1. Introduction
Lost circulation refers to the phenomenon that drilling fluid leaks into the formation through fracture caused by the pressure of the drilling fluid being higher than the formation pressure during the drilling process, including permeable lost circulation, fractural lost circulation, and cavernous lost circulation. Lost circulation may result in matrix permeability decreasing, oil and gas channels becoming choked, non-production time (NPT) increasing, etc. [
1,
2], and in severe cases, even secondary reservoir contamination and well collapse [
3,
4,
5]. The conventional countermeasures use lost circulation materials (LCM) to prevent and treat leakage. Nowadays, those LCMs include bridging materials [
6,
7,
8], high-filtration materials [
9,
10,
11], and polymer materials [
12,
13,
14]. Compared with other formations, reservoir fractures may lead to leakage of drilling fluid during well workover operations. They can improve recovery efficiency during subsequent oil and gas recovery operations. Due to the weak physical properties of the reservoir, traditional high-strength plugging materials may cause irreversible damage to the reservoir, and subsequent unblocking operations are cumbersome; therefore, they are unsuitable to be used in the reservoir.
The concept of a temporary plugging agent (TPA), which can plug the fractures to prevent lost circulation during the operations of well workover and dissolve and unblock the fracture after well completion, is proposed. When injected into the formation with drilling fluid, TPA will seal the fractures during the operations of well workover and dissolve and unblock under certain conditions after well completion, with the consequence of not affecting the gas and oil recovery efficiency. According to the different soluble conditions, TPA can be divided into three types: acid-soluble TPA, oil soluble TPA, and water-soluble TPA. Acid-soluble TPA mainly contains cellulose, calcium carbonate, etc. What causes the consequence of limited plugging ability is the poor strength of cellulose and the terrible adaptability of calcium carbonate, and an additional acid solution shall be injected to dissolve TPA after well completion, which may cause contamination of the reservoir [
15,
16]. Oil-soluble TPA mainly refers to resin, which has many lipophilic groups. The resin commonly has a high physical capacity but is brittle and deforms in high-temperature conditions, which may cause mass loss and increase the sealing cost [
17,
