Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Synthesis Conditions
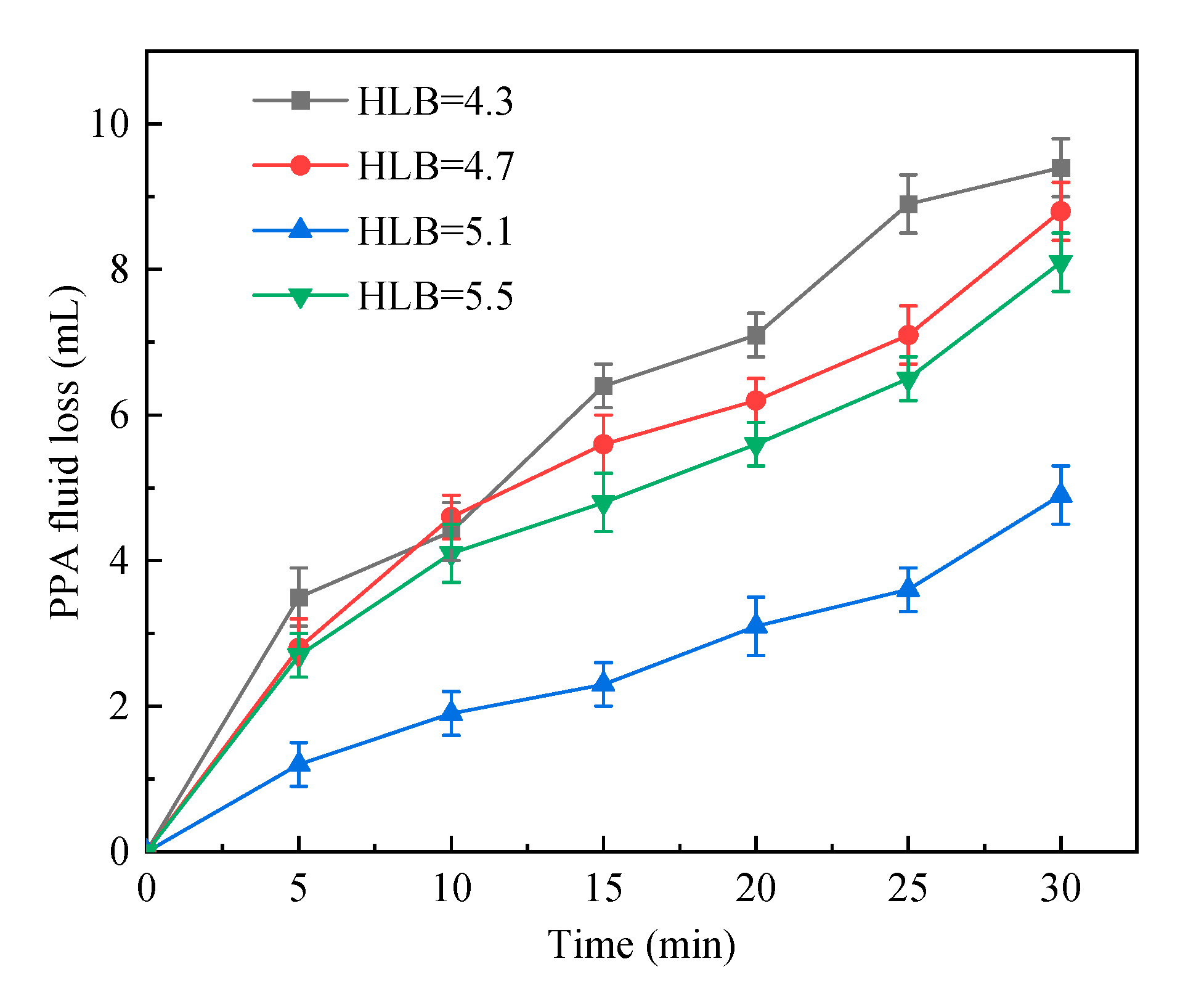
2.1.1. HLB
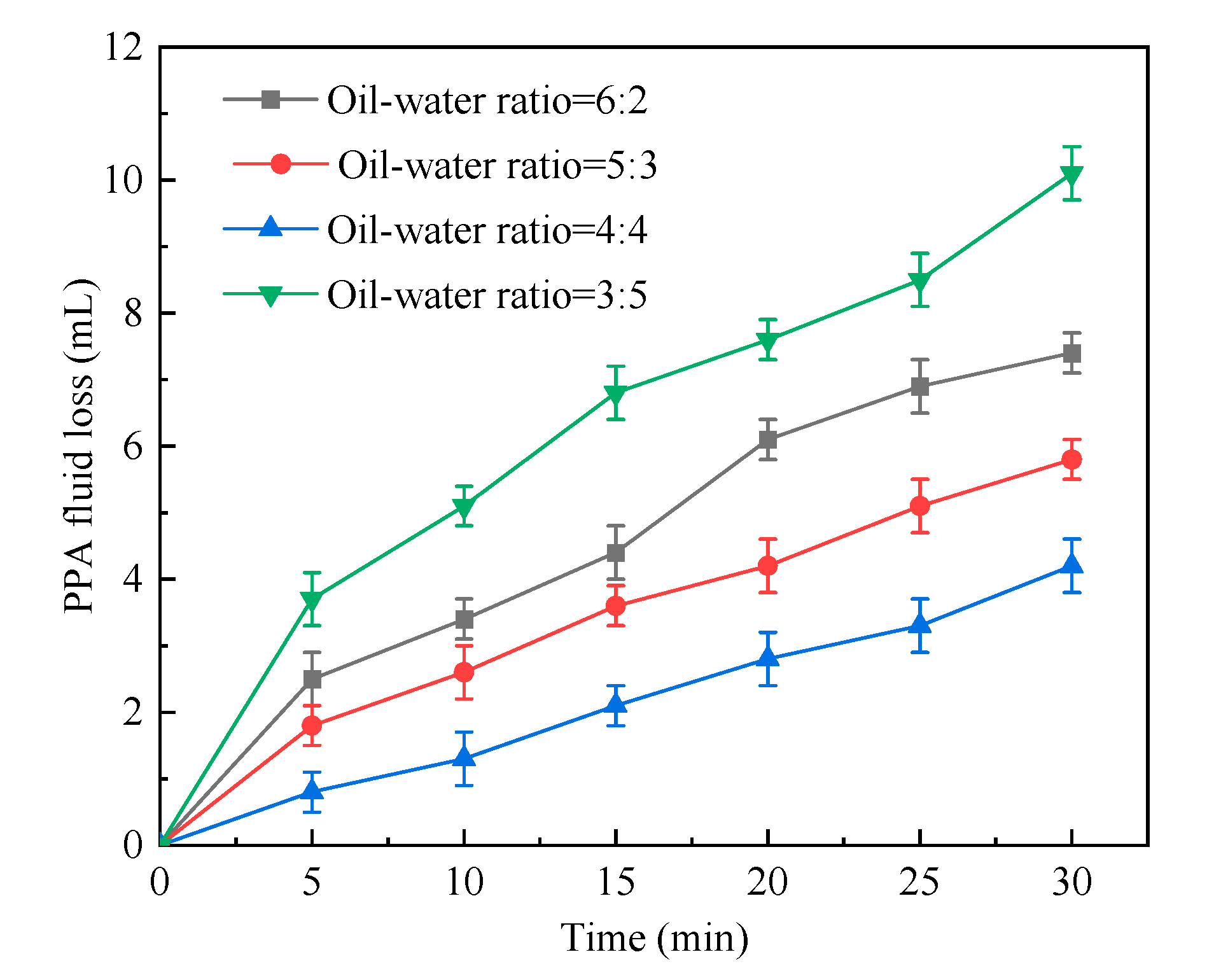
2.1.2. Oil–Water Ratio
2.1.3. Monomer ratio
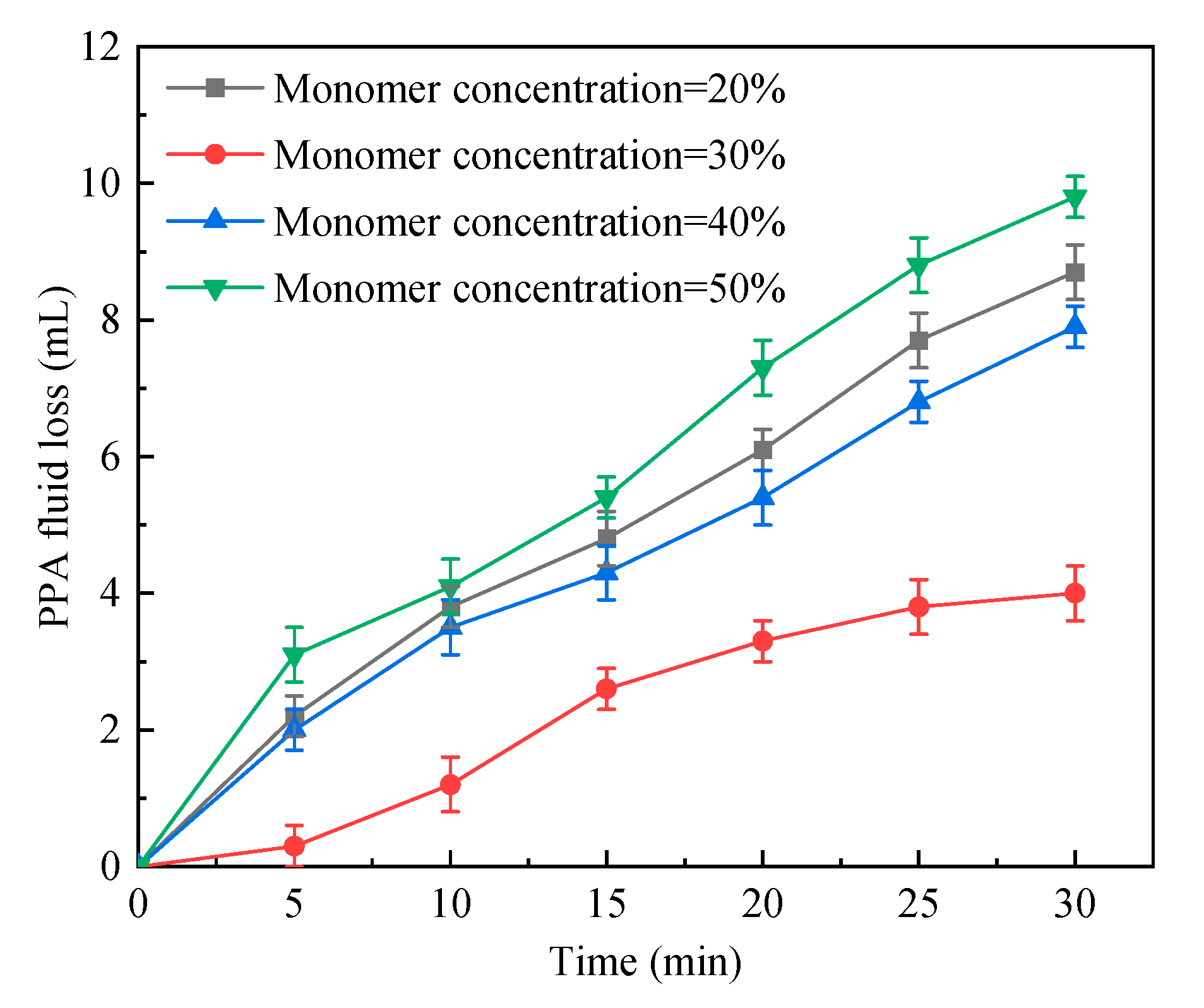
2.1.4. Total Monomer Concentration
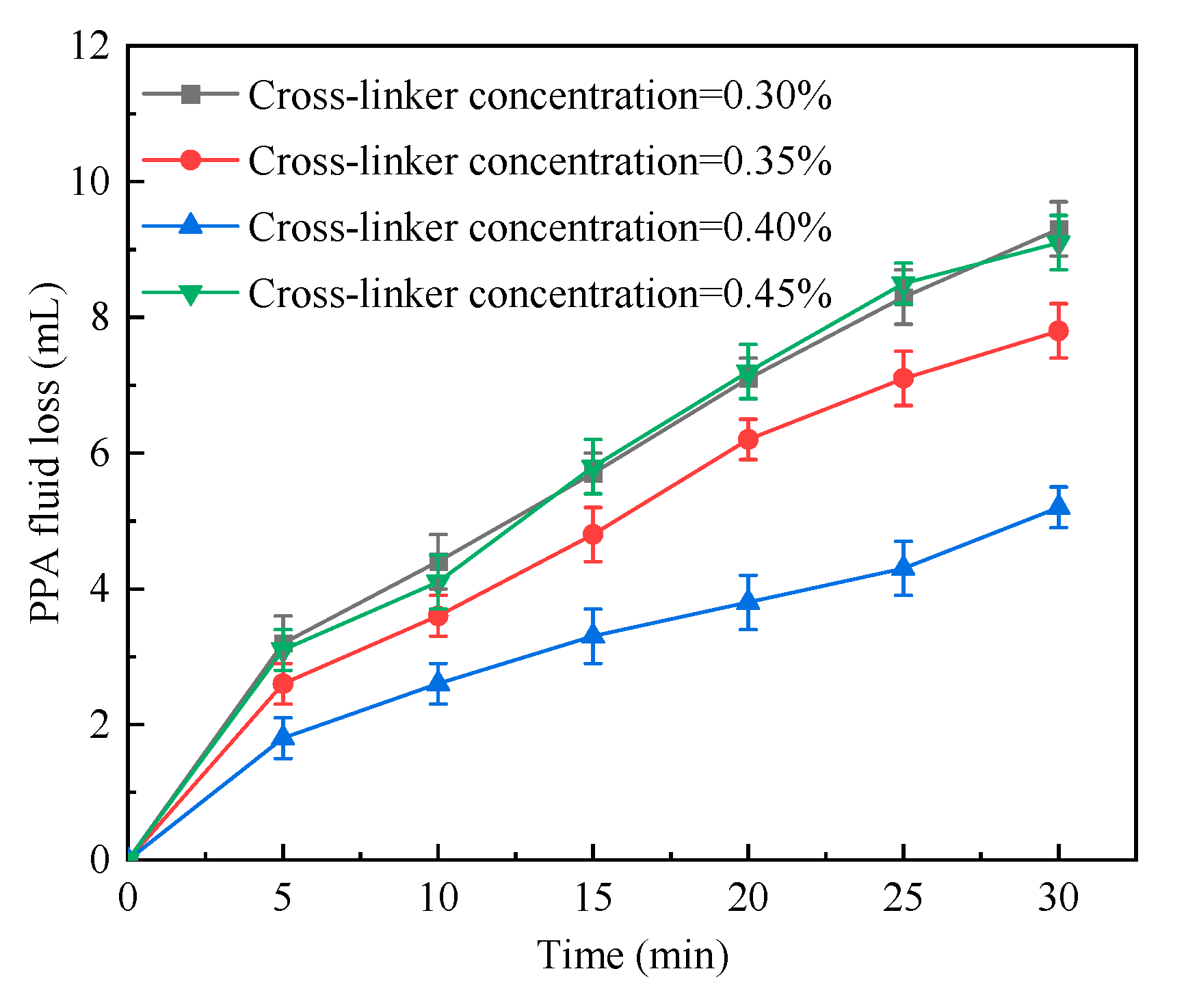
2.1.5. Cross-Linker Concentration
2.2. Material Characterization
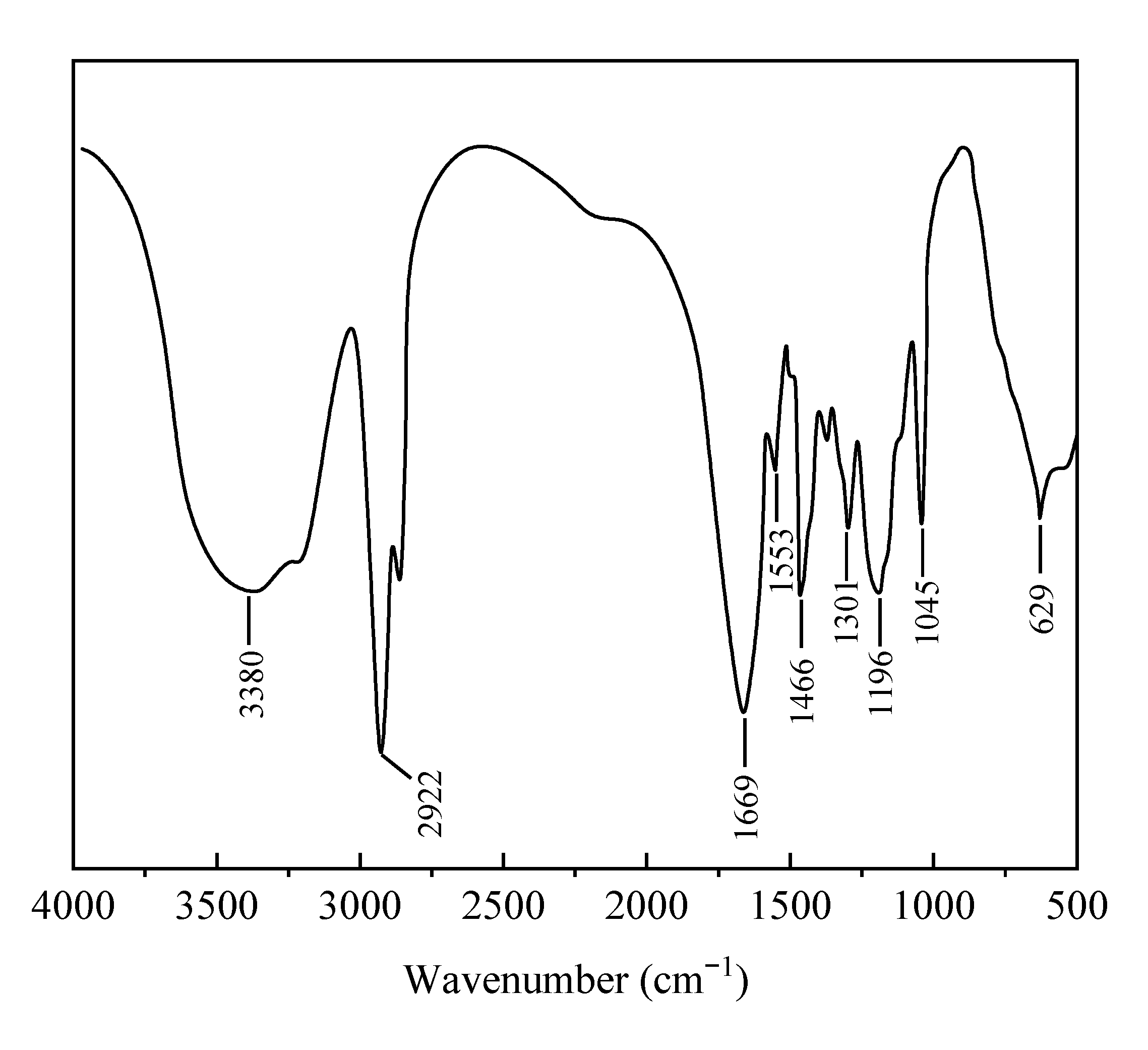
2.2.1. Infrared Spectroscopy
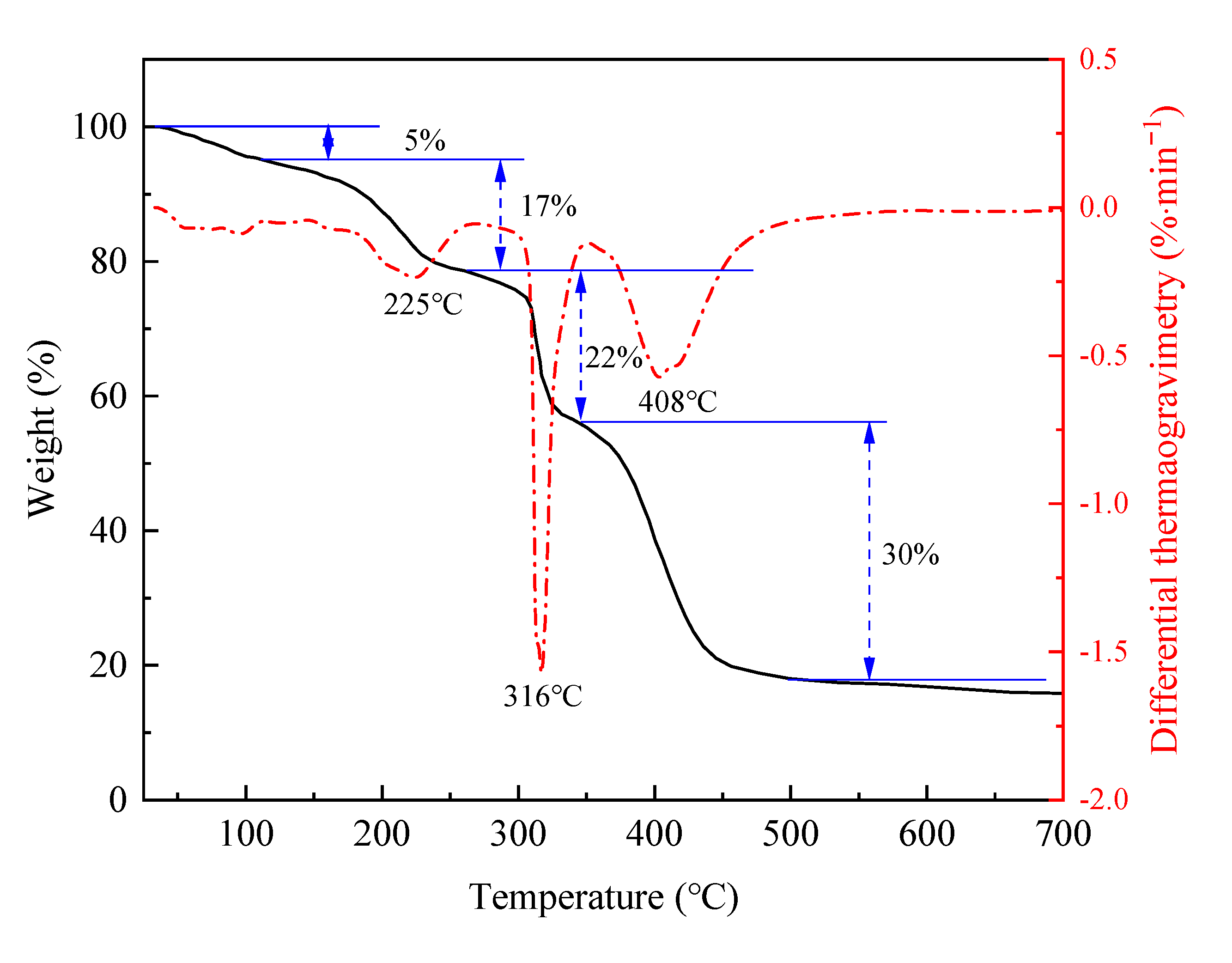
2.2.2. Thermal Stability Analysis
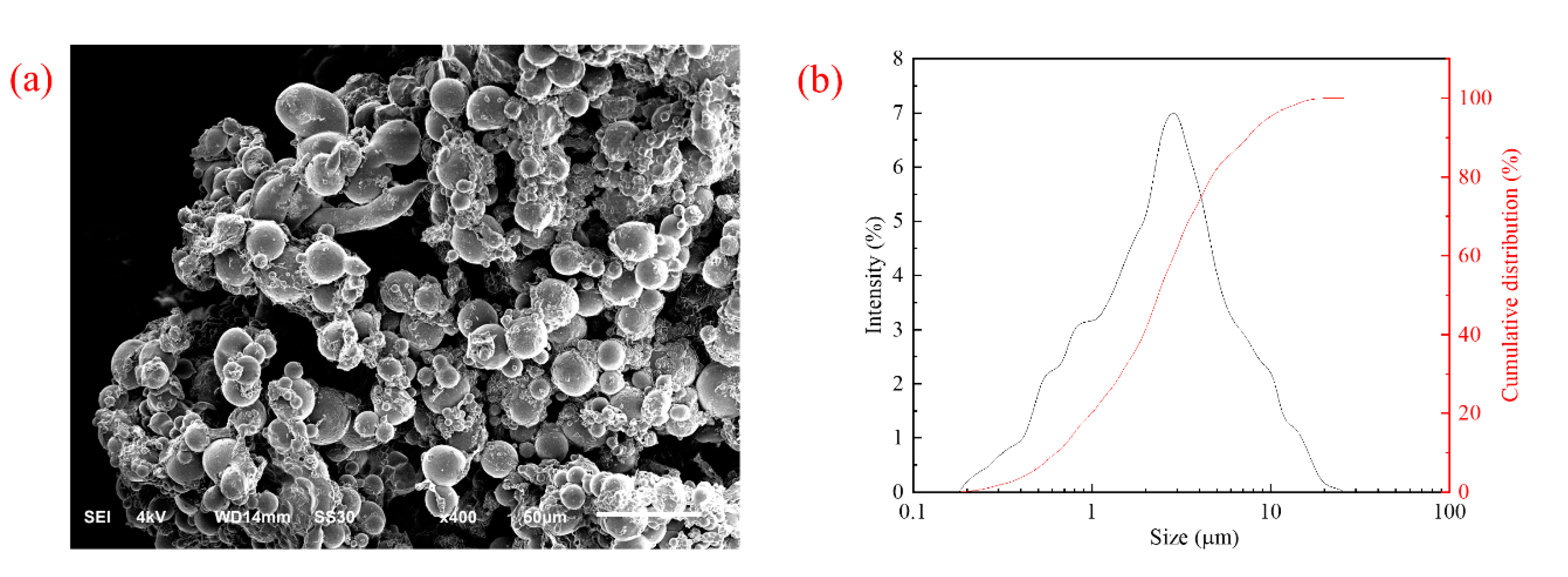
2.2.3. Scanning Electron Microscopy and Particle Size Distribution Analysis
2.3. Plugging Performance
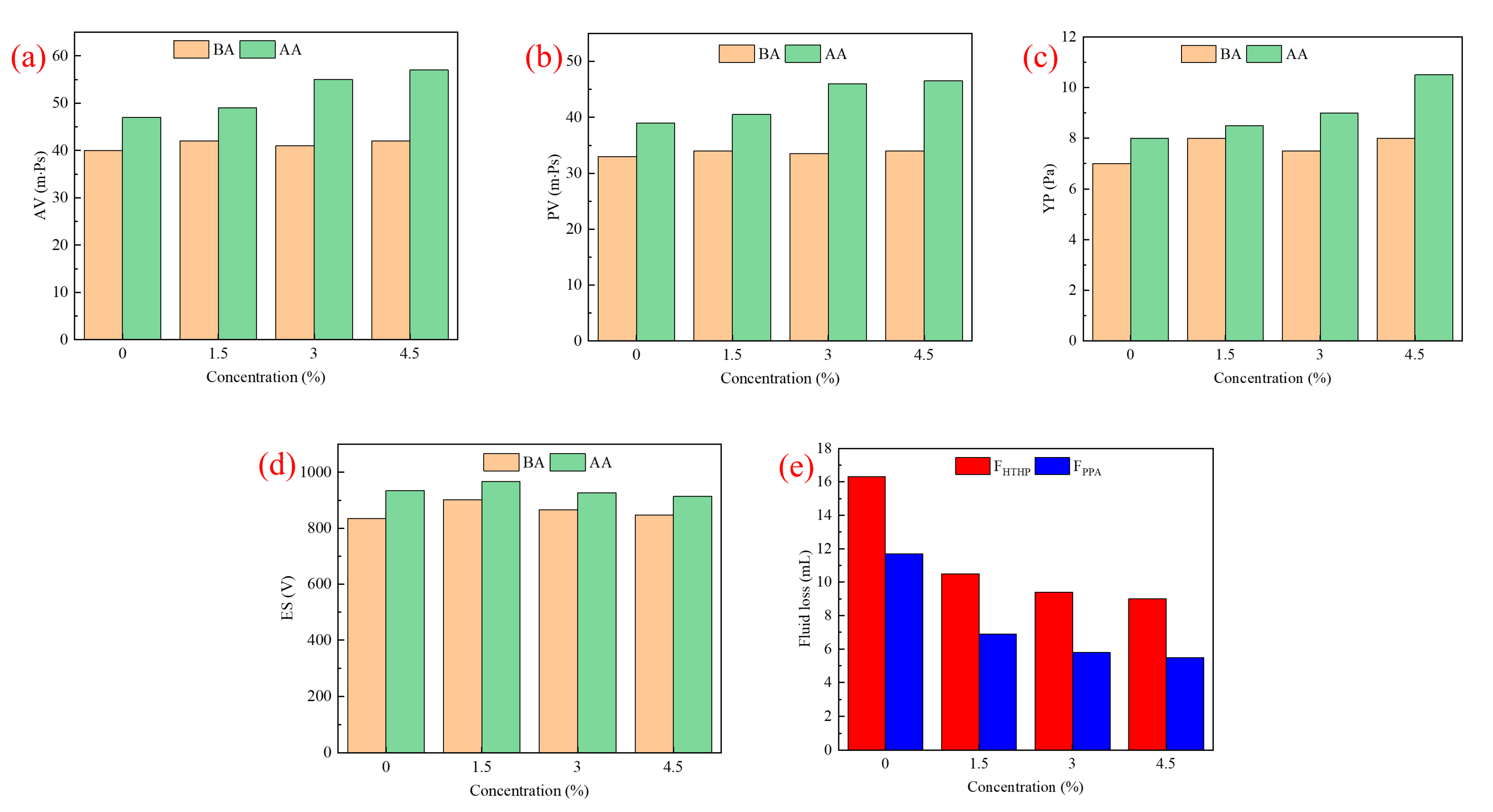
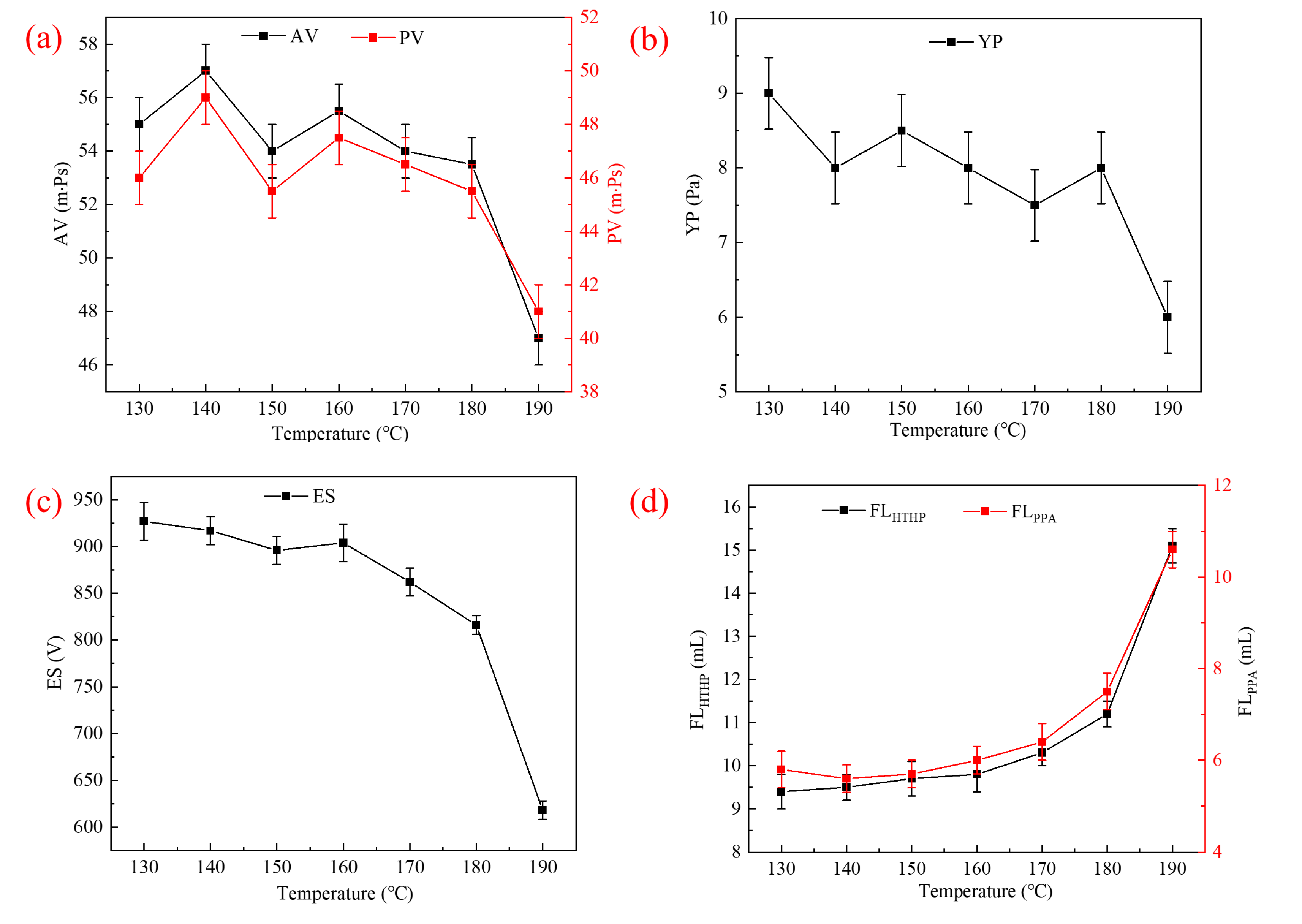
2.3.1. Rheological and Filtration Properties
2.3.2. Thermal Tolerance
2.3.3. Pressure Transfer
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Polymeric Microspheres(AMN)
4.3. Characterization Methods of AMN
4.4. Plugging Performance Test
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.; Zhou, D.; Cai, X.; Wang, F.; Feng, D.; Lu, T. Progress and prospects in shale gas development of Sinopec. China Pet. Explor. 2020, 25, 14–26. [Google Scholar]
