Surface Functionalization of Sugarcane-Bagasse-Derived Cellulose Nanocrystal for Pickering Emulsion Gel: Microstructural Properties and Stability Efficiency
Abstract
:1. Introduction
2. Results and Discussion
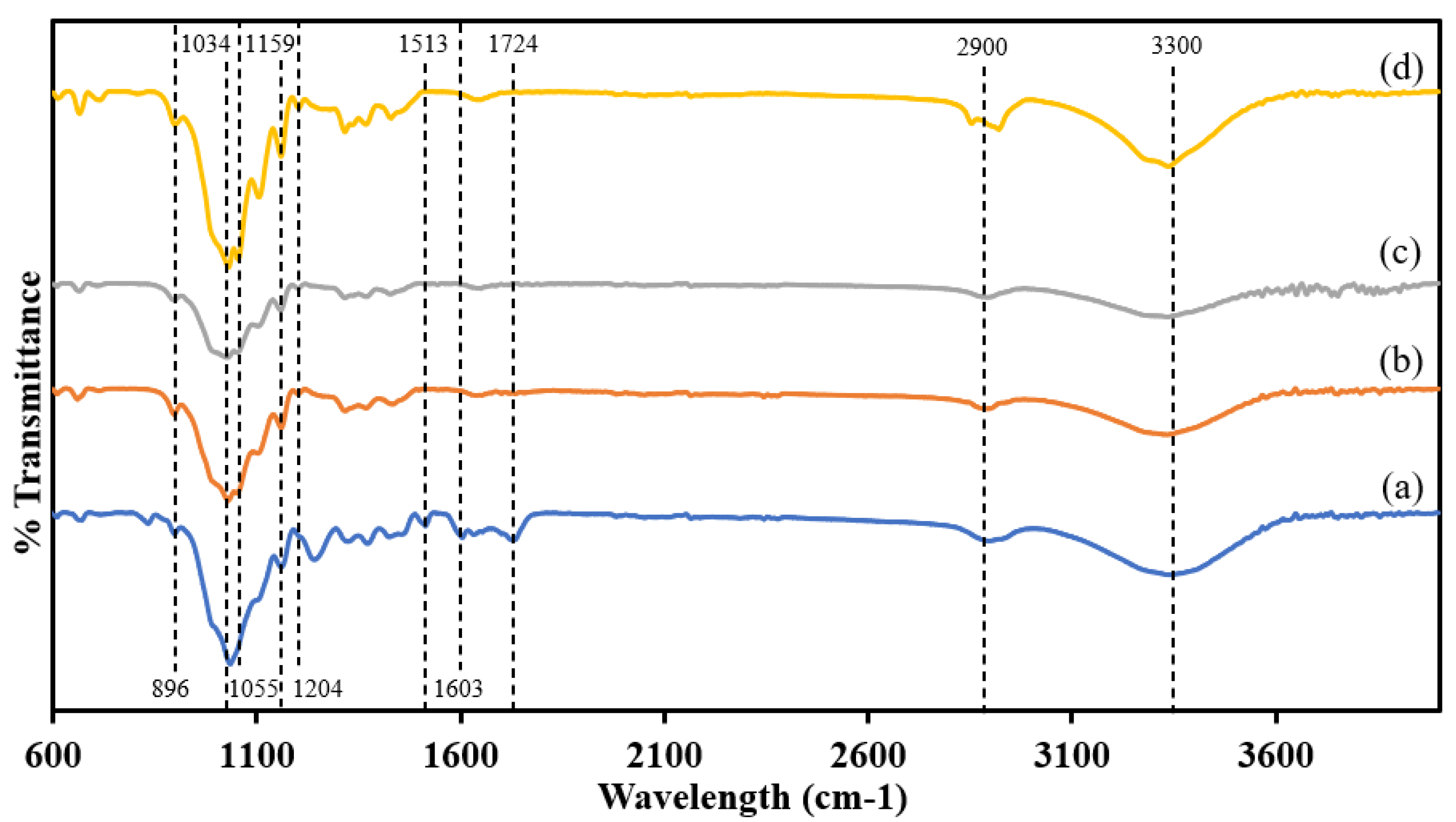
2.1. Characterization of m-CNC Pickering Stabilizer
Physicochemical Properties of m-CNC-Based Pickering Stabilizer
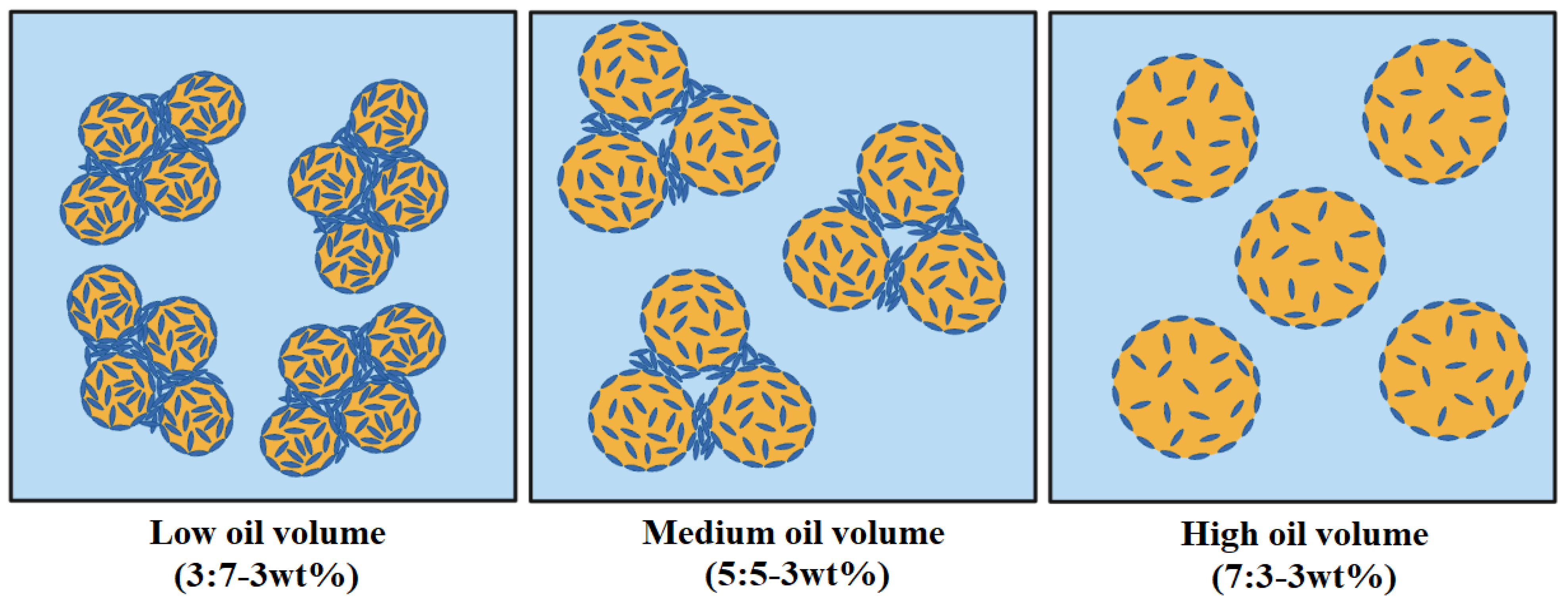
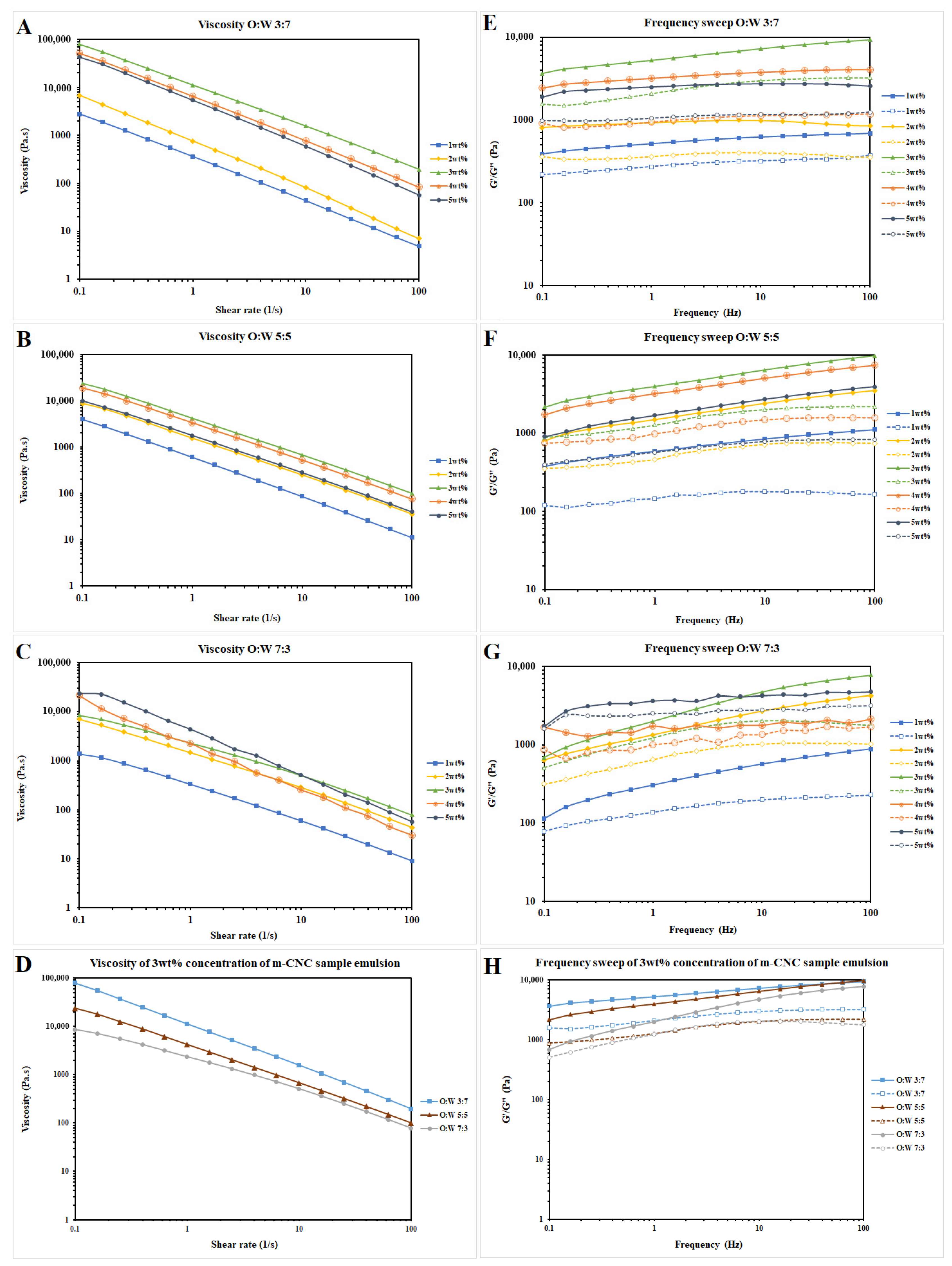
2.2. Characterization of m-CNC-Stabilised Oil–Water Emulsion Gels
Colloidal Stability Profile of Oil–Water Emulsion Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Pre-Treatment of Sugarcane Bagasse
4.2.2. Synthesis of Modified Cellulose Nanocrystal
4.2.3. Preparation of Pickering Emulsion Gels
4.3. Characterization of SCB, CPC, CNC, and m-CNC
4.4. Characterization of Pickering Emulsion Gels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raftery, A.E.; Sevcikova, H. Probabilistic population forecasting: Short to very long-term. Int. J. Forecast 2023, 39, 73–97. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. Npj Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Pereira, B.; Arantes, V. Nanocelluloses From Sugarcane Biomass. In Advances in Sugarcane Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 179–196. [Google Scholar]
- Faizal, M.; Ang, W.Y.; Amirah, A.; Tan, Y.H. Energy, Economic and Environmental Impact of Sugarcane Bagasse in Malaysia. Int. J. Adv. Sci. Res. Manag. 2019, 4, 104–110. [Google Scholar]
- Rocha, G.J.d.M.; Nascimento, V.M.; Gonçalves, A.R.; Silva, V.F.N.; Martín, C. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Ind. Crops Prod. 2015, 64, 52–58. [Google Scholar] [CrossRef]
- Gupta, P.K.; Raghunath, S.S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. Cellulose 2019, 201, 84727. [Google Scholar] [CrossRef]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2018, 7, 10. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Bertsch, P.; Fischer, P. Adsorption and interfacial structure of nanocelluloses at fluid interfaces. Adv. Colloid. Interface Sci. 2020, 276, 102089. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89. [Google Scholar] [CrossRef]
