Excellent Superhydrophobic Cone-Array Surfaces with Low Contact Time of Droplet Pancake Bouncing Under Various Conditions
Abstract
1. Introduction
2. Materials and Methods
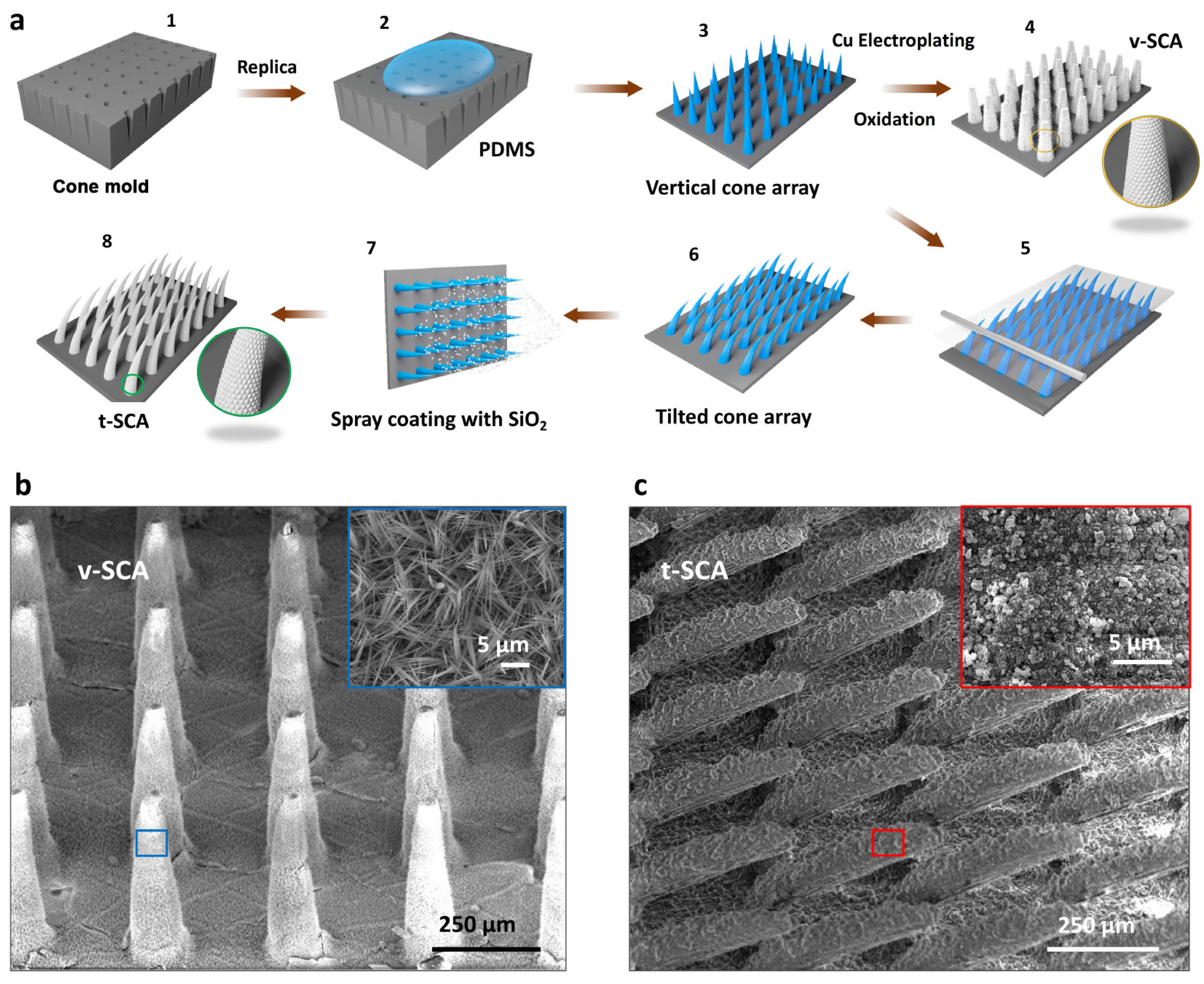
2.1. Fabrication of Vertical Superhydrophobic Cone Arrays (v-SCAs)
2.2. Fabrication of Superhydrophobic Cone Arrays with Tilt Angles (t-SCAs)
2.3. Characterization of Superhydrophobic Cone Arrays
2.4. Observation of Droplet Bouncing Behaviors
3. Results
3.1. Fabrication of Vertical SCAs (v-SCAs) and Tilted SCAs (t-SCAs)
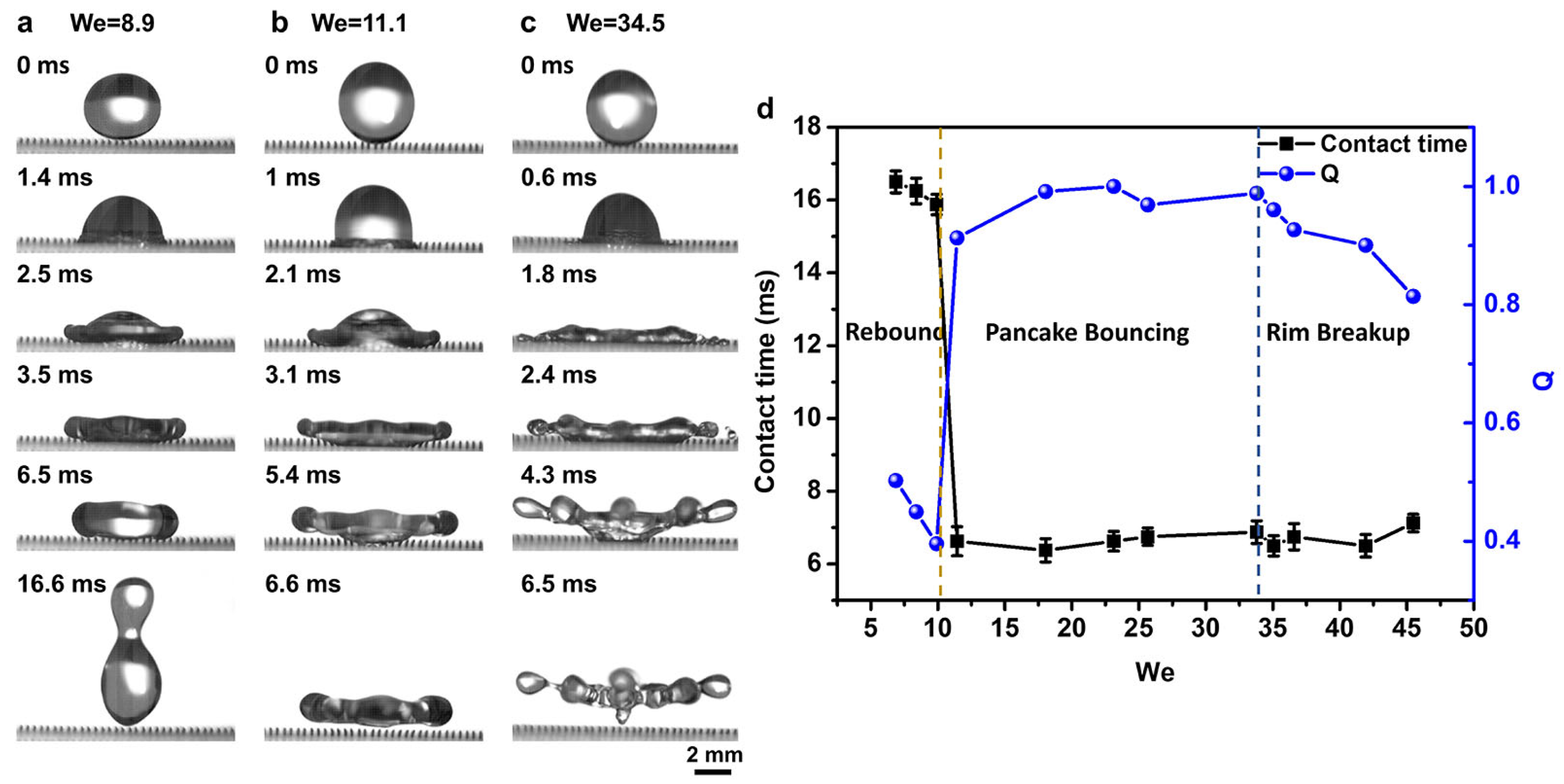
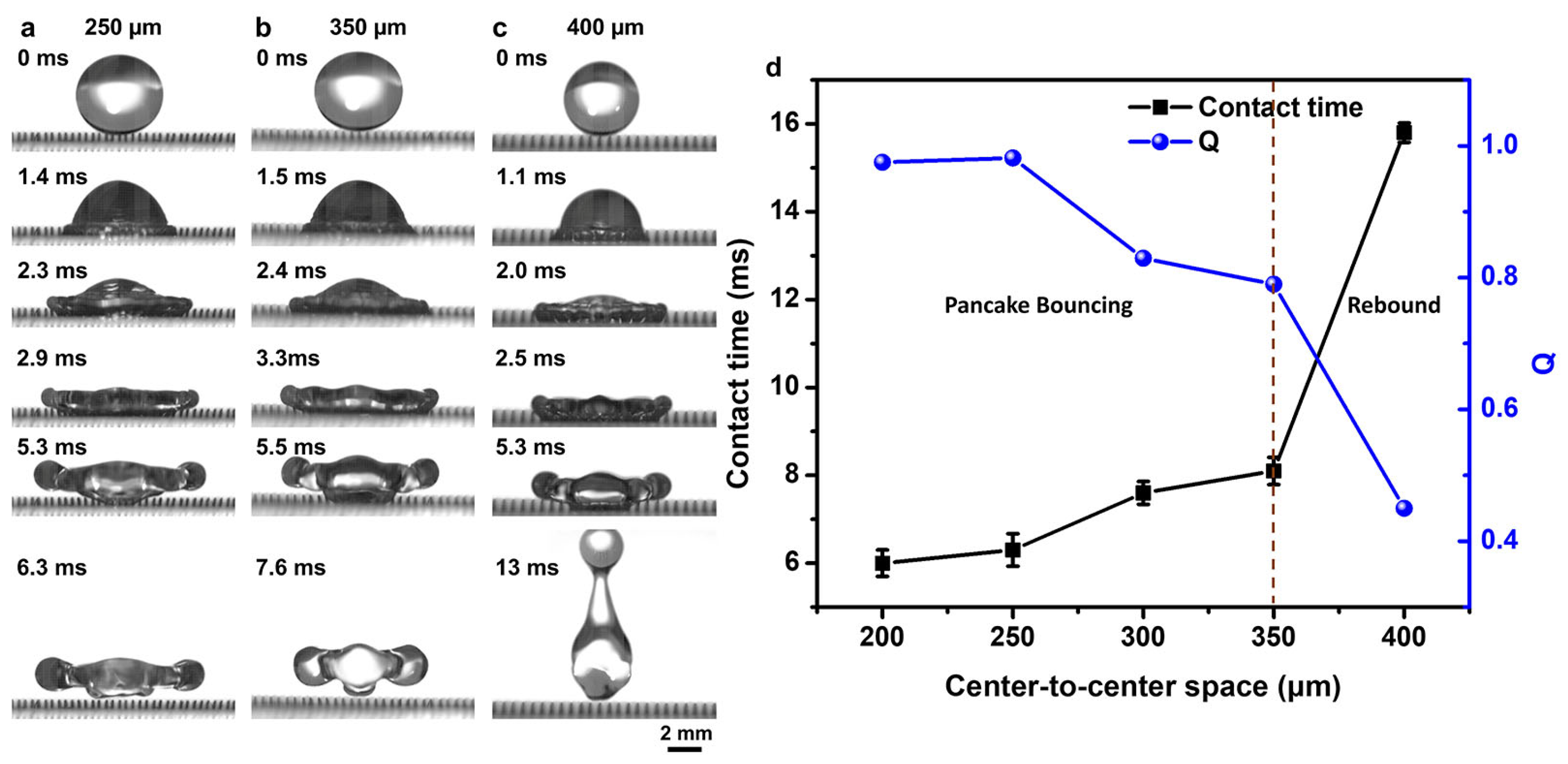
3.2. Structure Optimization for Droplet Pancake Bouncing
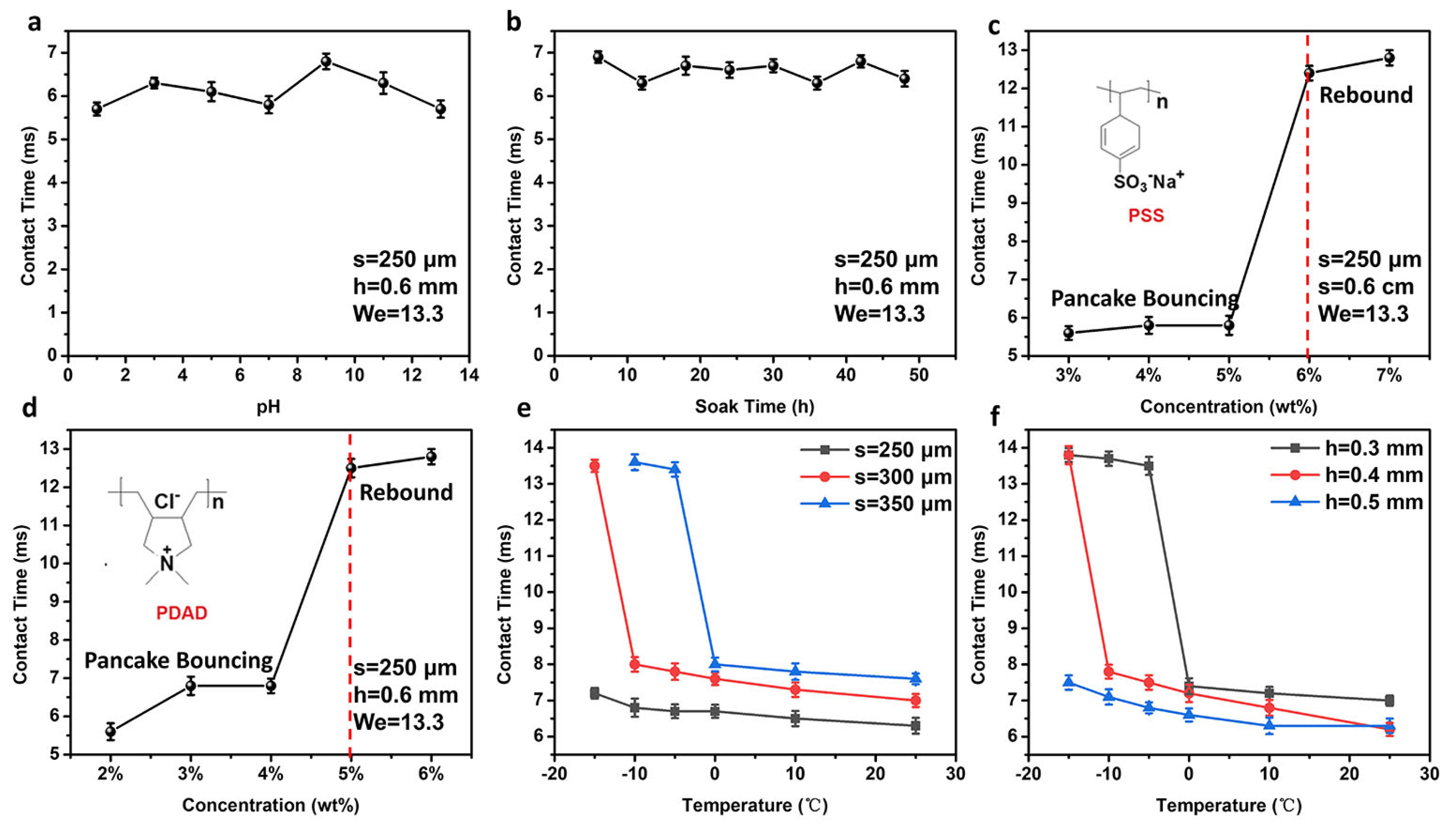
3.3. Influence of the Environmental Condition on the Droplet Bouncing Behavior
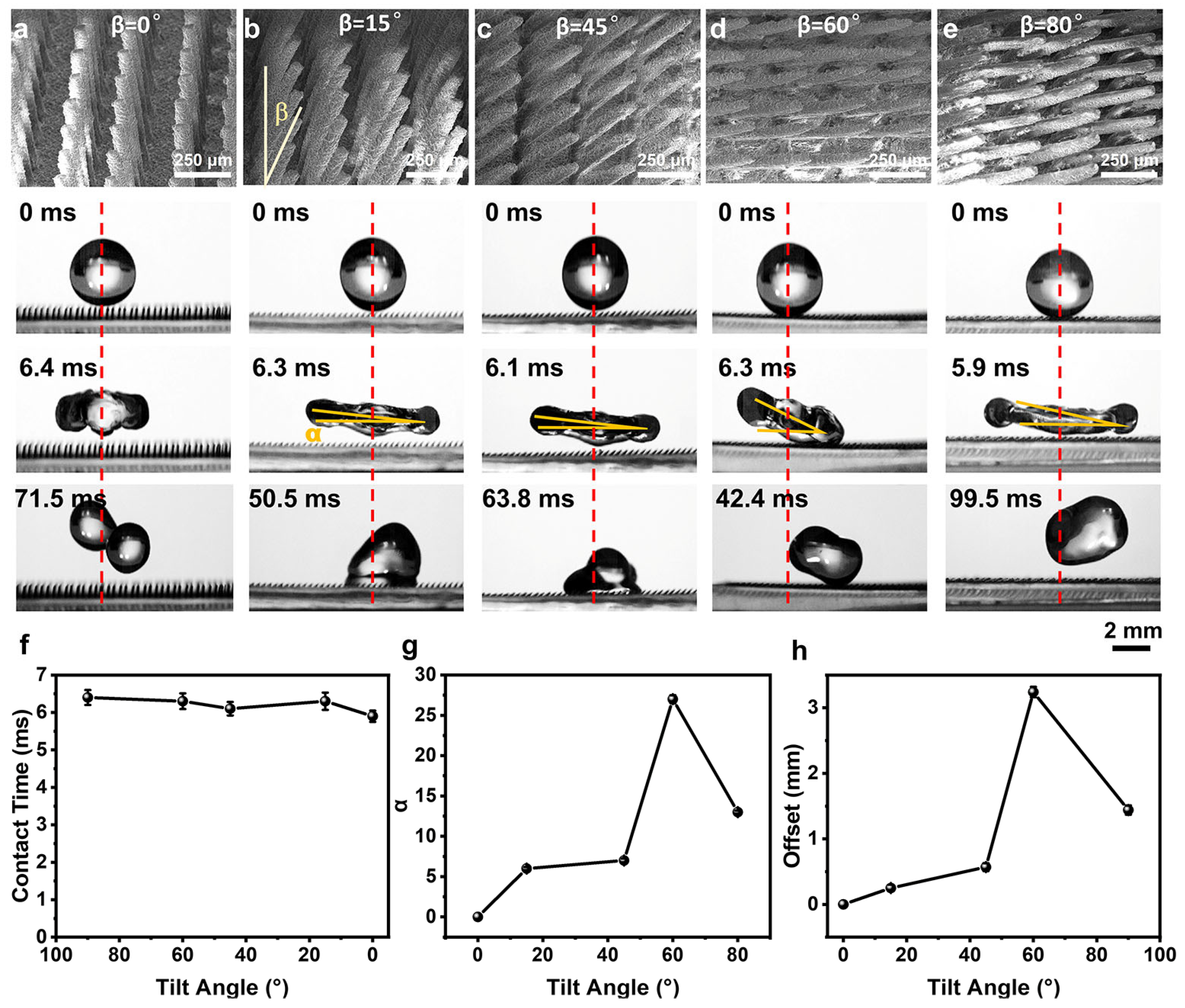
3.4. Directional Droplet Pancake Bouncing on t-SCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.-J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of Ice-free Nanostructured Surfaces Based on Repulsion of Impacting Water Droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Stone, H.A. Ice-Phobic Surfaces That Are Wet. ACS Nano 2012, 6, 6536–6540. