The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications
Abstract
1. Introduction
2. PDMS Properties
3. Recent Advances of PDMS Applications in Engineering
3.1. Application of PDMS in Biomicrofluidics
3.1.1. Application in Microfluidic Devices with Contractions
- Mold Preparation: After photomask creation, a master mold is made using photolithography, typically on a silicon wafer with a SU-8 photoresist.
- Mixing and Degassing: The PDMS base polymer and curing agent are mixed in a specified ratio (e.g., 10:1) and degassed to remove air bubbles.
- Casting: The PDMS mixture is poured into the mold and cured at 60–80 °C.
- Demolding: Once cured, the PDMS device is peeled off the mold.
- Bonding: Plasma treatment or other surface modification techniques are used to bond PDMS to itself or other materials, such as glass [39].
3.1.2. Application in Microfluidic Devices with Bifurcations
3.1.3. Application of PDMS Based Blood Analogues in Microfluidics
3.2. Application of PDMS to Produce In Vitro Biomodels
| Geometry | Fabrication Method and Material | Cast Material | Blood Analogue | Measurement Method | Ref. |
|---|---|---|---|---|---|
| Real intracranial aneurysms | Stereolithography (SLA); photopolymer resin | PDMS | Dimethyl sulfoxide (DMSO) in water | PTV | [65,134] |
| Real aneurysms | FDM 3D printer; ABS | PDMS | Water-Glycerin | PIV | [139] |
| Intracranial aneurysm | Digital light processing (DLP) printer; resin | PDMS | Water–Glycerin–Urea | [138] | |
| Carotid artery | Lost-core manufacturing technique | PDMS | Water–Glycerin–Sodium iodide | Stereoscopic PIV | [143,144,145] |
| Neck artery constriction | 3D printer | PDMS | Water–Glycerin | PIV | [146] |
| Coronary artery | Lost-core casting with sucrose | PDMS | Water–Glycerin | Micro-PIV | [20,140,141] |
| Intracranial aneurysm | FDM 3D printer; ABS | PDMS | Water–Glycerin | Digital Image Correlation | [136] |
| Porcine coronary arteries | 3D printer | PDMS | Water | Mobile phone | [142] |
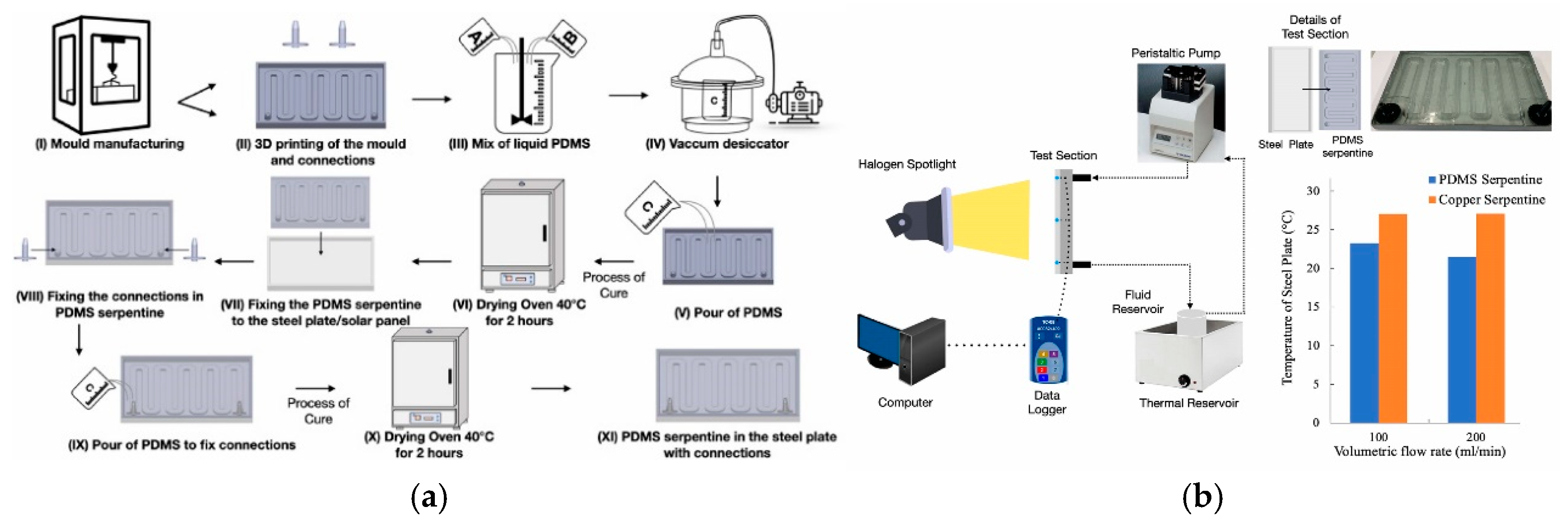
3.3. Application of PDMS for Heat Transfer Studies

3.4. Application of PDMS to Produce Face Masks
3.5. Additional PDMS Applications in Engineering
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with designer functionalities—Properties, modifications strategies, and applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, H.; Zhang, J.; Phan, H.-P.; Nguyen, N.-T. Flexible Microfluidics: Fundamentals, Recent Developments, and Applications. Micromachines 2019, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Salieb-Beugelaar, G.B.; Simone, G.; Arora, A.; Philippi, A.; Manz, A. Latest developments in microfluidic cell biology and analysis systems. Anal. Chem. 2010, 82, 4848–4864. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Nobrega, G.; Neves, L.B.; Barbosa, F.; Ribeiro, J.; Ferrera, C.; Lima, R.A. Recent Advances of PDMS In Vitro Biomodels for Flow Visualizations and Measurements: From Macro to Nanoscale Applications. Micromachines 2024, 15, 1317. [Google Scholar] [CrossRef]
- Clarson, S.J.; Dodgson, K.; Semlyen, J.A. Studies of Cyclic and Linear Poly(Dimethylsiloxanes): 19. Glass-Transition Temperatures and Crystallization Behavior. Polymer 1985, 26, 930–934. [Google Scholar] [CrossRef]
- Ruzi, M.; Celik, N.; Onses, M.S. Superhydrophobic Coatings for Food Packaging Applications: A Review. Food Packag. Shelf Life 2022, 32, 100823. [Google Scholar] [CrossRef]
- Zhou, J.; Khodakov, D.A.; Ellis, A.V.; Voelcker, N.H. Surface Modification for PDMS-Based Microfluidic Devices. Electrophoresis 2012, 33, 89–104. [Google Scholar] [CrossRef]
- van Poll, M.L.; Zhou, F.; Ramstedt, M.; Hu, L.; Huck, W.T.S. A Self-Assembly Approach to Chemical Micropatterning of Poly(Dimethylsiloxane). Angew. Chem. 2007, 119, 6754–6757. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas Sorption, Diffusion, and Permeation in Poly(Dimethylsiloxane). J. Polym. Sci. B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Giri, K.; Tsao, C.-W. Recent Advances in Thermoplastic Microfluidic Bonding. Micromachines 2022, 13, 486. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2022, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.D.; Tracey, M.C.; Davis, J.B.; Tan, C.K.L. Micro throttle pump employing displacement amplification in an elastomeric substrate. J. Micromech. Microeng. 2005, 15, 1831–1839. [Google Scholar] [CrossRef]
- Wu, X.; Kim, S.-H.; Ji, C.-H.; Allen, M.G. A solid hydraulically amplified piezoelectric microvalve. J. Micromech. Microeng. 2011, 21, 095003. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takama, N.; Kim, B.; Sharma, K.; Paul, O.; Ruther, P. Microfluidic Chip to Interface Porous Microneedles for ISF Collection. Biomed. Microdevices 2019, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.; Sousa, P.; Pinto, V.; Soares, D.; Lima, R.; Minas, G.; Rodrigues, R.O. PDMS porous microneedles used as engineered tool in advanced microfluidic devices and their proof-of-concept for biomarker detection. Chem. Eng. J. 2024, 485, 149725. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujii, T.; Nojima, T. PDMS–Glass Hybrid Microreactor Array with Embedded Temperature Control Device. Application to Cell-Free Protein Synthesis. Lab Chip 2002, 2, 197–202. [Google Scholar] [CrossRef]
- Bozukova, D.; Pagnoulle, C.; Jérôme, R.; Jérôme, C. Polymers in modern ophthalmic implants—Historical background and recent advances. Mater. Sci. Eng: R Rep. 2010, 69, 63–83. [Google Scholar] [CrossRef]
- Yazdi, S.G.; Geoghegan, P.H.; Docherty, P.D.; Jermy, M.; Khanafer, A. A Review of Arterial Phantom Fabrication Methods for Flow Measurement Using PIV Techniques. Ann. Biomed. Eng. 2018, 46, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Doutel, E.; Viriato, N.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Geometrical effects in the hemodynamics of stenotic and non-stenotic left coronary arteries—Numerical and in vitro approaches. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3207. [Google Scholar] [CrossRef] [PubMed]