- Chen, T.; Feng, X.; Cui, G.; Tan, Y.; Pan, Z. Engineering. Experimental study of permeability change of organic-rich gas shales under high effective stress. J. Nat. Gas Sci. Eng. 2019, 64, 1–14. [Google Scholar] [CrossRef]
- He, S.; Li, H.; Qin, Q.; Long, S. Influence of mineral compositions on shale pore development of Longmaxi Formation in the Dingshan Area, Southeastern Sichuan Basin, China. Energy Fuels 2021, 35, 10551–10561. [Google Scholar] [CrossRef]
- Wilson, M.; Wilson, L.J. Clay mineralogy and shale instability: An alternative conceptual analysis. Clay Miner. 2014, 49, 127–145. [Google Scholar] [CrossRef]
- Chen, G.; Chenevert, M.; Sharma, M.; Yu, M. A study of wellbore stability in shales including poroelastic, chemical, and thermal effects. J. Pet. Sci. Eng. 2003, 38, 167–176. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.; Shen, F.; Li, W.; Zhang, W. Mechanism of wellbore instability in continental shale gas horizontal sections and its water-based drilling fluid countermeasures. Nat. Gas Ind. B 2020, 7, 680–688. [Google Scholar] [CrossRef]
- Murtaza, M.; Alarifi, S.; Kamal, M.; Onaizi, S.; Al-Ajmi, M.; Mahmoud, M. Experimental investigation of the rheological behavior of an oil-based drilling fluid with rheology modifier and oil wetter additives. Molecules 2021, 26, 4877. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Kang, Y. Engineering. Investigation of drill-in fluids damage and its impact on wellbore stability in Longmaxi shale reservoir. J. Pet. Sci. Eng. 2017, 159, 702–709. [Google Scholar] [CrossRef]