- Ahmad Khorairi, A.N.S.; Sofian-Seng, N.-S.; Othaman, R.; Abdul Rahman, H.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. A Review on Agro-industrial Waste as Cellulose and Nanocellulose Source and Their Potentials in Food Applications. Food Rev. Int. 2021, 39, 663–688. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.; Aquino, A.; Neves, M.A.D.; Conte-Junior, C.A. Nanoemulsions: Using emulsifiers from natural sources replacing synthetic ones—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Satpute, S.K.; Zinjarde, S.S.; Banat, I.M. Recent updates on biosurfactant/s in Food industry. In Microbial Cell Factories; Taylor & Francis: Abingdon, UK, 2018; pp. 1–20. [Google Scholar]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid. Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Togawa, E.; Kuroda, K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci. Technol. Adv. Mater. 2017, 18, 959–971. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New Pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Medronho, B.; Lindman, B.; Edlund, H.; Norgren, M. Cellulose as a Natural Emulsifier: From Nanocelluloses to Macromolecules. In Cellulose Science and Derivatives; IntechOpen: London, UK, 2021. [Google Scholar]
- Tang, S.; Chen, Z.; Chen, F.; Lai, X.; Wei, Q.; Chen, X.; Jiang, C. Extraction and Surface Functionalization of Cellulose Nanocrystals from Sugarcane Bagasse. Molecules 2023, 28, 5444. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. In Progress in Polymer Science; Elsevier: Amsterdam, The Netherlands, 2021; Volume 119. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Du Le, H.; Loveday, S.M.; Singh, H.; Sarkar, A. Pickering emulsions stabilised by hydrophobically modified cellulose nanocrystals: Responsiveness to pH and ionic strength. Food Hydrocoll. 2020, 99, 105344. [Google Scholar] [CrossRef]
- Parajuli, S.; Dorris, A.L.; Middleton, C.; Rodriguez, A.; Haver, M.O.; Hammer, N.I.; Urena-Benavides, E. Surface and Interfacial Interactions in Dodecane/Brine Pickering Emulsions Stabilized by the Combination of Cellulose Nanocrystals and Emulsifiers. Langmuir 2019, 35, 12061–12070. [Google Scholar] [CrossRef]
