Development of Thixotropic Molecular Oleogels Comprising Alkylanilide Gelators by Using a Mixing Strategy
Abstract
:1. Introduction
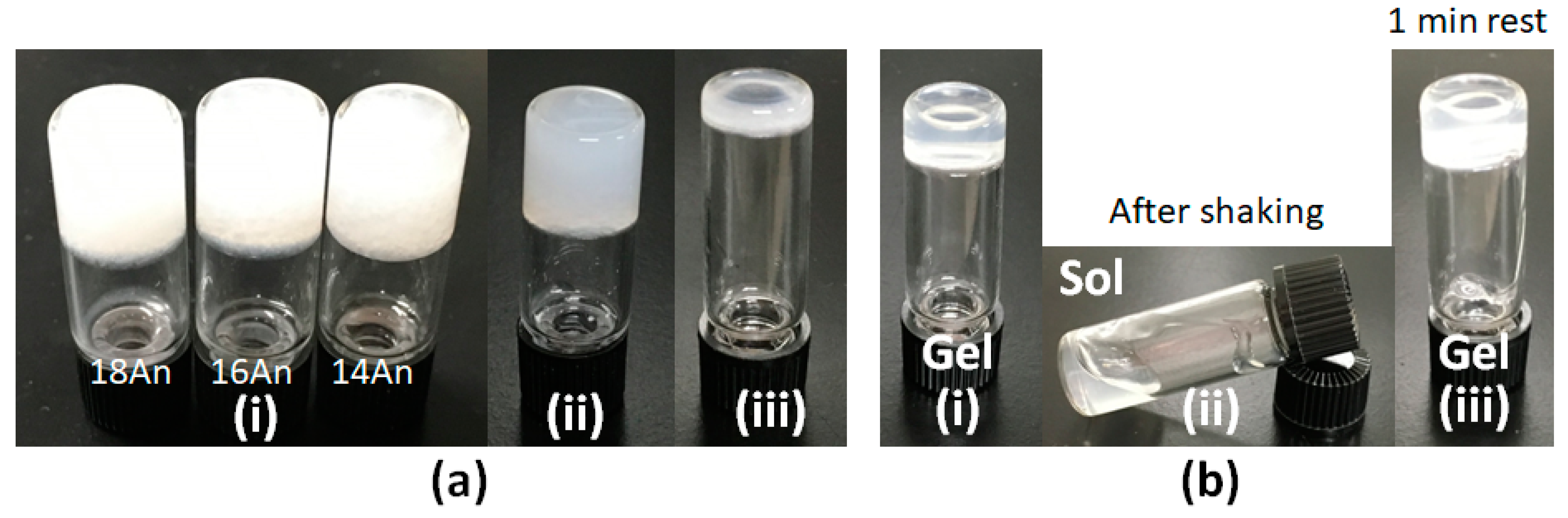
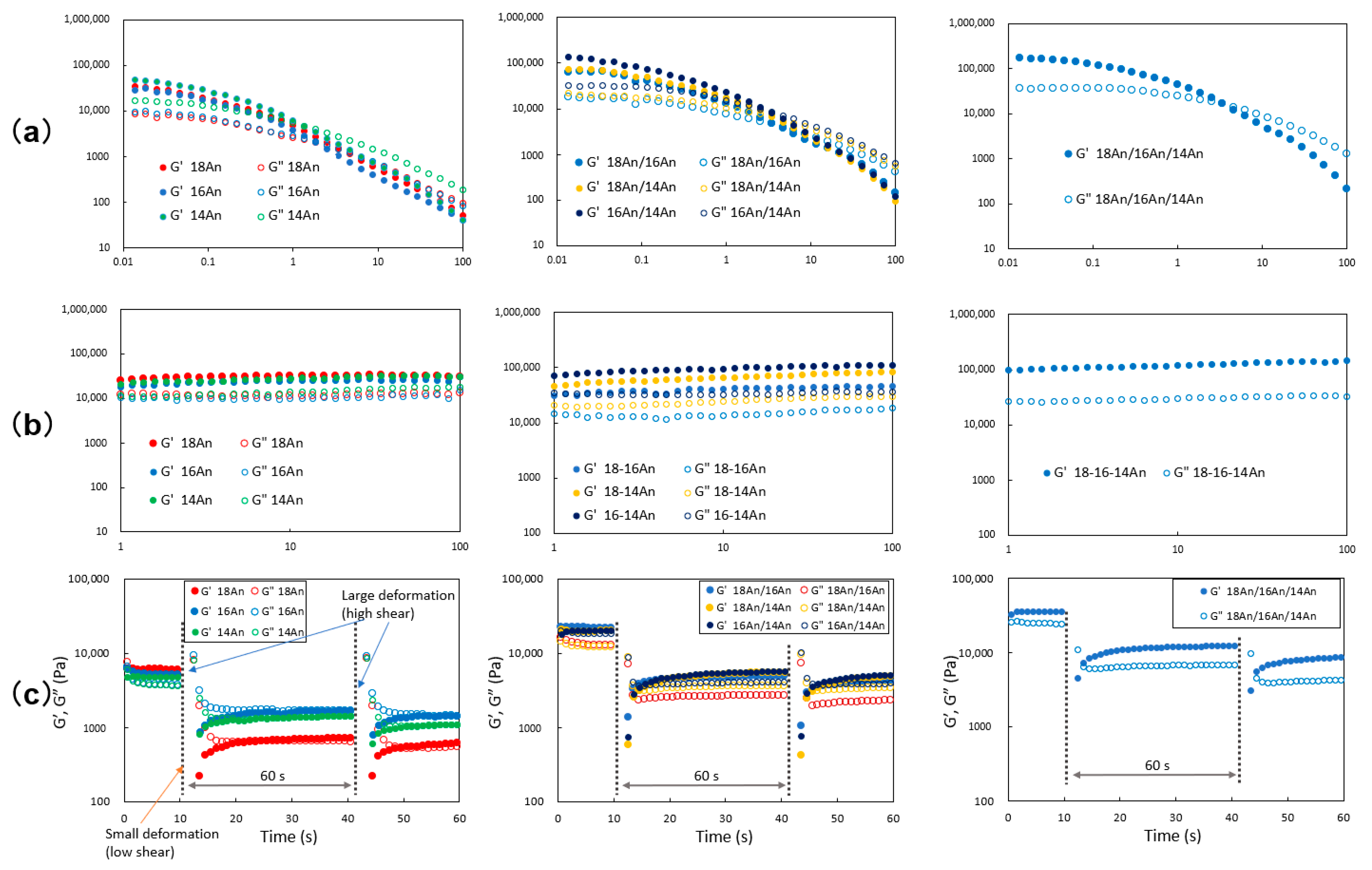
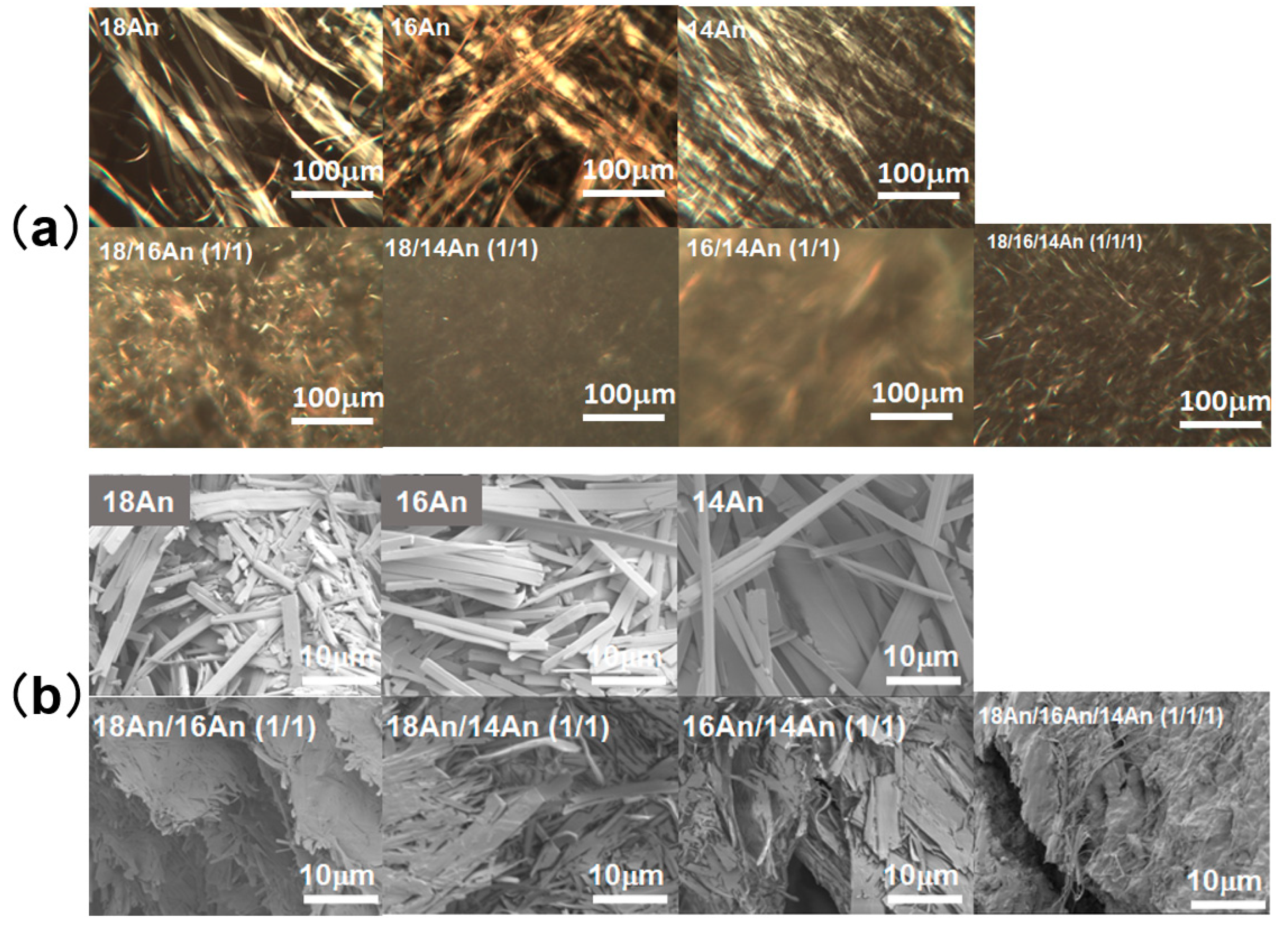
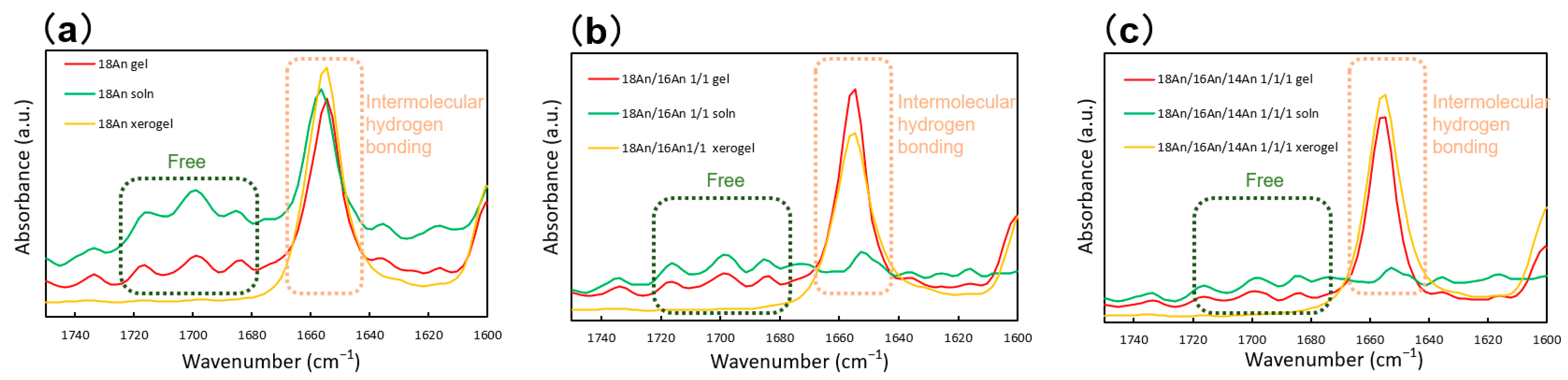
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, R.G.; Terech, P. (Eds.) Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Seiffert, S. (Ed.) Supramolecular Polymer Networks and Gels; Springer International Publishing AG: Cham, Switzerland, 2015. [Google Scholar]
- Guenet, J.-M. Organogels Thermodynamics, Structure, Solvent Role, and Properties; Springer International Publishing AG: Cham, Switzerland, 2016. [Google Scholar]
- Weiss, R.G. (Ed.) Molecular Gels, Structure and Dynamics; The Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Dawn, A.; Shiraki, T.; Haraguchi, S.; Tamaru, S.; Shinkai, S. What Kind of “Soft Materials” Can We Design from Molecular Gels? Chem. Asian J. 2011, 6, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.G. The Past, Present, and Future of Molecular Gels. What Is the Status of the Field, and Where Is It Going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Weiss, R.G. Systematic Modifications of Alkane-Based Molecular Gelators and the Consequences to the Structures and Properties of Their Gels. New J. Chem. 2015, 39, 785–799. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-Molecular-Weight Organogelators as Functional Materials for Oil Spill Remediation. Polym. Adv. Technol. 2016, 27, 704–711. [Google Scholar] [CrossRef]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular Materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Peng, J.; Fang, Y. Molecular Gels as Intermediates in the Synthesis of Porous Materials and Fluorescent Films: Concepts and Applications. Langmuir 2017, 33, 10419–10428. [Google Scholar] [CrossRef]
