Evaluation of In Vitro Antioxidant, Anti-Obesity, and Anti-Diabetic Activities of Opuntia ficus Cladodes Gel and Its Application as a Preservative Coating for Shrimp during Refrigerated Storage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Analysis
2.2. Sugars Identification for OFC Gel
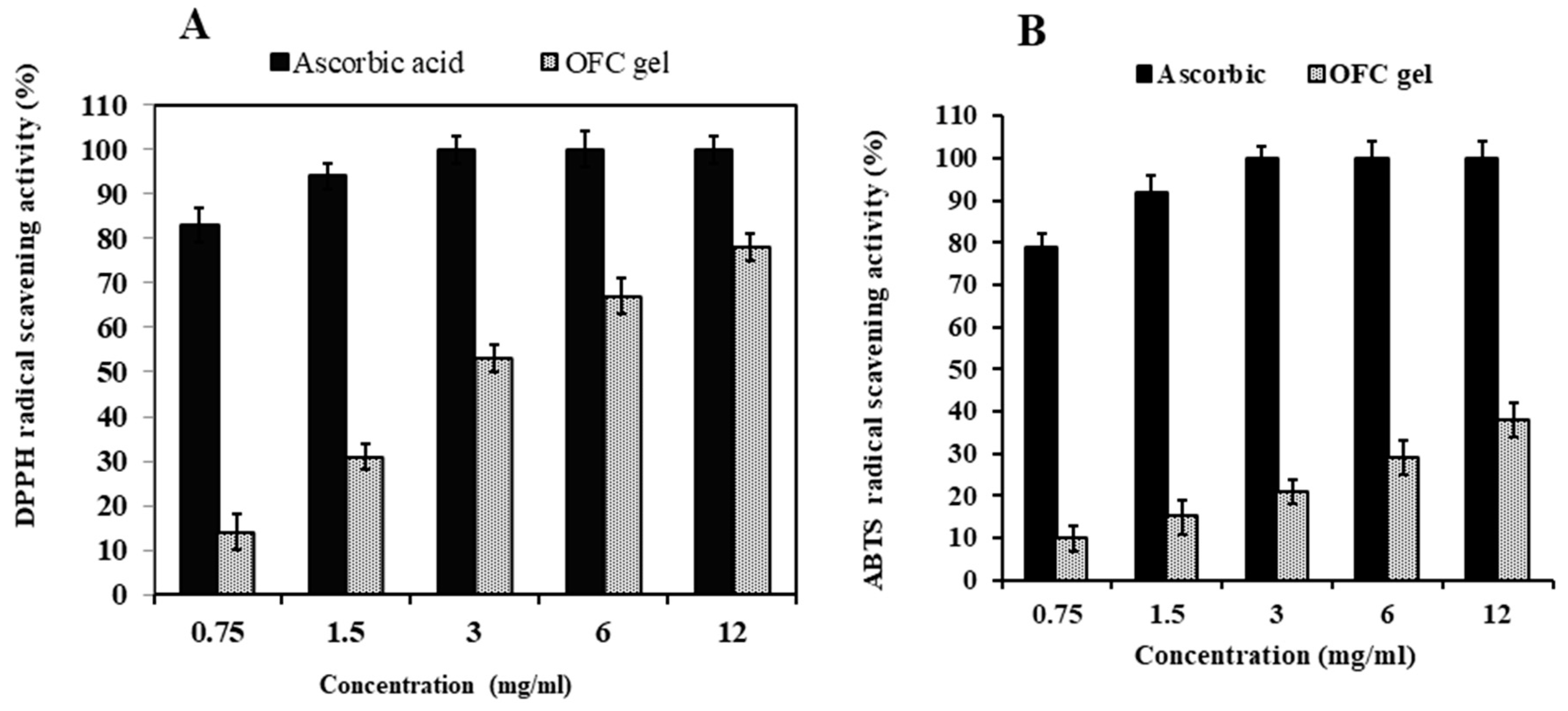
2.3. OFC Gel Antioxidant Activity
2.4. OFC Gel Anti-Lipase Activity
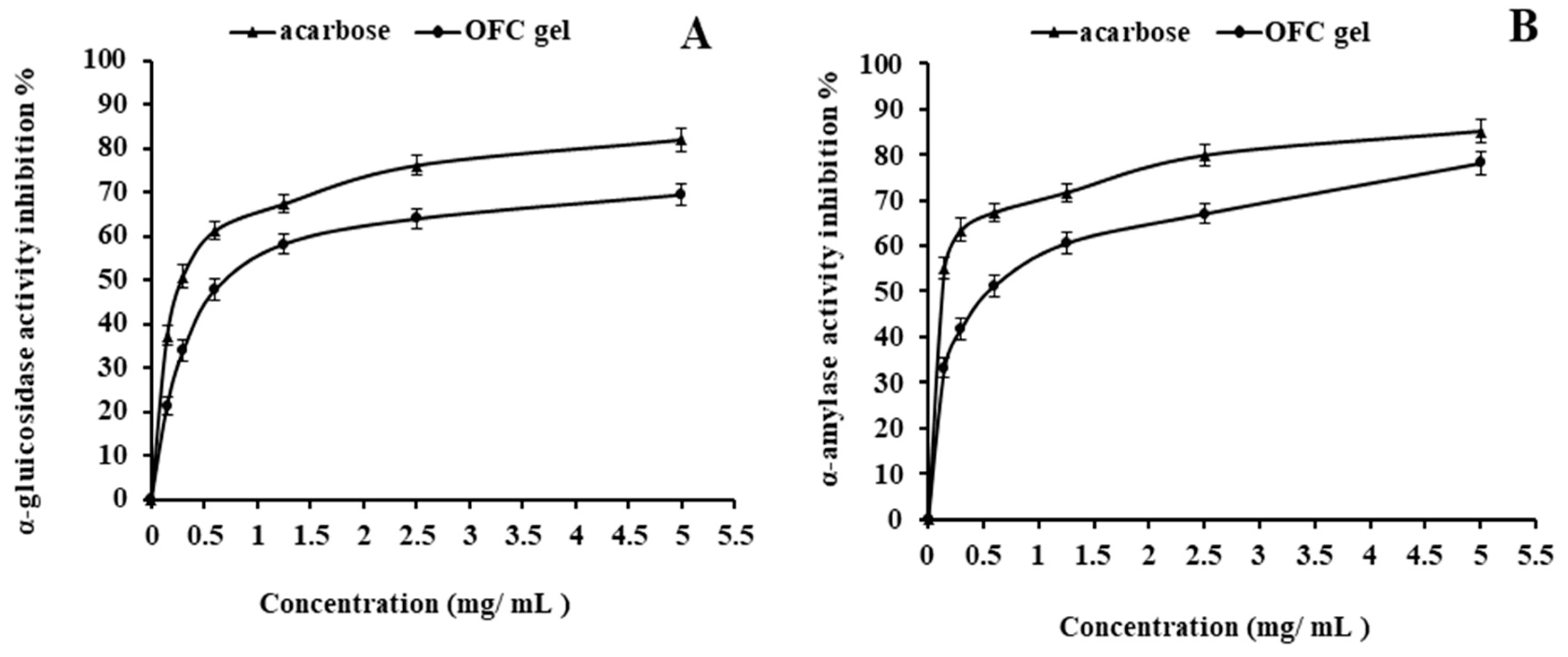
2.5. OFC Gel Anti-Diabetic Activity
2.5.1. α-Glucosidase Inhibition
2.5.2. α-Amylase Inhibition
2.6. Shrimp Quality during Cold Storage
2.6.1. Drip Loss
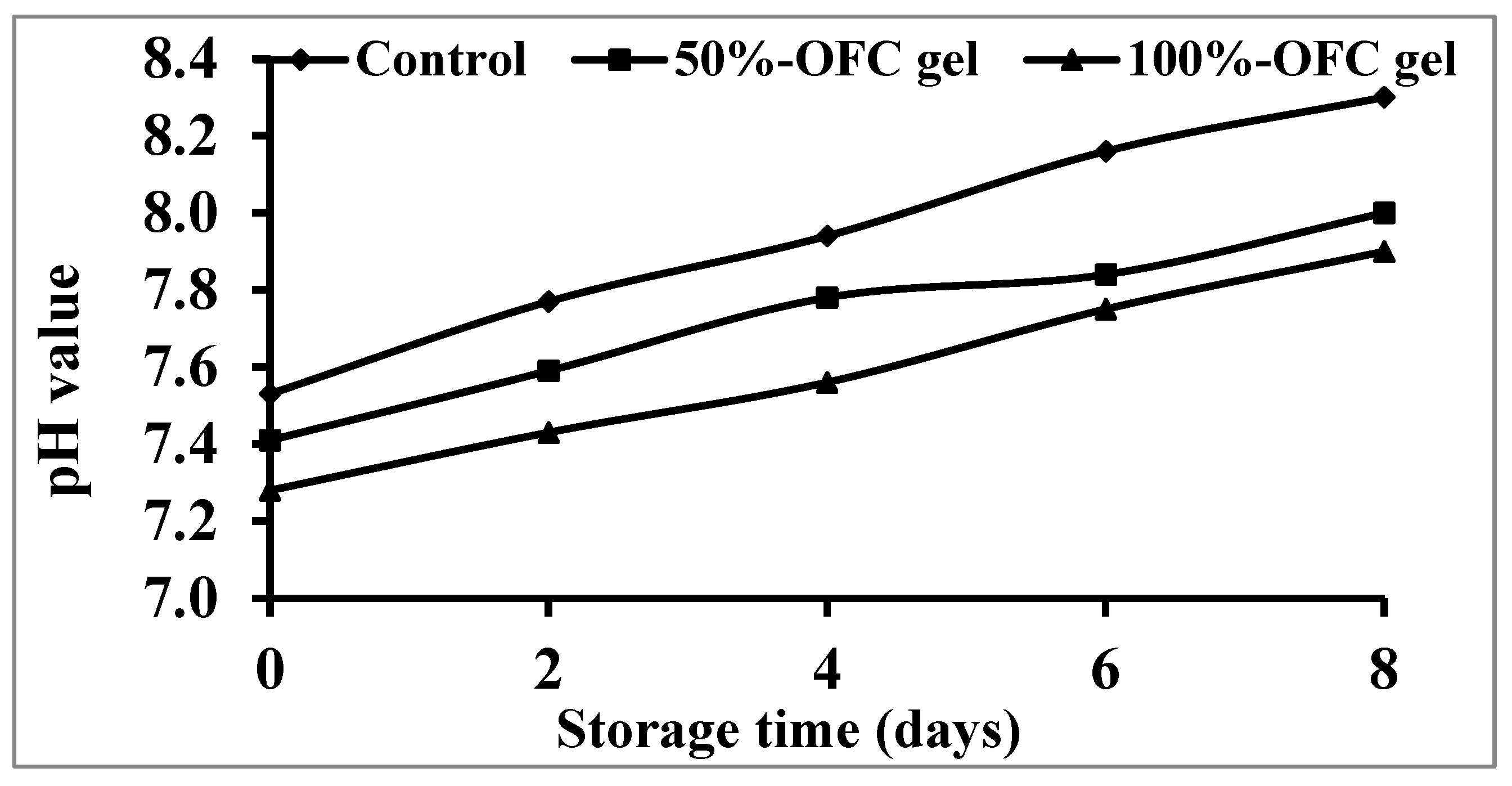
2.6.2. pH Value
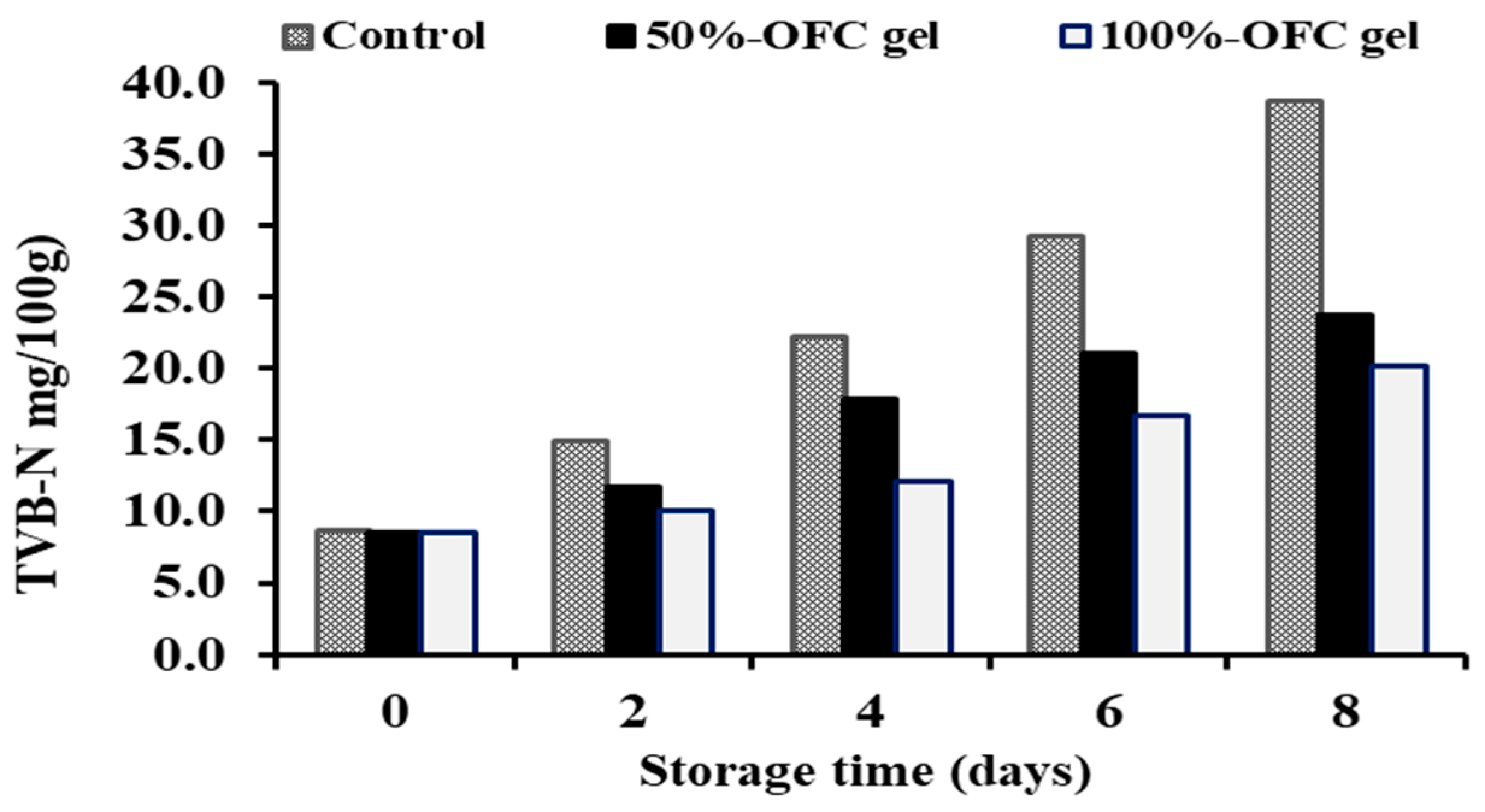
2.6.3. Total Volatile Base Nitrogen (TVB-N)
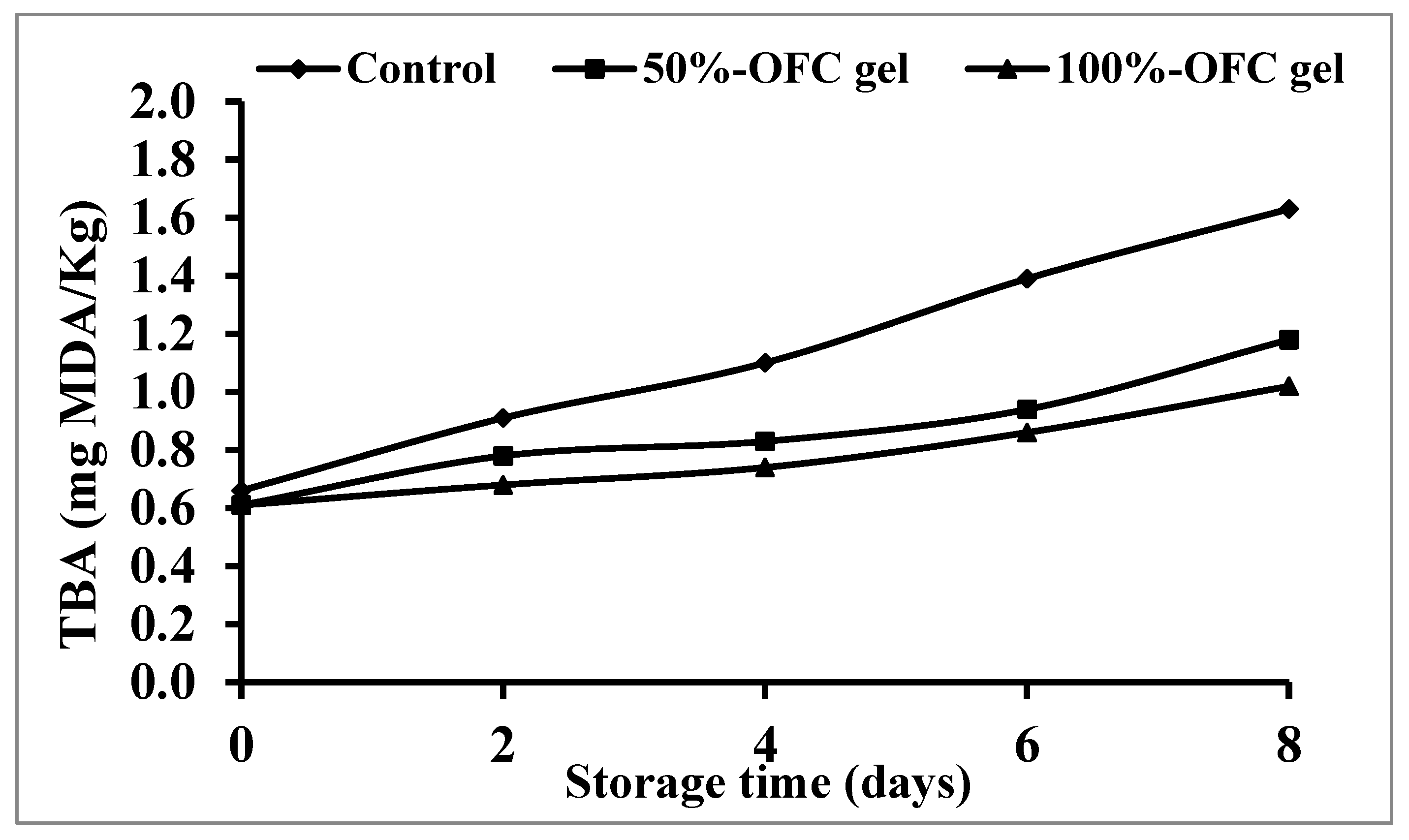
2.6.4. Thio-barbituric Acid Reactive Substances (TBARS)
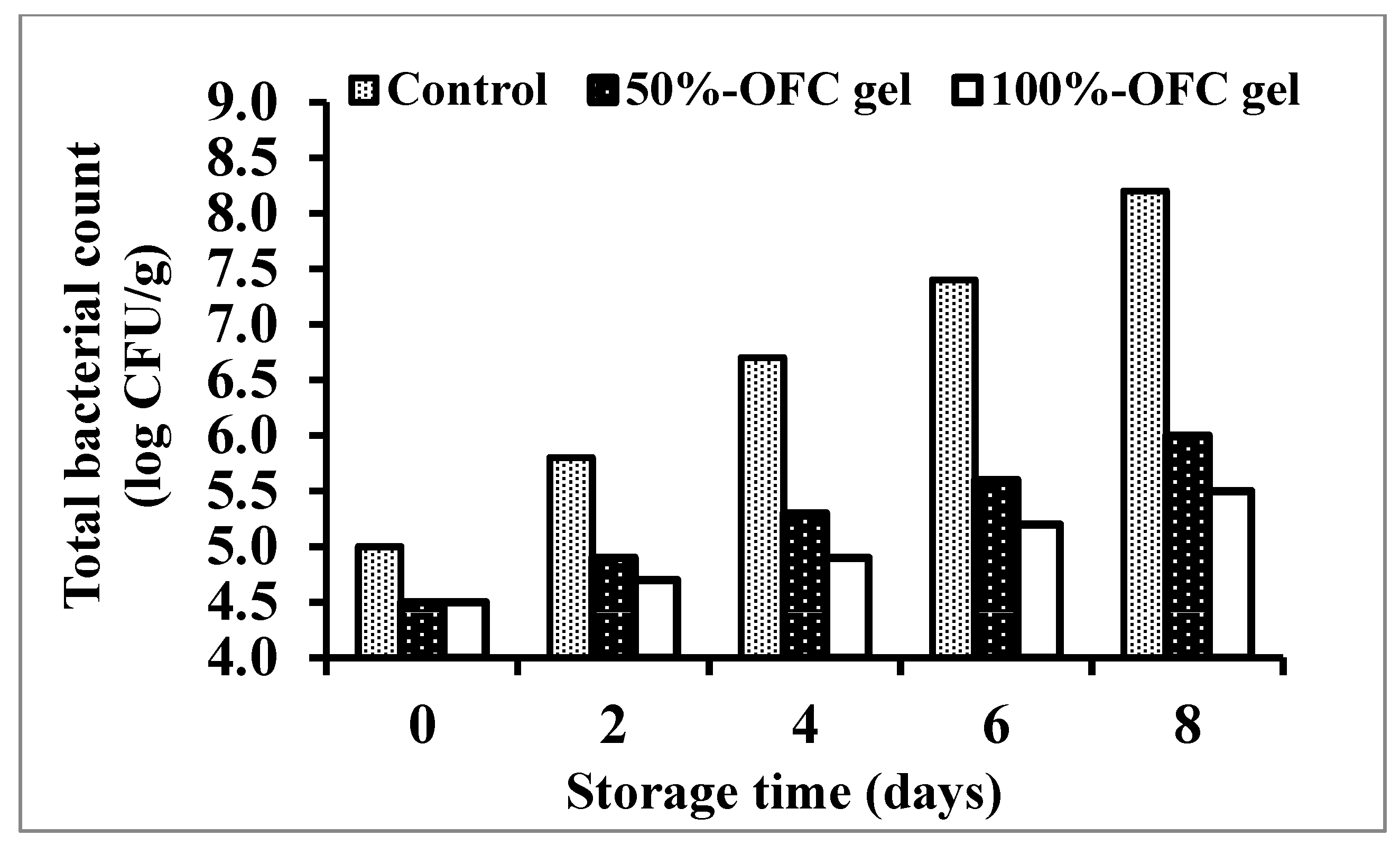
2.6.5. Total Bacterial Count Assessment
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. OFC Gel Extraction
4.3. Determination of OGC Gel Chemical Composition
4.4. OFC Gel Polyphenols
OFC Gel Antioxidant Activity
4.5. OFC Gel Sugar Composition
4.6. In Vitro Anti-Obesity Activity
4.7. In Vitro Anti-Diabetic Activity
4.7.1. α-Glucosidase Inhibition Assay
4.7.2. α-Amylase Inhibition Assay
4.8. Shrimp Coating with OFC Gel
4.9. Shrimp Quality Indicators
4.9.1. Drip Loss
4.9.2. pH Determination
4.9.3. TVBN Determination
4.9.4. TBARS Determination
4.9.5. Total Bacterial Count Measurment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. Agroecol. Sustain. Food Syst. 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Chahdoura, H.; Barreira, J.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.; Achour, L. Seeds of Opuntia spp. as a novel high potential by-product: Phytochemical characterization and antioxidant activity. Ind. Crops Prod. 2015, 65, 383–389. [Google Scholar] [CrossRef]
- Swart, W.J.; Oelofse, R.M.; Labuschagne, M.T. Susceptibility of South African cactus pear varieties to four fungi commonly associated with disease symptoms. J. Prof. Assoc. Cactus Dev. 2003, 5, 86–97. [Google Scholar]
- Ammar, M.; Shltout, A.M.; Kamhawy, M. Cladode and fruit rots of prickly pear (Opuntia ficus-indica L. Mill.) in Egypt. Egypt. J. Phytopathol. 2004, 32, 119–128. [Google Scholar]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Noguera-Artiaga, L.; Lipan, L.; Carbonell-Barrachina, Á.A.; Rocamora-Montiel, B.; Legua, P.; Hernández, F.; López-Lluch, D. Economic estimation of cactus pear production and its feasibility in Spain. Trends Food Sci. Technol. 2020, 103, 379–385. [Google Scholar] [CrossRef]