- Wang, B.; Luo, C.; Wang, Y. Gas. Research and Application of Fuling Shale Gas Anti-Collapse and Anti-Leakage Drilling Fluid System. Open J. Yangtze Oil Gas 2021, 6, 60–71. [Google Scholar] [CrossRef]
- Tang, X.; Yang, H.; Gao, Y.; Lashari, Z.A.; Cao, C.; Kang, W. Preparation of a micron-size silica-reinforced polymer microsphere and evaluation of its properties as a plugging agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 8–18. [Google Scholar] [CrossRef]
- Tan, Q.; Yu, B.; Deng, J.; Zhao, K.; Chen, J. Study on wellbore stability and instability mechanism in Piedmont structures. Open Pet. Eng. J. 2015, 8, 208–213. [Google Scholar] [CrossRef]
- Lu, T.; Ye, C.; Lu, X.; Rong, K.; Liu, K.; Yao, X.; Zhou, Z. Wellbore Instability Mechanism and Countermeasures of High-Temperature Organic-Matter-Rich Reservoirs in the Southern Margin of Junggar Basin. Xinjiang Oil Gas 2022, 18, 26–31. [Google Scholar]
- Xie, J.; Liao, X.; Cao, G.; Xu, X.; Zhao, L. Study on Huoerguosi anticline ultra-high density oil-based drilling fluid technology. Xinjiang Oil Gas 2018, 14, 53–55. [Google Scholar]
