Optimising Air Change Rates: A CFD Study on Mitigating Pathogen Transmission in Aircraft Cabins
Abstract
1. Introduction
2. Materials and Methods
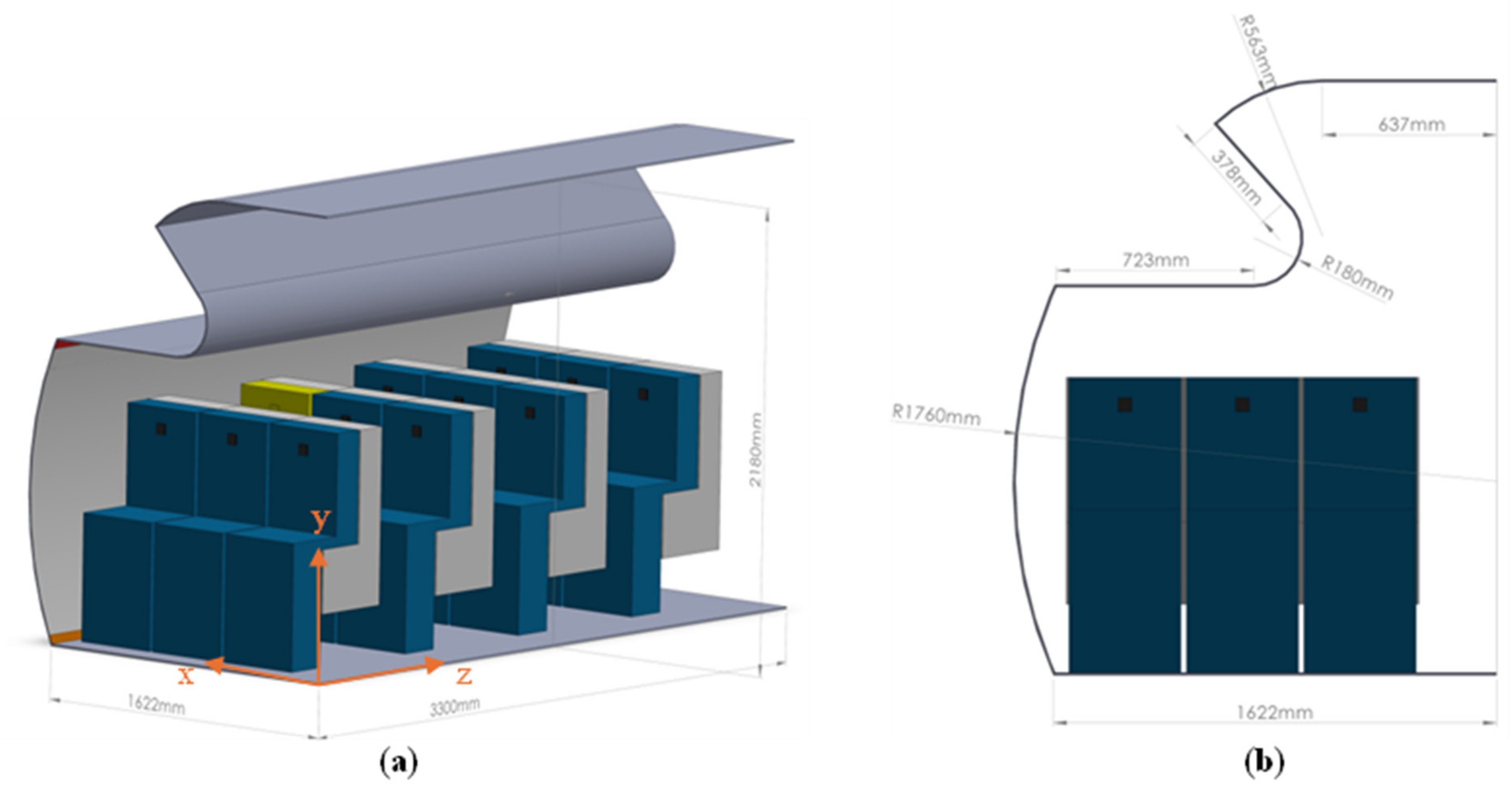
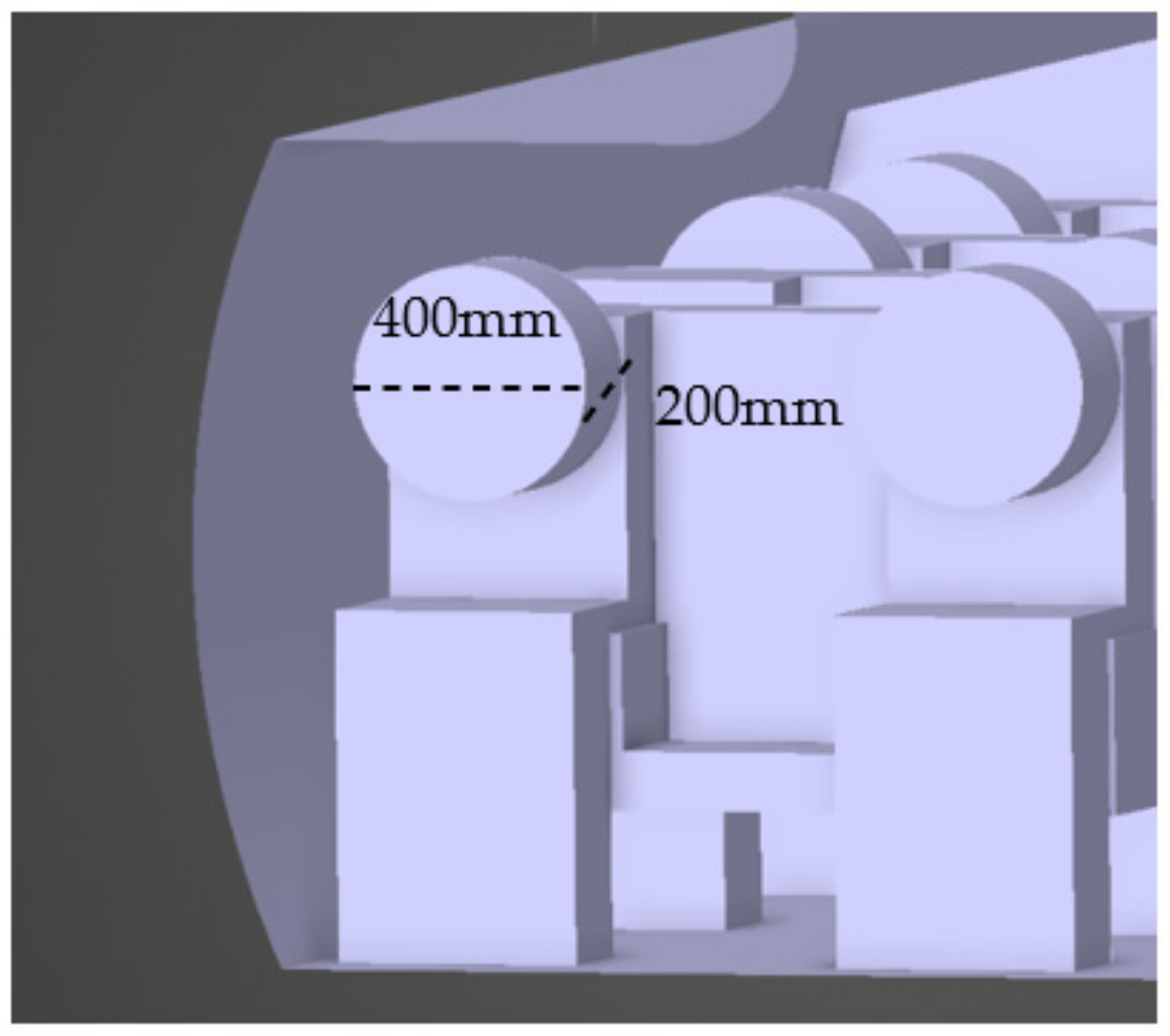
2.1. Airplane Cabin Model
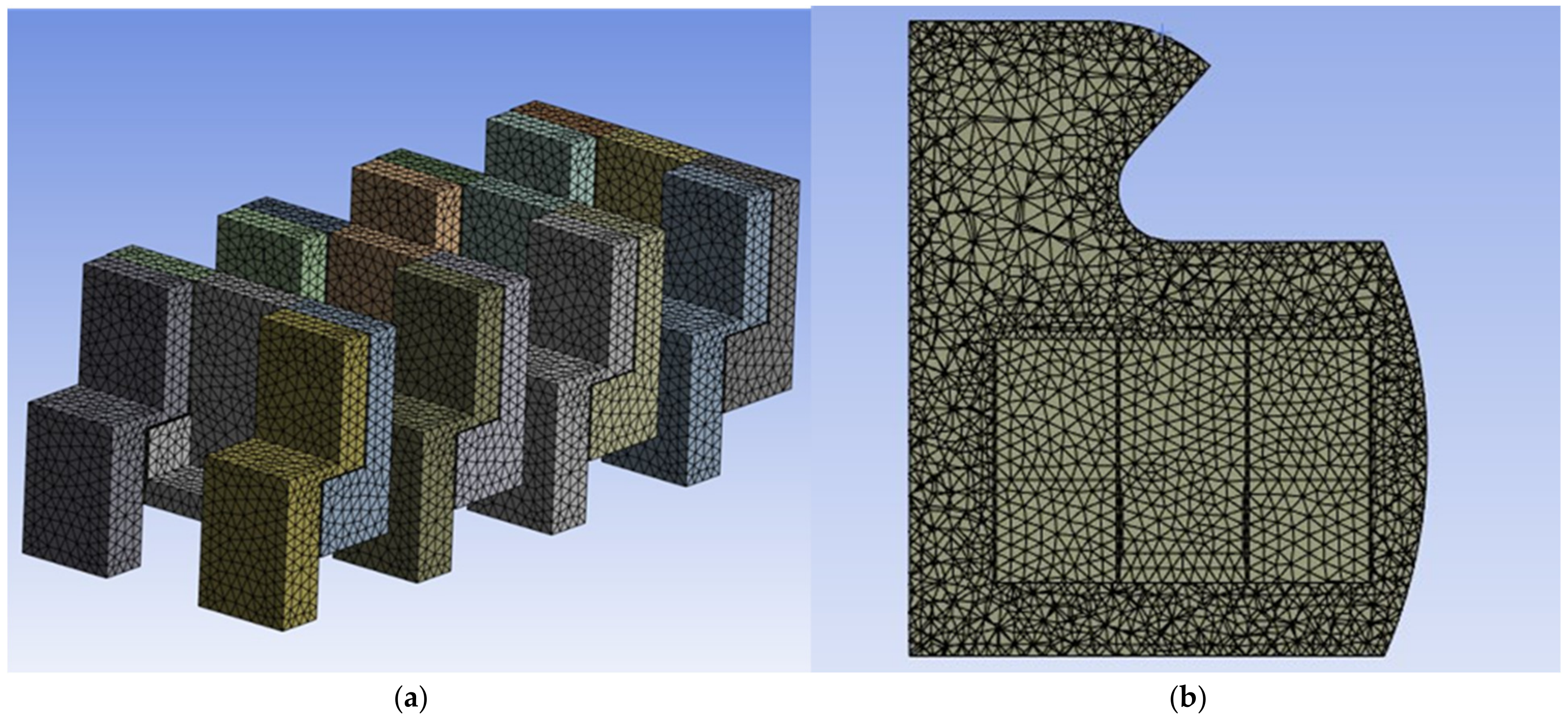
2.2. Meshing
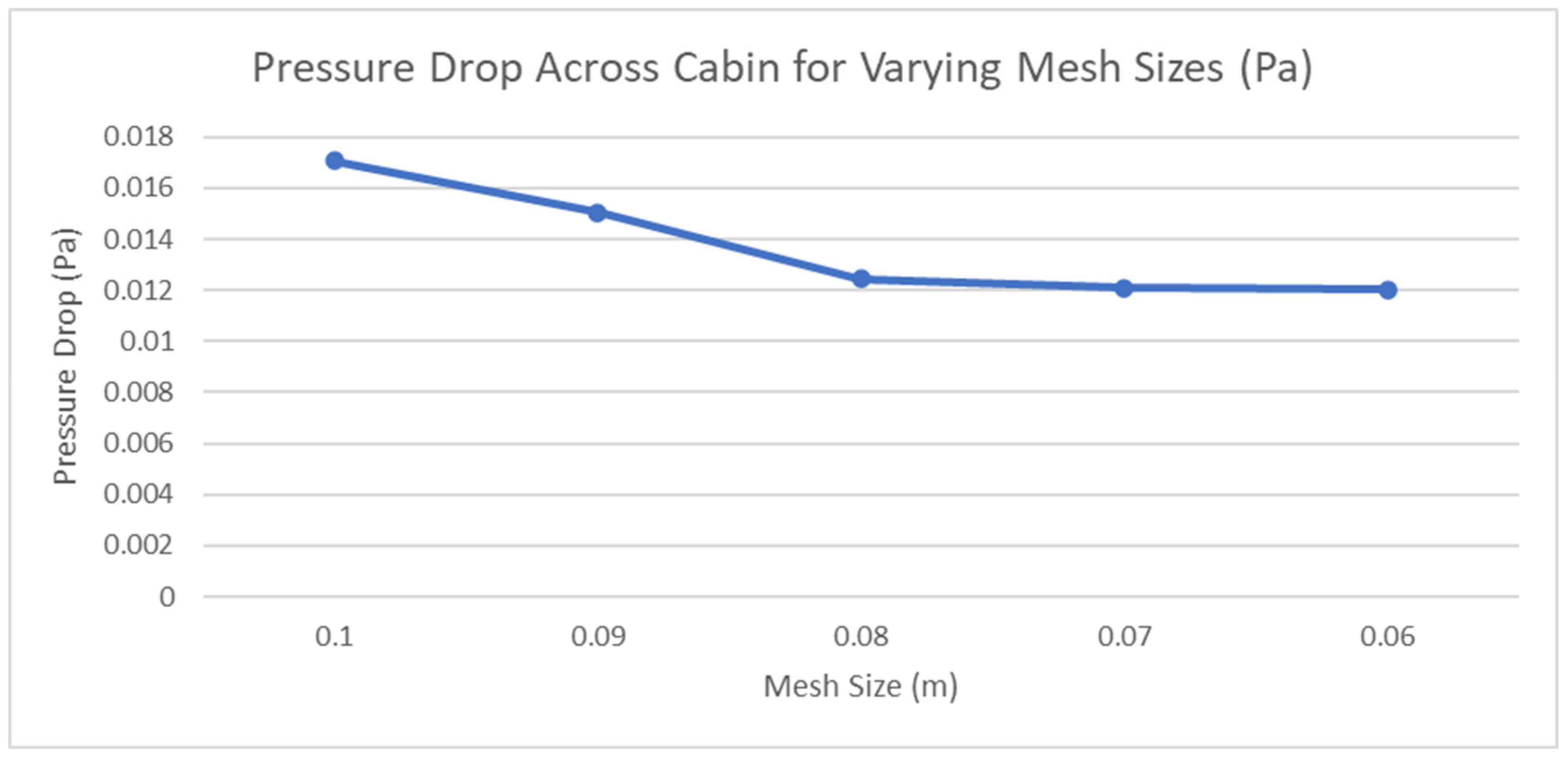
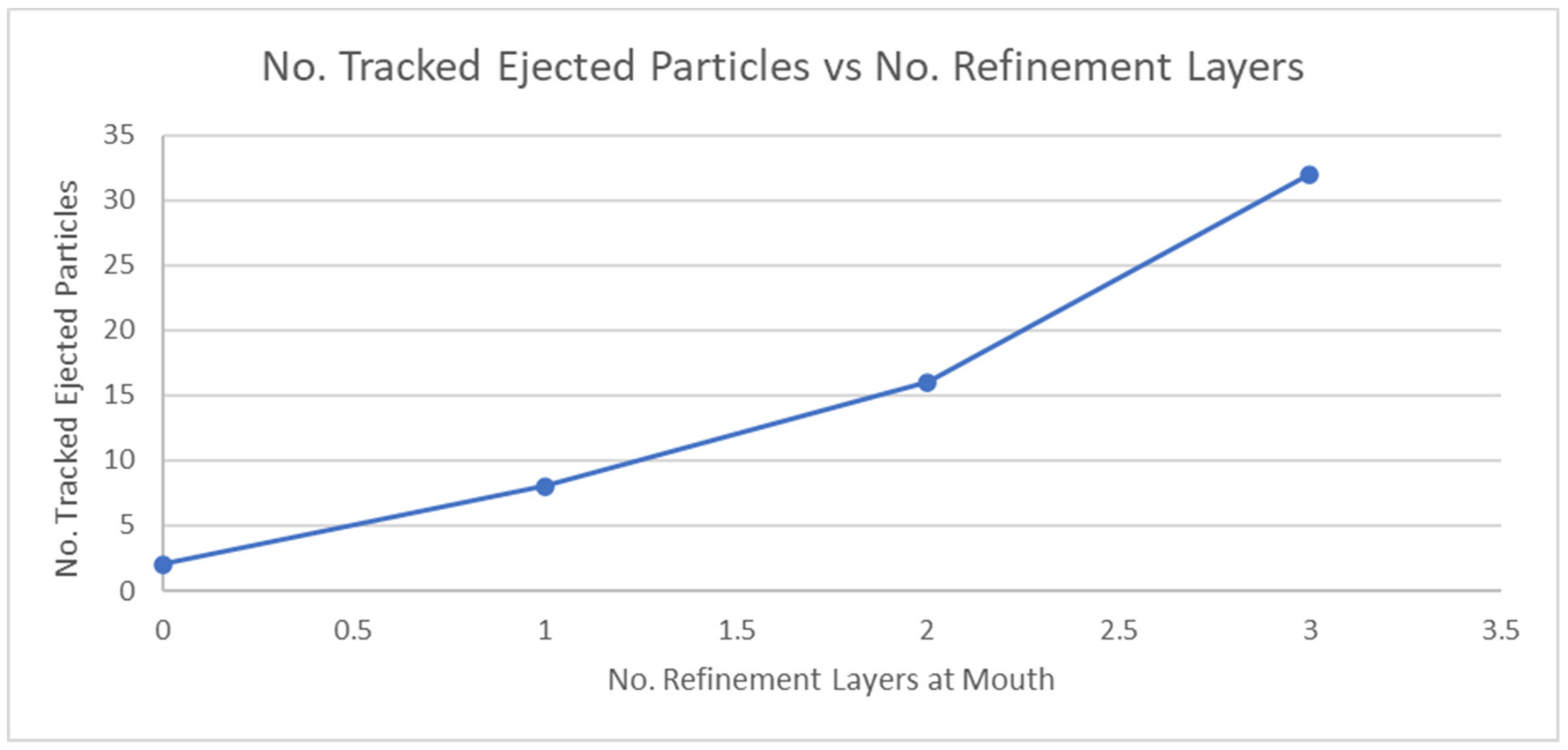
2.2.1. Meshing Sensitivity Analysis
2.3. Governing Equations
2.3.1. Airflow Simulation
- 1.
- Continuity Equation (Conservation of Mass):
- 2.
- Momentum Equations (Conservation of Momentum):
- 3.
- Energy Equation (Conservation of Energy):
- 4.
- Turbulence Transport Equations:
- a.
- Turbulent Kinetic Energy Equation:
