A Non-Hydrolytic Sol–Gel Route to Organic-Inorganic Hybrid Polymers: Linearly Expanded Silica and Silsesquioxanes
Abstract
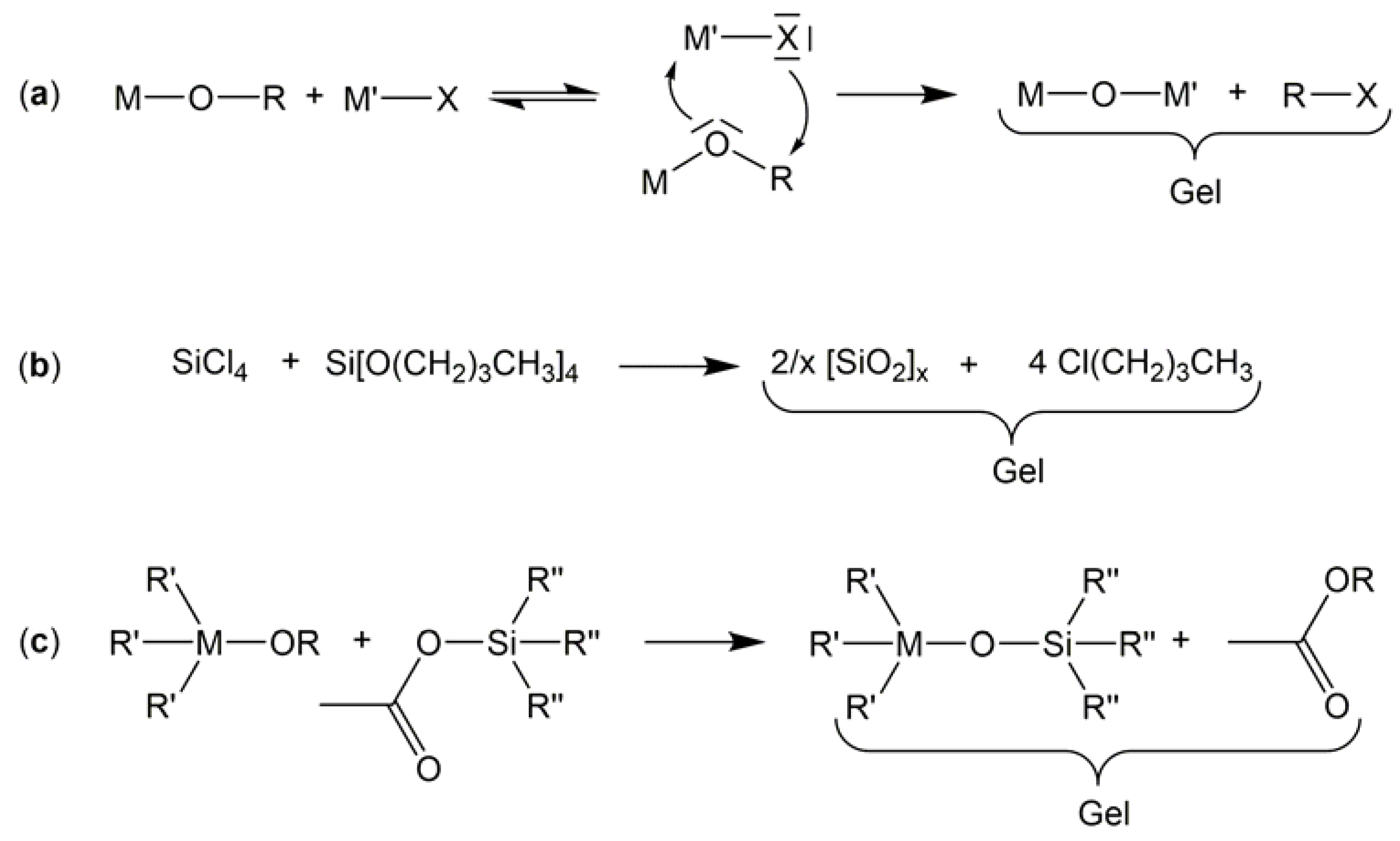
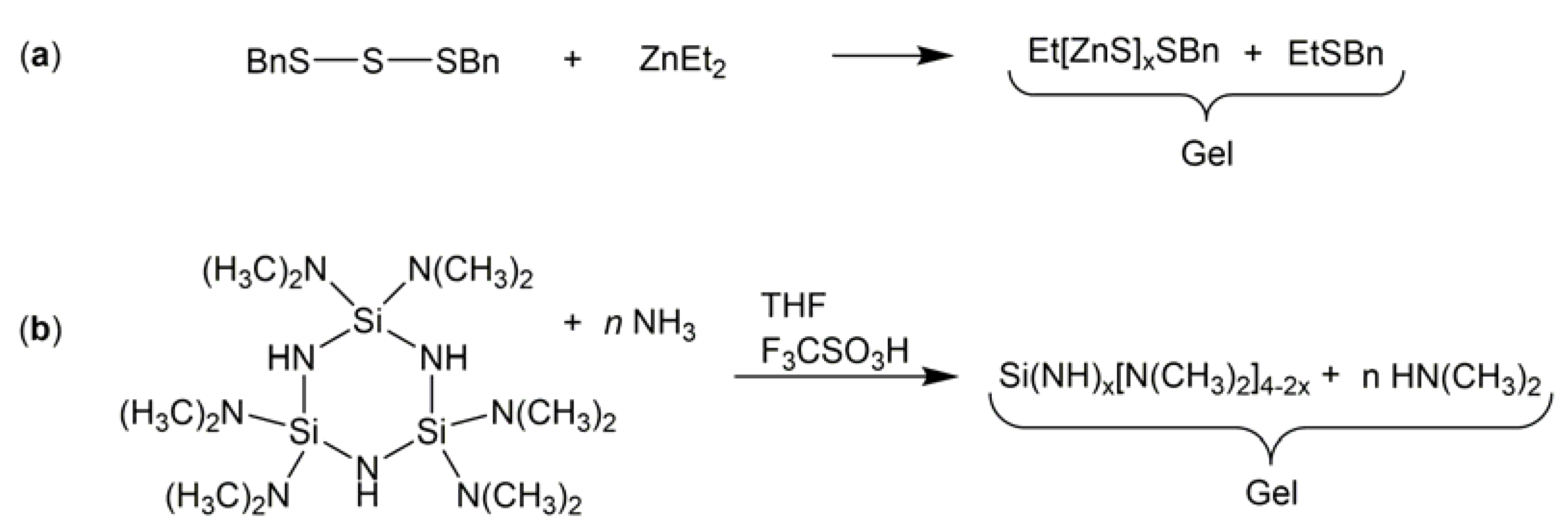
1. Introduction
2. Results and Discussion
2.1. Selection, Synthesis and Characterisation of the Starting Materials
2.2. Oligomer and Polymer Formation in Solution
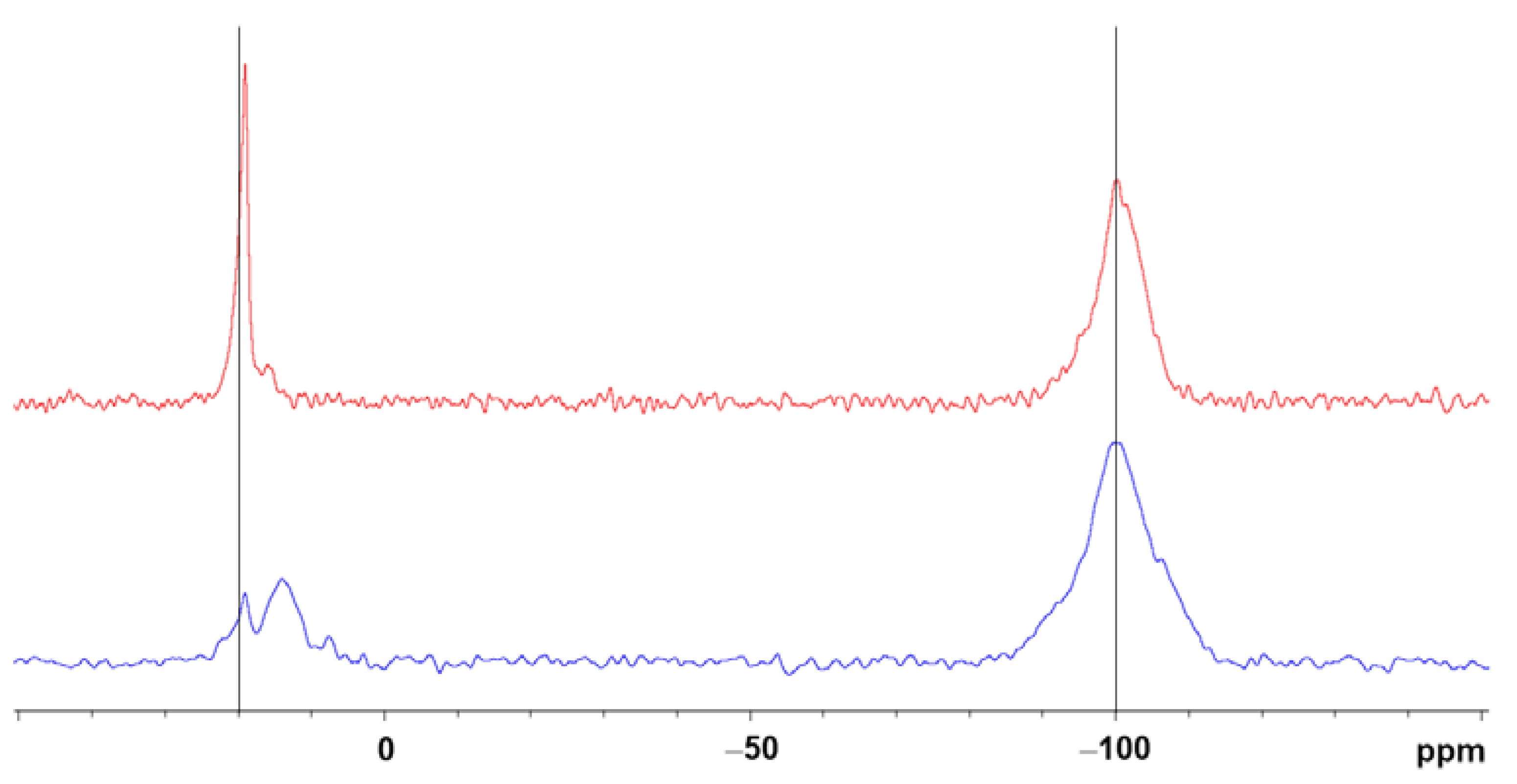
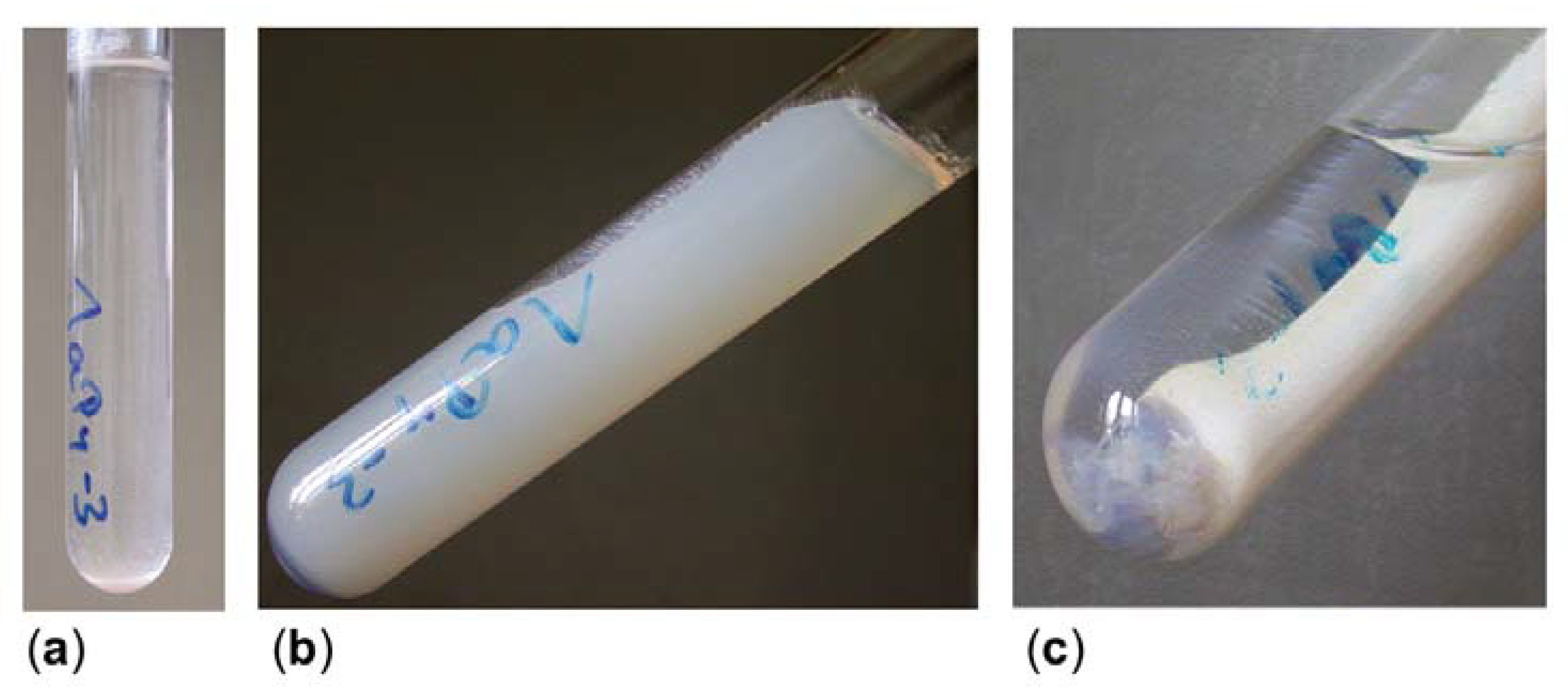
2.3. Gel Synthesis and Characterisation
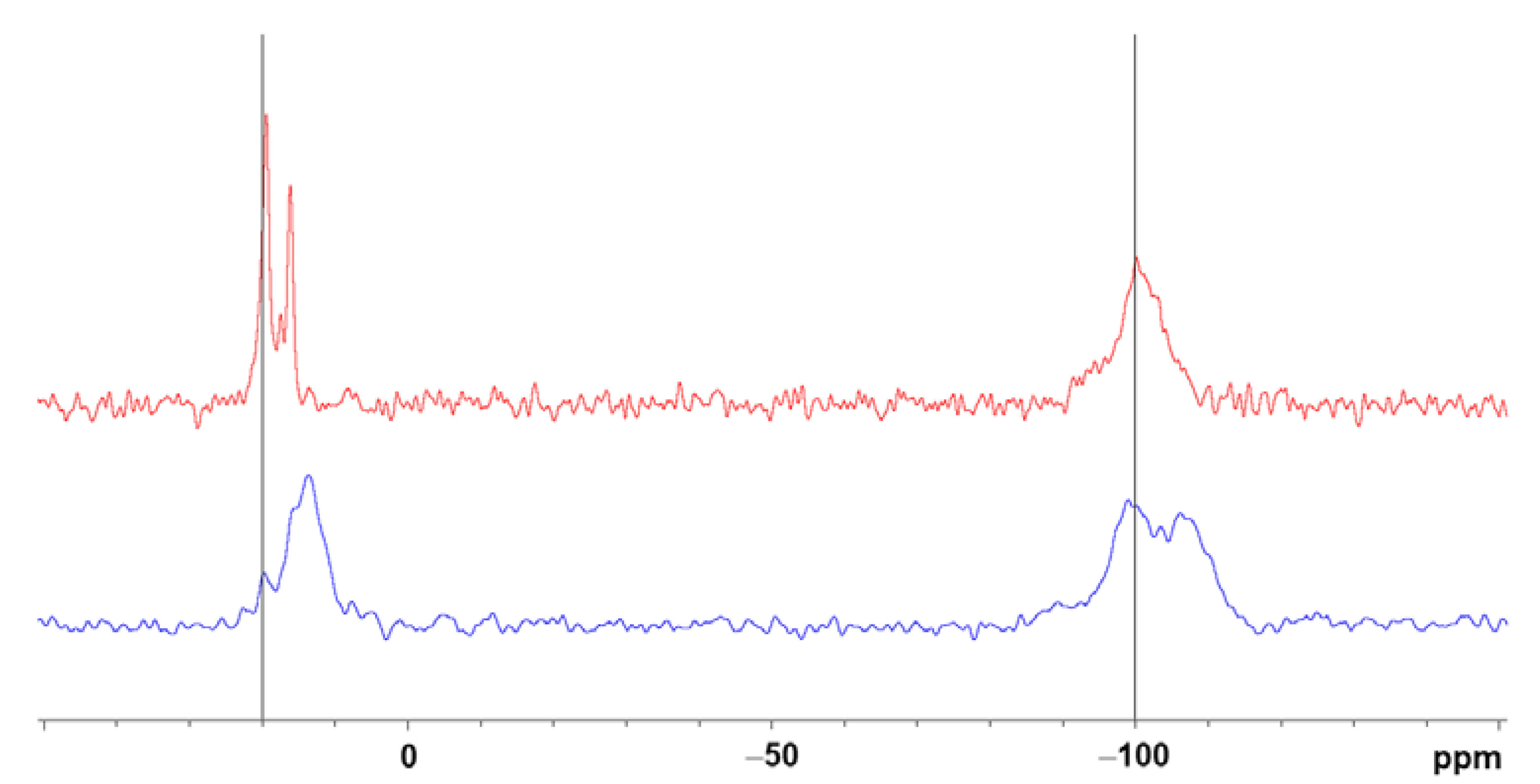
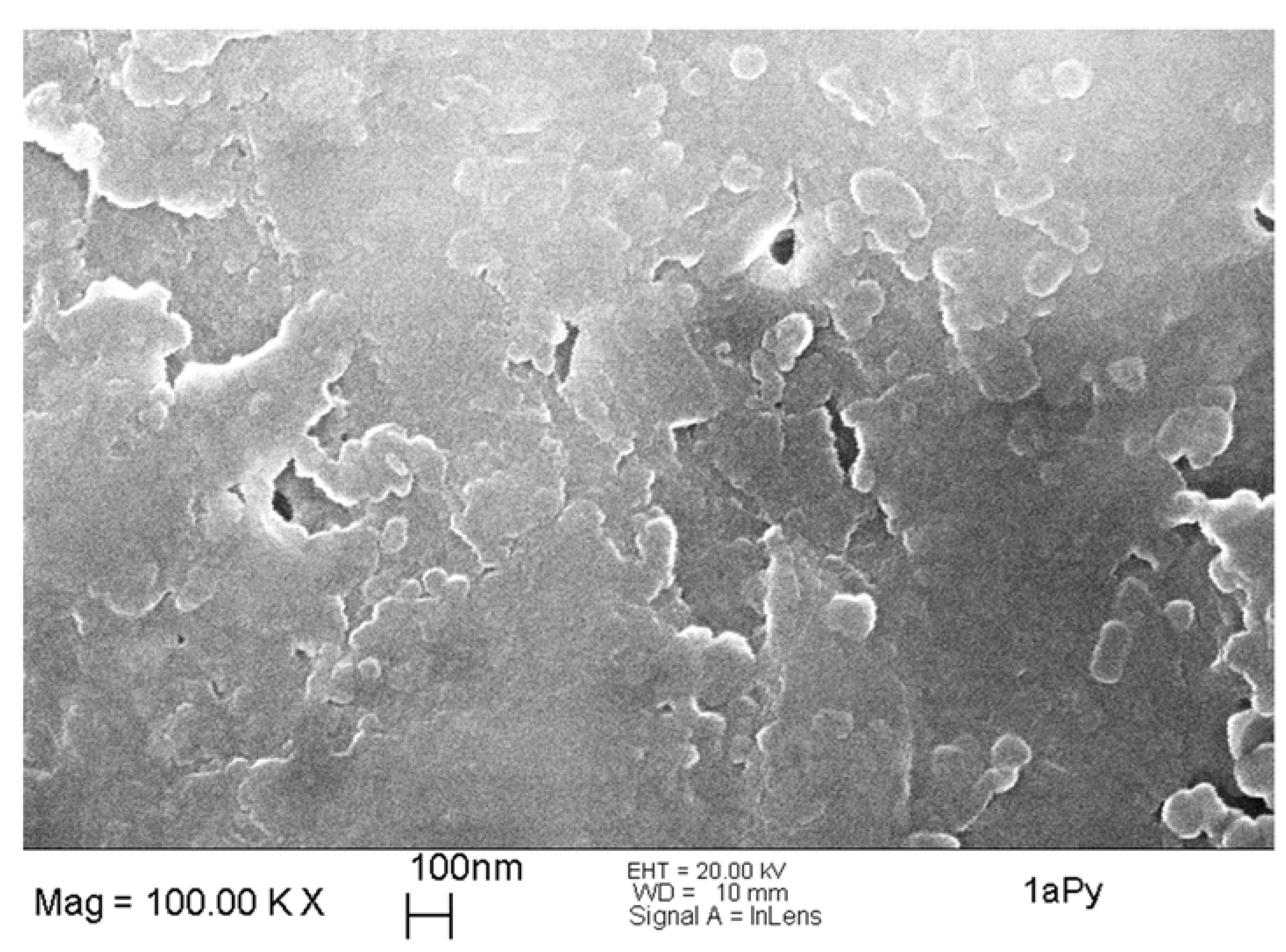
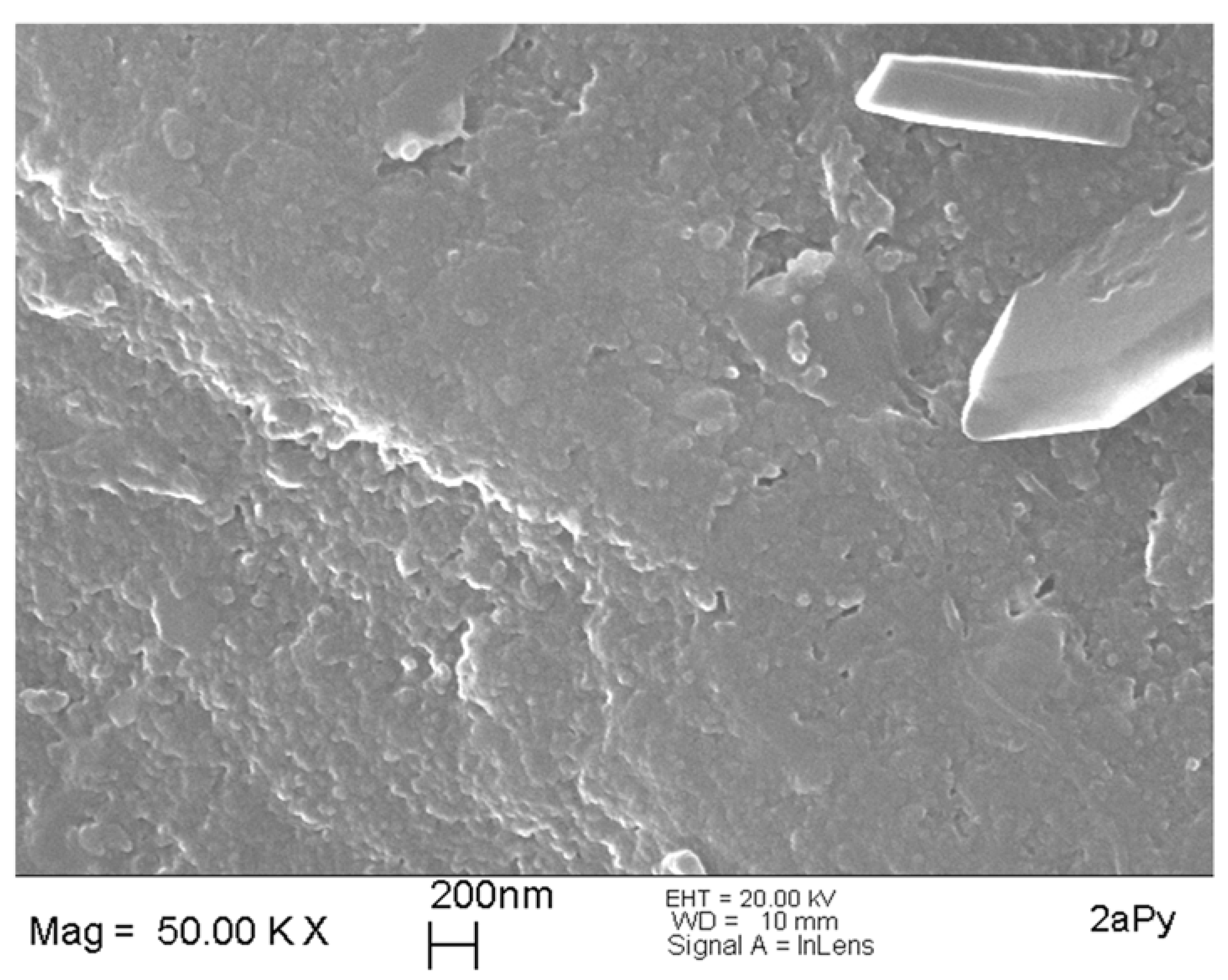
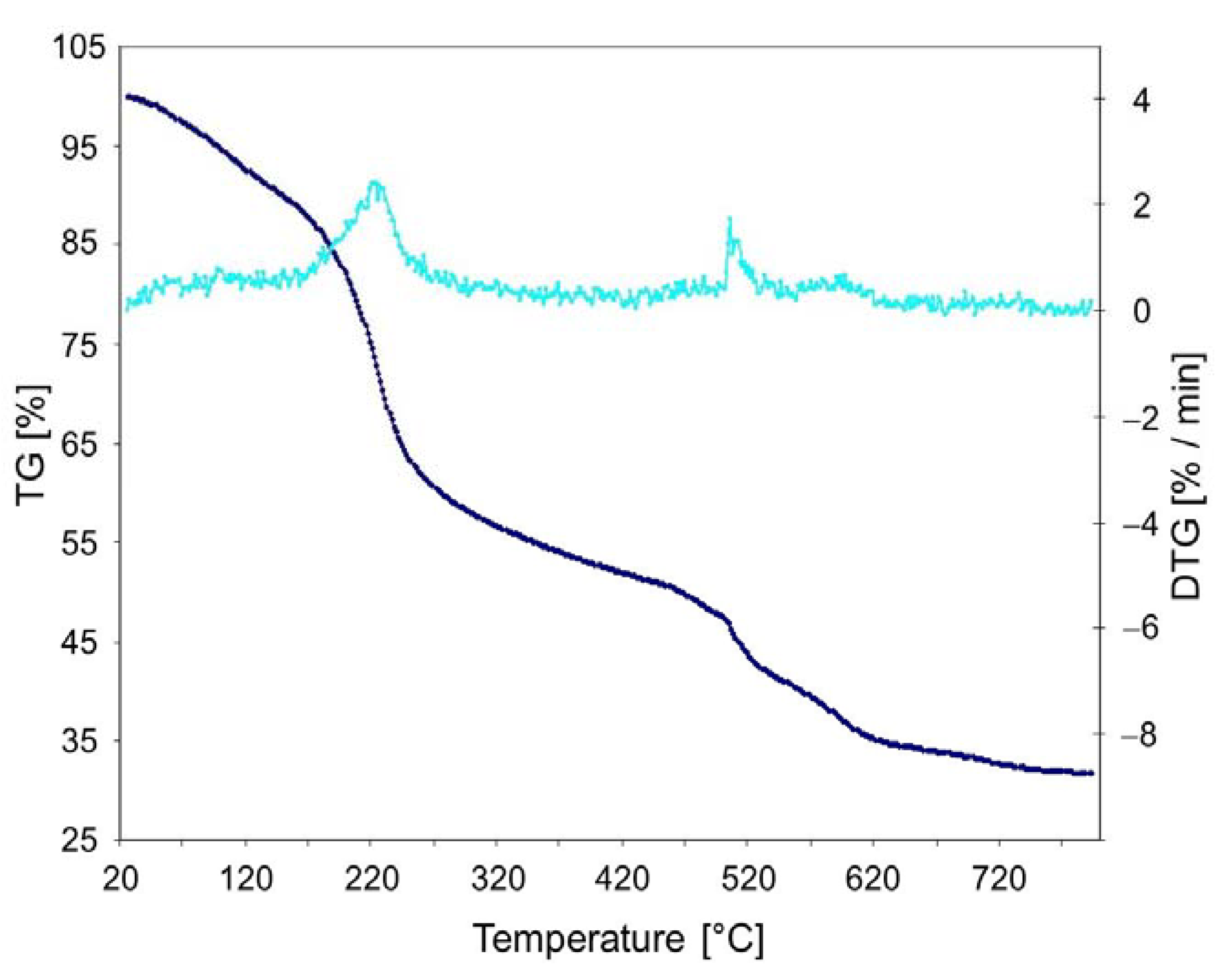
2.4. Morphological and Thermal Characterization of the Xerogels
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Faustini, M.; Nicole, L.; Ruiz-Hitzky, E.; Sanchez, C. History of Organic–Inorganic Hybrid Materials: Prehistory, Art, Science, and Advanced Applications. Adv. Funct. Mater. 2018, 28, 1704158. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Hench, L.L.; West, J.K. The Sol–Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclerq, D. Recent developments of molecular chemistry for Sol–Gel processes. Angew. Chem. Int. Ed. 1996, 35, 1420–1436. [Google Scholar] [CrossRef]
- Sakka, S. (Ed.) Sol–Gel Science and Technology; 4 volume set; Springer: New York, NY, USA, 2003. [Google Scholar]
- Vioux, A. Nonhydrolytic Sol−Gel Routes to Oxides. Chem. Mater. 1997, 9, 2292–2299. [Google Scholar] [CrossRef]
- Hay, J.N.; Raval, H.M. Synthesis of Organic−Inorganic Hybrids via the Non-hydrolytic Sol−Gel Process. Chem. Mater. 2001, 13, 3396–3403. [Google Scholar] [CrossRef]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol–gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef]