- Yan, X.; Kang, Y.; You, L. Wellbore instability induced by the coupling of high-pH fluid–shale reaction and fracture surface sliding in shale gas wells: Experimental and field studies. Energy Fuels 2020, 34, 5578–5588. [Google Scholar] [CrossRef]
- Deng, S.; Huang, Y.; Hu, X.; Wang, H.; Zhao, H.; He, J. Nano-Film-Forming Plugging Drilling Fluid and Bridging Cross-Linking Plugging Agent Are Used to Strengthen Wellbores in Complex Formations. ACS Omega 2022, 7, 22804–22810. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Voncken, J.; Opstal, T.; Dams, R.; Zitha, P. Investigation of the mitigation of lost circulation in oil-based drilling fluids using additives. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012. [Google Scholar]
- Ma, C.; Li, L.; Lu, H.; Yuan, X.; Wang, G. Study on the effect of humic acid acetamide on the rheological properties of diesel oil-based drilling fluids. Appl. Mech. Mater. 2014, 620, 449–452. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Qi, Y.; Huang, X.; She, H. Engineering. High-performance shale plugging agent based on chemically modified graphene. J. Nat. Gas Sci. Eng. 2016, 32, 347–355. [Google Scholar]
- Xie, G.; Luo, P.; Deng, M.; Wang, Z. Nanoplugging Performance of Hyperbranched Polyamine as Nanoplugging Agent in Oil-Based Drilling Fluid. J. Nanomater. 2015, 2015, 821910. [Google Scholar] [CrossRef]