- b.
- Turbulent Dissipation Rate Equation:
2.3.2. Particle Tracking
2.4. Setup and Boundary Conditions
2.4.1. Material Properties
2.4.2. Boundary Conditions and Flow Parameters
2.4.3. Variables and Metrics
3. Results and Discussions
3.1. Airflow Pathlines
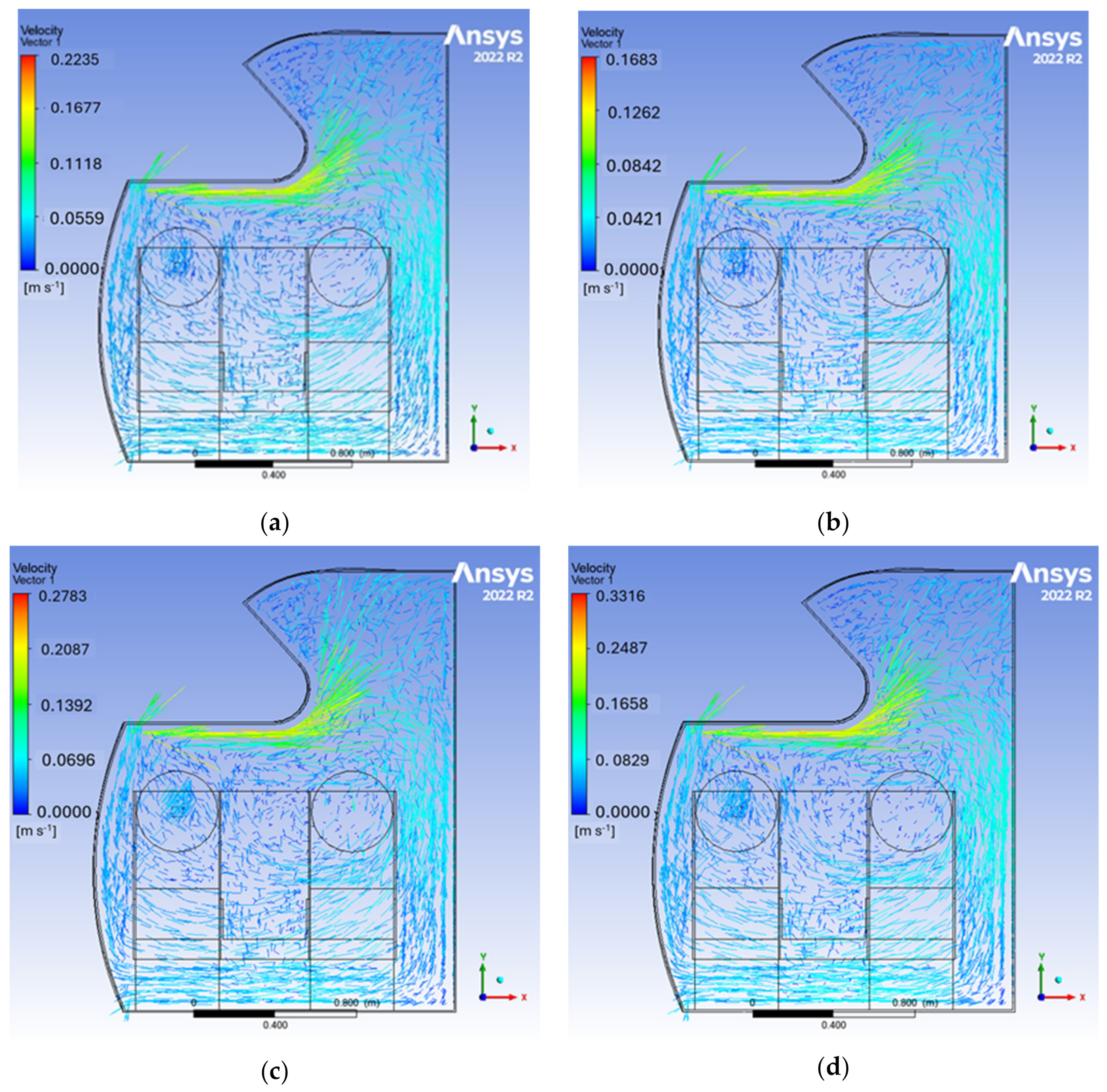
3.2. Velocity Distribution
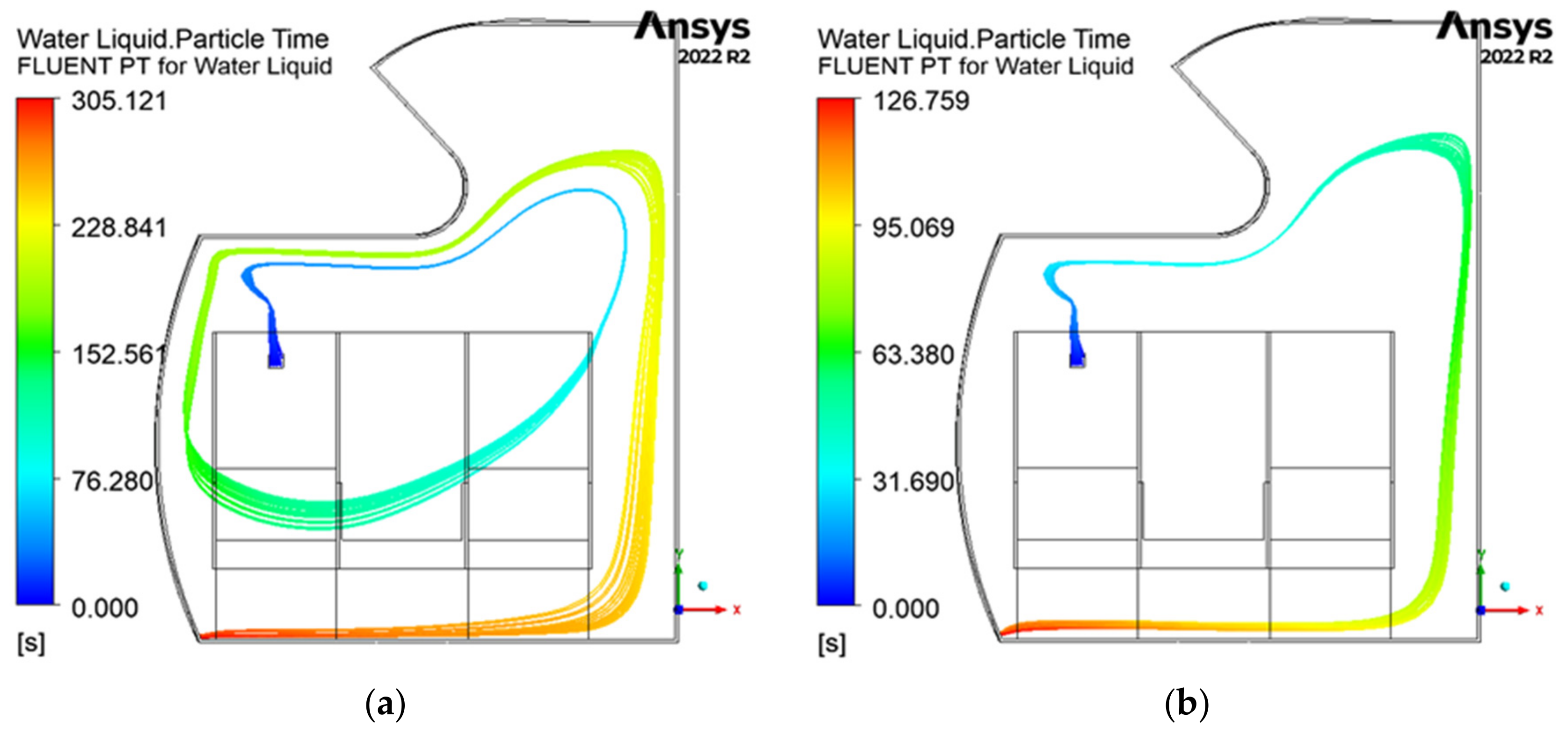
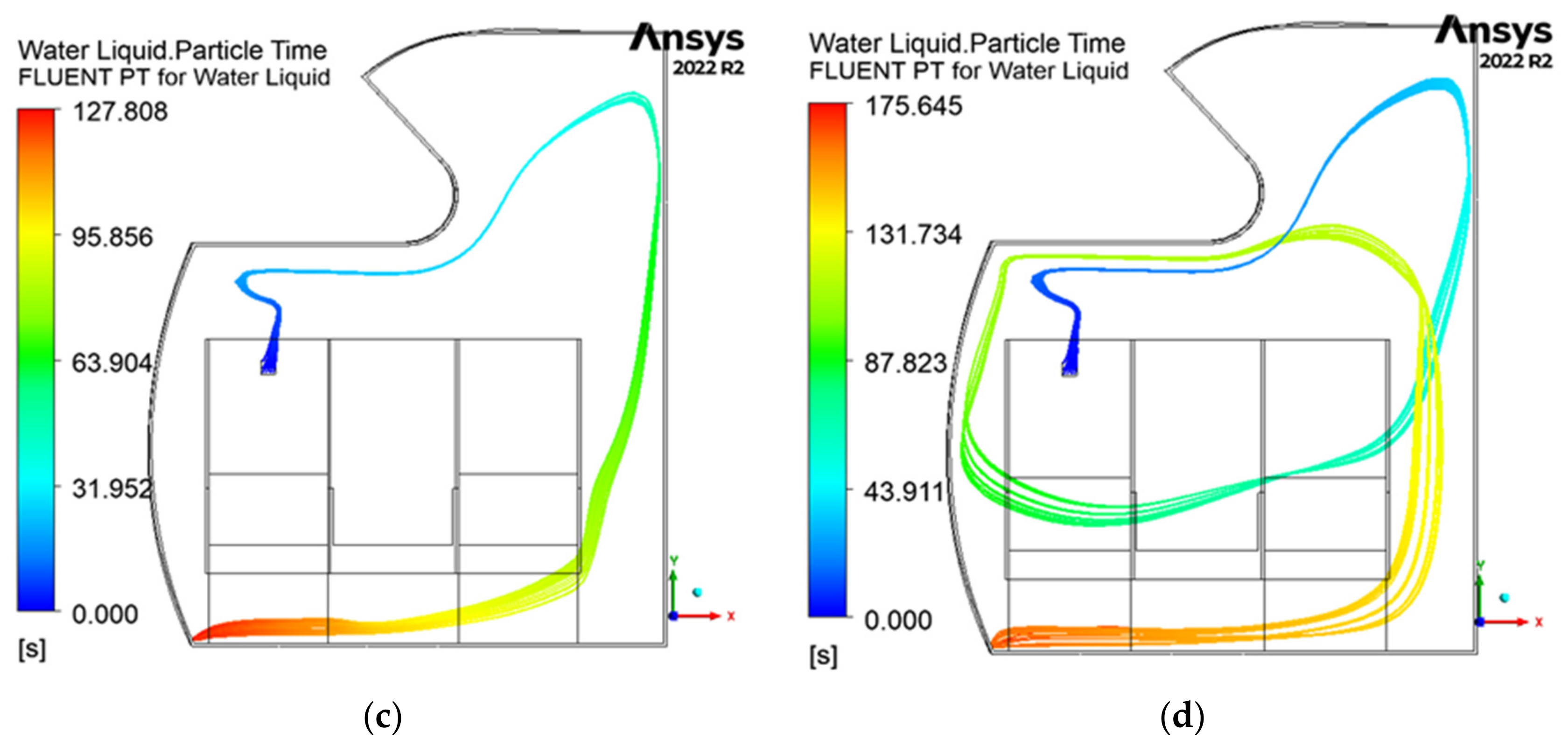
3.3. Pathogen Residence Time
3.4. Summary of the Results
- The pathogen residence time is the longest at 15 ACH, while the shortest residence times are observed at 20 and 25 ACH.
- At lower ACH rates, airflow stagnation is more prominent, increasing the potential for pathogen buildup in certain areas.
