1. Introduction
Amoxicillin (AMX) as a β–Lactam antibiotic has a wide range of applications in medicine and veterinary medicine [
1]. AMX has been found in groundwater, surface water, and soil, which pose serious risks, including human endocrine disorders and antibiotic resistance [
2,
3,
4]. Due to its complex structure, high chemical stability and good solubility in aquatic environments, it is challenging to remove AMX from wastewater using traditional wastewater treatment plants. Therefore, the advanced oxidation process (AOP) is an effective and promising technology for purifying various water pollutants, but it is limited by the high cost of intensive chemical inputs and post-treatment.
Photocatalysis technology is considered one of the most attractive and promising technologies to achieve green production. High-efficiency photocatalysts play an important role in catalytic activities. There are many common photocatalytic materials, such as TiO
2 [
5], g-C
3N
4 [
6,
7], ZnO [
8], and their nanostructure components. According to dimensions, nanophotocatalysts include 0D nanomaterials [
9,
10], 1D nanotubes [
11], 2D nanosheets [
12] and 3D aerogel [
13,
14] materials. Among them, g-C
3N
4 is favored due to its suitable band structure, diverse morphology, chemical and thermal stability, low cost and environmental friendliness [
15,
16,
17]. Particularly, g-C
3N
4-based aerogels have been widely studied. For example, a 3D porous g-C
3N
4/GO (p-CNG) skeleton was constructed via a thermal treatment aided by the template technique. Then, precious metal (Au, Pd, and Pt) cocatalysts were immobilized to the 3D p-CNG skeleton to construct 3D p-CNG-M (Au, Pd and Pt) composite catalysts. Due to the typical 3D porous structure and bonding interaction between g-C
3N
4 and GO, 3D p-CNG-M (Au, Pd and Pt) has a large specific surface area and stable physicochemical properties. Meanwhile, precious metal cocatalysts acting as electron acceptors remarkably increase the number of active sites and promote electron–hole separation. Therefore, 3D p-CNG-M catalysts present outstanding enhanced hydrogen evolution reaction activities under simulated solar light [
16]. Furthermore, Xu et al. prepared a novel 3D graphene aerogel composite Nb
2O
5-g-C