- Fouche, H.; Coetzer, G. Response of cactus pear (Opuntia spp.) biomass production to fruit load. In Proceedings of the VIII International Congress on Cactus Pear and Cochineal 1067, Palermo, Italy, 28–31 October 2013; pp. 199–204. [Google Scholar]
- Hernández-Becerra, E.; de los Angeles Aguilera-Barreiro, M.; Contreras-Padilla, M.; Pérez-Torrero, E.; Rodriguez-Garcia, M.E. Nopal cladodes (Opuntia Ficus Indica): Nutritional properties and functional potential. J. Funct. Foods 2022, 95, 105183. [Google Scholar] [CrossRef]
- Sciacca, F.; Palumbo, M.; Pagliaro, A.; Di Stefano, V.; Scandurra, S.; Virzì, N.; Melilli, M.G. Opuntia cladodes as functional ingredient in durum wheat bread: Rheological, sensory, and chemical characterization. CyTA-J. Food 2021, 19, 96–104. [Google Scholar] [CrossRef]
- Abbas, E.Y.; Ezzat, M.I.; El Hefnawy, H.M.; Abdel-Sattar, E. An overview and update on the chemical composition and potential health benefits of Opuntia ficus-indica (L.) Miller. J. Food Biochem. 2022, 46, e14310. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Castaño, J.A.G. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef]
- Nuñez-López, M.A.; Paredes-López, O.; Reynoso-Camacho, R. Functional and hypoglycemic properties of nopal cladodes (O. ficus-indica) at different maturity stages using in vitro and in vivo tests. J. Agric. Food Chem. 2013, 61, 10981–10986. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Miglani, N.; Bains, K. Interplay between proteins and metabolic syndrome–A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.R.; Jong-Anurakkun, N.; Hong, G.; Kawabata, J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.). Food Chem. 2008, 106, 247–252. [Google Scholar] [CrossRef]
- Chiasson, J.-L.; Josse, R.G.; Gomis, R.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomised trial. Lancet 2002, 359, 2072–2077. [Google Scholar] [CrossRef]
- Lustig, R.H.; Collier, D.; Kassotis, C.; Roepke, T.A.; Kim, M.J.; Blanc, E.; Barouki, R.; Bansal, A.; Cave, M.C.; Chatterjee, S. Obesity I: Overview and molecular and biochemical mechanisms. Biochem. Pharmacol. 2022, 199, 115012. [Google Scholar] [CrossRef] [PubMed]