- Chen, Q.-H.; Zheng, J.; Xu, Y.-T.; Yin, S.-W.; Liu, F.; Tang, C.-H. Surface modification improves fabrication of pickering high internal phase emulsions stabilized by cellulose nanocrystals. Food Hydrocoll. 2018, 75, 125–130. [Google Scholar] [CrossRef]
- Jusoh, N.; Ko, J.; Jeon, N.L. Microfluidics-based skin irritation test using in vitro 3D angiogenesis platform. APL Bioeng. 2019, 3, 036101. [Google Scholar] [CrossRef]
- Saidane, D.; Perrin, E.; Cherhal, F.; Guellec, F.; Capron, I. Some modification of cellulose nanocrystals for functional Pickering emulsions. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150139. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Lam, N.T.; Chollakup, R.; Smitthipong, W.; Nimchua, T.; Sukyai, P. Characterization of Cellulose Nanocrystals Extracted from Sugarcane Bagasse for Potential Biomedical Materials. Sugar Tech 2017, 19, 539–552. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Cheng, T.-Y.; Yang, W.-G.; Ma, P.-T.; He, H.-Z.; Yin, X.-C.; Yu, X.-X. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Ind. Crops Prod. 2018, 111, 285–291. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M. Physico-Chemical Characterization of Cellulose Extracted from Ficus Leaves. J. Biobased Mater. Bioenergy 2013, 7, 496–499. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hasanulbasori, M.A.; Chiat, P.F.; Lee, H.V. Pyrus pyrifolia fruit peel as sustainable source for spherical and porous network based nanocellulose synthesis via one-pot hydrolysis system. Int. J. Biol. Macromol. 2019, 123, 1305–1319. [Google Scholar] [CrossRef]
- Evans, S.K.; Wesley, O.N.; Nathan, O.; Moloto, M.J. Chemically purified cellulose and its nanocrystals from sugarcane baggase: Isolation and characterization. Heliyon 2019, 5, e02635. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Salajková, M.; Berglund, L.A.; Zhou, Q. Hydrophobic cellulose nanocrystals modified with quaternary ammonium salts. J. Mater. Chem. 2012, 22, 19798–19805. [Google Scholar] [CrossRef]