- Sadek, S.H.; Rubio, M.; Lima, R.; Vega, E.J. Blood Particulate Analogue Fluids: A Review. Materials 2021, 14, 2451. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of Microfluidic Systems in Poly(Dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Fukui, T.; Funamoto, K.; Tanaka, M.; Tanaka, M.; Murata, S.; Miyauchi, S.; Hayase, T. Viscosity Estimation of a Suspension with Rigid Spheres in Circular Microchannels Using Particle Tracking Velocimetry. Micromachines 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ono, D.; Sugita, S. Mechanophenotyping of B16 Melanoma Cell Variants for the Assessment of the Efficacy of (-)-Epigallocatechin Gallate Treatment Using a Tapered Microfluidic Device. Micromachines 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Pitts, K.L.; Mehri, R.; Mavriplis., C.; Fenech, M. Micro-particle image velocimetry measurement of blood flow: Validation and analysis of data pre-processing and processing methods. Meas Sci Technol. 2012, 23, 105302. [Google Scholar] [CrossRef]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, H. Red cell motions and wall interactions in tube flow. Fed. Proc. 1971, 30, 1578–1588. [Google Scholar] [PubMed]
- Goldsmith, H. Deformation of human red cells in tube flow. Biorheology 1971, 7, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef]
- Zhao, J.; Sheadel, D.A.; Xue, W. Surface treatment of polymers for the fabrication of all-polymer MEMS devices. Sens. Actuators A Phys. 2012, 187, 43–49. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of Polydimethylsiloxane (PDMS) Properties for Biomedical Micro/Nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Morbioli, G.G.; Speller, N.C.; Stockton, A.M. A practical guide to rapid-prototyping of PDMS-based microfluidic devices: A tutorial. Anal. Chim. Acta 2020, 1135, 150–174. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J. Microelectromech. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Gharib, G.; Bütün, İ.; Muganlı, Z.; Kozalak, G.; Namlı, İ.; Sarraf, S.S.; Ahmadi, V.E.; Toyran, E.; van Wijnen, A.J.; Koşar, A. Biomedical Applications of Microfluidic Devices: A Review. Biosensors 2022, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Bhushan, B. Transparent, wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid. Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Abkarian, M.; Faivre, M.; Horton, R.; Smistrup, K.; Best-Popescu, C.A.; Stone, H.A. Cellular-scale hydrodynamics. Biomed. Mater. 2008, 3, 034011. [Google Scholar] [CrossRef]
- Bento, D.; Rodrigues, R.O.; Faustino, V.; Pinho, D.; Fernandes, C.S.; Pereira, A.I.; Garcia, V.; Miranda, J.M.; Lima, R. Deformation of Red Blood Cells, Air Bubbles, and Droplets in Microfluidic Devices: Flow Visualizations and Measurements. Micromachines 2018, 9, 151. [Google Scholar] [CrossRef]
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593. [Google Scholar] [CrossRef]
- Cierpka, C.; Kähler, C.J. Particle imaging techniques for volumetric three-component (3D3C) velocity measurements in microfluidics. J. Vis. 2012, 15, 1–31. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, Y.V.B.V.; Prabhakar, A.; Joshi, S.S.; Agrawal, A. Passive blood plasma separation at the microscale: A review of design principles and microdevices. J. Micromech. Microeng. 2015, 25, 083001. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H.M.N. Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; Rodrigues, R.O.; Moita, A.S.; Hori, T.; Kaji, H.; Lima, R.A.; Minas, G. Recent trends of biomaterials and biosensors for organ-on-chip platforms. Bioprinting 2022, 26, e00202. [Google Scholar] [CrossRef]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gomez-Sjoberg, R.; Fleming, R.M.T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, Y.; Khandurina, J.; Guttman, A.; Lo, Y.-H. UV/Ozone Modification of Poly (Dimethylsiloxane) Microfluidic Channels. Sens. Actuators B Chem. 2004, 97, 402–408. [Google Scholar] [CrossRef]
- Fatona, A.; Chen, Y.; Reid, M.; Brook, M.A.; Moran-Mirabal, J.M. One-Step in-Mould Modification of PDMS Surfaces and Its Application in the Fabrication of Self-Driven Microfluidic Channels. Lab Chip 2015, 15, 4322–4330. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.B.; Afonso, I.S.; Nobrega, G.; Barbosa, L.G.; Lima, R.A.; Ribeiro, J.E. A Review of Methods to Modify the PDMS Surface Wettability and Their Applications. Micromachines 2024, 15, 670. [Google Scholar] [CrossRef]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Petrou, P.S.; Kakabakos, S.E.; Tserepi, A.; Beltsios, K.; Gogolides, E. Effect of surface nanostructuring of PDMS on wetting properties, hydrophobic recovery and protein adsorption. Microelectron. Eng. 2009, 86, 1321–1324. [Google Scholar] [CrossRef]
- Cai, D.K.; Neyer, A.; Kuckuk, R.; Heise, H.M. Optical absorption in transparent PDMS materials applied for multimode waveguides fabrication. Opt. Mater. 2008, 30, 1157–1161. [Google Scholar] [CrossRef]
- Stankova, N.E.; Atanasov, P.A.; Nikov, R.G.; Nikov, R.G.; Nedyalkov, N.N.; Stoyanchov, T.R.; Fukata, N.; Kolev, K.N.; Valova, E.I.; Georgieva, J.S.; et al. Optical properties of polydimethylsiloxane (PDMS) duringnanosecond laser processing. Appl. Surf. Sci. 2016, 374, 96–103. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Gokaltun, A.; Yarmush, M.L.; Asatekin, A.; Usta, O.B. Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology. Technology 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Mark, J.E. Polymer Data Handbook; Mark, J.E., Ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Souza, R.R.; Barbosa, F.M.S.; Nobrega, G.; Cardoso, E.M.; Teixeira, J.C.F.; Moita, A.S.; Lima, R. Experimental study of an innovative elastomer-based heat exchanger. Case Stud. Therm. Eng. 2023, 49, 103365. [Google Scholar] [CrossRef]
- Hong, J.; Lee, J.; Hong, C.; Shim, S. Effect of dispersion state of carbon nanotube on the thermal conductivity of poly(dimethyl siloxane) composites. Curr. Appl. Phys. 2010, 10, 359–363. [Google Scholar] [CrossRef]
- Xu, G.; Ni, Z.; Chen, X.; Tu, J.; Guo, X.; Bruus, H.; Zhang, D. Acoustic characterization of polydimethylsiloxane for microscale Acoustofluidics. Phys. Rev. Appl. 2020, 13, 54069. [Google Scholar] [CrossRef]
- Tsou, J.K.; Liu, J.; Barakat, A.I.; Insana, M.F. Role of ultrasonic shear rate estimation errors in assessing inflammatory response and vascular risk. Ultrasound Med. Biol. 2008, 34, 963. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Sathoff, H.J.; Zagzebski, J.A. Ultrasonic shear wave properties of soft tissues and tissuelike materials. J. Acoust. Soc. Am. 1983, 74, 1346. [Google Scholar] [CrossRef]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. J. Micromech Microeng 2014, 24, 035017. [Google Scholar] [CrossRef]