18]. Water-soluble TPA mainly refers to those polymers that are composed of natural biodegradable material and have the advantages of good compatibility with drilling fluid and less damage to the environment compared with others. Conversely, the disadvantage of water-soluble TPA is that it is poor in stability and physical properties in high-temperature high-pressure (HTHP) [
19,
20].
Based on the above, a new plugging material named self-degradable pre-formed plugging gel (s-DPPG), which can degrade and unblock the fractures after well completion spontaneously, is supposed to be developed. It is currently reported that introducing hydrolyzable long molecular chains or crosslinkers achieves degradation spontaneously. The hydrolysis reaction of ester bonds is reversible. Under the high temperature and alkaline conditions of the reservoir, the hydrolysis of ester bonds is promoted, and the reaction between the generated carboxylic acid and the OH
− in the environment continues to promote the hydrolysis reaction. The “bridge” connecting different molecular chains breaks, and the spatial three-dimensional structure collapses into a linear structure. The repulsive force between molecular chains dominates as the distance between molecular chains increases and finally unblocks under pressure differences. Xiong et al. [
21] used polylactic acid (PLA), polyglycolic acid (PGA), polybutylene succinate (PBS), etc. prepolymers as row materials to prepare a polyester material whose degradation rate can reach 99% at 150 °C. Furthermore, the acidic environment can accelerate the process of degradation. Li et al. [
22] used PLA, acrylamide (AM), and N,N-Methylenebis(2-propenamide) (MBA) as row materials to prepare the degradable temporary material TZDJ with a core-shell structure. When the shell, which acts as the sacrificial agent and the shielding agent, is hydrolyzed, the core of PLA is exposed to the environment and attacked by water molecules, leading to hydrolysis with the consequence of unblocking the fractures. On the other hand, to achieve degradation, you can also introduce hydrolyzable crosslinkers. Zheng et al. [
23] used AM and acrylic acid (AA) as polymeric monomers and an azo-type thermosensitive crosslinker, which is prepared from azobisisobutyramidine hydrochloride (V-50) and maleic anhydride, to prepare a thermosensitive self-degradable gel that is more stable in HTHP compared with conventional TPA and degrades completely within 5 h at 100 °C. Additionally, high temperatures, an alkaline/acid environment, etc. can efficiently accelerate degradation. Zhang et al. [