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust Anti-Icing Performance of a Flexible Superhydrophobic Surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
- He, Z.K.; Lan, X.R.; Hu, Q.S.; Li, H.M.; Li, L.M.; Mao, J.Y. Antifouling strategies based on super-phobic polymer materials. Prog. Org. Coat 2021, 157, 106285. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, X.Y.; Wang, D.P.; Zhao, D.Q.; Ding, D.W.; Liu, K.; Wang, W.H. Superhydrophobic metallic glass surface with superior mechanical stability and corrosion resistance. Appl. Phys. Lett. 2014, 104, 173701. [Google Scholar] [CrossRef]
- Cottin-Bizonne, C.; Barrat, J.L.; Bocquet, L.; Charlaix, E. Low-friction flows of liquid at nanopatterned interfaces. Nat. Mater. 2003, 2, 237–240. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, J.; Dodiuk, H.; Kenig, S.; Barry, C.; Sun, H.; Mead, J. Effect of Superhydrophobic Composite Coatings on Drag Reduction in Laminar Flow. ACS Appl. Polym. Mater. 2020, 2, 1614–1622. [Google Scholar] [CrossRef]
- Ngo, C.V.; Davaasuren, G.; Oh, H.S.; Chun, D.M. Transparency and superhydrophobicity of cone-shaped micropillar array textured polydimethylsiloxane. Inter. J. Precis. Eng. Manuf. 2015, 16, 1347–1353. [Google Scholar] [CrossRef]