- Yin, Y.; Hong, Z.; Tian, X.; Zhu, Q.; Jiang, X.; Wang, H.; Gao, W. Cellulose nanocrystals modified with quaternary ammonium salts and its reinforcement of polystyrene. Polym. Bull. 2017, 75, 2151–2166. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Fernandez Perez, A.; Reyes, G.; Oviedo, C.; Gacitua, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Mariano, M.; Rabelo, S.C.; Gouveia, R.F.; Lona, L.M.F. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro- to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Tuerxun, D.; Pulingam, T.; Nordin, N.I.; Chen, Y.W.; Kamaldin, J.B.; Julkapli, N.B.M.; Lee, H.V.; Leo, B.F.; Johan, M.R.B. Synthesis, characterization and cytotoxicity studies of nanocrystalline cellulose from the production waste of rubber-wood and kenaf-bast fibers. Eur. Polym. J. 2019, 116, 352–360. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, Y.; Chen, H.; Gao, M.; Yue, X.; Ni, Y. Redispersion of dried plant nanocellulose: A review. Carbohydr. Polym. 2022, 294, 119830. [Google Scholar] [CrossRef] [PubMed]
- Hamad, W.Y.; Hu, T.Q. Structure-process-yield interrelations in nanocrystalline cellulose extraction. Can. J. Chem. Eng. 2010, 88, 392–402. [Google Scholar] [CrossRef]
- Shafeiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Influence of degree of sulfation on the rheology of cellulose nanocrystal suspensions. Rheol. Acta 2013, 52, 741–751. [Google Scholar] [CrossRef]
- Saffarionpour, S. Nanocellulose for Stabilization of Pickering Emulsions and Delivery of Nutraceuticals and Its Interfacial Adsorption Mechanism. Food Bioprocess Technol. 2020, 13, 1292–1328. [Google Scholar] [CrossRef]
- Varanasi, S.; Henzel, L.; Mendoza, L.; Prathapan, R.; Batchelor, W.; Tabor, R.; Garnier, G. Pickering Emulsions Electrostatically Stabilized by Cellulose Nanocrystals. Front. Chem. 2018, 6, 409. [Google Scholar] [CrossRef]