24] used AM and 2-Acrylamido-2-methyl-1-propane sulfonic acid (AMPS) as polymeric monomers and polyethylene glycol diacrylate (PEGDA) as the crosslinker to prepare a self-degradable gel material. A series of experiments was carried out to investigate the influence of the molecular weight of PEGDA on the performance of self-degradable gel and found that when the molecular weight of PEGDA decreased, the stability and hydrolysis time of gel increased, and the swelling volume rate decreased. Zhao et al. [
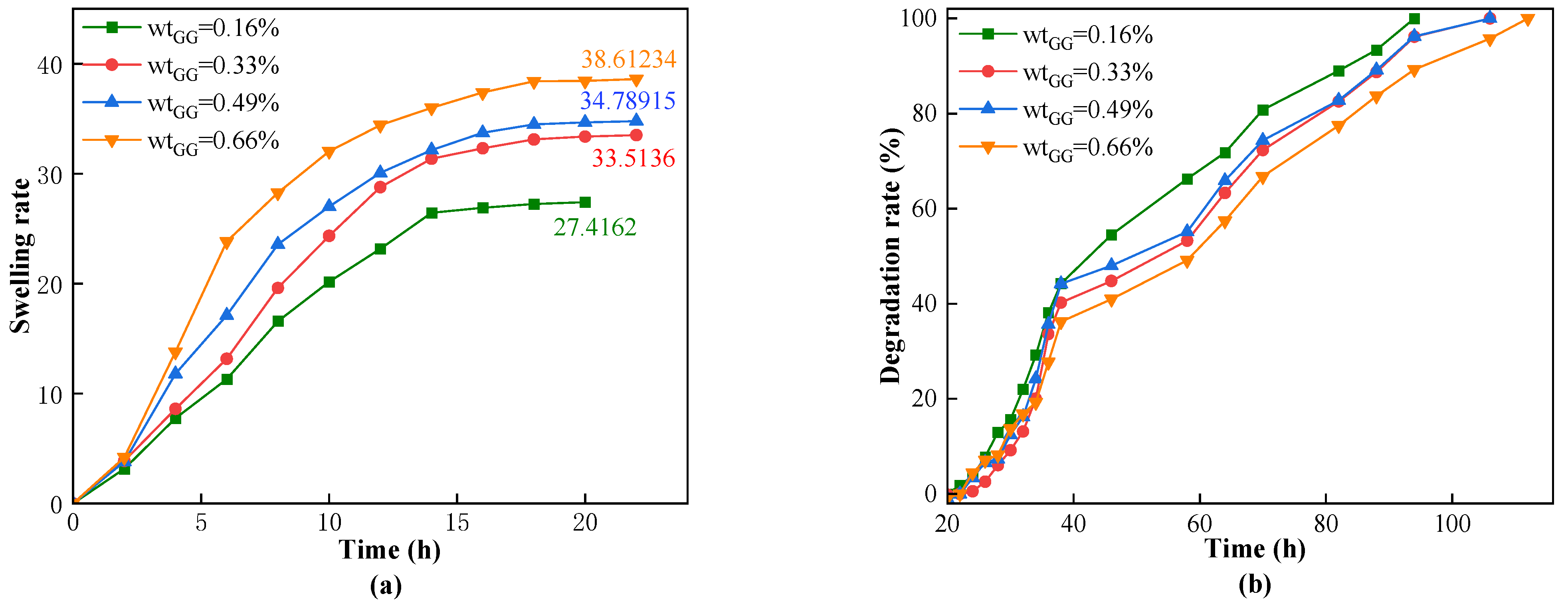
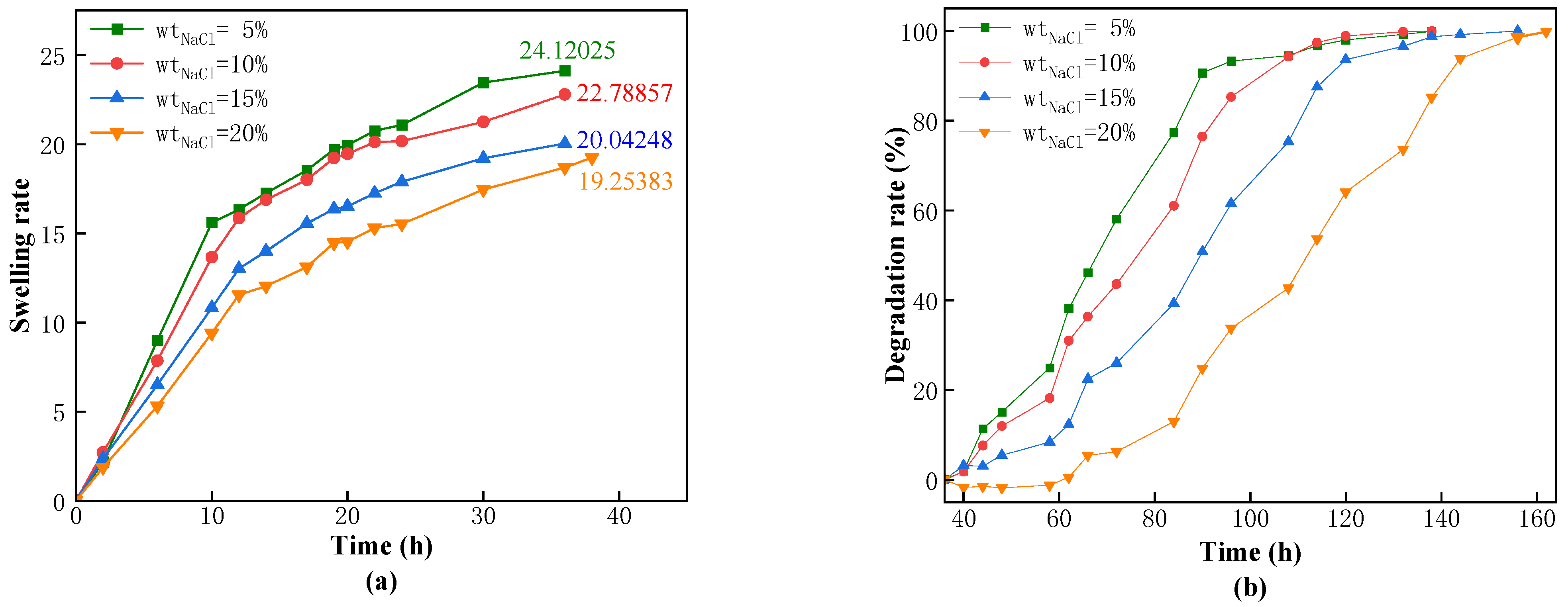
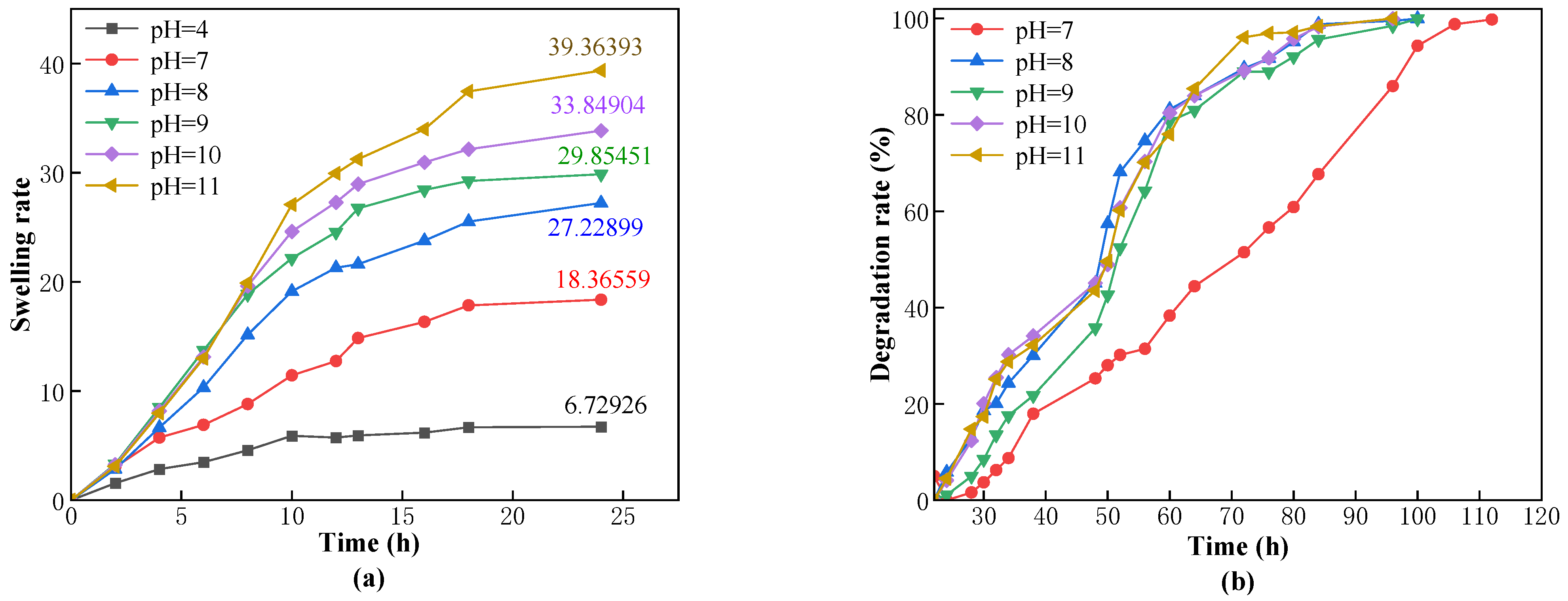
25] investigated the influence of different component concentrations on the swelling volume rate and found that the swelling volume rate decreased with the increase in concentrations of monomer and crosslinker. Currently, the main challenges faced by s-DPPG are short degradation times and low strength. The self-degrading plugging material CSK-DPPG prepared in this paper has a complete degradation time of more than 120 h under the conditions of low temperature and high mineralization of the reservoir, which can provide sufficient time for the subsequent operation.
In this paper, a self-degradable pre-formed particle gel (CKS-DPPG) suitable for the reservoir was prepared by using AM as the monomer, PEGDA (molecular weight 200) as a cross-linker, nano-silica as rigid filler particles, and ceric ammonium nitrate as the initiator to react with guar gum to form guar gum radicals, followed by the free radical polymerization reaction. CKS-DPPG was characterized by Fourier infrared spectral analysis (FT-IR), scanning electron microscope (SEM), and thermogravimetric analyzer (TGA) for its chemical structure, microscopic morphology, and thermal stability. Then, the effects of the concentration of guar gum, nano-silica, temperature, salinity, pH, and other factors on the performance of CKS-DPPG were investigated, and the degradation reaction mechanism was analyzed. The evaluation of fracture plugging performance was also carried out.
4. Materials and Methods
4.1. Materials
Acrylamide (AM, AR 99%), guar gum (GG, AR 99%), polyethylene glycol diacrylate (PEGDA, molecular weight 200), Ammonium cerium (IV) nitrate (CAN, AR 99%), sodium hydroxide (AR 99%), NaCl (AR 99%), CaCl2 (AR 99%), and hydrochloric acid (AR 99%) were all purchased from Shanghai Macklin Biological Co., Ltd., Shanghai, China.
AM and GG were employed as monomers, PEGDA as the crosslinker, and APS as the initiator. Deionized water was prepared in the laboratory. The frequency of the ultrasound was 40 KHz.