- Jin, M.; Feng, X.; Xi, J.; Zhai, J.; Cho, K.; Feng, L.; Jiang, L. Super-hydrophobic PDMS surface with ultra-low adhesive force. Micromol. Rapid Communi. 2005, 26, 1805–1809. [Google Scholar] [CrossRef]
- Davaasuren, G.; Ngo, C.V.; Oh, H.S.; Chun, D.M. Geometric study of transparent superhydrophobic surfaces of molded and grid patterned polydimethysiloxane (PDMS). Appl. Surf. Sci. 2014, 314, 530–536. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B. Influence of surface roughness on superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, C.; Zhao, W.; Wang, S.; Zhu, P. Suppression of hollow droplet rebound on super-repellent surfaces. Nat. Commun. 2023, 14, 5386. [Google Scholar] [CrossRef]
- Richard, D.; Clanet, C.; Quere, D. Contact time of a bouncing drop. Nature 2002, 417, 811. [Google Scholar] [CrossRef]
- Bartolo, D.; Bouamrirene, F.; Verneuil, E.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplet: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Liu, Y.; Whyman, G.; Bormashenko, E.; Hao, C.; Wang, Z. Controlling drop bouncing using surfaces with gradient features. Appl. Phys. Lett. 2015, 107, 051604. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathy, A.; Nam, Y.; Lee, C.; Sen, P. Effect of geometrical parameters on rebound of impacting droplets on leaky superhydrophobic meshes. Soft Matter 2018, 14, 1571–1580. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Hao, P.; He, F. Internal rupture and rapid bouncing of impacting drops induced by submillimeter-scale textures. Phys. Rev. E 2017, 95, 063104. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, M.; Zhao, C.; Lu, Y.; Huang, L.; Liu, X.; Carmalt, C.J.; Deng, X.; Parkin, I.P. Large-Area Fabrication of Droplet Pancake Bouncing Surface and Control of Bouncing State. ACS Nano 2017, 11, 9259–9267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef]
- Moevius, L.; Liu, Y.; Wang, Z.; Yeomans, J.M. Pancake bouncing: Simulations and theory and experimental verification. Langmuir 2014, 30, 13021–13032. [Google Scholar] [CrossRef]
- Bird, J.C.; Dhiman, R.; Kwon, H.M.; Varanasi, K.K. Reducing the contact time of a bouncing drop. Nature 2013, 503, 385–388. [Google Scholar] [CrossRef]
