Copper Ion Removal Using a Waste-Plastic-Derived Hydrogel Adsorbent Prepared via Microwave-Assisted PET Aminolysis
Abstract
:1. Introduction
2. Results and Discussion
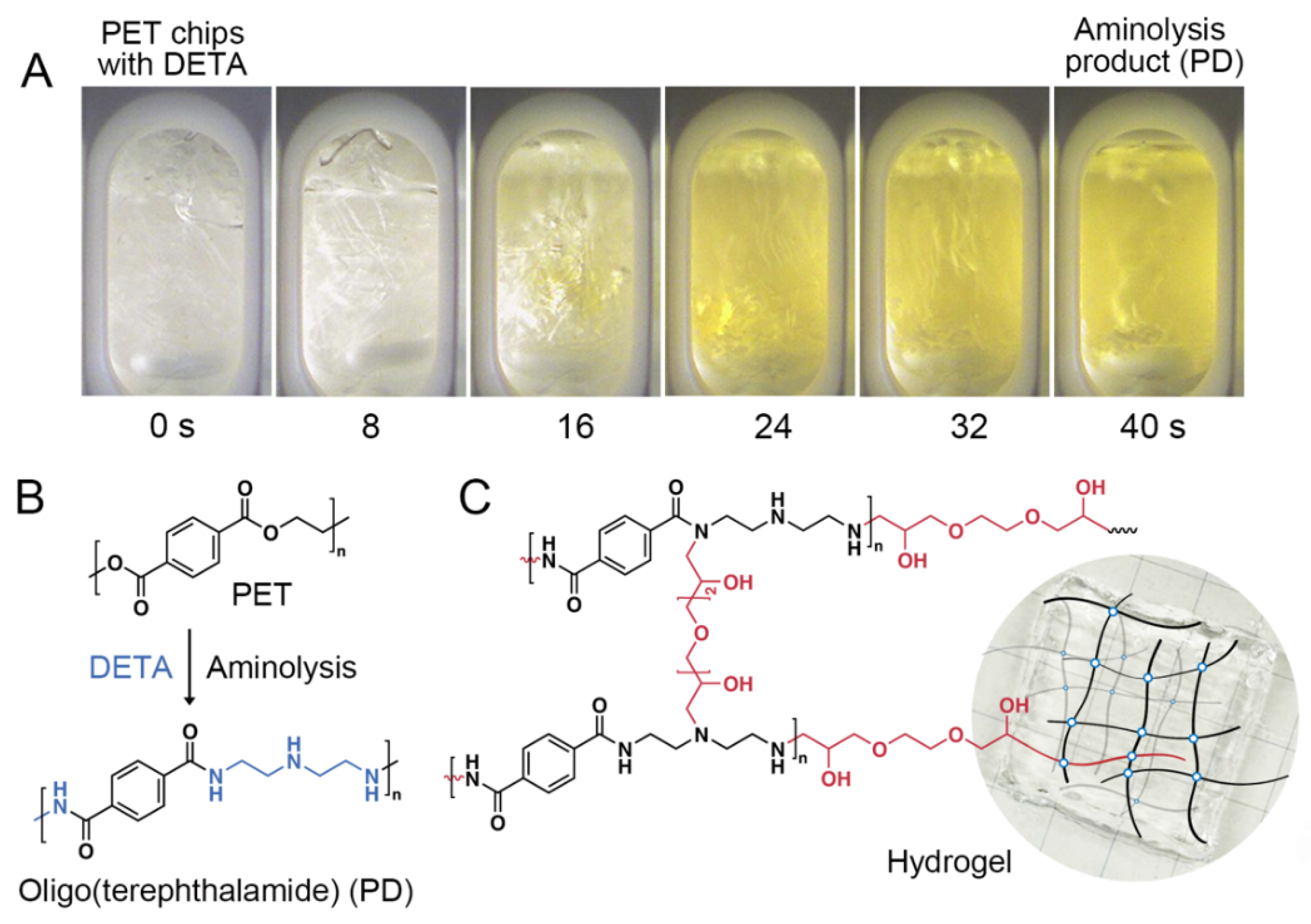
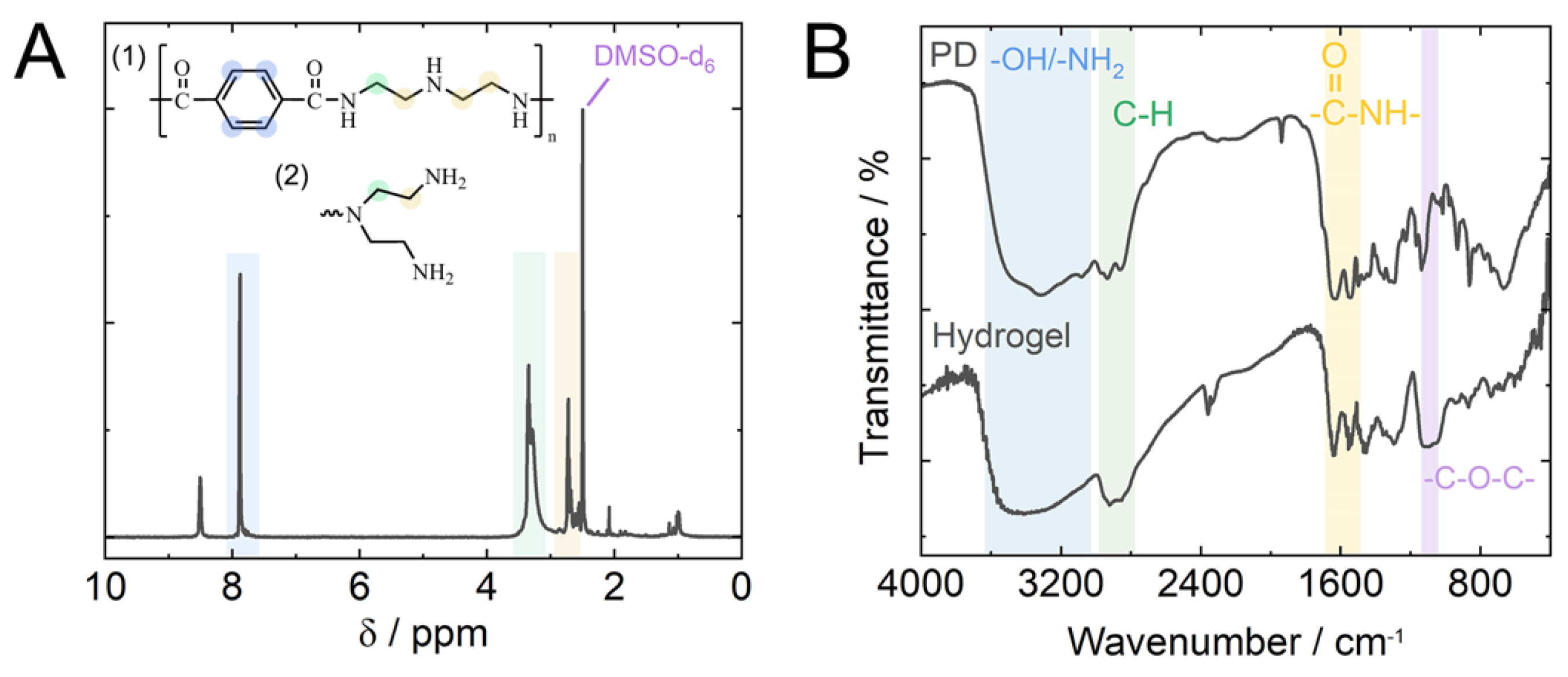
2.1. Formation and Characterization of Hydrogel Adsorbent via Microwave-Assisted Aminolysis of PET
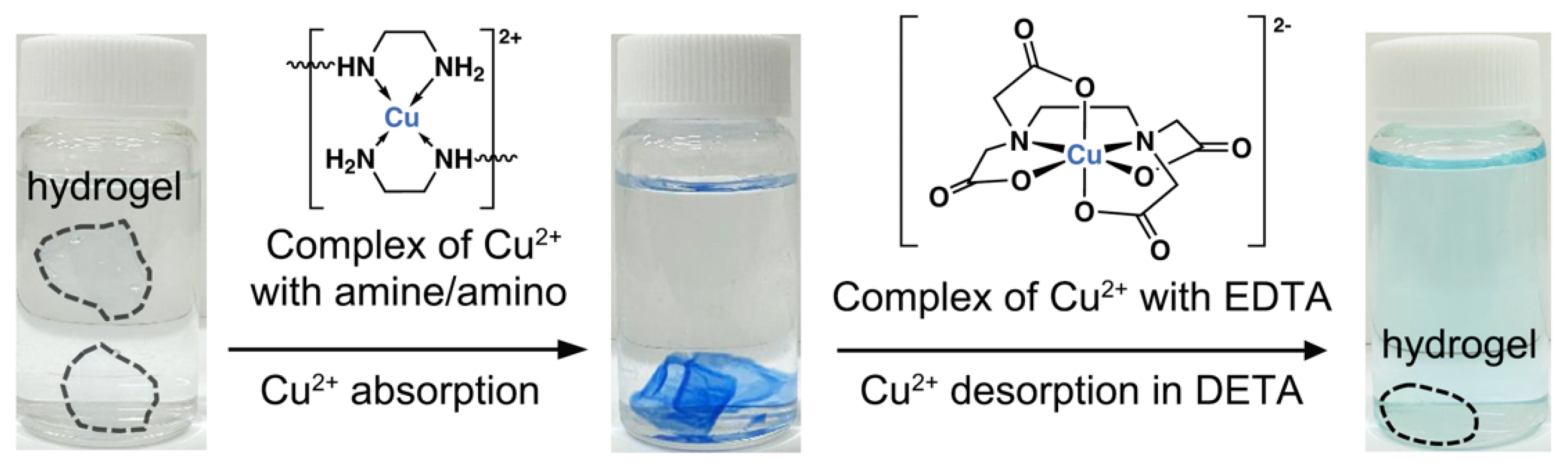
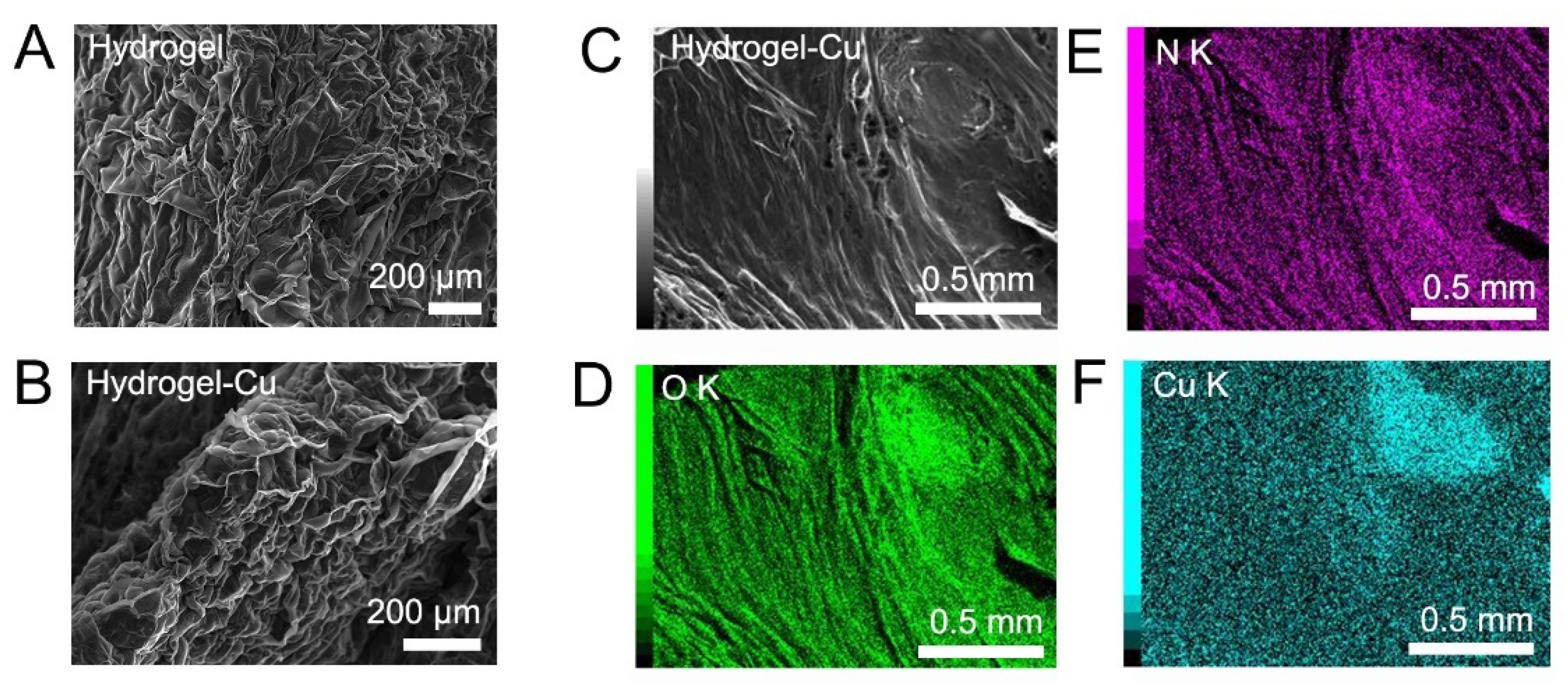
2.2. Removal of Cu2+ Ions from Aqueous Solution by the PET-Derived Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Sample Preparation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xie, Y.; Wang, Q.; Yi, X.; Shamshina, J.L.; Rogers, R.D. Enhanced heavy metal adsorption ability of lignocellulosic hydrogel adsorbents by the structural support effect of lignin. Cellulose 2019, 26, 4005–4019. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, V.; Bhatnagar, A. Utilization of industrial waste products as adsorbents for the removal of dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste material adsorbents for zinc removal from wastewater: A comprehensive review. Int. J. Chem. Eng. 2014, 2014, 347912. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Adewuyi, Y.G. State-of-the-art review on capture of CO2 using adsorbents prepared from waste materials. Process. Saf. Environ. Prot. 2020, 139, 1–25. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel adsorbents for the removal of hazardous pollutants—Requirements and available functions as adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Hydrogel adsorbent in industrial wastewater treatment and ecological environment protection. Environ. Technol. Innov. 2020, 20, 101107. [Google Scholar] [CrossRef]