- Alston, M.C.; Redman, L.M.; Sones, J.L. An overview of obesity, cholesterol, and systemic inflammation in preeclampsia. Nutrients 2022, 14, 2087. [Google Scholar] [CrossRef] [PubMed]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Holmbäck, U.; Grudén, S.; Litorp, H.; Willhems, D.; Kuusk, S.; Alderborn, G.; Söderhäll, A.; Forslund, A. Effects of a novel weight-loss combination product containing orlistat and acarbose on obesity: A randomized, placebo-controlled trial. Obesity 2022, 30, 2222–2232. [Google Scholar] [CrossRef]
- Dong, X.; Raghavan, V. Recent advances of selected novel processing techniques on shrimp allergenicity: A review. Trends Food Sci. Technol. 2022, 124, 334–344. [Google Scholar] [CrossRef]
- Das, J.; Mishra, H.N. A comprehensive review of the spoilage of shrimp and advances in various indicators/sensors for shrimp spoilage monitoring. Food Res. Int. 2023, 173, 113270. [Google Scholar] [CrossRef]
- Peng, S.; Wei, H.; Zhan, S.; Yang, W.; Lou, Q.; Deng, S.; Yu, X.; Huang, T. Spoilage mechanism and preservation technologies on the quality of shrimp: An overview. Trends Food Sci. Technol. 2022, 129, 233–243. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Della Tan, S.L.; Shiekh, K.A.; Benjakul, S.; Nirmal, N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021, 341, 128251. [Google Scholar] [CrossRef] [PubMed]
- Hocaoğlu, A.; Demirci, A.S.; Gümüs, T.; Demirci, M. Effects of gamma irradiation on chemical, microbial quality and shelf life of shrimp. Radiat. Phys. Chem. 2012, 81, 1923–1929. [Google Scholar] [CrossRef]
- Bindu, J.; Ginson, J.; Kamalakanth, C.; Asha, K.; Gopal, T.S. Physico-chemical changes in high pressure treated Indian white prawn (Fenneropenaeus indicus) during chill storage. Innov. Food Sci. Emerg. Technol. 2013, 17, 37–42. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Younis, E.R.; Mohamed, E.A. Impact of atmospheric cold plasma (ACP) on maintaining bolti fish (Tilapia nilotica) freshness and quality criteria during cold storing. J. Food Process. Preserv. 2021, 45, e15442. [Google Scholar] [CrossRef]
- Lin, D.; Sun, L.-C.; Chen, Y.-L.; Liu, G.-M.; Miao, S.; Cao, M.-J. Shrimp spoilage mechanisms and functional films/coatings used to maintain and monitor its quality during storage. Trends Food Sci. Technol. 2022, 129, 25–37. [Google Scholar] [CrossRef]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntia ficus indica fruit gum: Extraction, characterization, antioxidant activity and functional properties. Carbohydr. Polym. 2019, 206, 565–572. [Google Scholar] [CrossRef]