- Müller, A.; Wapler, M.C.; Wallrabe, U. A quick and accurate method to determine the Poisson’s ratio and the coefficient of thermal expansion of PDMS. Soft Matter 2019, 15, 779–784. [Google Scholar] [CrossRef] [PubMed]
- The Dow SYLGARDTM 184 Silicone Elastomer Technical Datasheet. Silicone Elastomer Technical Data Sheet. 2017. Available online: https://consumer.dow.com/en-us/document-viewer.html?ramdomVar=3835418757322904567&docPath=/documents/en-us/productdatasheet/11/11-31/11-3184-sylgard-184-elastomer.pdf (accessed on 9 December 2024).
- Zhang, G.; Sun, Y.; Qian, B.; Gao, H.; Zuo, D. Experimental study on mechanical performance of polydimethylsiloxane (PDMS) at various temperatures. Polym. Test. 2020, 90, 106670. [Google Scholar] [CrossRef]
- Souza, A.; Lopes, D.; Souza, S.; Ribeiro, J.; Lima, R.A.; Ferrera, C. Experimental and numerical analyses of the hemodynamics impact on real intracranial aneurysms: A particle tracking approach. Results Eng. 2024, 24, 103566. [Google Scholar] [CrossRef]
- Garg, N.; Westerhof, T.M.; Liu, V.; Liu, R.; Nelson, E.L.; Lee, A.P. Whole-blood sorting, enrichment and in situ immunolabeling of cellular subsets using acoustic microstreaming. Microsyst. Nanoeng. 2018, 4, 17085. [Google Scholar] [CrossRef]
- Tang, T.; Yuan, Y.; Yalikun, Y.; Hosokawa, Y.; Li, M.; Tanaka, Y. Glass based micro total analysis systems: Materials, fabrication methods, and applications. Sens. Actuators B Chem. 2021, 339, 129859. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Connacher, W.; Zhang, N.; Huang, A.; Mei, J.; Zhang, S.; Gopesh, T.; Friend, J. Micro/nano acoustofluidics: Materials, phenomena, design, devices, and applications. Lab Chip 2018, 18, 1952. [Google Scholar] [CrossRef]
- Park, J.; Cha, B.; Almus, F.G.; Sahin, M.A.; Kang, H.; Kang, Y.; Destgeer, G.; Park, J. Acoustic Waves Coupling with Polydimethylsiloxane in Reconfigurable Acoustofluidic Platform. Adv. Sci. 2024, 11, 202407293. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L.Y. Microscale acoustofluidics: Microfluidics driven via acoustics and ultrasonics. Rev. Mod. Phys. 2011, 83, 647. [Google Scholar] [CrossRef]
- Lenshof, A.; Magnusson, C.; Laurell, T. Acoustofluidics 8: Applications of acoustophoresis in continuous flow microsystems. Lab Chip 2012, 12, 1210. [Google Scholar] [CrossRef]
- Ozcelik, A.; Rufo, J.; Guo, F.; Gu, Y.; Li, P.; Lata, J.; Huang, T.J. Acoustic tweezers for the life sciences. Nat. Methods 2018, 15, 1021. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Qiu, H. Acoustically driven micro-thermal-bubble dynamics in a microspace. J. Micromech. Microeng. 2010, 20, 095012. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Q.; Du, S.; Chen, Z.C.; Fu, J.Z.; Chen, B.; Liu, Z.J.; He, Y. Fabrication of cerebral aneurysm simulator with a desktop 3D printer. Sci. Rep. 2017, 7, 44301. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, H.; Woidt, C.; Istock, A.; Kobylinskiy, A.; Nguyen, D.T.; Ahmed, N.; Brunner, R.; Kusserow, T. Role of Nanoimprint Lithography for Strongly Miniaturized Optical Spectrometers. Nanomaterials 2021, 11, 164. [Google Scholar] [CrossRef]
- Unno, N.; Mäkelä, T. Thermal Nanoimprint Lithography—A Review of the Process, Mold Fabrication, and Material. Nanomaterials 2023, 13, 2031. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, F. Recent progress on femtosecond laser micro-/nano-fabrication of functional photonic structures in dielectric crystals: A brief review and perspective. APL Photonics 2023, 8, 090901. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Wang, T.; Zhang, L. Recent Developments of Femtosecond Laser Direct Writing for Meta-Optics. Nanomaterials 2023, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Long, H.P.; Lai, C.C.; Chung, C.K. Polyethylene Glycol Coating for Hydrophilicity Enhancement of Polydimethylsiloxane Self-Driven Microfluidic Chip. Surf. Coat. Technol. 2017, 320, 315–319. [Google Scholar] [CrossRef]
- Gökaltun, A.; Kang, Y.B.; Yarmush, M.L.; Usta, O.B.; Asatekin, A. Simple Surface Modification of Poly(dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics. Sci. Rep. 2019, 9, 7377. [Google Scholar] [CrossRef]
- Vickers, J.A.; Caulum, M.M.; Henry, C.S. Generation of Hydrophilic Poly(Dimethylsiloxane) for High-Performance Microchip Electrophoresis. Anal. Chem. 2006, 78, 7446–7452. [Google Scholar] [CrossRef]
- Peterson, S.L.; McDonald, A.; Gourley, P.L.; Sasaki, D.Y. Poly(Dimethylsiloxane) Thin Films as Biocompatible Coatings for Microfluidic Devices: Cell Culture and Flow Studies with Glial Cells. J. Biomed. Mater. Res. Part A 2005, 72A, 10–18. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent Developments in PDMS Surface Modification for Microfluidic Devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chung, C.-K. PDMS Microfabrication and Design for Microfluidics and Sustainable Energy Application: Review. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic Surface Modification of PDMS for Droplet Microfluidics Using a Simple, Quick, and Robust Method via PVA Deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef] [PubMed]
- Holczer, E.; Fürjes, P. Effects of Embedded Surfactants on the Surface Properties of PDMS; Applicability for Autonomous Microfluidic Systems. Microfluid. Nanofluid. 2017, 21, 81. [Google Scholar] [CrossRef]
- Vilčáková, J.; Moučka, R.; Svoboda, P.; Ilčíková, M.; Kazantseva, N.; Hřibová, M.; Mičušík, M.; Omastová, M. Effect of Surfactants and Manufacturing Methods on the Electrical and Thermal Conductivity of Carbon Nanotube/Silicone Composites. Molecules 2012, 17, 13157–13174. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hjort, K. Surface Modification of PDMS by Gradient-Induced Migration of Embedded Pluronic. Lab Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef]
- Gonçalves, I.M.; Borges, J.; Faustino, V.; Soares, D.; Vaz, F.; Minas, G.; Lima, R.; Pinho, D. Polydimethylsiloxane Surface Modification of Microfluidic Devices for Blood Plasma Separation. Polymers 2024, 16, 1416. [Google Scholar] [CrossRef]
- Bubendorfer, A.; Liu, X.; Ellis, A.V. Microfabrication of PDMS microchannels using SU-8/PMMA moldings and their sealing to polystyrene substrates. Smart Mater. Struct. 2007, 16, 367–371. [Google Scholar] [CrossRef]
- Pinho, D.; Faustino, V.; Catarino, S.O.; Pereira, A.I.; Minas, G.; Pinho, F.T.; Lima, R. Label-free multi-step microfluidic device for mechanical characterization of blood cells: Diabetes type II. Micro Nano Eng. 2022, 16, 100149. [Google Scholar] [CrossRef]