- Song, M.; Liu, Z.; Ma, Y.; Dong, Z.; Wang, Y.; Jiang, L. Reducing the contact time using macro anisotropic superhydrophobic surfaces-effect of parallel wire spacing on the drop impact. NPG Asia Mater. 2017, 9, e415. [Google Scholar] [CrossRef]
- Liu, Y.; Andrew, M.; Li, J.; Yeomans, J.M.; Wang, Z. Symmetry breaking in drop bouncing on curved surfaces. Nat. Commun. 2015, 6, 10034. [Google Scholar] [CrossRef]
- Gauthier, A.; Symon, S.; Clanet, C.; Quere, D. Water impacting on superhydrophobic macrotextures. Nat. Commun. 2015, 6, 8001. [Google Scholar] [CrossRef]
- Wu, H.P.; Jiang, K.P.; Xu, Z.X.; Yu, S.H.; Peng, X.; Zhang, Z.; Bai, H.; Liu, A.P.; Chai, G.Z. Theoretical and Experimental Studies on the Controllable Pancake Bouncing Behavior of Droplets. Langmuir 2019, 35, 17000–17008. [Google Scholar] [CrossRef]
- Song, J.; Huang, L.; Zhao, C.; Wu, S.; Liu, H.; Lu, Y.; Deng, X.; Carmalt, C.J.; Parkin, I.P.; Sun, Y. Robust Superhydrophobic Conical Pillars from Syringe Needle Shape to Straight Conical Pillar Shape for Droplet Pancake Bouncing. ACS Appl. Mater. Interfaces 2019, 11, 45345–45353. [Google Scholar] [CrossRef]