- Smeets, V.; Styskalik, A.; Debecker, D.P. Non-hydrolytic sol–gel as a versatile route for the preparation of hybrid heterogeneous catalysts. J. Sol–Gel Sci. Technol. 2021, 97, 505–522. [Google Scholar] [CrossRef]
- Kroke, E. Novel Sol–Gel Routes to Non-Oxide Ceramics. In 9th CIMTEC—World Ceramics Congress, Ceramics: Getting into the 2000‘s—Part C; Vincenzini, P., Ed.; Techna: Faenza, Italy, 1999; pp. 123–134. [Google Scholar]
- Hector, A.L. Synthesis and processing of silicon nitride and related materials using preceramic polymer and non-oxide Sol–Gel approaches. Coord. Chem. Rev. 2016, 323, 120–137. [Google Scholar] [CrossRef]
- Rex, A.; dos Santos, J.H.Z. The use of Sol–Gel processes in the development of supported catalysts. J. Sol–Gel Sci. Technol. 2023, 105, 30–49. [Google Scholar] [CrossRef]
- Almeida, R.M.; Martucci, A.; Santos, L.; Rojas-Hernandez, R.E. (Eds.) Sol–Gel Derived Optical and Photonic Materials; Elsevier Ltd.: London, UK, 2021. [Google Scholar]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011. [Google Scholar]
- Hu, P.; Liu, L.; Zhao, M.; Wang, J.; Ma, X.; Wang, J. Review on design, synthesis, and use of high temperature resistant aerogels exceeding 800 °C. ES Mater. Manuf. 2022, 15, 14–33. [Google Scholar]
- Eychmueller, A. Nanoparticle-Based Aerogels and Their Prospective Future Applications. J. Phys. Chem. C 2022, 126, 19011–19023. [Google Scholar] [CrossRef]
- Gerrard, W.; Jones, J.V. Stability of isomeric butoxysilanes with respect to silicon tetrachloride and hydrogen chloride. J. Chem. Soc. 1952, 1690–1693. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclercq, D.; Lefèvre, P.; Mutin, P.H.; Vioux, A. Preparation of monolithic gels from silicon halides by a non-hydrolytic Sol–Gel process. J. Non-Cryst. Solids 1992, 146, 301–303. [Google Scholar] [CrossRef]