3N
4/rGA. The 1D–2D NbNR-CN heterojunction was first prepared via a simple grinding and calcination method. Then, different amounts of NbNR-CN (30%, 60% and 90%) were loaded onto graphene nanosheets via a chemical reduction self-assembly method to obtain the 3D composites Nb
2O
5-g-C
3N
4/rGA (NbNR-CN/rGA) composites. Compared with g-C
3N
4, NbNR and NbNR-CN, NbNR-CN/rGA showed the lowest charge transfer resistance and the highest photocurrent density. Photodegradation experiments showed that the NbNR-CN/rGA composites exhibited remarkably enhanced visible-light photocatalytic activity for the degradation of Rhodamine B (RhB). The removal rate for the 60% NbNR-CN/rGA composite was 94.8% within 100 min. This enhanced photoactivity could be explained by a prolonged lifetime of photo-generated carriers due to the formation of heterojunctions as well as the good electron transfer ability of rGA [
11]. On the one hand, aerogels with a 3D network structure can avoid aggregation and provide convenient transfer channels. On the other hand, the block structure of aerogels not only can prevent itself from dispersing in water, but also simplifies the process of operation, collection and separation of materials from water. More importantly, combinations with other photocatalysts to construct g-C
3N
4-based aerogel can not only greatly improve the specific surface area, but also expand the spectral utilization range and hinder the rapid recombination of photo-generated electrons and holes. A large number of studies and reports have proven that aerogels have outstanding advantages in adsorption and catalysis due to their massive pores, high catalytic activity, low density and other properties [
18,
19,
20,
21]. Carbon dots (CDs), as a new 0D material, are often used as light energy conversion components to construct high-efficiency photocatalysts [
12,
13,
22]. CDs have tunable fluorescence properties, flexible surface states and excellent electron transport channels. Moreover, CDs themselves are also photocatalysts. In previous work, we successfully synthesized novel porous nitrogen-doped carbon dots of (NCDs)@g-C
3N
4 composite via a facile hydrothermal approach. The degradation rate of 2.0 wt% NCDs@g-C
3N
4 is about twice that of pristine g-C