- Boruah, A.K.; Nath, L.K. Extraction, purification and physicochemical evaluation of mucilage of Chrysophyllum lanceolatum (blume) dc fruits: An investigation for bioadhesive property. Int. J. Pharm. Pharm. Sci. 2016, 8, 282–288. [Google Scholar]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.; Chacón-García, L. Extraction and characterization of mucilage from wild species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Salisbury, F.B. Plant Physiology; 0534980031; Wadsworth Pub. Co.: Belmont, CA, USA, 1978. [Google Scholar]
- Du Toit, A.; De Wit, M.; Hugo, A. Cultivar and harvest month influence the nutrient content of Opuntia spp. cactus pear cladode mucilage extracts. Molecules 2018, 23, 916. [Google Scholar] [CrossRef]
- Gebresamuel, N.; Gebre-Mariam, T. Comparative physico-chemical characterization of the mucilages of two cactus pears (Opuntia spp.) obtained from Mekelle, Northern Ethiopia. J. Biomater. Nanobiotechnology 2011, 3, 79–86. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Muller, G.; Grisel, M. Prickly pear nopals pectin from Opuntia ficus-indica physico-chemical study in dilute and semi-dilute solutions. Carbohydr. Polym. 2001, 46, 69–79. [Google Scholar] [CrossRef]
- Quintero-García, M.; Gutiérrez-Cortez, E.; Bah, M.; Rojas-Molina, A.; Cornejo-Villegas, M.d.l.A.; Del Real, A.; Rojas-Molina, I. Comparative analysis of the chemical composition and physicochemical properties of the mucilage extracted from fresh and dehydrated Opuntia ficus indica cladodes. Foods 2021, 10, 2137. [Google Scholar] [CrossRef]
- de Andrade Vieira, É.; Alcântara, M.A.; Dos Santos, N.A.; Gondim, A.D.; Iacomini, M.; Mellinger, C.; de Magalhães Cordeiro, A.M.T. Mucilages of cacti from Brazilian biodiversity: Extraction, physicochemical and technological properties. Food Chem. 2021, 346, 128892. [Google Scholar] [CrossRef]
- Procacci, S.; Bojórquez-Quintal, E.; Platamone, G.; Maccioni, O.; Vecchio, V.L.; Morreale, V.; Alisi, C.; Balducchi, R.; Bacchetta, L. Opuntia ficus-indica pruning waste recycling: Recovery and characterization of mucilage from cladodes. Nat. Resour. 2021, 12, 91–107. [Google Scholar]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of plasticized edible films from Opuntia ficus-indica mucilage: A comparative study of various polyol plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.M.d.O.; Silva, N.H.d.; Lima Filho, J.L.d.; Brito, J.Z.d.; Silva, M.d.P.C.d. Study of carbohydrates present in the cladodes of Opuntia ficus-indica (fodder palm), according to age and season. Food Sci. Technol. 2010, 30, 933–939. [Google Scholar] [CrossRef]