- Acosta, S.; Corriu, R.J.P.; Leclerq, D.; Mutin, P.H.; Vioux, A. Novel non-hydrolytic Sol–Gel route to metal oxides. J. Sol–Gel Sci. Technol. 1994, 2, 25–28. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclerq, D.; Mutin, P.H.; Vioux, A. A Solution Chemistry Study of Nonhydrolytic Sol−Gel Routes to Titania. Chem. Mater. 1997, 9, 694–698. [Google Scholar] [CrossRef]
- Oakton, E.; Siddiqi, G.; Fedorov, A.; Coperet, C. Tungsten oxide by non-hydrolytic sol–gel: Effect of molecular precursor on morphology, phase and photocatalytic performance. New J. Chem. 2016, 40, 217–222. [Google Scholar] [CrossRef]
- Andriamananarivelo, M.; Corriu, R.J.P.; Leclerq, D.; Mutin, P.H.; Vioux, A. Mixed oxides SiO2–ZrO2 and SiO2–TiO2 by a non-hydrolytic sol–gel route. J. Mater. Chem. 1996, 6, 1665–1671. [Google Scholar] [CrossRef]
- Andriamananarivelo, M.; Corriu, R.J.P.; Leclerq, D.; Mutin, P.H.; Vioux, A. Nonhydrolytic Sol−Gel Process: Aluminum Titanate Gels. Chem. Mater. 1997, 9, 1098–1102. [Google Scholar] [CrossRef]
- Jansen, M.; Guenther, E. Oxide gels and ceramics prepared by a nonhydrolytic sol- gel process. Chem. Mater. 1995, 7, 2110–2114. [Google Scholar] [CrossRef]
- Nassar, E.J.; dos Santos Pereira, P.F.; de Oliveira Nassor, E.C.; Ávila, L.R.; Ciuffi, K.J.; Calefi, P.S. Nonhydrolytic Sol–Gel synthesis and characterization of YAG. J. Mater. Sci. 2007, 42, 2244–2249. [Google Scholar] [CrossRef]
- Janković-Častvan, I.; Lazarević, S.; Jordović, B.; Petrović, R.; Tanasković, D.; Janaćković, D. Electrical properties of cordierite obtained by non-hydrolytic sol–gel method. J. Eur. Ceram. Soc. 2007, 27, 3659–3661. [Google Scholar] [CrossRef]
- Gao, Y.; Hamana, D.; Iwasaki, R.; Iihama, J.; Honda, S.; Kumari, M.; Hayakawa, T.; Bernard, S.; Thomas, P.; Iwamoto, Y. Chemical route for synthesis of β-SiAlON:Eu2+ phosphors combining polymer-derived ceramics route with non-hydrolytic Sol–Gel chemistry. J. Sol–Gel Sci. Technol. 2022, 104, 711–723. [Google Scholar] [CrossRef]