- At 30 ACH, the residence time slightly increases, breaking the downward trend from 15 to 25 ACH.
- At 25 and 30 ACH, there is increased pathogen movement between the rows.
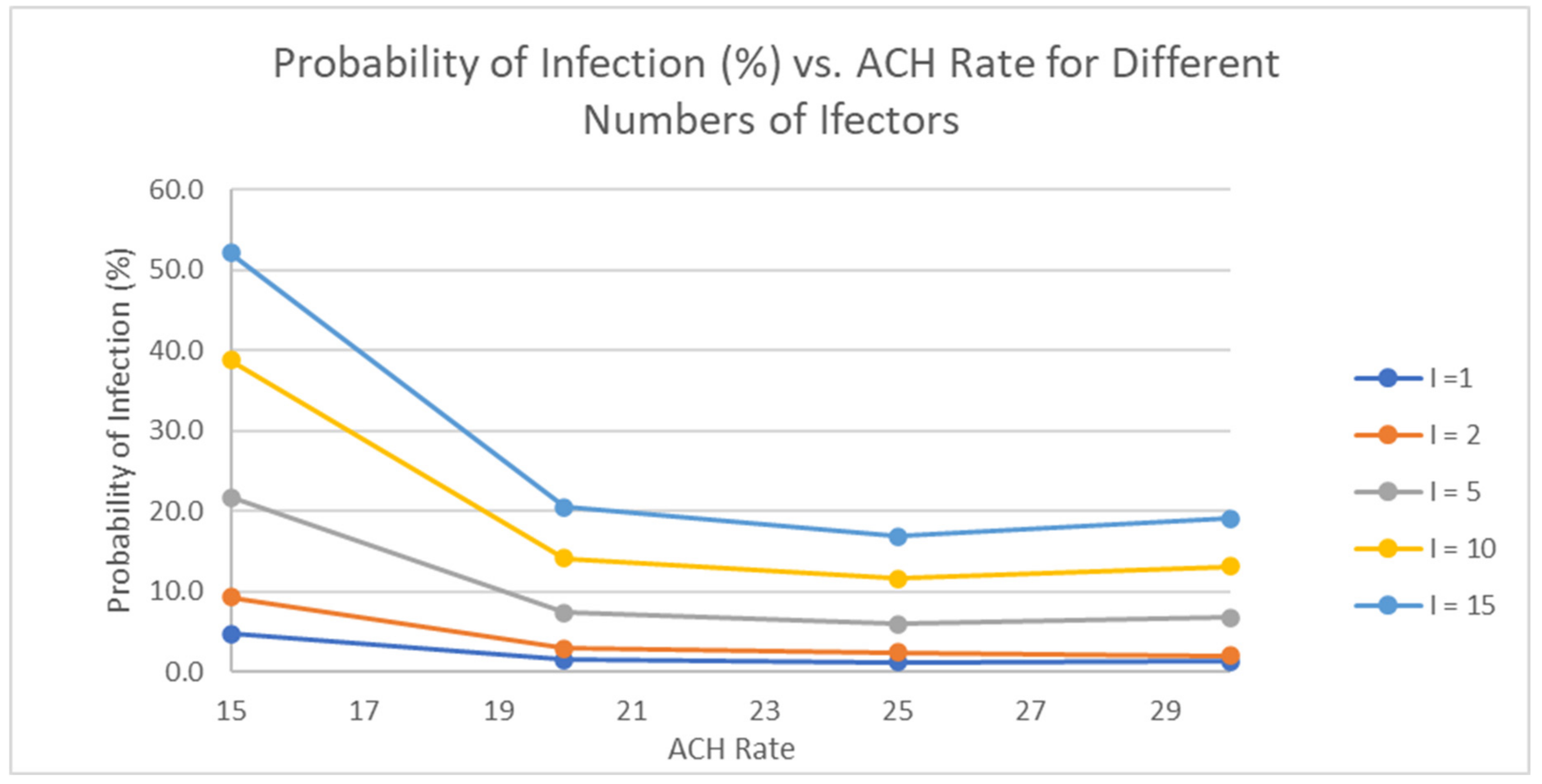
3.4.1. Infection Risk Assessment
3.4.2. Optimisation
4. Conclusions
- (a)
- Validate the ACH impact: Conduct further studies with more incremental ACH rates to determine the specific point at which the transmission risk is most effectively reduced.
- (b)
- Explore the ACH beyond 30: Investigate whether ACH rates above 30 continue to show diminishing returns or offer further reductions in the transmission risk.
- (c)