- Elshewy, A.; Blando, F.; Bahlol, H.; El-Desouky, A.; De Bellis, P.; Khalifa, I. Egyptian Opuntia ficus-indica (OFI) Residues: Recovery and Characterization of Fresh Mucilage from Cladodes. Horticulturae 2023, 9, 736. [Google Scholar] [CrossRef]
- Abu Hafsa, S.; Ibrahim, S. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2018, 102, 268–275. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Depolymerization of polysaccharides from Opuntia ficus indica: Antioxidant and antiglycated activities. Int. J. Biol. Macromol. 2015, 79, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.M.; Arena, R.; Morghese, M.; Santulli, A.; Liguori, G.; Inglese, P. Seasonal characterization of nutritional and antioxidant properties of Opuntia ficus-indica [(L.) Mill.] mucilage. Food Hydrocoll. 2021, 111, 106398. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, Y.S.; Lee, Y.; Kim, J.; Sun, H.; Kim, J.H.; Kim, J.S. Inhibitory activities of pancreatic lipase and phosphodiesterase from Korean medicinal plant extracts. Phytother. Res. 2012, 26, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M. Inhibitory activities of some mucilage’s and gums against certain intestinal disaccharidases. Aust. J. Basic Appl. Sci. 2009, 3, 2741–2746. [Google Scholar]
- Uebelhack, R.; Busch, R.; Alt, F.; Beah, Z.-M.; Chong, P.-W. Effects of cactus fiber on the excretion of dietary fat in healthy subjects: A double blind, randomized, placebo-controlled, crossover clinical investigation. Curr. Ther. Res. 2014, 76, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef]
- Saravanan, S.; Parimelazhagan, T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Li, C.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Comparative study on the physicochemical properties and bioactivities of polysaccharide fractions extracted from Fructus Mori at different temperatures. Food Funct. 2019, 10, 410–421. [Google Scholar] [CrossRef]
- Wu, M.; Li, W.; Zhang, Y.; Shi, L.; Xu, Z.; Xia, W.; Zhang, W. Structure characteristics, hypoglycemic and immunomodulatory activities of pectic polysaccharides from Rosa setate x Rosa rugosa waste. Carbohydr. Polym. 2021, 253, 117190. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, C.; Yu, Y.-B.; Wu, L.-X.; Chen, T.-T.; Wang, Z.-W. Physicochemical characteristics and in vitro biological activities of polysaccharides derived from raw garlic (Allium sativum L.) bulbs via three-phase partitioning combined with gradient ethanol precipitation method. Food Chem. 2021, 339, 128081. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, Y.