4.2. Preparation of CKS-DPPG
A certain amount of AM (30% mass fraction) solid powder and PEGDA (0.24% mass fraction) was weighed into a beaker filled with deionized water and mixed thoroughly with mechanical stirring for 20 min to form a homogeneous solution. A certain amount of GG (0.33% mass fraction) powder was weighed and slowly added into the solution while stirred, and the solution was guaranteed to be stirred at a constant mix speed for 30 min after the GG solid powder was completely added to form a homogeneous solution. After adding nano silica (0.33% mass fraction) and continuing mechanical stirring for 10 min, the homogeneous polymer base solution was obtained by ultrasonic dispersion treatment for 10 min. Nitrogen was introduced into the beaker, which was sealed with cling film for 20 min to remove the original oxygen. Then, a certain amount of Ammonium cerium (IV) nitrate (0.1% mass fraction) was added into a beaker in an ice water bath and stirred to form a homogeneous solution, and then the beaker was transferred to a water bath at 40 °C for a full reaction of at least 6 h. The preparation process is shown in
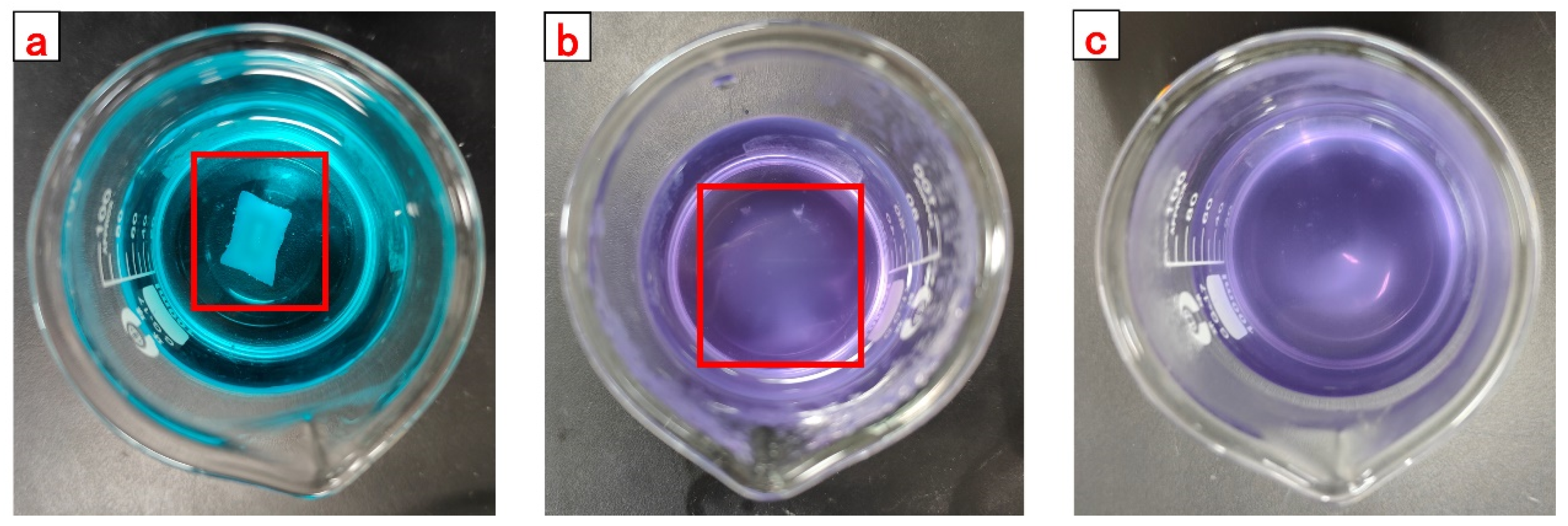
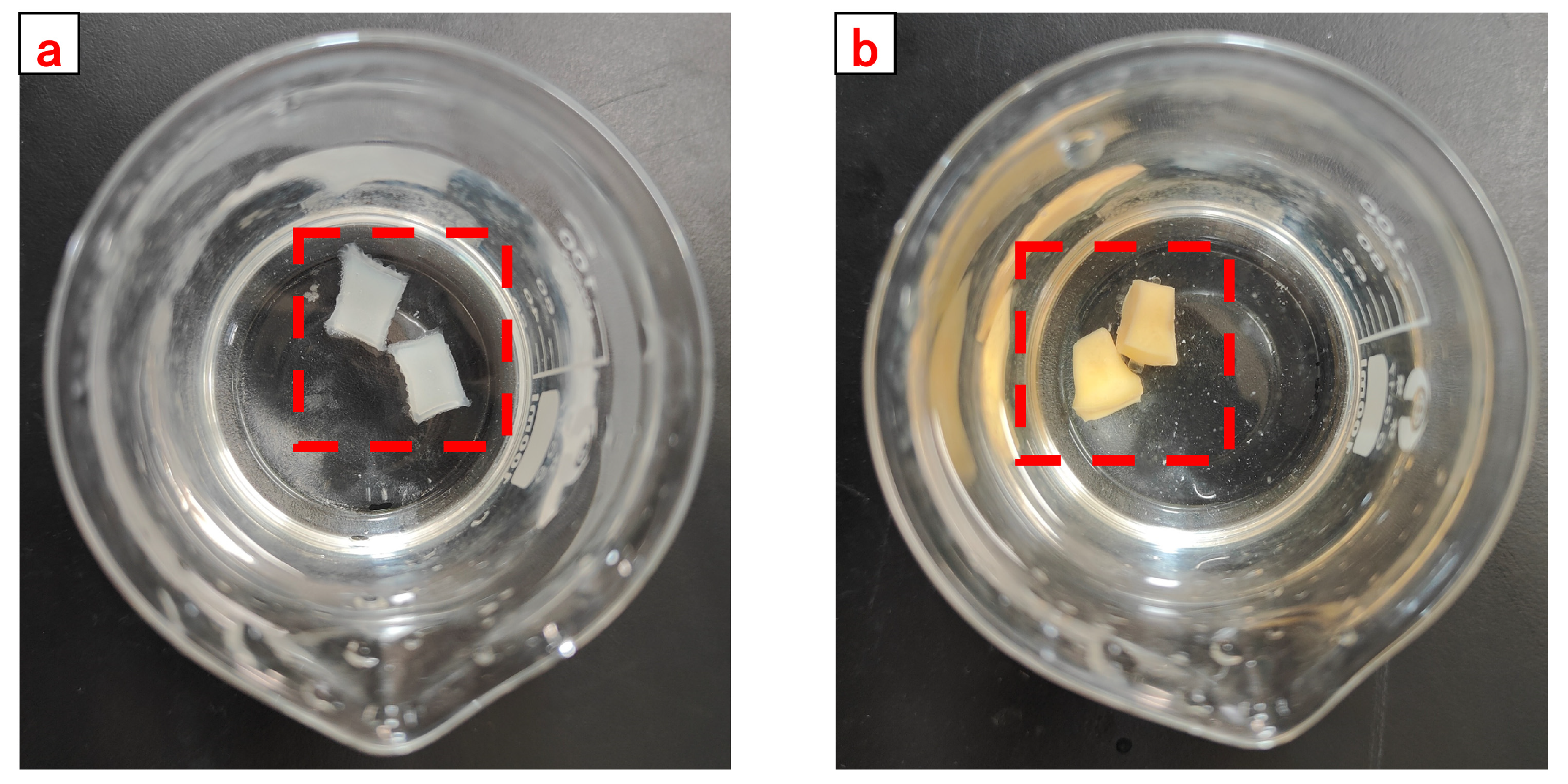