- Koshevaya, E.; Mikhaylov, V.; Sitnikov, P.; Krivoshapkina, E.; Krivoshapkin, P. Electrosurface properties and acid-base equilibria of Ta2O5 and Ta2O5:Eu nanoparticles in NaCl solutions. Surf. Interfaces 2022, 29, 101713. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle processing: Understanding and controlling aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Perez, A.M.; Louvain, N.; Boury, B.; Brun, N.; Mutin, P.H. Ethers as Oxygen Donor and Carbon Source in Non-hydrolytic Sol–Gel: One-Pot, Atom-Economic Synthesis of Mesoporous TiO2-Carbon Nanocomposites. Chem. Eur. J. 2018, 24, 4982–4990. [Google Scholar] [CrossRef]
- Corriu, R.J.P.; Leclerq, D.; Lefevre, P.; Mutin, P.H.; Vioux, A. Preparation of monolithic binary oxide gels by a nonhydrolytic Sol–Gel process. Chem. Mater. 1992, 4, 961–963. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yasumori, A.; Shibata, S.; Yamane, M. Preparation of high homogeneity BaO-TiO2-SiO2 gel. J. Sol–Gel Sci. Technol. 1994, 2, 387–391. [Google Scholar] [CrossRef]
- Goel, S.C.; Chiang, M.Y.; Gibbons, P.C.; Buhro, W.F. New Chemistry for the Sol–Gel Process: Acetone as a New Condensation Reagent. Mater. Res. Soc. Symp. Proc. 1992, 271, 3–13. [Google Scholar] [CrossRef]
- Mehrotra, R.C. Present status and future potential of the Sol–Gel process. In Chemistry, Spectroscopy and Applications of Sol–Gel Glasses; Reisfeld, R., Jorgensen, C.K., Eds.; Springer: Berlin, Germany, 1992; p. 20. [Google Scholar]
- Guiton, T.A.; Czekaj, C.L.; Pantano, C.G. Organometallic Sol/Gel Chemistry of Metal Sulfides. J. Non-Cryst. Solids 1990, 121, 7–15. [Google Scholar] [CrossRef]