-T.; Deng, J.-L.; Zhao, P.; Mao, Y.-F.; Chen, Z.-X. Rapid unfolding of pig pancreas α-amylase: Kinetics, activity and structure evolution. Food Chem. 2022, 368, 130795. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.T.; Staerk, D.; Jäger, A.K. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. J. Ethnopharmacol. 2016, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-F.; Gan, C.-Y. Polysaccharide with antioxidant, α-amylase inhibitory and ACE inhibitory activities from Momordica charantia. Int. J. Biol. Macromol. 2016, 85, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, Y.; Gao, Y.; Niu, X.; Wang, L.; Li, X.; Chen, H.; Yu, Z.; Yang, Y. Seperation, characterization and inhibition on α-glucosidase, α-amylase and glycation of a polysaccharide from blackcurrant fruits. LWT 2018, 93, 16–23. [Google Scholar] [CrossRef]
- Kasipandi, M.; Manikandan, A.; Sreeja, P.S.; Suman, T.; Saikumar, S.; Dhivya, S.; Parimelazhagan, T. Effects of in vitro simulated gastrointestinal digestion on the antioxidant, α-glucosidase and α-amylase inhibitory activities of water-soluble polysaccharides from Opilia amentacea roxb fruit. LWT 2019, 111, 774–781. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Tian, J.; Wang, Y.; Xing, L.; Wang, J. Chemical modification, antioxidant and α-amylase inhibitory activities of corn silk polysaccharides. Carbohydr. Polym. 2013, 98, 428–437. [Google Scholar] [CrossRef]
- Paidari, S.; Zamindar, N.; Tahergorabi, R.; Kargar, M.; Ezzati, S.; Shirani, N.; Musavi, S.H. Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 2021, 15, 4205–4214. [Google Scholar] [CrossRef]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The influence of Opuntia ficus-indica mucilage edible coating on the quality of ‘Hayward’kiwifruit slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Shen, H.; You, J.; Luo, Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala). Food Control 2011, 22, 608–615. [Google Scholar] [CrossRef]
- Lu, S. Effects of bactericides and modified atmosphere packaging on shelf-life of Chinese shrimp (Fenneropenaeus chinensis). LWT-Food Sci. Technol. 2009, 42, 286–291. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Kyrana, V.R.; Lougovois, V.P.; Valsamis, D.S. Assessment of shelf-life of maricultured gilthead sea bream (Sparus aurata) stored in ice. Int. J. Food Sci. Technol. 1997, 32, 339–347. [Google Scholar] [CrossRef]