Figure 16. With the completion of the reaction in the beaker, the gel was fished out and rinsed with acetone. The rinsed gel was transferred into the oven to deprive the body of water and then crushed to obtain a self-degradable plugging particle gel. The product images are shown in
Figure 17. The reaction mechanism is shown in
Figure 1. The optimal formulation is AM (30% mass fraction) + PEGDA (molecular weight 200, 0.24% mass fraction) + GG (0.33% mass fraction) + nano silica (0.33% mass fraction) + ammonium cerium (IV) nitrate (0.1% mass fraction) + deionized water.
4.3. Reaction Mechanism
The adjacent double hydroxyl in guar gum is oxidized by the cerium ion (IV) to form guar gum radicals. Guar gum radicals as initiators induce free radical polymerization of acrylamide monomer and PEGDA crosslinker to produce AM-GG polymer. The reaction mechanism is shown in
Figure 18.
4.4. Characterization of CKS-DPPG
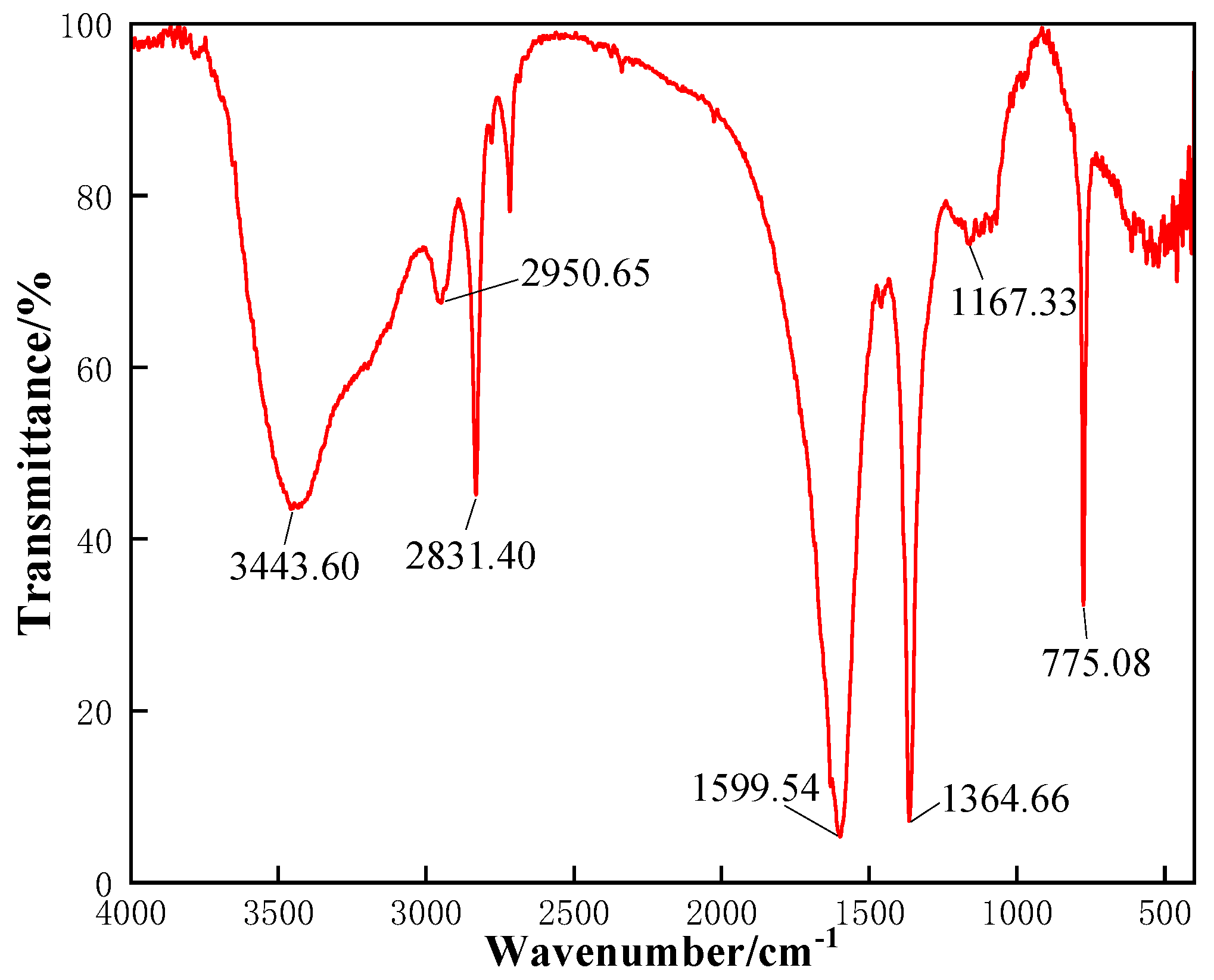
After being placed into a vacuum oven for 1 d, the CKS-DPPG particles prepared in
Section 4.2 were ground into 200–300 mesh powder and mixed with dry KBr homogeneously, then pressed into sheets under 20 MPa pressure by a tablet press, which would be used to be tested by a Fourier transform infrared spectrometer.