- Gacion, T.; Malier, L.; Boilot, J.-P. New Transparent Chalcogenide Materials Using a Sol–Gel Process. Chem. Mater. 1997, 9, 1502–1504. [Google Scholar]
- Ptatschek, V.; Schreder, B.; Herz, K.; Hilbert, U.; Ossau, W.; Schottner, G.; Rahäuser, O.; Bischof, T.; Lermann, G.; Materny, A.; et al. Sol–Gel Synthesis and Spectroscopic Properties of Thick Nanocrystalline CdSe Films. J. Phys. Chem. B 1997, 101, 8898–8906. [Google Scholar] [CrossRef]
- Rusch, P.; Luebkemann, F.; Borg, H.; Eckert, J.G.; Dorfs, D.; Bigall, N.C. Influencing the coupling between network building blocks in CdSe/CdS dot/rod aerogels by partial cation exchange. J. Chem. Phys. 2022, 156, 234701. [Google Scholar] [CrossRef]
- Seddon, A.B.; Hodgson, S.N.B.; Scott, M.G. Sol–Gel approach to preparing germanium disulphide. J. Mater. Sci. 1991, 26, 2599–2602. [Google Scholar] [CrossRef]
- Leidich, S.; Buechele, D.; Lauenstein, R.; Kluenker, M.; Lind, C. “Non-hydrolytic” sol–gel synthesis of molybdenum sulfides. J. Solid State Chem. 2016, 242, 175–181. [Google Scholar] [CrossRef]
- Gerwig, M.; Schwarz, M.; Brendler, E.; Kroke, E. [SiSxHy]n—Perhydridopolysilathianes—Cross-linked thio-analogs of polysiloxanes. Eur. J. Inorg. Chem. 2016, 2016, 5028–5035. [Google Scholar] [CrossRef]
- Rovai, R.; Lehmann, C.W.; Bradley, J.S. Non-Oxide Sol–Gel Chemistry: Preparation from Tris(dialkylamino)silazanes of a Carbon-Free, Porous, Silicon Diimide Gel. Angew. Chem. Int. Ed. 1999, 38, 2036–2038. [Google Scholar] [CrossRef]
- Kitney, S.P.; Sajedin, S.M.; Rocher, V.; Cheng, F.; Kelly, S.M. Silicon diimide gel as an efficient stationary phase in thin layer chromatography for acid-sensitive organic compounds. Chem. Commun. 2017, 53, 11080–11082. [Google Scholar] [CrossRef]
- Paine, R.T. Processing of boron-nitrogen preceramic polymers. J. Inorg. Organomet. Polym. 1992, 2, 183–195. [Google Scholar] [CrossRef]