3N
4 at 60 min under the same conditions. In addition, at 120 min, MB was almost degraded completely by 2.0 wt% NCDs@g-C
3N
4 with a degradation rate as high as 97.9%, while that of pristine g-C
3N
4 was only 47%. Moreover, NCDs were found to have better photocatalytic performance than that of g-C
3N
4. At 120 min, the degradation rate of NCDs is 72%. This improved photocatalytic activity can be attributed to the fact that the addition of NCDs not only broadens the photoresponse range, but also suppresses the photo-generated electron and hole recombination of g-C
3N
4. Meanwhile, NCDs themselves are also excellent photocatalysts [
12]. Furthermore, N-modified carbon dots/graphite carbon nitride (NCDs/g-C
3N
4) aerogels were successfully prepared by a simple electrostatic self-assembly method. Among them, NCDs carry a large amount of –NH– from polyethyleneimine (PEI), exhibiting positivity. g-C
3N
4 nanosheets exhibit a negative charge. The hydrogen evolution rate of NCDs/g-C
3N
4 aerogel can reach 13,499 μ mol h
−1 g
−1, which is 1.6 times that of pure g-C
3N
4 [
14]. Generally speaking, in the process of constructing aerogel, high-molecular polymers such as agar, polyethylene, chitosan, etc., often play a key role [
13,
23,
24].
Herein, the agar, CDs, and g–C3N4 aqueous solutions were transformed into mixed hydrogels with a 3D network structure by heating–cooling polymerization. The Agar/CDs/g-C3N4 aerogel was obtained by further freeze drying. Agar can maintain the aerogel structure well and adsorb organic pollutants. Meanwhile, CDs can effectively expand the spectral utilization range of aerogels and reduce the recombination rate of photo-generated electrons and holes. Finally, the Agar/CDs/g-C3N4 aerogel showed high photocatalytic degradation for AMX, implying that it has enormous potential as a photocatalyst for degrading antibiotic pollutants. The preparation process of aerogel only involves simple agitation and heating and freeze drying, which are very friendly to industrialization. It is also suitable for the preparation of almost all organic aerogels. g-C3N4 nanosheets are obtained by multiple thermal sintering processes, which is also suitable for extending to other 2D materials, such as graphene. The CDs are prepared through the hydrothermal method, which can be used for the synthesis of almost all nanocrystals/microcrystals. Therefore, the experimental methods are universal, easy to operate, and suitable for large-scale industrial production.