- Hou, H.W.; Li, Q.S.; Lee, G.Y.H.; Kumar, A.P.; Ong, C.N.; Lim, C.T. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 2009, 11, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Shelby, J.P.; White, J.; Ganesan, K.; Rathod, P.K.; Chiu, D.T. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum -infected erythrocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 14618–14622. [Google Scholar] [CrossRef]
- Le, A.V.; Fenech, M. Image-Based Experimental Measurement Techniques to Characterize Velocity Fields in Blood Microflows. Front. Physiol. 2022, 13, 886675. [Google Scholar] [CrossRef]
- Chenouard, N.; Smal, I.; de Chaumont, F.; Maška, M.; Sbalzarini, I.F.; Gong, Y.; Cardinale, J.; Carthel, C.; Coraluppi, S.; Winter, M. Objective comparison of particle tracking methods. Nat. Method. 2014, 11, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Antaki, J.F.; Naik, T.; Bachman, T.N.; Kameneva, M.V.; Wu, Z.J. Microscopic investigation of erythrocyte deformation dynamics. Biorheology 2006, 43, 747–765. [Google Scholar] [CrossRef]
- Forsyth, A.M.; Wan, J.; Ristenpart, W.D.; Stone, H.A. The dynamic behavior of chemically “stiffened” red blood cells in microchannel flows. Microvasc. Res. 2010, 80, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Tse, H.T.K.; Lee, S.A.; Ying, Y.; Lindgren, A.G.; Yang, O.O.; Rao, J.; Clark, A.T.; Carlo, D.D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl. Acad. Sci. USA 2012, 109, 7630–7635. [Google Scholar] [CrossRef] [PubMed]
- Faustino, V.; Rodrigues, R.O.; Pinho, D.; Costa, E.; Santos-Silva, A.; Miranda, V.; Amaral, J.S.; Lima, R. A Microfluidic Deformability Assessment of Pathological Red Blood Cells Flowing in a Hyperbolic Converging Microchannel. Micromachines 2019, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Reale, R.; De Ninno, A.; Nepi, T.; Bisegna, P.; Caselli, F. Extensional-Flow Impedance Cytometer for Contactless and Optics-Free Erythrocyte Deformability Analysis. IEEE Trans. Biomed. Eng. 2023, 70, 565–572. [Google Scholar] [CrossRef]
- Lima, R.; Vega, E.J.; Moita, A.S.; Miranda, J.M.; Pinho, D.; Moreira, A.L.N. Fast, flexible and low-cost multiphase blood analogue for biomedical and energy applications. Exp. Fluids 2020, 61, 231. [Google Scholar] [CrossRef]
- Lee, S.S.; Yim, Y.; Ahn, K.H.; Lee, S.J. Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed. Microdevices 2009, 11, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zografos, K.; Pimenta, F.; Alves, M.A.; Oliveira, M.S.N. Microfluidic converging/diverging channels optimised for homogeneous extensional deformation. Biomicrofluidics 2016, 10, 043508. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.F.; Ristenpart, W.D. Mechanical response of red blood cells entering a constriction. Biomicrofluidics 2014, 8, 064123. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Sakai, Y.; Fujii, T. Cell Culture in 3-Dimensional Microfluidic Structure of PDMS (polydimethylsiloxane). Biomed. Microdevices 2003, 5, 109–114. [Google Scholar] [CrossRef]
- Ohashi, T.; Sato, M. Endothelial Cell Responses to Fluid Shear Stress: From Methodology to Applications. In Single and Two-Phase Flows on Chemical and Biomedical Engineering; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 579–599. [Google Scholar] [CrossRef]
- Torino, S.; Corrado, B.; Iodice, M.; Coppola, G. PDMS-Based Microfluidic Devices for Cell Culture. Inventions 2018, 3, 65. [Google Scholar] [CrossRef]
- Fiddes, L.K.; Raz, N.; Srigunapalan, S.; Tumarkan, E.; Simmons, C.A.; Wheeler, A.R.; Kumacheva, E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials 2010, 31, 3459–3464. [Google Scholar] [CrossRef]
- Shin, M.; Matsuda, K.; Ishii, O.; Terai, H.; Kaazempur-Mofrad, M.; Borenstein, J.; Detmar, M.; Vacanti, J.P. Endothelialized Networks with a Vascular Geometry in Microfabricated Poly(dimethyl siloxane). Biomed. Microdevices 2004, 6, 269–278. [Google Scholar] [CrossRef]
- Shevkoplyas, S.S.; Yoshida, T.; Gifford, S.C.; Bitensky, M.W. Direct measurement of the impact of impaired erythrocyte deformability on microvascular network perfusion in a microfluidic device. Lab Chip 2006, 6, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Noh, S.; Kim, H. A multiple-channel, multiple-assay platform for characterization of full-range shear stress effects on vascular endothelial cells. Lab Chip 2014, 14, 1880–1890. [Google Scholar] [CrossRef]
- Zhou, Q.; Fidalgo, J.; Bernabeu, M.O.; Oliveira, M.S.N.; Krüger, T. Emergent cell-free layer asymmetry and biased haematocrit partition in a biomimetic vascular network of successive bifurcations. Soft Matter 2021, 17, 3619–3633. [Google Scholar] [CrossRef] [PubMed]
- Belenkovich, M.; Veksler, R.; Kreinin, Y.; Mekler, T.; Flores, M.; Sznitman, J.; Holinstat, M.; Korin, N. Clot Accumulation in 3D Microfluidic Bifurcating Microvasculature Network. Micromachines 2024, 15, 988. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, J. Two-dimensional lattice Boltzmann study of red blood cell motion through microvascular bifurcation: Cell deformability and suspending viscosity effects. Biomech. Model. Mechanobiol. 2012, 11, 575–583. [Google Scholar] [CrossRef]
- Kodama, Y.; Aoki, H.; Yamagata, Y.; Tsubota, K. In vitro analysis of blood flow in a microvascular network with realistic geometry. J. Biomech. 2019, 88, 88–94. [Google Scholar] [CrossRef]
- Kaliviotis, E.; Sherwood, J.M.; Balabani, S. Partitioning of red blood cell aggregates in bifurcating microscale flows. Sci. Rep. 2017, 7, 44563. [Google Scholar] [CrossRef]
- Popel, A.S.; Johnson, P.C. Microcirculation and hemorheology. Annu. Rev. Fluid. Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Fujiwara, H.; Matsuki, N.; Yoshimoto, T.; Imai, Y.; Ueno, H.; Yamaguchi, T. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence. Biomed. Microdevices 2011, 13, 159–167. [Google Scholar] [CrossRef]
- Bento, D.; Fernandes, C.S.; Miranda, J.M.; Lima, R. In vitro blood flow visualizations and cell-free layer (CFL) measurements in a microchannel network. Exp. Therm. Fluid. Sci. 2019, 109, 109847. [Google Scholar] [CrossRef]