- Weiss, R.G. Controlling Variables in Molecular Gel Science: How Can We Improve the State of the Art? Gels 2018, 4, 25. [Google Scholar] [CrossRef]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of Small Bioactive Molecules from Physical Gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Chivers, P.R.A.; Smith, D.K. Shaping and structuring supramolecular gels. Nat. Rev. Mater. 2019, 4, 463–478. [Google Scholar] [CrossRef]
- Dawn, A. Supramolecular Gel as the Template for Catalysis, Inorganic Superstructure, and Pharmaceutical Crystallization. Int. J. Mol. Sci. 2019, 20, 781. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, M.; Kimura, S.; Yamanaka, M. Urea Derivatives as Functional Molecules: Supramolecular Capsules, Supramolecular Polymers, Supramolecular Gels, Artificial Hosts, and Catalysts. Chem.-Eur. J. 2021, 27, 5601–5614. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Adams, D.J. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Hughes, R. Rheology for Chemists: An Introduction, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Larson, R.G.; Wei, Y. A Review of Thixotropy and Its Rheological Modeling. J. Rheol. 2019, 63, 477–501. [Google Scholar] [CrossRef]
- Dayan, N. Delivery System Design in Topically Applied Formulations: An Overview. In Delivery System Handbook for Personal Care and Cosmetic Products, Technology, Applications, and Formulations; Rosen, M.R., Ed.; William Andrew, Inc.: New York, NY, USA, 2005; pp. 101–118. [Google Scholar]
- Sugibayashi, K.; Morimoto, Y. Transdermal Patches. In Gels Handbook, The Fundamentals; Osada, Y., Kajiwara, K., Fushimi, T., Irasa, O., Hirokawa, Y., Matsunaga, T., Shimomura, T., Wang, L., Ishida, H., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2001; Volume 3, Section 6, pp. 201–210. [Google Scholar]
- Boekhoven, J.; Stupp, S.I. 25th Anniversary Article: Supramolecular Materials for Regenerative Medicine. Adv. Mater. 2014, 26, 1642–1659. [Google Scholar] [CrossRef]