2. Results and Discussion
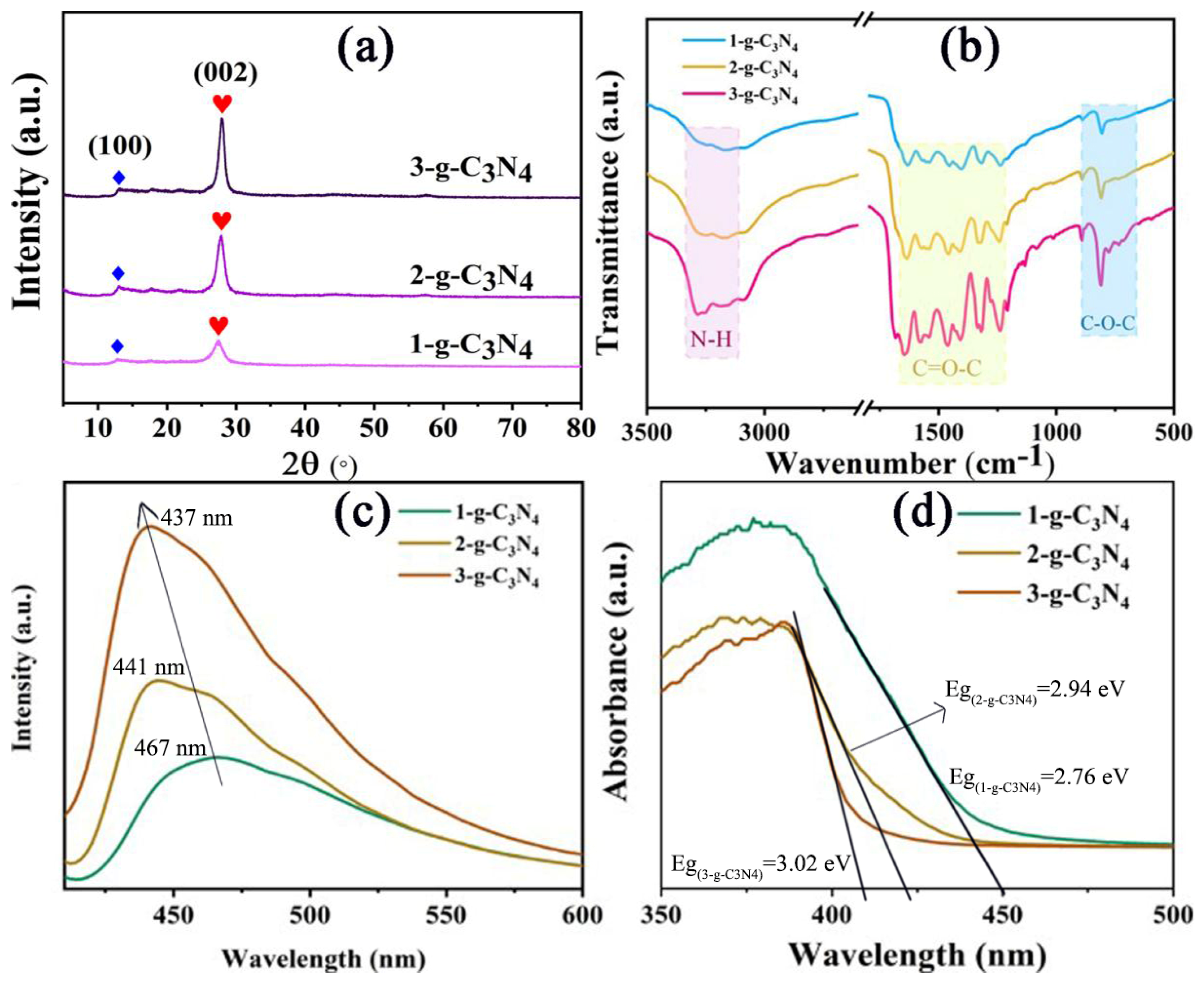
As shown in
Figure S1, multiple thermal sintering is an effective method to obtain g-C
3N
4 nanosheets. The 1-g-C
3N
4 shows thick micron-scale blocks (
Figure S1a). After secondary calcination, the particle size of obtained 2-g-C
3N
4 significantly decreased (200 nm–1 µm). What’s more, layered structures of 2-g-C
3N
4 can be observed clearly (
Figure S1b). 3-g-C
3N
4 nanosheets with curled edges were obtained by thermal stripping of 2-g-C
3N
4. The particle size of 3-g-C
3N
4 is around 200 nm and there is no obvious aggregation (
Figure S1c). Ultra-thin 3-g-C
3N
4 nanosheets have a larger specific surface area and abundant active sites, which is beneficial for subsequent photocatalytic. The g-C
3N
4 nanosheets with relatively complete crystallization and stable luminescence were obtained through the thermal stripping method. As shown in
Figure 1a, the XRD results show that g-C
3N
4 nanosheets have two characteristic peaks, which are 13.2° and 27.5°, respectively. Among them, the diffraction peak at 27.5° is the strongest, which is the characteristic peak of interlayer stacking of aromatic compounds [
20]. The crystal plane index is marked as (002), corresponding layer spacing is d = 0.324 nm, indicating that the g-C
3N
4 nanosheets have a layered structure similar to graphite [
21]. The other diffraction peak appears at 13.2°, belonging to the characteristic peak of the melon-like substance [
25]. The nitrogen pore spacing corresponding to the 3-s-triazine structure is d=0.670 nm, and the crystal plane index is marked as (100) [
26]. With the increase in thermal stripping times, the diffraction peak near 27.5° shifts to a higher angle, indicating that the layer spacing of g-C
3N
4 becomes smaller. What’s more, with the increase in sintering times, the diffraction peaks of g-C
3N
4 become stronger and maintain high symmetry, indicating that the crystallinity of g-C
3N
4 nanosheets become more and more complete. In the FTIR spectra of g-C