- Bento, D.; Lopes, S.; Maia, I.; Lima, R.; Miranda, J.M. Bubbles Moving in Blood Flow in a Microchannel Network: The Effect on the Local Hematocrit. Micromachines 2020, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Bessho, S.; Wada, S. Spring-network-based model of a red blood cell for simulating mesoscopic blood flow. Int. J. Numer. Method. Biomed. Eng. 2013, 29, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Kawaguchi, M.; Morinishi, K. A two-way coupling scheme to model the effects of particle rotation on the rheological properties of a semidilute suspension. Comput. Fluids 2018, 173, 6–16. [Google Scholar] [CrossRef]
- Kamada, H.; Tsubota, K.; Nakamura, M.; Wada, S.; Ishikawa, T.; Yamaguchi, T. A three-dimensional particle simulation of the formation and collapse of a primary thrombus. Int. J. Numer. Method. Biomed. Eng. 2010, 26, 488–500. [Google Scholar] [CrossRef]
- Gracka, M.; Lima, R.; Miranda, J.M.; Student, S.; Melka, B.; Ostrowski, Z. Red blood cells tracking and cell-free layer formation in a microchannel with hyperbolic contraction: A CFD model validation. Comput. Methods Programs Biomed. 2022, 226, 107117. [Google Scholar] [CrossRef]
- Carvalho, V.; Gonçalves, I.M.; Rodrigues, N.; Sousa, P.; Pinto, V.; Minas, G.; Kaji, H.; Shin, S.R.; Rodrigues, R.O.; Teixeira, S.F.C.F.; et al. Numerical evaluation and experimental validation of fluid flow behavior within an organ-on-a-chip model. Comput. Methods Programs Biomed. 2024, 243, 107883. [Google Scholar] [CrossRef]
- Ensley, A.E.; Lynch, P. Toward Designing the Optimal Total Cavopulmonary Connection: An In Vitro Study. Ann. Thorac. Surg. 1999, 68, 1384–1390. [Google Scholar] [CrossRef]
- Helgeson, Z.L. Particle Trajectories and Agglomeration/Accumulation in Branching Arteries Subjected to Orbital Atherectom. Open Biomed. Eng. J. 2011, 5, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Siebes, M.; Campbell, C.S.; D’argenio, D.Z. Fluid dynamics of a partially collapsible stenosis in a flow model of the coronary circulation. J. Biomech. Eng. 1996, 118, 489–497. [Google Scholar] [CrossRef]
- Porenta, G.; Schima, H.; Pentaris, A.; Tsangaris, S.; Moertl, D.; Probst, P.; Maurer, G.; Baumgartner, H. Assessment of coronary stenoses by Doppler wires: A validation study using in vitro modeling and computer simulations. Ultrasound Med. Biol. 1999, 25, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Benard, N.; Coisne, D.; Donal, E.; Perrault, R. Experimental study of laminar blood flow through an artery treated by a stent implantation: Characterisation of intra-stent wall shear stress. J. Biomech. 2003, 36, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Araci, I.E.; Quake, S.R. Microfluidic very large scale integration (mVLSI) with integrated micromechanical valves. Lab. Chip 2012, 12, 2803–2806. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Souza, M.S.; Pinho, D.; Agujetas, R.; Ferrera, C.; Lima, R.; Puga, H.; Ribeiro, J. 3D manufacturing of intracranial aneurysm biomodels for flow visualizations: Low cost fabrication processes. Mech. Res. Commun. 2020, 107, 103535. [Google Scholar] [CrossRef]
- Parlea, L.; Fahrig, R.; Holdsworth, D.W.; Lownie, S.P. An Analysis of the Geometry of Saccular Intracranial Aneurysms. Am. J. Neuroradiol. 1999, 20, 1079–1089. [Google Scholar] [PubMed]
- Rodrigues, R.O.; Pinho, D.; Bento, D.; Lima, R.; Ribeiro, J. Wall expansion assessment of an intracranial aneurysm model by a 3D Digital Image Correlation System. Measurement 2016, 88, 262–270. [Google Scholar] [CrossRef]
- Falk, K.L.; Medero, R.; Roldán-Alzate, A. Fabrication of Low-Cost Patient-Specific Vascular Models for Particle Image Velocimetry. Cardiovasc. Eng. Technol. 2019, 10, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Karam, S.; Shirdade, N.; Madden, B.; Rheinstadter, J.; Church, E.W.; Brindise, M.C.; Manograhan, G. Additive manufacturing of patient-specific high-fidelity and thickness-controlled cerebral aneurysm geometries. Manuf. Lett. 2023, 35, 770–777. [Google Scholar] [CrossRef]
- Ford, M.D.; Milner, J.S.; Lownie, S.P.; Demont, E.M.; Holdsworth, D.W.; Steinman, D.A. PIV-Measured Versus CFD-Predicted Flow Dynamics in Anatomically Realistic Cerebral. J. Biomech. Eng. 2008, 130, 021015. [Google Scholar] [CrossRef]
- Doutel, E.; Carneiro, J.; Oliveira, M.S.N.; Campos, J.B.L.M.; Miranda, J.M. Fabrication of 3d mili-scale channels for hemodynamic studies. J. Mech. Med. Biol. 2015, 15, 1550004. [Google Scholar] [CrossRef]
- Doutel, E.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Artificial stenoses for computational hemodynamics. Appl. Math. Model. 2018, 59, 427–440. [Google Scholar] [CrossRef]
- Jewkes, R.; Burton, H.E.; Espino, D.M. Towards additive manufacture of functional, spline-based morphometric models of healthy and diseased coronary arteries: In vitro proof-of-concept using a porcine template. J. Funct. Biomater. 2018, 9, 15. [Google Scholar] [CrossRef]
- Kefayati, S.; Poepping, T.L. Transitional flow analysis in the carotid artery bifurcation by proper orthogonal decomposition and particle image velocimetry. Med. Eng. Phys. 2013, 35, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Kefayati, S.; Holdsworth, D.W.; Poepping, T.L. Turbulence intensity measurements using particle image velocimetry in diseased carotid artery models: Effect of stenosis severity, plaque eccentricity, and ulceration. J. Biomech. 2014, 47, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kefayati, S.; Milner, J.S.; Holdsworth, D.W.; Poepping, T.L. In vitro shear stress measurements using particle image velocimetry in a family of carotid artery models: Effect of stenosis severity, plaque eccentricity, and ulceration. PLoS ONE 2014, 9, e98209. [Google Scholar] [CrossRef]
- Choi, W.; Park, J.H.; Byeon, H.; Lee, S.J. Flow characteristics around a deformable stenosis under pulsatile flow condition. Phys. Fluids 2018, 30, 011902. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Kang, S.-W.; Wu, T.-Y. Fabrication of polydimethylsiloxane (PDMS) pulsating heat pipe. Appl. Therm. Eng. 2009, 29, 573–580. [Google Scholar] [CrossRef]
- Lima, R.; Catarino, S.O.; Minas, G.M.H.; De Lima, R.A.M.M.; Souza, R.R.; Moita, A.; Bañobre-López, M.; Moreira, A.L.N.; Barbosa, F.M.S.; Teixeira, J.C.; et al. Elastomer Composite Serpentine for a Heat Exchanger, Method for Obtaining it and Its Uses. Patent Number PT118128, 26 January 2024. Available online: https://pt.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=2&ND=3&adjacent=true&locale=pt_PT&FT=D&date=20240126&CC=PT&NR=118128A&KC=A (accessed on 10 December 2024).