- Ohsedo, Y. Low-Molecular-Weight Gelators as Base Materials for Ointments. Gels 2016, 2, 13. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Du, X.; Xu, B. Supramolecular Biofunctional Materials. Biomaterials 2017, 129, 1–27. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Two-Component Gel-Phase Materials—Highly Tunable Self-Assembling Systems. Chem.-Eur. J. 2005, 11, 5496–5508. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Rowan, S.J. Supramolecular Gels Formed from Multi-Component Low Molecular Weight Species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef]
- Draper, E.R.; Wallace, M.; Schweins, R.; Poole, R.J.; Adams, D.J. Nonlinear Effects in Multicomponent Supramolecular Hydrogels. Langmuir 2017, 33, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Adams, D.J. How Should Multicomponent Supramolecular Gels Be Characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.R.; Sproules, S.; Schweins, R.; Draper, E.R.; Adams, D.J. Controlled Tuning of the Properties in Optoelectronic Self-Sorted Gels. J. Am. Chem. Soc. 2018, 140, 8667–8670. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, W.; Shigemitsu, H.; Fujisaku, T.; Kubota, R.; Minami, S.; Urayama, K.; Hamachi, I. Post-Assembly Fabrication of a Functional Multicomponent Supramolecular Hydrogel Based on a Self-Sorting Double Network. J. Am. Chem. Soc. 2019, 141, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.J.; Smith, D.K. Photo-Patterned Multi-Domain Multi-Component Hybrid Hydrogels. Chem. Commun. 2020, 56, 7029–7032. [Google Scholar] [CrossRef]
- Vasilyev, G.; Koifman, N.; Shuster, M.; Gishvoliner, M.; Cohen, Y.; Zussman, E. Synergistic Effect of Two Organogelators for the Creation of Bio-Based, Shape-Stable Phase-Change Materials. Langmuir 2020, 36, 15572–15582. [Google Scholar] [CrossRef]
- Dasgupta, D.; Srinivasan, S.; Rochas, C.; Ajayaghosh, A.; Guenet, J.M. Hybrid Thermoreversible Gels from Covalent Polymers and Organogels. Langmuir 2009, 25, 8593–8598. [Google Scholar] [CrossRef]
- Zoukal, Z.; Elhasri, S.; Carvalho, A.; Schmutz, M.; Collin, D.; Vakayil, P.K.; Ajayaghosh, A.; Guenet, J.M. Hybrid Materials from Poly(Vinyl Chloride) and Organogels. ACS Appl. Polym. Mater. 2019, 1, 1203–1208. [Google Scholar] [CrossRef]