- Huang, L.; Pan, W.H.; Chen, Y.; Ming, P.M.; Song, J.L.; Wang, X.Y.; Hua, S.G. Drop impact on elastic superhydrophobic films: From pancake bouncing to saucer bouncing. Mater. Lett. 2021, 285, 129076. [Google Scholar] [CrossRef]
- Qian, C.; Zhou, F.; Wang, T.; Li, Q.; Hu, D.; Chen, X.; Wang, Z. Pancake Jumping of Sessile Droplets. Adv. Sci. 2022, 9, 2103834. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Liang, G.; Dou, B.; Wu, J.; Li, B.; Hao, C. Oblique pancake bouncing. Cell Rep. Phys. Sci. 2022, 3, 100721. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Gao, X.F.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Wen, J.; Zhan, F.; Shi, M.; Zhou, N.; Huang, C.; Wang, L.; Mao, H. Oriented bouncing of droplet with a small Weber number on inclined one-dimensional nanoforests. Nanoscale 2024, 16, 5343–5351. [Google Scholar] [CrossRef]
- Jin, Y.K.; He, Z.Y.; Guo, Q.; Wang, J.J. Control of Ice Propagation by Using Polyelectrolyte Multilayer Coatings. Angew. Chem. Int. Ed. 2017, 56, 11436–11439. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Z.; Gao, C.; Guo, Z.; Li, C.; Zheng, Y. Directional Droplet Spreading Transport Controlled on Tilt-Angle Pillar Arrays. Adv. Mater. Interfaces 2018, 5, 1800962. [Google Scholar] [CrossRef]