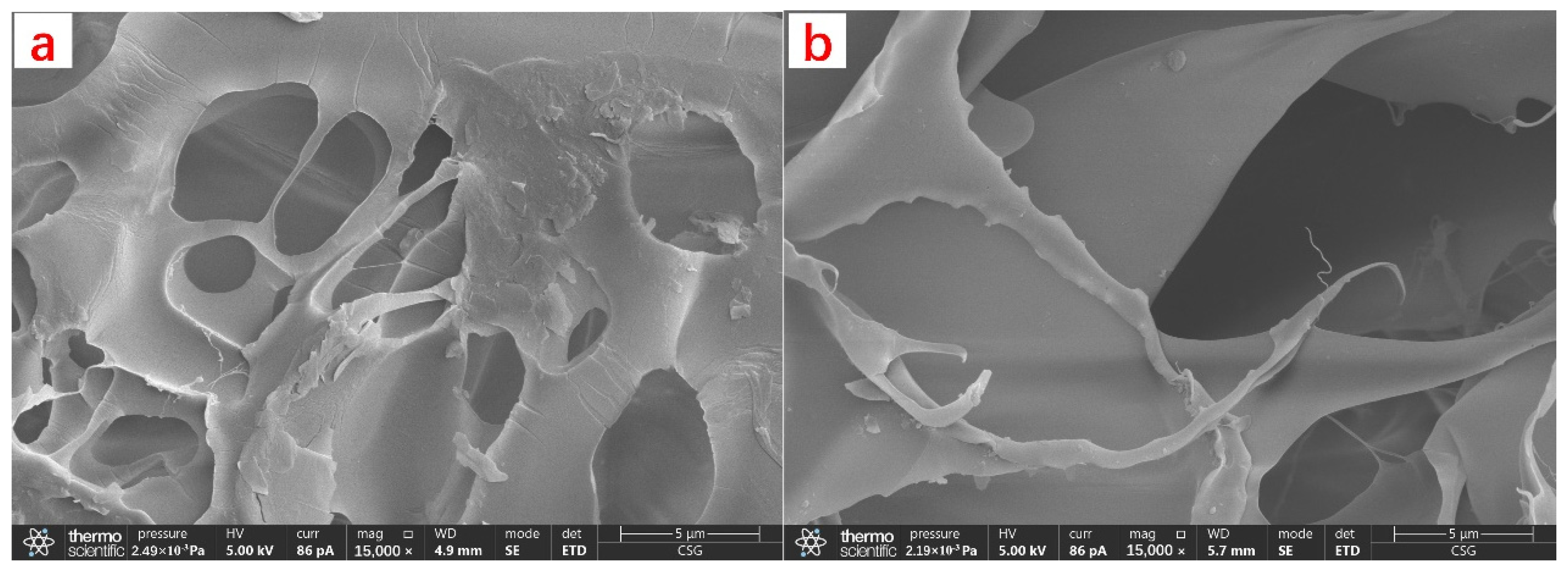
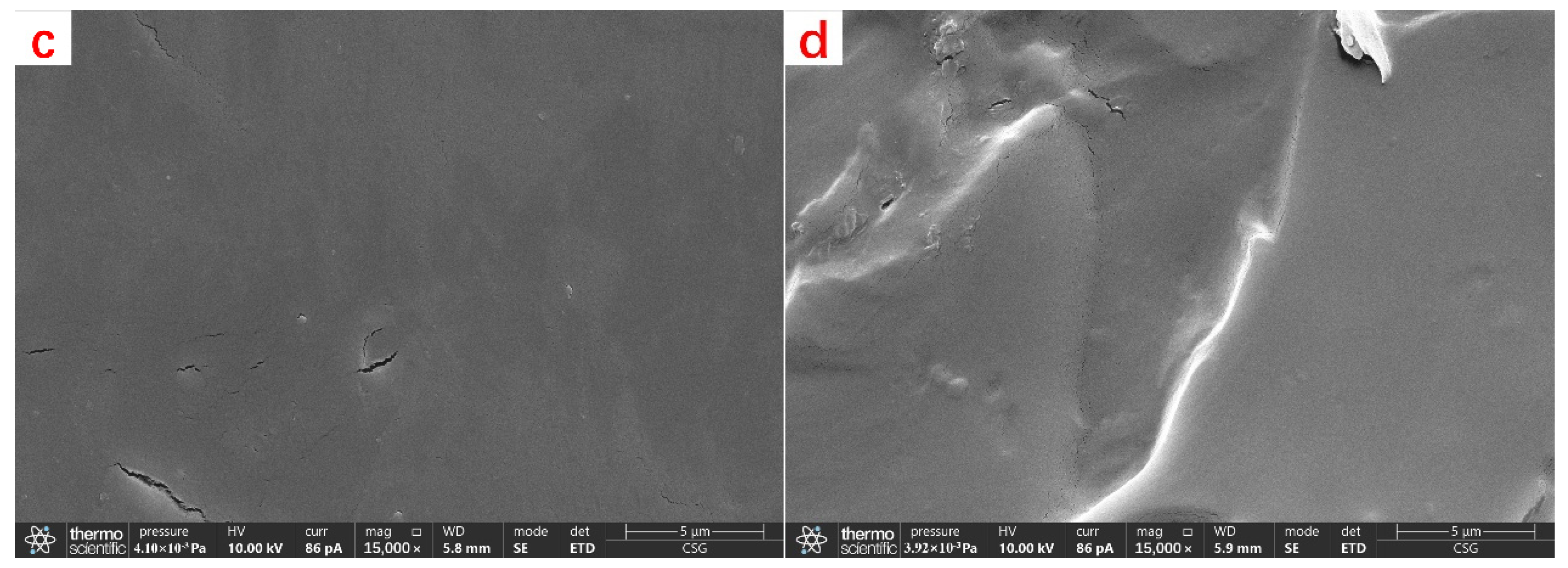
The pre-degradation CKS-DPPG was lyophilized and then gold-plated before being morphologically characterized using SEM. The CKS-DPPG was degraded for 24 h, then lyophilized and gold plated, and the gels were characterized using SEM.