- Kilincceker, O.; Dogan, İ.S.; Kucukoner, E. Effect of edible coatings on the quality of frozen fish fillets. LWT-Food Sci. Technol. 2009, 42, 868–873. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Danza, A.; Conte, A.; Muratore, G.; Del Nobile, M.A. Shelf life of ready to use peeled shrimps as affected by thymol essential oil and modified atmosphere packaging. Int. J. Food Microbiol. 2010, 144, 250–256. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Agricultural Chemists). The Official Methods of Analysis of AOAC International, 20th ed.; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Kochert, G. Carbohydrate determination by the phenol-sulfuric acid method. Handb. Phycol. Methods Physiol. Biochem. Methods 1978, 95, 136–145. [Google Scholar]
- Wuttisin, N.; Nararatwanchai, T.; Sarikaphuti, A. Total phenolic, flavonoid, flavonol contents and antioxidant activity of Inca peanut (Plukenetia volubilis L.) leaves extracts. Food Res. 2021, 5, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, M.A.; Hafsa, J.; Rihouey, C.; Le Cerf, D.; Majdoub, H. Effect of extraction conditions on the antioxidant and antiglycation capacity of carbohydrates from Opuntia robusta cladodes. Int. J. Food Sci. Technol. 2016, 51, 929–937. [Google Scholar] [CrossRef]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from bauhinia t arapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and bioactive properties of commonly consumed wild greens: Potential sources for new trends in modern diets. Food Res. Int. 2011, 44, 2634–2640. [Google Scholar] [CrossRef]
- Nakai, M.; Fukui, Y.; Asami, S.; Toyoda-Ono, Y.; Iwashita, T.; Shibata, H.; Mitsunaga, T.; Hashimoto, F.; Kiso, Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J. Agric. Food Chem. 2005, 53, 4593–4598. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G. Aqueous extracts of Roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J. Med. Food 2013, 16, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425. [Google Scholar] [PubMed]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Essa, R.; Elsebaie, E. Effect of using date pits powder as a fat replacer and anti-oxidative agent on beef burger quality. J. Food Dairy Sci. 2018, 9, 91–96. [Google Scholar] [CrossRef]
- Elsebaie, E.; Elsanat, S.; Gouda, M.; Elnemr, K. Studies on antimicrobial and antioxidant efficiency of glasswort (Salicornia fruticosa) herb juice and methanolic extract in minced beef. Int. J. Mod. Agric 2013, 2, 2. [Google Scholar]


| Component | Mean Value ± SD |
|---|---|
| Moisture (%) | 7.98 ± 0.56 |
