- Ruoppolo, G.; Cante, A.; Chirone, R.; Miccio, F.; Stanzione, V. Fluidized bed gasification of coal/biomass slurries. In Proceedings of the 20th International Conference on Fluidized Bed Combustion, Xi’an, China, 18–21 May 2009; Istituto di Ricerche sulla Combustione–CNR: Napoli, Italy, 2012; pp. 1–10. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).