The formation temperature where CKS-DPPG works is often high, which requires CKS-DPPG to have better thermal stability. The dried sample was heated to 800 °C at a heating rate of 20 °C/min in a nitrogen purge environment, and the mass change curve was recorded.
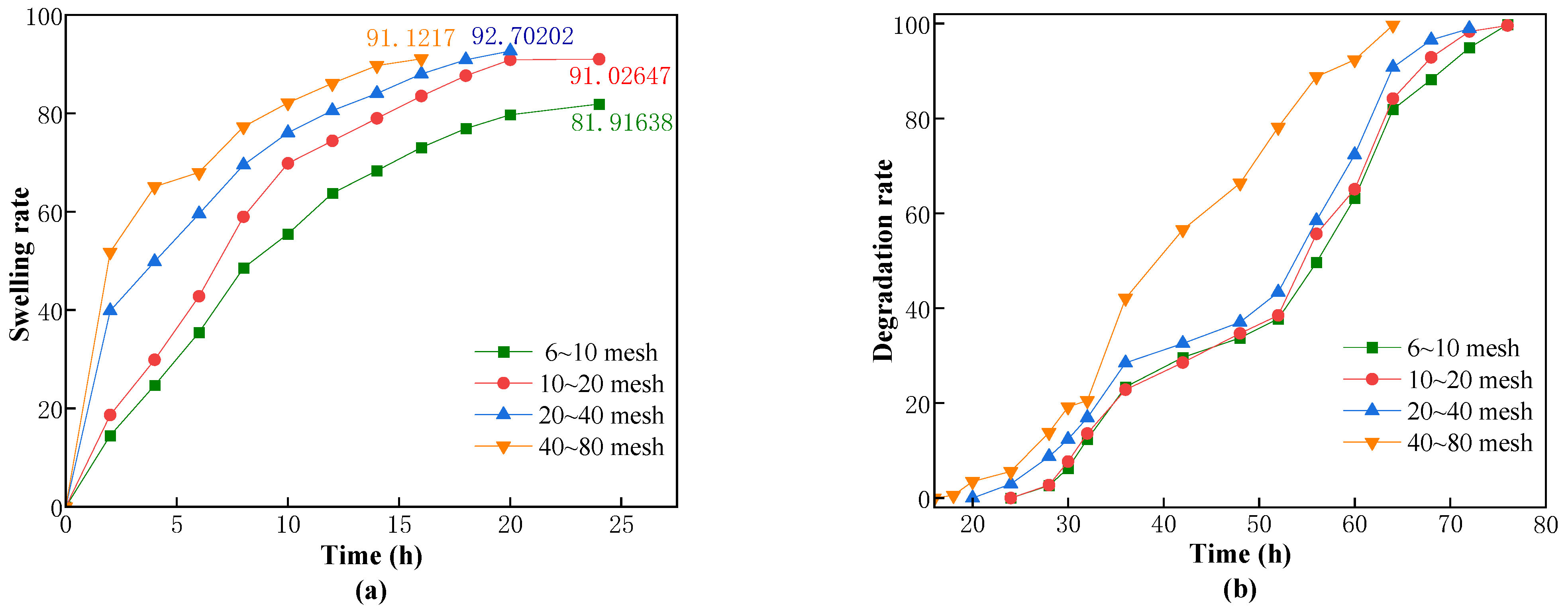
4.5. Measurement of Swelling and Degradation Properties of CKS-DPPG
The swelling and degradation processes of the dried CKS-DPPG in solution are simultaneous. The swelling phase is considered when the swelling rate is greater than the degradation rate, and vice versa for the degradation phase. Since the edges of CKS-DPPG are gradually degrading during the process of degradation, the traditional method of weighing the reduction of mass may lead to a large error due to the inability to accurately weigh the softened layer at the edges. Therefore, the swelling rate (
ηs) test is carried out by placing the dry CKS-DPPG (
m0) into a pocket in which small molecule chains can pass and then weighing the mass change of the pocket. The pockets are placed in solutions from different environments to investigate the effects of the concentration of guar gum, the concentration of nano silica, salinity, pH value, temperature, and particle size on the swelling and degradation properties. The pockets are completely submerged in the solution, and then the pockets are removed at certain time intervals. The surface of the pockets is dried up and weighed, and the mass of that moment is recorded (
mt). The swelling rate is calculated according to Equation (1), and the swelling rate is considered to have reached a maximum when the mass starts to stabilize or decrease.
where
is the swelling rate;
mt is the mass of CKS-DPPG at
t moment;
m0 is the mass of initial dry CKS-DPPG;
mw is the mass of wet pocket.