- Jung, S.Y.; Park, J.H.; Lee, S.J.; Park, H. Heat Transfer and Flow Characteristics of Forced Convection in PDMS Microchannel Heat Sink. Exp. Therm. Fluid. Sci. 2019, 109, 109904. [Google Scholar] [CrossRef]
- Chuang, H.-S.; Wereley, S. Design, fabrication and characterization of a conducting PDMS for microheaters and temperature sensors. J. Micromech. Microeng. 2009, 19, 045010. [Google Scholar] [CrossRef]
- Hashimoto, M.; Chen, P.-C.; Mitchell, M.W.; Nikitopoulos, D.E.; Soperb, S.A.; Murphy, M.C. Rapid PCR in a continuous flow device. Lab Chip 2004, 4, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Awang, R.A.; Rowe, W.S.T.; Kalantar-zadeh, K.; Khoshmanesh, K. PDMS Nanocomposites for Heat Transfer Enhancement in Microfluidic Platforms. Lab Chip 2014, 14, 3419. [Google Scholar] [CrossRef]
- Souza, R.R.; Sá Barbosa, F.M.; Nobrega, G.; Cardoso, E.M.; Teixeira, J.C.F.; Moita, A.S.; Lima, R. An Innovative PDMS Cell to Improve the Thermal Conductivity Measurements of Nanofluids. Therm. Sci. Eng. Progress. 2023, 42, 101926. [Google Scholar] [CrossRef]
- Assael, M.J.; Antoniadis, K.D.; Wakeham, W.A. Historical evolution of the transient hot-wire technique. Int. J. Thermophys. 2010, 31, 1051–1072. [Google Scholar] [CrossRef]
- Mbunge, E.; Simelane, S.; Fashoto, S.G.; Akinnuwesi, B.; Metfula, A.S. Application of deep learning and machine learning models to detect COVID-19 face masks—A review. Sustain. Oper. Comput. 2021, 2, 235–245. [Google Scholar] [CrossRef]
- Wang, W.; Chen, T.; Li, Z.; Tan, Q.; Meng, Z.; Qiu, H.; Liu, X.; Zheng, J. Comparison of filtration efficiency and respiratory resistance of COVID-19 protective masks by multi-national standards. Am. J. Infect. Control 2022, 50, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.A.; Teixeira, S.; Minas, G.; Rodrigues, C.; Carvalho, V. i9MASKS Workshop: Extended Abstracts; UMinho Editora: Braga, Portugal, 2022. [Google Scholar] [CrossRef]
- EN 14683:2019; Medical Face Masks—Requirements and Test Methods. European Union: Brussels, Belgium, 2019.