- Personalised exhaust systems: Research how personalised exhaust systems might prevent cross-contamination between passengers and complement the existing ventilation strategies.
- (d)
- ACH for different airplane models: Analyse ACH rates across various airplane models, considering differences in volume and cabin geometry, to assess the generalisability of the current findings.
- (e)
- Optimisation analysis: Perform a full optimisation study to balance the ACH rates with factors like draft, economic feasibility, energy consumption, and passenger comfort to find the most practical solution.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernstrom, A.; Goldblatt, M. Aerobiology and its role in the transmission of infectious diseases. J. Pathog. 2013, 2013, 493960. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.L. A Method to Assess COVID-19 Transmission Risks in Indoor Settings. MIT News. 2021. Available online: https://news.mit.edu/2021/covid-19-risks-indoor-0415 (accessed on 1 June 2024).
- Rajendran, R.R.; Țurcanu, F.E.; Tawfiqur, R.M.D.; Askarpour, H. Computational fluid dynamic analysis of corona virus patients breathing in an airplane. Phys. Fluids 2023, 35, 035129. [Google Scholar] [CrossRef]
- Lin, C.H.; Horstman, R.H.; Ahlers, M.F.; Sedgwick, L.M.; Dunn, K.H.; Topmiller, J.L.; Bennett, J.S.; Wirogo, S. Numerical Simulation of Airflow and Airborne Pathogen Transport in Aircraft Cabins—Part 