- Talebpour, P.; Heinrich, B.; Gavat, O.; Carvalho, A.; Moulin, E.; Giuseppone, N.; Guenet, J.M. Modulation of the Molecular Structure of Tri-Aryl Amine Fibrils in Hybrid Poly[Vinyl Chloride] Gel/Organogel Systems. Macromolecules 2021, 54, 8104–8111. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Watanabe, H.; Oono, M.; Tanaka, A. Mixing Enhancement Effect of Low-Molecular-Weight Organogelators for Thixotropic Organogel Creation. Chem. Lett. 2013, 42, 363–365. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Tanaka, A.; Watanabe, H. Mixing Induced Thixotropy of a Two-Component System of Alkylurea Organogelators Having Different Alkyl Chains. New J. Chem. 2013, 37, 2250–2253. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Watanabe, H.; Oono, M.; Tanaka, A. Improved Mechanical Properties of Alkylamide Organogels via a Mixing Enhancement Effect. RSC Adv. 2013, 3, 5803–5806. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Taniguchi, M.; Oono, M.; Saruhashi, K.; Watanabe, H. Creation of Thixotropic Multicomponent Alkylamide Organogels Containing Non-Volatile Oil as Potential Drug Release Host Materials. RSC Adv. 2014, 4, 35484–35488. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H. Onset of mixing-induced thixotropy in hydrogels by mixing two homologues of low-molecular-weight hydrogelators. RSC Adv. 2014, 4, 43560–43563. [Google Scholar] [CrossRef]
- Ohsedo, Y. N-Alkylhydantoins as New Organogelators and Their Ability to Create Thixotropic Mixed Molecular Organogels. Gels 2022, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Rebaka, V.P.; Rachamalla, A.K.; Batra, S.; Subbiah, N. State of the Art and New Perspectives in Oleogels and Applications. In Sustainable Green Chemical Processes and Their Allied Applications; Inamuddin, Asiri, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2020. [Google Scholar]
- Sagiri, S.S.; Singh, V.K.; Pal, K.; Banerjee, I.; Basak, P. Stearic acid based oleogels: A study on the molecular, thermal and mechanical properties. Mater. Sci. Eng. C 2015, 48, 688–699. [Google Scholar] [CrossRef]