- Jäger, L.; Köhler, H. Pseudochalkogene. Sulfur Reports 1992, 12, 159–203. [Google Scholar] [CrossRef]
- Riedel, R.; Kroke, E.; Greiner, A.; Gabriel, A.O.; Ruwisch, L.; Nicolich, J.; Kroll, P. Inorganic Solid State Chemistry with Main Group Element Carbodiimides. Chem. Mater. 1998, 10, 2964–2979. [Google Scholar] [CrossRef]
- Lippe, K.; Wagler, J.; Kroke, E.; Herkenhoff, S.; Ischenko, V.; Woltersdorf, J. Cyclic Silylcarbodiimides as Precursors for Porous Si/C/N Materials: Formation, Structures, and Stabilities. Chem. Mater. 2009, 21, 3941–3949. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Kroke, E.; Herkenhoff, S. In situ synthesis of Si2N2O/Si3N4 composite ceramics using polysilyloxycarbodiimide precursors. J. Eur. Ceram. Soc. 2013, 33, 2181–2189. [Google Scholar] [CrossRef]
- Voelger, K.W.; Hauser, R.; Kroke, E.; Riedel, R.; Ikuhara, Y.H.; Iwamoto, Y. Synthesis and characterization of novel non-oxide Sol–Gel derived mesoporous amorphous Si-CN membranes. J. Ceram. Soc. Japan 2006, 114, 567–570. [Google Scholar] [CrossRef]
- Krupinski, K.; Brendler, E.; Gericke, R.; Wagler, J.; Kroke, E. A new aspect of the “pseudo water” concept of bis(trimethylsilyl)carbodiimide—“pseudohydrates” of aluminum. Z. Naturforsch. 2018, B73, 911–918. [Google Scholar] [CrossRef]
- Kroke, E.; Völger, K.W.; Klonczynski, A.; Riedel, R. A Sol–Gel Route to B4C. Angew. Chem. Int. Ed. 2001, 40, 1698–1700. [Google Scholar] [CrossRef]
- El-Gamel, N.E.A.; Schwarz, M.; Brendler, E.; Kroke, E. s-Triazine and tri-s-triazine based organic-inorganic hybrid gels prepared from chlorosilanes by exchange reactions. Chem. Commun. 2006, 4741–4743. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Wang, Q.; Meti, P.; Lee, K.-Y.; Gong, Y.-D.; Park, H.-H. Synthesis of multi-functional porous superhydrophobic trioxybenzene cross-linked silica aerogels with improved textural properties. Ceram. Internat. 2020, 46, 17969–17977. [Google Scholar] [CrossRef]