The degradation process starts to be calculated when the swelling rate reaches its maximum. The mass of the pockets (
mt) is weighed at certain time intervals, and the degradation is considered complete when there is no obvious residue in the pockets and the mass is stable. The swelling rate is calculated according to Equation (2).
where
is the degradation rate;
mmax is the max mass of CKS-DPPG;
mt is the mass of CKS-DPPG at
t moment; and
m0 is the mass of initial dry CKS-DPPG.
4.6. Plugging Property Evaluation
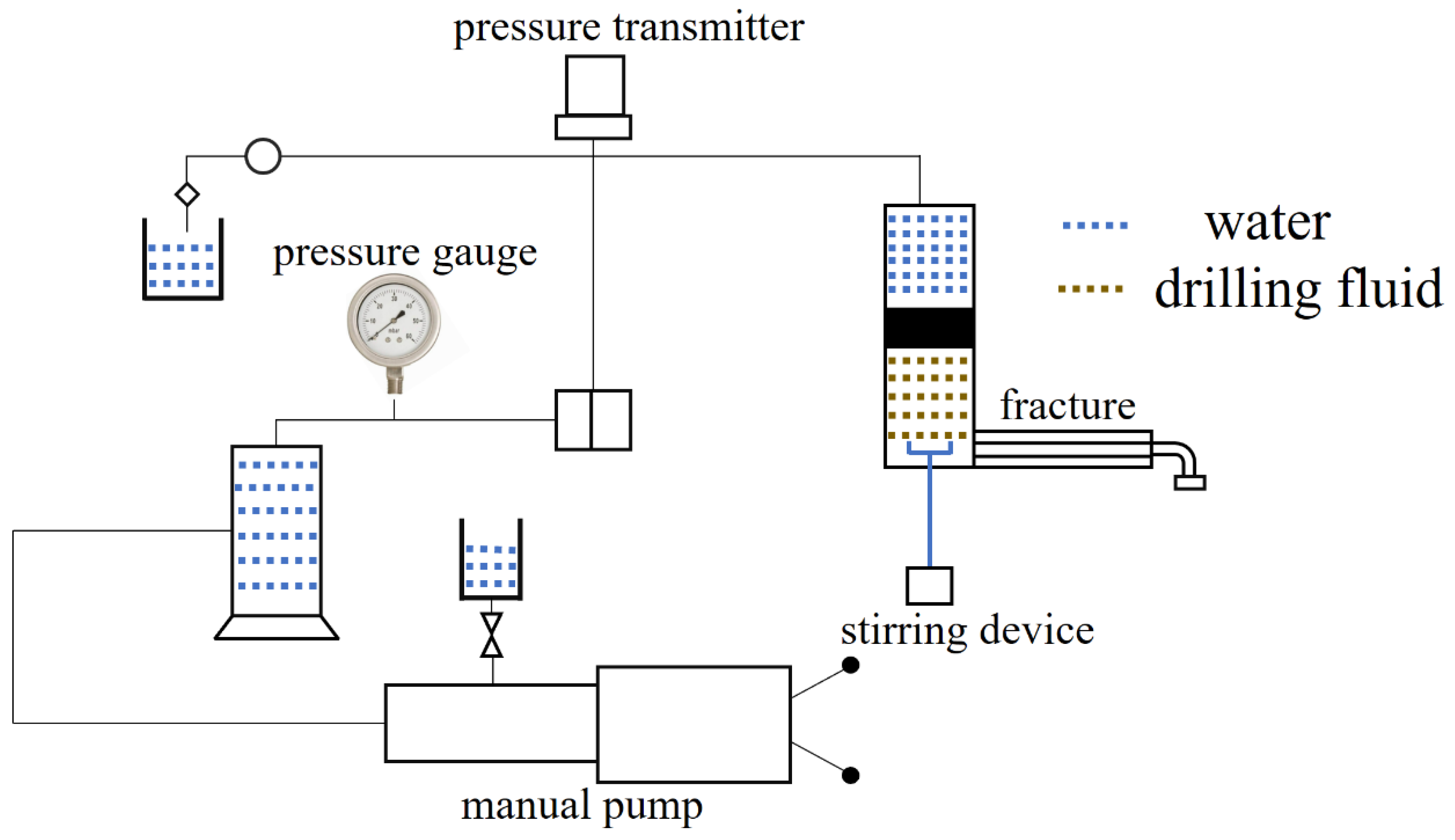
The simulated fracture plugging equipment is shown in
Figure 19. Add the compound CKS-DPPG to the simulated fracture equipment and set the maximum pressure to 6 MPa with the manual pump. If the pressure reaches 6 MPa, the device will stop pressurizing. If not, then the maximum breaching pressure will be recorded. Turn on the equipment, and the pressure gradually increases.
The composition of the drilling fluid is 2000 mL of slurry (3%) + 0.2% CMC-LV, +0.5% KPAM, +3% SPNH, +3% superfine calcium carbonate. The composition of the compound CKS-DPPG is 1 g CKS-DPPG (10~20 mesh) + 1 g CKS-DPPG (20~40 mesh) + 1 g CKS-DPPG (40~80 mesh) + 1.5 g CKS-DPPG (>80 mesh) + 1 g walnut shell (10~12 mesh) + 1 g walnut shell (12~16 mesh) + 1 g quartz (12~16 mesh) + 1 g superfine calcium carbonate + 0.5 g fiber.