- Chan, K.; Morikawa, K.; Shibata, N.; Zinchenko, A. Adsorptive removal of heavy metal ions, organic dyes, and pharmaceuticals by dna–chitosan hydrogels. Gels 2021, 7, 112. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Conversion of waste bottles’ PET to a hydrogel adsorbent via PET aminolysis. J. Environ. Chem. Eng. 2021, 9, 106129. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Templating of catalytic gold and silver nanoparticles by waste plastic PET-derived hydrogel playing a dual role of a reductant and a matrix. Waste Manag. 2023, 164, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Hu, Y.; Chen, S.; Zhuang, L.; Ma, B.; Wu, Q. Preparation and Properties of a Hyperbranch-Structured Polyamine adsorbent for Carbon Dioxide Capture. Sci. Rep. 2017, 7, 3913. [Google Scholar] [CrossRef] [PubMed]
- Mannan, A.; Hirano, Y.; Quitain, A.T.; Koinuma, M.; Kida, T. Graphene oxide to B, N co-doped graphene through tris-dimethylaminoborane complex by hydrothermal implantation. Am. J. Mater. Sci 2019, 9, 22–28. [Google Scholar]
- Blight, M.M.; Curtis, N. Transition-metal complexes with aliphatic Schiff bases. Part III. Compounds formed by reaction of some 1, 2-diamine complexes of copper (II) with some ketones. J. Chem. Soc. 1962, 86, 3016–3020. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef]