- Silva, R.C.; Ferdaus, M.J.; Foguel, A.; da Silva, T.L.T. Oleogels as a Fat Substitute in Food: A Current Review. Gels 2023, 9, 180. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.; Gong, D.; Zhang, G. Oleogel-structured emulsions: A review of formation, physicochemical properties and applications. Food Chem. 2023, 404, 134553. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Liu, H.; Chen, D.; Li, H.; Chen, Z.; Xu, W. Synthesis and antimicrobial bioassays of 1,3,4-thiadiazole sulfone derivatives containing amide moiety: A study based on molecular dynamics (MD) simulations, MM/GBSA, and molecular docking. J. Saudi Chem. Soc. 2022, 26, 101415. [Google Scholar] [CrossRef]
- Ballari, M.S.; Cano, N.H.; Wunderlin, D.A.; Feresin, G.E.; Santiago, A.N. One-pot sequential synthesis and antifungal activity of 2-(benzylsulfonyl)benzothiazole derivatives. RSC Adv. 2019, 9, 29405–29413. [Google Scholar] [CrossRef]
- Meng, Y.-B.; Yang, Y.-J. Gelation of the organic liquid electrolytes and the conductivities as gel electrolytes. Electrochem. Commun. 2007, 9, 1428–1433. [Google Scholar] [CrossRef]
- Xue, P.-C.; Sun, J.-B.; Xu, Q.-X.; Lu, R.; Takafuji, M.; Ihara, H. Anion response of organogels: Dependence on intermolecular interactions between gelators. Org. Biomol. Chem. 2013, 11, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Collin, D.; Gavat, O.; Carvalho, A.; Moulin, E.; Giuseppone, N.; Guenet, J.-M. Effect of solvent isomers on the gelation properties of tri-aryl amine organogels and their hybrid thermoreversible gels with poly[vinyl chloride]. Soft Matter 2022, 18, 5575–5584. [Google Scholar] [CrossRef]
- Tadros, T.F. Formulations: In Cosmetic and Personal Care; Walter de Gruyter GmbH: Berlin/Boston, Germany, 2016. [Google Scholar]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure–Function Correlation. Chem.-Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological Characterisation of Polymer Gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]


| Solvent | 18An | 16An | 14An |
|---|---|---|---|
| Propylene carbonate | 2 | 2 | 2 |