- Shirota, M.; Kato, M.; Ishio, A. Rim breakup of imparting drops on a superhydrophobic surface and a superheated surface. Fluids 2022, 7, 79. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lin, Y.; Feng, S.; Zheng, Y. Excellent Superhydrophobic Cone-Array Surfaces with Low Contact Time of Droplet Pancake Bouncing Under Various Conditions. Fluids 2025, 10, 144. https://doi.org/10.3390/fluids10060144
Chen Y, Lin Y, Feng S, Zheng Y. Excellent Superhydrophobic Cone-Array Surfaces with Low Contact Time of Droplet Pancake Bouncing Under Various Conditions. Fluids. 2025; 10(6):144. https://doi.org/10.3390/fluids10060144
Chicago/Turabian StyleChen, Yuanjie, Yucai Lin, Shile Feng, and Yongmei Zheng. 2025. "Excellent Superhydrophobic Cone-Array Surfaces with Low Contact Time of Droplet Pancake Bouncing Under Various Conditions" Fluids 10, no. 6: 144. https://doi.org/10.3390/fluids10060144
APA StyleChen, Y., Lin, Y., Feng, S., & Zheng, Y. (2025). Excellent Superhydrophobic Cone-Array Surfaces with Low Contact Time of Droplet Pancake Bouncing Under Various Conditions. Fluids, 10(6), 144. https://doi.org/10.3390/fluids10060144