- Geng, Y.; Sun, J.; Wang, J.; Wang, R.; Yang, J.; Wang, Q.; Ni, X. Modified Nanopolystyrene as a Plugging Agent for Oil-Based Drilling Fluids Applied in Shale Formation. Energy Fuels 2021, 35, 16543–16552. [Google Scholar] [CrossRef]
- Du, H.; Lv, K.; Sun, J.; Huang, X.; Shen, H. Styrene-Lauryl Acrylate Rubber Nanogels as a Plugging Agent for Oil-Based Drilling Fluids with the Function of Improving Emulsion Stability. Gels 2022, 9, 23. [Google Scholar] [CrossRef]
- Geng, J.; Ding, H.; Han, P.; Wu, Y.; Bai, B. Transportation and Potential Enhanced Oil Recovery Mechanisms of Nanogels in Sandstone. Energy Fuels 2018, 32, 8358–8365. [Google Scholar] [CrossRef]
- Jamali, A.; Moghbeli, M.R.; Ameli, F.; Roayaie, E.; Karambeigi, M.S. Synthesis and characterization of pH-sensitive poly(acrylamide-co-methylenebisacrylamide-co-acrylic acid) hydrogel microspheres containing silica nanoparticles: Application in enhanced oil recovery processes. J. Appl. Polym. Sci. 2019, 137, 48491. [Google Scholar] [CrossRef]
- Wang, H.; Lin, M.; Chen, D.; Dong, Z.; Yang, Z.; Zhang, J. Research on the rheological properties of cross-linked polymer microspheres with different microstructures. Powder Technol. 2018, 331, 310–321. [Google Scholar] [CrossRef]