- Ni, Y.; Li, J.; Fan, L. Production of nanocellulose with different length from ginkgo seed shells and applications for oil in water Pickering emulsions. Int. J. Biol. Macromol. 2020, 149, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, X.; Li, J.; Chen, Y.; Liang, H.; Li, Y.; Li, B.; Luo, X.; Pei, Y.; Liu, S. Nanocellulose from bamboo shoots as perfect Pickering stabilizer: Effect of the emulsification process on the interfacial and emulsifying properties. Food Biosci. 2022, 46, 101596. [Google Scholar] [CrossRef]
- Lu, Y.; Li, J.; Ge, L.; Xie, W.; Wu, D. Pickering emulsion stabilized with fibrous nanocelluloses: Insight into fiber flexibility-emulsifying capacity relations. Carbohydr. Polym. 2021, 255, 117483. [Google Scholar] [CrossRef]
- Tian, Y.; Yuan, C.; Cui, B.; Lu, L.; Zhao, M.; Liu, P.; Wu, Z.; Li, J. Pickering emulsions stabilized by beta-cyclodextrin and cinnamaldehyde essential oil/beta-cyclodextrin composite: A comparison study. Food Chem. 2022, 377, 131995. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 1. Formation and stability. Food Hydrocoll. 2019, 96, 699–708. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, Y.; Shi, A.; Zhong, J.; Rao, P.; Wang, Q.; Zhang, H. Correlation between interfacial layer properties and physical stability of food emulsions: Current trends, challenges, strategies, and further perspectives. Adv. Colloid. Interface Sci. 2023, 313, 102863. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Mirvakili, M.N.; Hamad, W.Y. A rheological investigation of oil-in-water Pickering emulsions stabilized by cellulose nanocrystals. J. Colloid. Interface Sci. 2022, 608, 2820–2829. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, Y.; Luo, X.; Li, Y.; Li, B.; Wang, J.; Liu, S. Surface modification of cellulose nanofibrils with protein nanoparticles for enhancing the stabilization of O/W pickering emulsions. Food Hydrocoll. 2019, 97, 105180. [Google Scholar] [CrossRef]