- Lima, R.; Catarino, S.O.; Minas, G.M.H.; De Lima, R.A.M.M.; Souza, R.R.; Moita, A.; Bañobre-López, M.; Moreira, A.L.N.; Barbosa, F.M.S.; Teixeira, J.C.; et al. Face Mask, Methods for obtaining and Using it. Patent Number PT117823, 28 August 2023. Available online: https://pt.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=4&ND=3&adjacent=true&locale=pt_PT&FT=D&date=20230828&CC=PT&NR=117823A&KC=A (accessed on 10 December 2024).
- Hashemzadeh, H.; Allahverdi, A.; Sedghi, M.; Vaezi, Z.; Tohidi Moghadam, T.; Rothbauer, M.; Fischer, M.B.; Ertl, P.; Naderi-Manesh, H. PDMS Nano-Modified Scaffolds for Improvement of Stem Cells Proliferation and Differentiation in Microfluidic Platform. Nanomaterials 2020, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, B.; Sun, D.; Lin, H.; Xiao, R.; Liu, H. Pervaporation of the polydimethylsiloxane composite membranes filled with hydroxy-terminated silicone oil modified nano-silica. Desalination Water Treat. 2023, 282, 23–32. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef] [PubMed]
- Majidi, C. Soft-Matter Engineering for Soft Robotics. Adv. Mater. Technol. 2019, 4, 1800477. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; He, J.; Liu, W.; Wang, Y.; Huang, Z.; Pang, H.; Chen, Y. Functional PDMS Elastomers: Bulk Composites, Surface Engineering, and Precision Fabrication. Adv. Sci. 2023, 10, 2304506. [Google Scholar] [CrossRef] [PubMed]
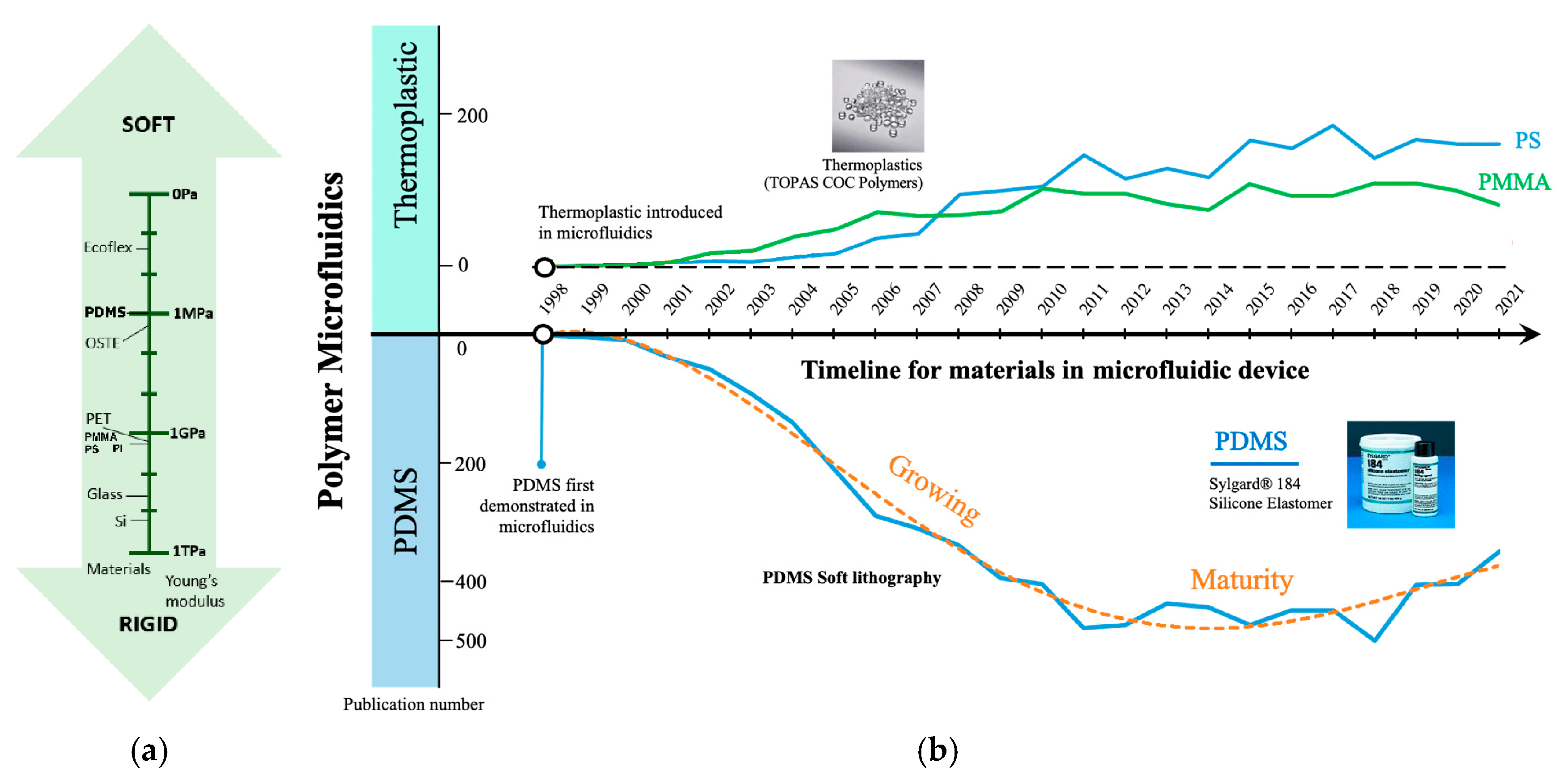
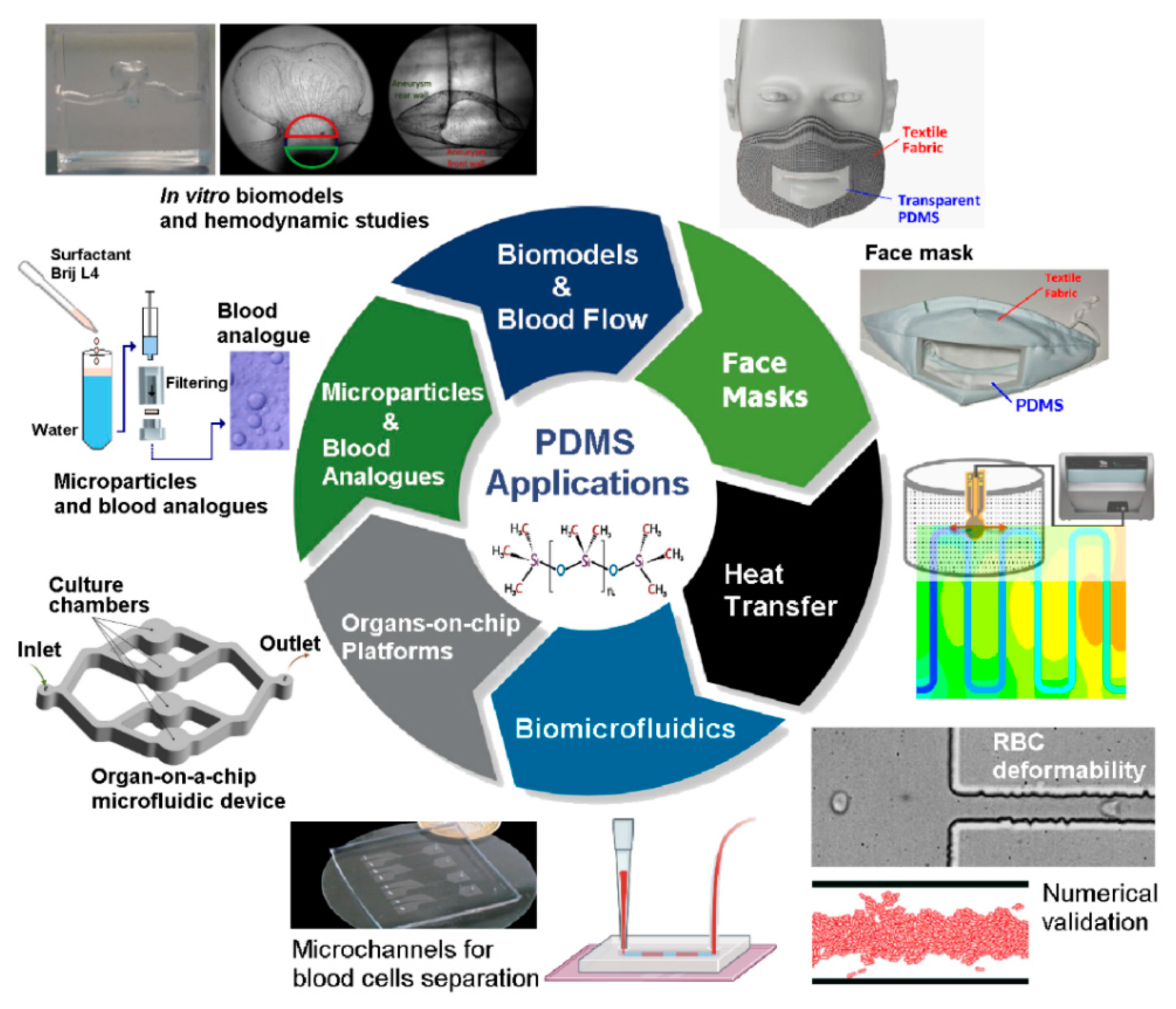
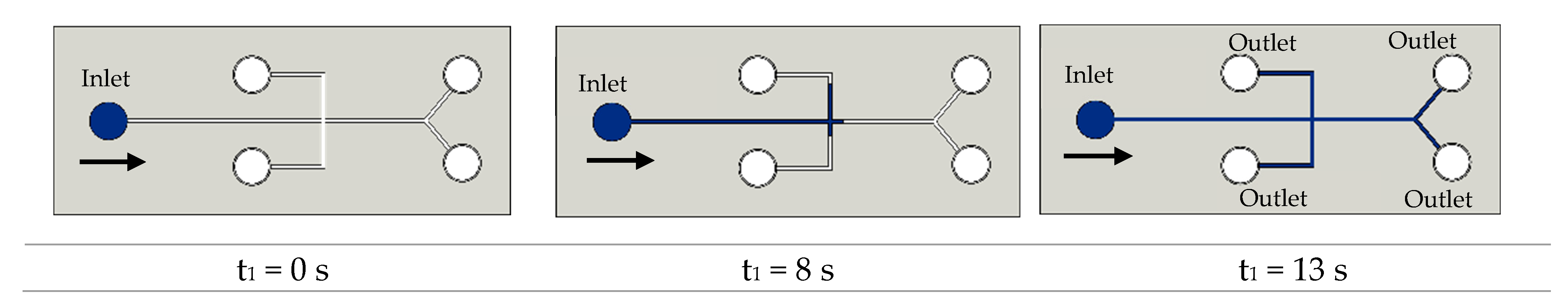


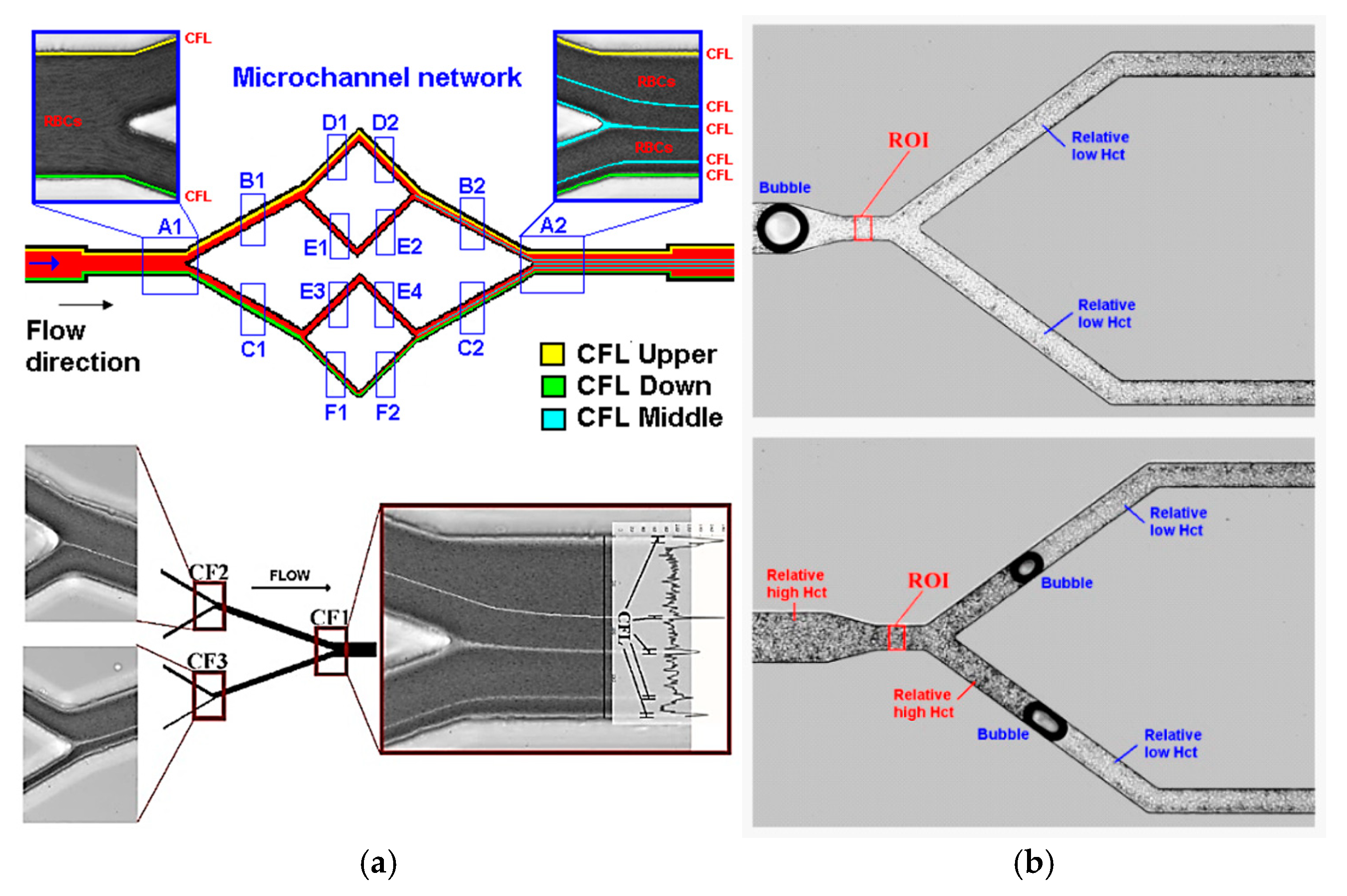




| Property | Value | References |
|---|---|---|
| Optical transparency | 240–1100 (nm) | [51,52,53] |
| Hydrophobicity—contact angle | ~108 ± 7 (°) | [54] |
| Refraction index | 1.4 | [55] |
| Thermal conductivity | 0.2–0.27 (W/m∙K) | [56,57] |
| Specific heat | 1.46 (kJ/kg∙K) | [55] |
| Electrical conductivity | 4 × 1013 (ohm∙m) | [55] |
| Longitudinal wave velocity | 1028.3–1119.1 (m/s) | [58,59] |
| Shear wave velocity | 75–124.3 (m/s) | [58,60] |
| Young’s elastic modulus | ~1–3 (MPa) | [29,61] |
| Poisson ratio | 0.5 | [62] |
| Tensile strength | 2.24–6.7 (MPa) | [55,63] |
| Hardness | 41–43 (Shore A) | [64] |