- Dai, B.; Xu, P.; Xu, M.; Jiang, Q.; Liu, Q.; Wang, S. Synthesis and plugging effect of inverse emulsion polymerization microspheres (OPME) for oil-based drilling fluids. Arab. J. Chem. 2023, 16, 104577. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Jiang, G.; Deng, Z.; Li, W.; Ye, Y.; Zhou, H. Gel microsphere: Synthesis through inverse emulsion polymerization and evaluation of its plugging capacity. Drill. Fluid Complet. Fluid 2020, 37, 275–281. [Google Scholar]
- Liu, F.; Yang, L.; Ni, X.; Jiang, G.; Cui, X.; Wang, J. Preparation and plugging performance of zwitterionic polymer gel micro spheres. Drill. Fluid Complet. Fluid 2021, 38, 435–441. [Google Scholar]
- Zhao, Z.; Bai, Y.; Sun, J.; Lv, K.; Lei, S.; Qiu, J. Tough and self-healing hydrophobic association hydrogels with cationic surfactant. J. Appl. Polym. Sci. 2021, 138, 50645. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, J.; Liu, F.; Bai, Y.; Wang, R. A laboratory study of self-healing hydrophobic association gels used as lost circulation material. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 646, 128964. [Google Scholar] [CrossRef]


| Synthesis Conditions | Various Parameters |
|---|---|
| Oil–water ratio | 1:1 |
| Monomer ratio (AMPS:AM:NVP) | 1:4:5 |
| Total monomer concentration | 30% |