| N,N-Dimethyl formamide | 6 | 7 | S 1 |
| Methanol | 5 | 5 | 6 |
| Ethanol | 3 | 2 | 7 |
| 1-Butanol | 4 | 8 | S 1 |
| Dichloroethane | 3 | 3 | 4 |
| Tetrahydrofuran | 10 | S 1 | S 1 |
| Ethyl acetate | 3 | 3 | 4 |
| Toluene | 2 | 2 | 4 |
| n-Octane | 1 | 1 | 1 |
| Olive oil | 3 | 3 | 4 |
| Squalane | 1 | 1 | 1 |
| Mixed Squalane—Oleogel Samples 1 | ||||
|---|---|---|---|---|
| Mixed Molar Ratio | 18An/16An | 18An/14An | 16An/14An | 18An/16An/14An |
| 5/1 | 0.25 (0.10) 2 | 0.50 (0.10) 2 | 0.25 (0.10) 2 | |
| 2/1 | 0.25 (0.10) 2 | 0.50 (0.10) 2 | 0.25 (0.10) 2 | |
| 1/1 | 0.50 (0.10) 2 | 0.50 (0.10) 2 | 0.50 (0.10) 2 | |
| 1/2 | 1.00 (0.10) 2 | 0.50 (0.10) 2 | 0.50 (0.10) 2 | |
| 1/5 | 1.00 (0.10) 2 | 0.50 (0.10) 2 | 1.00 (0.10) 2 | |
| 1/1/1 | 0.25 (0.10) 2 | |||
| Samples 1 | Tgel to sol on Heating/°C (ΔH/mJ mg−1) | Tsol to gel on Cooling/°C (ΔH/mJ mg−1) |
|---|---|---|
| 18An | 43.0 (13.4), 89.7 (1.2) | 41.4 (13.9), 86.5 (1.3) |
| 16An | 37.1 (5.7), 84.2 (3.2) | 79.5 (3.2) |
| 14An | 36.9 (11.1), 81.1 (5.7) | 33.9 (11.1), 71.2 (5.7) |
| 18An/16An 1/1 | 30.7 (4.4), 81.4 (7.9) | 27.2 (4.0), 76.9 (2.2) |
| 18An/14An 1/1 | 22.9 (5.7) | 23.1 (5.6) |
| 16An/14An 1/1 | 77.9 (8.3) | 71.7 (8.1) |
| 18An/16An/14An 1/1/1 | 76.4 (7.3) | 75.2 (7.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohsedo, Y. Development of Thixotropic Molecular Oleogels Comprising Alkylanilide Gelators by Using a Mixing Strategy. Gels 2023, 9, 717. https://doi.org/10.3390/gels9090717
Ohsedo Y. Development of Thixotropic Molecular Oleogels Comprising Alkylanilide Gelators by Using a Mixing Strategy. Gels. 2023; 9(9):717. https://doi.org/10.3390/gels9090717
Chicago/Turabian StyleOhsedo, Yutaka. 2023. "Development of Thixotropic Molecular Oleogels Comprising Alkylanilide Gelators by Using a Mixing Strategy" Gels 9, no. 9: 717. https://doi.org/10.3390/gels9090717
APA StyleOhsedo, Y. (2023). Development of Thixotropic Molecular Oleogels Comprising Alkylanilide Gelators by Using a Mixing Strategy. Gels, 9(9), 717. https://doi.org/10.3390/gels9090717