- Wang, Q.; Mahadik, D.B.; Meti, P.; Gong, Y.-D.; Lee, K.-Y.; Park, H.-H. Dioxybenzene-bridged hydrophobic silica aerogels with enhanced textural and mechanical properties. Micropor. Mesopor. Mater. 2020, 294, 109863. [Google Scholar] [CrossRef]
- Small, J.H.; Shea, K.J.; Loy, D.A. Arylene- and alkylene-bridged polysilsesquioxanes. J. Non-Cryst. Solids 1993, 160, 234–246. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A. Bridged Polysilsesquioxanes: Molecular Engineering of Hybrid Organic–Inorganic Materials. MRS Bulletin 2001, 26, 368–375. [Google Scholar] [CrossRef]
- Pfeifer, S.; Schwarzer, A.; Schmidt, D.; Brendler, E.; Veith, M.; Kroke, E. Precursors for pyromellit-bridged silica sol–gel hybrid materials. New J. Chem. 2013, 37, 169–180. [Google Scholar]
- Pfeifer, S.; Brendler, E.; Veith, M.; Kroke, E. Hybrid-Coatings Derived from Pyromellitic Acid Bridged Alkoxy-silylalkyl Precursors. J. Sol–Gel Sci. Technol. 2014, 70, 191–202. [Google Scholar] [CrossRef]
- Haas, K.-H. Hybrid Inorganic–Organic Polymers Based on Organically Modified Si-Alkoxides. Adv. Eng. Mater. 2000, 2, 571–582. [Google Scholar] [CrossRef]
- Croissant, J.G.; Cattoen, X.; Durand, J.-O.; Wong Chi Man, M.; Khashab, N.M. Organosilica hybrid nanomaterials with a high organic content: Syntheses and applications of silsesquioxanes. Nanoscale 2016, 8, 19945–19972. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs). From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.M. Silylierung mit Hexamethyldisilazan. Z. Naturforsch. 1963, 18b, 582. [Google Scholar] [CrossRef]
- Lippe, K.; Kroke, E.; Wagler, J. CSD Private Communication, 2016; CCDC 622212.