| Cross-linker concentration | 0.4% |
| Emulsifier concentration | 10% |
| Initiator concentration | 0.08% |
| Reaction temperature | 50 °C |
| Reaction time | 12 h |
| Rotation speed | 460 rotations per minute (RPM) |
| Synthesis Conditions | Various Parameters |
|---|---|
| HLB | 5.1 |
| Monomer ratio (AMPS:AM:NVP) | 1:4:5 |
| Total monomer concentration | 30% |
| Cross-linker concentration | 0.4% |
| Emulsifier concentration | 10% |
| Initiator concentration | 0.08% |
| Reaction temperature | 50 °C |
| Reaction time | 12 h |
| Rotation speed | 460 rotations per minute (RPM) |
| Synthesis Conditions | Various Parameters |
|---|---|
| HLB | 5.1 |
| Oil–water ratio | 1:1 |
| Total monomer concentration | 30% |
| Cross-linker concentration | 0.4% |
| Emulsifier concentration | 10% |
| Initiator concentration | 0.08% |
| Reaction temperature | 50 °C |
| Reaction time | 12 h |
| Rotation speed | 460 rotations per minute (RPM) |
| Synthesis Conditions | Various Parameters |
|---|---|
| HLB | 5.1 |
| Oil–water ratio | 1:1 |
| Monomer ratio (AMPS:AM:NVP) | 1:4:5 |
| Cross-linker concentration | 0.4% |
| Emulsifier concentration | 10% |
| Initiator concentration | 0.08% |
| Reaction temperature | 50 °C |
| Reaction time | 12 h |
| Rotation speed | 460 rotations per minute (RPM) |