| Ether extract (%) | 0.12 ± 0.00 |
| Proteins (%) | 5.08 ± 0.73 |
| Ash (%) | 10.42 ± 1.60 |
| Crude fibers (%) | 0.46 ± 0.03 |
| * Total carbohydrate (%) | 75.17 ± 2.39 |
| Total phenolic content (mg GAE/g) | 19.79 ± 0.62 |
| Total flavonoids (mg QE/g) | 9.05 ± 0.15 |
| Total flavonols (mg QE/g) | 2.54 ± 0.06 |
| pH | 4.58 ± 0.31 |
| Antioxidant activity | |
| DPPH (µmol TE/g) | 28.10 ± 4.05 |
| ABTS (µmol TE/g) | 23.59 ± 2.17 |
| Sugar Type | Content (%) |
|---|---|
| Arabinose | 3.82 |
| Xylose | 36.72 |
| Galactose | 13.69 |
| Rhamnose | 12.57 |
| Glucose | 4.62 |
| Uronic acid | 28.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.S.; Elsebaie, E.M.; Abdelrhman, W.M.; Abdulmaguid, N.Y.M.; Bahnasy, R.M.; Elgendy, M.S.A.; Elashry, A.M.M.M.; El-Hassanin, M.F.; El-Wakeil, N.H.M.; Khalil, A.M.M.; et al. Evaluation of In Vitro Antioxidant, Anti-Obesity, and Anti-Diabetic Activities of Opuntia ficus Cladodes Gel and Its Application as a Preservative Coating for Shrimp during Refrigerated Storage. Gels 2023, 9, 716. https://doi.org/10.3390/gels9090716
Mohamed AS, Elsebaie EM, Abdelrhman WM, Abdulmaguid NYM, Bahnasy RM, Elgendy MSA, Elashry AMMM, El-Hassanin MF, El-Wakeil NHM, Khalil AMM, et al. Evaluation of In Vitro Antioxidant, Anti-Obesity, and Anti-Diabetic Activities of Opuntia ficus Cladodes Gel and Its Application as a Preservative Coating for Shrimp during Refrigerated Storage. Gels. 2023; 9(9):716. https://doi.org/10.3390/gels9090716
Chicago/Turabian StyleMohamed, Alaa S., Essam Mohamed Elsebaie, Wesam Mohammed Abdelrhman, Nabila Yahia Mahmoud Abdulmaguid, Rasha M. Bahnasy, Manal Salah Abbas Elgendy, Arwa Mohamed Mohamed Mahmoud Elashry, Marwa Fawzy El-Hassanin, Nora Hamdy Mouhamed El-Wakeil, Azhar Mostafa Mohamed Khalil, and et al. 2023. "Evaluation of In Vitro Antioxidant, Anti-Obesity, and Anti-Diabetic Activities of Opuntia ficus Cladodes Gel and Its Application as a Preservative Coating for Shrimp during Refrigerated Storage" Gels 9, no. 9: 716. https://doi.org/10.3390/gels9090716
APA StyleMohamed, A. S., Elsebaie, E. M., Abdelrhman, W. M., Abdulmaguid, N. Y. M., Bahnasy, R. M., Elgendy, M. S. A., Elashry, A. M. M. M., El-Hassanin, M. F., El-Wakeil, N. H. M., Khalil, A. M. M., & Amin, H. F. (2023). Evaluation of In Vitro Antioxidant, Anti-Obesity, and Anti-Diabetic Activities of Opuntia ficus Cladodes Gel and Its Application as a Preservative Coating for Shrimp during Refrigerated Storage. Gels, 9(9), 716. https://doi.org/10.3390/gels9090716