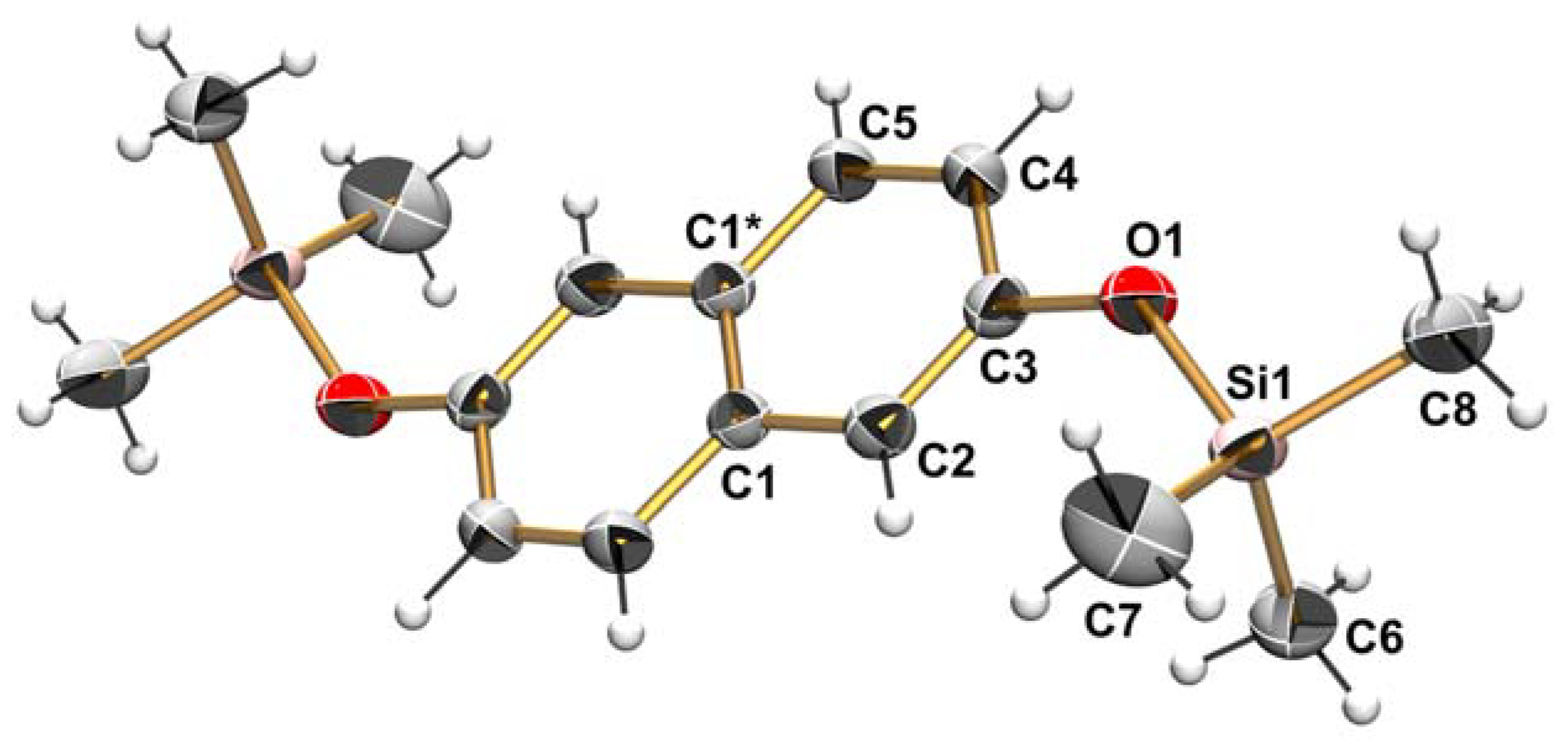

| Bond Lengths [Å] | Bond Angles [°] | ||
|---|---|---|---|
| C1–C2 | 1.424(2) | C1*–C1–C2 | 119.2(2) |
| C2–C3 | 1.364(2) | C1–C2–C3 | 120.5(1) |
| C3–C4 | 1.415(2) | C2–C3–C4 | 120.0(1) |
| C4–C5 | 1.364(2) | C3–C4–C5 | 120.6(1) |
| C5–C1* | 1.413(2) | C4–C5–C1* | 120.7(1) |
| C1–C1* | 1.413(2) | C5*–C1–C1* | 119.0(2) |
| C5*–C1–C2 | 121.9(1) | ||
| Reaction Mixture | n(1) [mmol] | THF [mL] | n(SiCl4) [mmol] | n(CH3SiCl3) [mmol] | nPyridine [mmol] | Observation |
|---|---|---|---|---|---|---|
| 1a | 1 | 5 | 0.5 | - | - | no gelation after three weeks at 60 °C |
| 1aPy | 1 | 5 | 0.5 | - | 0.1 | gelation after 1 day; strong syneresis in following weeks, linear shrinkage of about 57% for xerogel 1A |
| 1b | 0.5 | 4 | - | 0.33 | - | no gelation after three weeks at 60 °C |
| 1bPy | 0.5 | 4 | - | 0.33 | 0.05 |
| Reaction Mixture | n(2) [mmol] | THF [mL] | n(SiCl4) [mmol] | n(CH3SiCl3) [mmol] | nPyridine [mmol] | Observations |
|---|---|---|---|---|---|---|
| 2a | 1 | 5 | 0.5 | - | - | no gelation after three weeks at 60 °C |
| 2aPy | 1 | 5 | 0.5 | - | 0.1 | gelation after 1 day; strong syneresis in following weeks, linear shrinkage of 59% for xerogel 2A |
| 2b | 0.5 | 4 | - | 0.33 | - | no gelation after three weeks at 60 °C |
| 2bPy | 0.5 | 4 | - | 0.33 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupinski, K.; Wagler, J.; Brendler, E.; Kroke, E. A Non-Hydrolytic Sol–Gel Route to Organic-Inorganic Hybrid Polymers: Linearly Expanded Silica and Silsesquioxanes. Gels 2023, 9, 291. https://doi.org/10.3390/gels9040291
Krupinski K, Wagler J, Brendler E, Kroke E. A Non-Hydrolytic Sol–Gel Route to Organic-Inorganic Hybrid Polymers: Linearly Expanded Silica and Silsesquioxanes. Gels. 2023; 9(4):291. https://doi.org/10.3390/gels9040291
Chicago/Turabian StyleKrupinski, Katrin, Jörg Wagler, Erica Brendler, and Edwin Kroke. 2023. "A Non-Hydrolytic Sol–Gel Route to Organic-Inorganic Hybrid Polymers: Linearly Expanded Silica and Silsesquioxanes" Gels 9, no. 4: 291. https://doi.org/10.3390/gels9040291
APA StyleKrupinski, K., Wagler, J., Brendler, E., & Kroke, E. (2023). A Non-Hydrolytic Sol–Gel Route to Organic-Inorganic Hybrid Polymers: Linearly Expanded Silica and Silsesquioxanes. Gels, 9(4), 291. https://doi.org/10.3390/gels9040291