| Synthesis Conditions | Various Parameters |
|---|---|
| HLB | 5.1 |
| Oil–water ratio | 1:1 |
| Monomer ratio (AMPS:AM:NVP) | 1:4:5 |
| Total monomer concentration | 30% |
| Emulsifier concentration | 10% |
| Initiator concentration | 0.08% |
| Reaction temperature | 50 °C |
| Reaction time | 12 h |
| Rotation speed | 460 RPM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Wang, R.; Rong, K.; Yin, Z.; Lu, T.; Yu, Y.; Li, Y.; Yang, Z.; Yang, J.; Zhao, Z. Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids. Gels 2023, 9, 290. https://doi.org/10.3390/gels9040290
Liu K, Wang R, Rong K, Yin Z, Lu T, Yu Y, Li Y, Yang Z, Yang J, Zhao Z. Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids. Gels. 2023; 9(4):290. https://doi.org/10.3390/gels9040290
Chicago/Turabian StyleLiu, Kecheng, Ren Wang, Kesheng Rong, Zebin Yin, Tiemei Lu, Yongsheng Yu, Yingying Li, Zexing Yang, Jie Yang, and Zhen Zhao. 2023. "Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids" Gels 9, no. 4: 290. https://doi.org/10.3390/gels9040290
APA StyleLiu, K., Wang, R., Rong, K., Yin, Z., Lu, T., Yu, Y., Li, Y., Yang, Z., Yang, J., & Zhao, Z. (2023). Synthesis and Plugging Performance of Nano-Micron Polymeric Gel Microsphere Plugging Agents for Oil-Based Drilling Fluids. Gels, 9(4), 290. https://doi.org/10.3390/gels9040290