- Kinra, S.; Pal, R. Rheology of Pickering Emulsions Stabilized and Thickened by Cellulose Nanocrystals over Broad Ranges of Oil and Nanocrystal Concentrations. Colloids Interfaces 2023, 7, 36. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; J, J.G.M.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of trimoniums as used in cosmetics. Int. J. Toxicol. 2012, 31 (Suppl. S6), 296S–341S. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion of the SCCS on Alkyl (C16, C18, C22) Trimethylammonium Chloride, Other Uses Than as a Preservative; Scientific Committee on Consumer Safety: Luxembourg, 2009. [Google Scholar]





| Samples | Percentage Yield from Process a * | Percentage Yield from SCB b * |
|---|---|---|
| CPC (After pre-treatment) | 41.01 ± 1.48% | 41.01 ± 1.48% |
| CNC (After acid hydrolysis) | 37.81 ± 1.44% | 15.51 ± 3.93% |
| CPC | CNC | m-CNC | ||||||
|---|---|---|---|---|---|---|---|---|
| Element | Wt% | Atomic % | Element | Wt% | Atomic % | Element | Wt% | Atomic % |
| C | 50.28 | 57.39 | C | 49.52 | 56.78 | C | 55.28 | 62.36 |
| O | 49.72 | 42.61 | O | 49.96 | 42.98 | O | 44.18 | 37.41 |
| S | 0.00 | 0.00 | S | 0.55 | 0.24 | S | 0.54 | 0.23 |
| N | 0.00 | - | N | 0.00 | - | N | 0.16 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teo, S.H.; Ching, Y.C.; Fahmi, M.Z.; Lee, H.V. Surface Functionalization of Sugarcane-Bagasse-Derived Cellulose Nanocrystal for Pickering Emulsion Gel: Microstructural Properties and Stability Efficiency. Gels 2023, 9, 734. https://doi.org/10.3390/gels9090734
Teo SH, Ching YC, Fahmi MZ, Lee HV. Surface Functionalization of Sugarcane-Bagasse-Derived Cellulose Nanocrystal for Pickering Emulsion Gel: Microstructural Properties and Stability Efficiency. Gels. 2023; 9(9):734. https://doi.org/10.3390/gels9090734
Chicago/Turabian StyleTeo, Shao Hui, Yern Chee Ching, Mochamad Zakki Fahmi, and Hwei Voon Lee. 2023. "Surface Functionalization of Sugarcane-Bagasse-Derived Cellulose Nanocrystal for Pickering Emulsion Gel: Microstructural Properties and Stability Efficiency" Gels 9, no. 9: 734. https://doi.org/10.3390/gels9090734
APA StyleTeo, S. H., Ching, Y. C., Fahmi, M. Z., & Lee, H. V. (2023). Surface Functionalization of Sugarcane-Bagasse-Derived Cellulose Nanocrystal for Pickering Emulsion Gel: Microstructural Properties and Stability Efficiency. Gels, 9(9), 734. https://doi.org/10.3390/gels9090734