- Du, T.; Wang, J.; Zhang, T.; Zhang, L.; Yang, C.; Yue, T.; Sun, J.; Li, T.; Zhou, M.; Wang, J. An Integrating Platform of Ratiometric Fluorescent Adsorbent for Unconventional Real-Time Removing and Monitoring of Copper Ions. ACS Appl. Mater. Interfaces 2020, 12, 13189–13199. [Google Scholar] [CrossRef]
- Chen, L.; Hao, H.; Zhang, W.; Shao, Z. Adsorption mechanism of copper ions in aqueous solution by chitosan–carboxymethyl starch composites. J. Appl. Polym. Sci. 2020, 137, 48636. [Google Scholar] [CrossRef]
- Anbia, M.; Haqshenas, M. Adsorption studies of Pb(II) and Cu(II) ions on mesoporous carbon nitride functionalized with melamine-based dendrimer amine. Int. J. Environ. Sci. Technol. 2015, 12, 2649–2664. [Google Scholar] [CrossRef]
- Tang, S.; Shao, N.; Zheng, C.; Yan, F.; Zhang, Z. Amino-functionalized sewage sludge-derived biochar as sustainable efficient adsorbent for Cu(II) removal. Waste Manag. 2019, 90, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Q.; Liu, M.; Tian, J.; Zeng, G.; Li, Z.; Wang, K.; Zhang, Q.; Wan, Q.; Deng, F.; et al. Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2+ removal. Appl. Surf. Sci. 2015, 343, 19–27. [Google Scholar] [CrossRef]
- Da’na, E.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Equilibrium properties. Chem. Eng. J. 2011, 166, 445–453. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, H.; Lin, K.; Chen, B.; Chiou, C. Application of magnetic particles modified with amino groups to adsorb copper ions in aqueous solution. J. Environ. Sci. 2011, 23, 44–50. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A.; Lindo, M.; Gascón, V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009, 163, 213–221. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. A Novel Amine-Shielded Surface Cross-Linking of Chitosan Hydrogel Beads for Enhanced Metal Adsorption Performance. Ind. Eng. Chem. Res. 2005, 44, 6692–6700. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z. Enhanced selective removal of Cu(II) from aqueous solution by novel polyethylenimine-functionalized ion imprinted hydrogel: Behaviors and mechanisms. J. Hazard. Mater. 2015, 300, 18–28. [Google Scholar] [CrossRef]
- Choi, H.; Kim, T.; Kim, S.Y. Poly (Amidehydrazide) Hydrogel Particles for Removal of Cu2+ and Cd2+ Ions from Water. Gels 2021, 7, 121. [Google Scholar] [CrossRef]


| Kinetic Model | Pseudo-First Order | Pseudo-Second Order | ||||
| Qe (cal) (mg/g) | k1 (min−1) | R2 | Qe (cal) (mg/g) | k2 (g/mg·min) | R2 | |
| 44 | 0.0067 | 0.9873 | 58 | 1.02 × 10−4 | 0.9909 | |
| Isotherm model | Langmuir | Freundlich | ||||
| Qm (cal) (mg/g) | KL (L/mg) | R2 | KF (mg/g) | n | R2 | |
| 248 | 0.0054 | 0.9930 | 18 | 2.8 | 0.9488 | |
| Material | Adsorption Capacity/mg/g | Kinetics Model | Adsorption Model | Ref. |
|---|---|---|---|---|
| Amine-functionalized mesoporous C3N4 | 200 | Pseudo-second | Langmuir | [22] |
| Amine-functionalized biochar | 74.5 | Pseudo-second | Sips | [23] |