3N
4, the characteristic peaks are mainly distributed in the following three regions, 810 cm
−1, 1240–1643 cm
−1, and 3200 cm
−1 [
23,
24], which is consistent with the literature reports (
Figure 1b). Among them, the peak at 810 cm
−1 corresponds to the triazine structure. The 1240–1643 cm
−1 is attributed to a triangular C–N–C structure or bridging C–NH–C unit. The wide band at about 3200 cm
−1 is the N–H stretching vibration peak, which indicates that there are a lot of amino groups at the edge of g-C
3N
4 nanosheets. The thinner of g-C
3N
4 nanosheets, the more obvious the signal of amino groups becomes. The PL and UV–vis absorbance spectra display that, with the increase in sintering times, the g-C
3N
4 nanosheets are thinner, the layer spacing is smaller, the fluorescence emission is stronger, the luminescence shifts bluer (
Figure 1c), and the optical band gap is larger (
Figure 1d). The degradations of AMX by g-C
3N
4 with different sintering degrees under visible light were studied (
Figure S3). It can be seen that the photocatalytic performance gradually increases with the increase in sintering times. Among them, the g-C
3N
4 nanosheets sintered three times have the best degradation performance for AMX. The degradation of AMX is basically completed in 40 min, while 1-g-C
3N
4 only degrades 50% of AMX. This is due to the fact that thinner nanosheets can provide more reactive sites in the process of photocatalytic oxidation reduction [
27]. Therefore, 3-g-C
3N
4 was selected and compounded with CDs and agar to construct aerogel in the subsequent experiments.
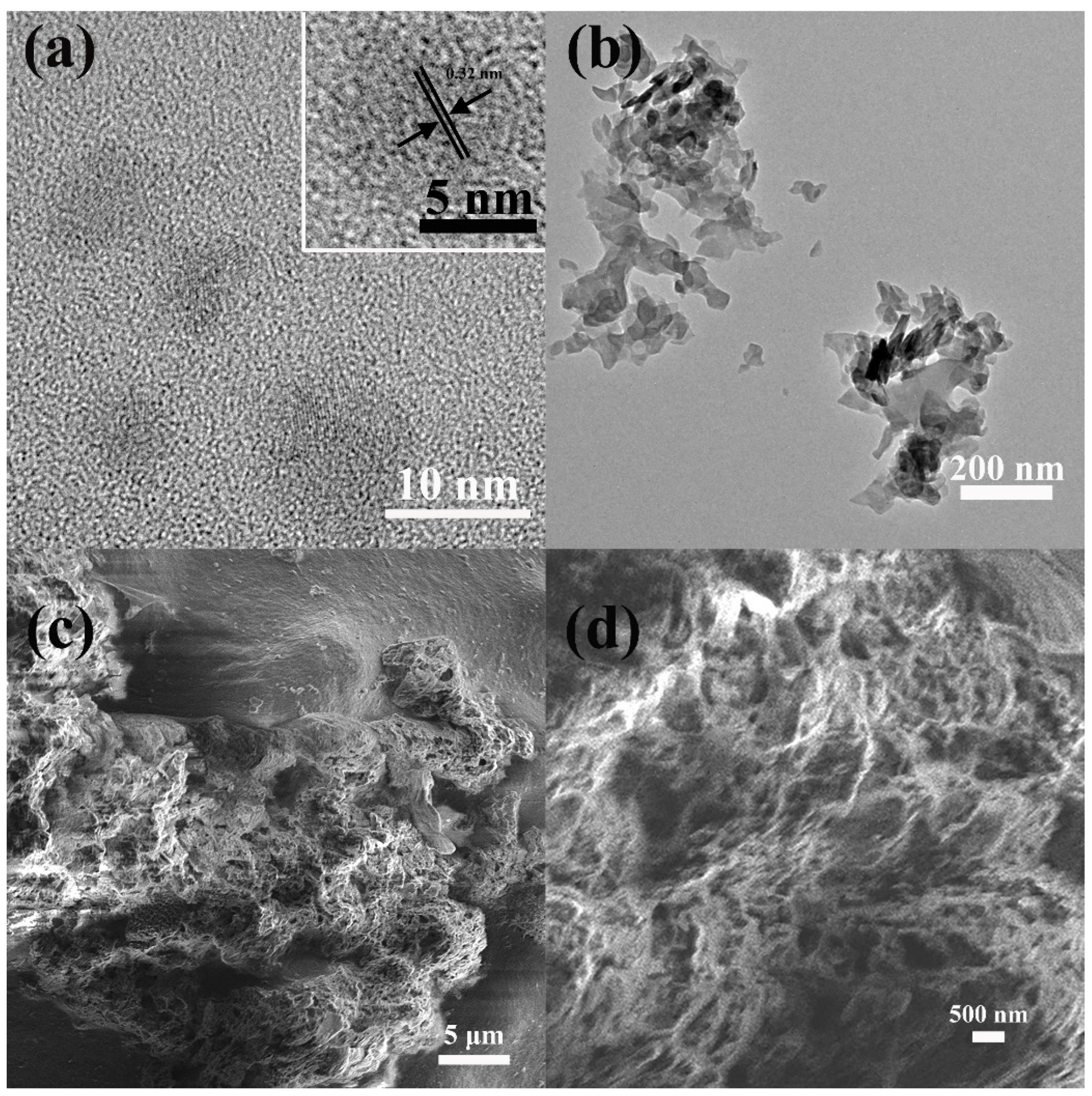
As shown in
Figure 2a, CDs are monodispersed, and their average lateral size determined by TEM is about 6.5 nm with a narrow size distribution (
Figure S2). It can be seen that CDs have a certain degree of crystallinity. The high resolution TEM (HRTEM) further proves that the lattice stripe spacing of CDs is 0.32 nm (inset in
Figure 2a), corresponding to the (002) crystal plane of graphene [
18]. In
Figure 2b, the 3-g-C
3N
4 nanosheets are about 200 nm. Agar is used as the support skeleton and adsorption module of aerogel, g-C
3N
4 is used as the photocatalysis main material, and CDs are used as light energy conversion components. The Agar/CDs/g-C