1. AIVC. 2005. Available online: https://www.aivc.org/resource/numerical-simulation-airflow-and-airborne-pathogen-transport-aircraft-cabins-part-1 (accessed on 13 June 2024).
- Boeing Commercial Airplanes. ‘737NG Airplane Characteristics for Airport Planning (Document No. D6-58329, Rev. A). Boeing. 2023. Available online: https://www.boeing.com/commercial/airports/plan-manuals (accessed on 20 April 2024).
- Tagliaferri, D.; Capsoni, C. SNIR predictions for on-aircraft VLC systems. In Proceedings of the 2016 Conference on Broadband Communications (COBCOM), Graz, Austria, 12–14 July 2016; pp. 1–7. [Google Scholar] [CrossRef]
- Topp, C.; Nielsen, P.V.; Sorensen, D. Application of computer simulated persons in indoor environmental modeling/Discussion. ASHRAE Trans. 2002, 108, 1084. [Google Scholar]
- Skill-Lync. CFD: Tackling Boundary Layers and Near Wall Regions in CFD. Skill-Lync. Available online: https://skill-lync.com/blogs/technical-blogs/cfd-tackling-boundary-layers-and-near-wall-regions-in-cfd (accessed on 2 September 2024).
- ASHRAE. Chapter 13, Aircraft. In ASHRAE Handbook—HVAC Applications; American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2019. [Google Scholar]
- The Engineering ToolBox. Air—Density vs. Pressure and Temperatures. 2004. Available online: https://www.engineeringtoolbox.com/air-temperature-pressure-density-d_771.html (accessed on 11 April 2024).