| Amine-functionalized carbon nanotubes | 26.4 | Pseudo-first | Langmuir | [24] |
| Amine-functionalized SBA-15 | 34.3 | - | Sips | [25] |
| Amine-functionalized magnetic particles | 10.4 | Pseudo-second | Langmuir | [26] |
| Amine-functionalized mesoporous silica | 52.7 | Pseudo-second | Langmuir | [27] |
| Chitosan hydrogel beads | 163.9 | - | Langmuir | [28] |
| Polyethylenimine-functionalized hydrogel | 40.0 | Pseudo-second | Langmuir | [29] |
| Poly(amidehydrazide) hydrogel | 85 | - | Sips | [30] |
| This study | 213 | Pseudo-second | Langmuir | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.; Kawai, M.; Yamake, M.; Zinchenko, A. Copper Ion Removal Using a Waste-Plastic-Derived Hydrogel Adsorbent Prepared via Microwave-Assisted PET Aminolysis. Gels 2023, 9, 874. https://doi.org/10.3390/gels9110874
Chan K, Kawai M, Yamake M, Zinchenko A. Copper Ion Removal Using a Waste-Plastic-Derived Hydrogel Adsorbent Prepared via Microwave-Assisted PET Aminolysis. Gels. 2023; 9(11):874. https://doi.org/10.3390/gels9110874
Chicago/Turabian StyleChan, Kayee, Masami Kawai, Mina Yamake, and Anatoly Zinchenko. 2023. "Copper Ion Removal Using a Waste-Plastic-Derived Hydrogel Adsorbent Prepared via Microwave-Assisted PET Aminolysis" Gels 9, no. 11: 874. https://doi.org/10.3390/gels9110874
APA StyleChan, K., Kawai, M., Yamake, M., & Zinchenko, A. (2023). Copper Ion Removal Using a Waste-Plastic-Derived Hydrogel Adsorbent Prepared via Microwave-Assisted PET Aminolysis. Gels, 9(11), 874. https://doi.org/10.3390/gels9110874