| Density | 1029.4–1031.4 (kg/m3) | [58] |
| Viscosity | 3.5 (Pa∙s) | [63] |
| Materials | Main Advantages | Main Disadvantages |
|---|---|---|
| PDMS | Optical transparency, gas permeability, simple and low-cost fabrication, biocompatibility, variable elasticity, and cell culture. | Can absorb hydrophobic molecules, hydrophobic nature, difficult mass production, and its attenuation of acoustic waves. |
| Hydrogel | Low cost, allows diffusion of small molecules, biocompatibility, and cells can be loaded on the surface or to the bulk. | Degradable, weak mechanical strength, and requires freezing or drying for long-term storage. |
| Thermoplastics | Low-cost fabrication, optical transparency, and mass production. | Rigid, thermal degradation and thermal oxidative degradation in the presence of oxygen, and permeability inability. |
| 3D printing resins | Simple and low-cost fabrication, variable mechanical properties, and ability to create complex geometries. | Inadequate optical transparency, low gas permeability, surface roughness, and limited material choices depending on printer technology. |
| Glass | Optical transparency, inert, and excellent roughness. | Rigid, fragile, expensive, and difficult to reproduce complex geometries. |
| Silicon | Ability to create complex geometries at both micro and nano level, thermal stability, and chemical resistance. | High-cost fabrication, need for clean-room facilities, permeability inability, and no optical transparency. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.A. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids 2025, 10, 41. https://doi.org/10.3390/fluids10020041
Lima RA. The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids. 2025; 10(2):41. https://doi.org/10.3390/fluids10020041
Chicago/Turabian StyleLima, Rui A. 2025. "The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications" Fluids 10, no. 2: 41. https://doi.org/10.3390/fluids10020041
APA StyleLima, R. A. (2025). The Impact of Polydimethylsiloxane (PDMS) in Engineering: Recent Advances and Applications. Fluids, 10(2), 41. https://doi.org/10.3390/fluids10020041