- Sanmark, E.; Oksanen, L.A.H.; Rantanen, N.; Lahelma, M.; Anttila, V.J.; Lehtonen, L.; Hyvärinen, A.; Geneid, A. Aerosol generation during coughing: An observational study. J. Laryngol. Otol. 2023, 137, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Gaspar, P.D.; Hamandosh, A.; Aguiar, E.B.; Guerra, A.C.; Souza, H.J. Best practices on HVAC design to minimize the risk of COVID-19 infection within indoor environments. Braz. Arch. Biol. Technol. 2020, 63, e20200335. [Google Scholar] [CrossRef]
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Shang, Y.; Tu, J. Evaluation of airborne disease infection risks in an airliner cabin using the Lagrangian-based Wells-Riley approach. Build. Environ. 2017, 121, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.; Abuhegazy, M.; Mahfoze, O.A.; Anderoglu, O.; Poroseva, S.V. Simulation of aerosol transmission on a Boeing 737 airplane with intervention measures for COVID-19 mitigation. Phys. Fluids 2021, 33, 033312. [Google Scholar] [CrossRef]
- Isukapalli, S.S.; Mazumdar, S.; George, P.; Wei, B.; Jones, B.; Weisel, C.P. Computational fluid dynamics modeling of transport and deposition of pesticides in an aircraft cabin. Atmos. Environ. 2013, 68, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Sze To, G.N.; Chao, C.Y. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Burkett, B. Ventilation Strategies to Reduce Airborne Infection Risk: Part 3. ASHRAE Journal, August 2021. pp. 18–25. Available online: https://www.ashrae.org/file%20library/technical%20resources/ashrae%20journal/2021journaldocuments/august2021_18-25_burkett--pt3.pdf (accessed on 11 October 2024).

| Air Property | Unit | Assumption | Value |
|---|---|---|---|
| Compressibility | - | Low-speed flow | Incompressible |
| Density | & 78.187 kPa | 0.91668 | |
| Dynamic Viscosity | @ | 18.32 × | |
| Gas Constant | 287 | ||
| Specific Heat Capacity | Isobaric @ & 1 bar | 1006 | |
| Thermal Conductivity | Isobaric @ & 1 bar | 0.02617 |
| Boundary | Boundary Type | Boundary Condition | Value | Unit | Velocity Condition |
|---|---|---|---|---|---|
| Human Mouth Surface —Temperature | Inlet | Escape | 35 | °C | N/A |
| Particle Injection—Velocity | Inlet | Escape | 11.7 | m/s | N/A |
| Particle Injection—Mass Flow Rate | Inlet | Escape | 8.38 × 10−9 | kg/s | N/A |
| Return Air Pressure | Outlet | Escape | 0 | Pa | N/A |
| Supply Air—Temperature | Inlet | Escape | 20 | °C | N/A |
| Supply Air—Velocity | Inlet | Escape | @30° to wall tangent. | m/s | N/A |
| Supply Air—Water vapour content | Inlet | Escape | 0.001008 | kg of Water vapor/kg of dry air | N/A |
| Symmetry Plane (y-z plane) | Symmetry | N/A | N/A | N/A | Symmetry |
| Chair and Manikin Surfaces | Wall | Undefined | N/A | N/A | N/A |
| Cabin Wall | Wall | Reflect | N/A | N/A | N/A |
| Thermal Boundary | |||||
| Ceiling | Adiabatic Wall | No heat transfer (adiabatic). | N/A | N/A | No-slip |
| Floor | Adiabatic Wall | No heat transfer (adiabatic). | N/A | N/A | No-slip |
| Passengers | Isothermal Wall | Fixed Temperature | 30.3 | °C | No-slip |
| Seats | Adiabatic Wall | No heat transfer (adiabatic). | No heat transfer (thermally insulated). | N/A | No-slip |
| Side walls | Isothermal Wall | Fixed Temperature | 18 | °C | No-slip |
| Case Number | Air Changes/Hour | Inlet Velocity (m/s) |
|---|---|---|
| 1 | 15 | 0.6051 |
| 2 | 20 | 0.8069 |
| 3 | 25 | 1.0086 |
| 4 | 30 | 1.2103 |
| ACH Rate | Total Time in Cabin (s) |
|---|---|
| 15 | 305.1 |
| 20 | 126.8 |
| 25 | 127.8 |
| 30 | 175.6 |
| ACH Rate | t (Exposure Time in s) | P (Probability of Infection in %) |
|---|---|---|
| 15 | 305.1 | 4.7875 |
| 20 | 126.8 | 1.5176 |
| 25 | 127.8 | 1.2254 |
| 30 | 175.6 | 1.4019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benn, J.; Tian, L. Optimising Air Change Rates: A CFD Study on Mitigating Pathogen Transmission in Aircraft Cabins. Fluids 2025, 10, 74. https://doi.org/10.3390/fluids10030074
Benn J, Tian L. Optimising Air Change Rates: A CFD Study on Mitigating Pathogen Transmission in Aircraft Cabins. Fluids. 2025; 10(3):74. https://doi.org/10.3390/fluids10030074
Chicago/Turabian StyleBenn, Jaydon, and Lin Tian. 2025. "Optimising Air Change Rates: A CFD Study on Mitigating Pathogen Transmission in Aircraft Cabins" Fluids 10, no. 3: 74. https://doi.org/10.3390/fluids10030074
APA StyleBenn, J., & Tian, L. (2025). Optimising Air Change Rates: A CFD Study on Mitigating Pathogen Transmission in Aircraft Cabins. Fluids, 10(3), 74. https://doi.org/10.3390/fluids10030074