3N
4 aerogel was formed by self-assembly of the three components. The 3D interconnected network stacked by g-C
3N
4 and agar has porous, wrinkled, and fluffy microstructures (
Figure 2c). The enlarged image in
Figure 2d clearly shows that the holes of aerogel are of different sizes and interconnected. The 3D structure of Agar/CDs/g-C
3N
4 aerogel effectively prevents the stacking of g-C
3N
4 nanosheets. What’s more, the rich pore structure further effectively increases the specific surface area of materials, providing abundant active sites for adsorption and catalytic processes in photocatalytic reactions.
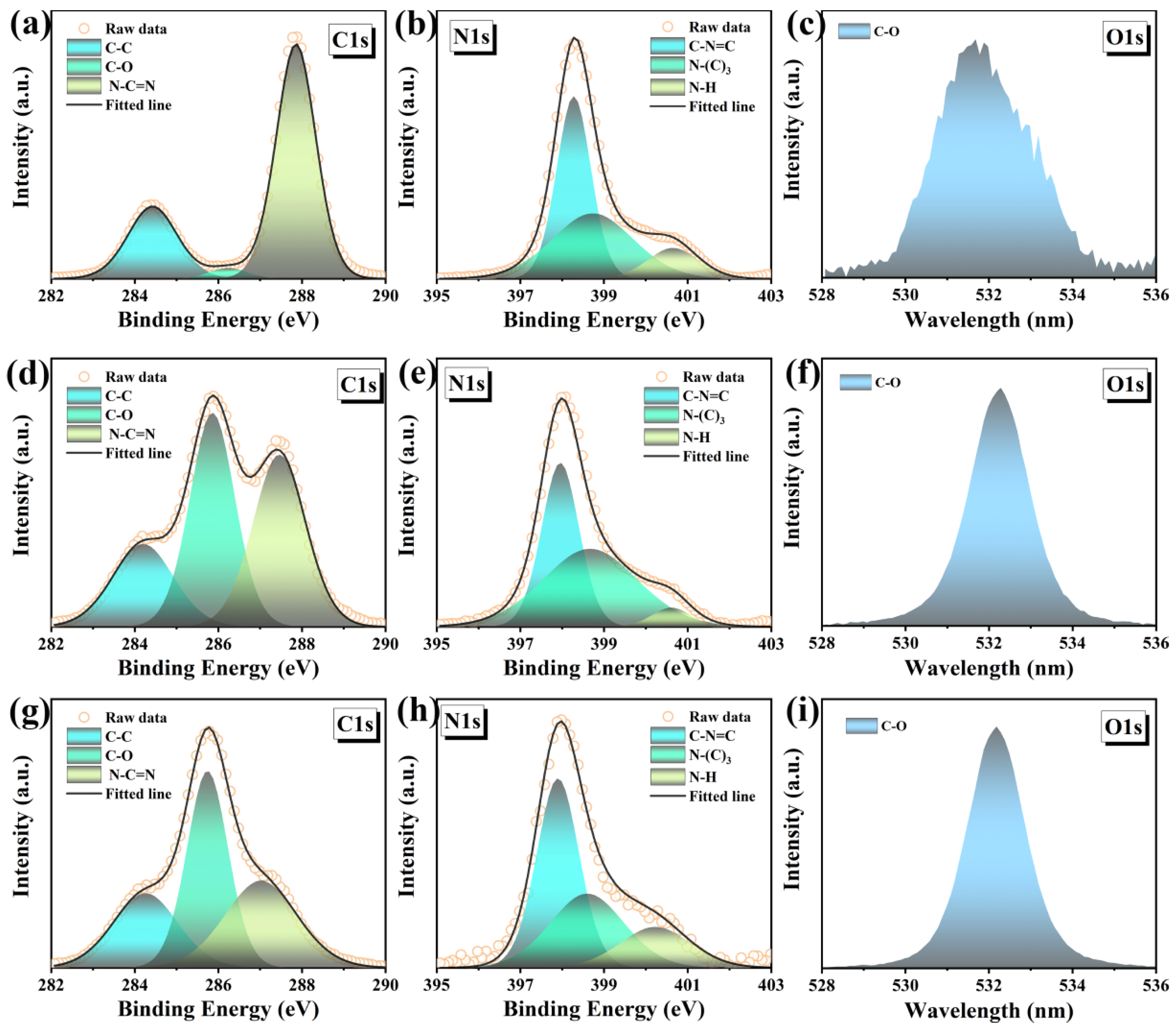
The chemical structures of the samples were carefully studied by XPS and FTIR. The g-C
3N
4, Agar/g-C
3N
4 and Agar/CDs/g-C
3N
4 only contain C, N and O three elements (
Figure S4). The high-resolution C1s spectra (
Figure 3a,d,g) show that the peaks of g-C
3N
4, Agar/g-C
3N
4 aerogel, and Agar/CDs/g-C
3N
4 aerogel can be fitted to three components, belonging to C–C (284.8 eV), C–O (286.45 eV) and N–C=N (288.15 eV), respectively [
15,
16,
17]. It can be seen that the integrated area of the C–O peak in the Agar/g-C
3N
4 aerogel is greatly increased (
Figure 3d), compared with that of g-C
3N
4 (
Figure 3a), which is attributed to successful compounding of agar. At the same time, due to the introduction of CDs, the C–C and C–O peaks of Agar/CDs/g-C
3N
4 aerogel increased significantly (
Figure 3g). As shown in
Figure 3b,e,h, the N1s high-resolution spectra show the main structural component at 398.3 eV, which is attributed to the
sp2-bonded nitrogen in the tris-triazine ring (C–N=C) [
15], while the weak peak at 398.9 eV is usually attributed to N–(C)
3 [
16]. In addition, the peak at 400.7 eV indicates the presence of amino groups (N–H) [
17]. Among them, the Agar/CDs/g-C
3N
4 aerogel has the strongest N–H peak (
Figure 3h) due to the abundant amino groups on the surface of CDs (
Figure S5). Finally, the high-resolution O1s spectra of all samples show the peak at 532.9 eV, corresponding to the C–O bond [
28]. A large number of functional groups can serve as active sites for photocatalysis, transferring electrons effectively during the redox reaction process.
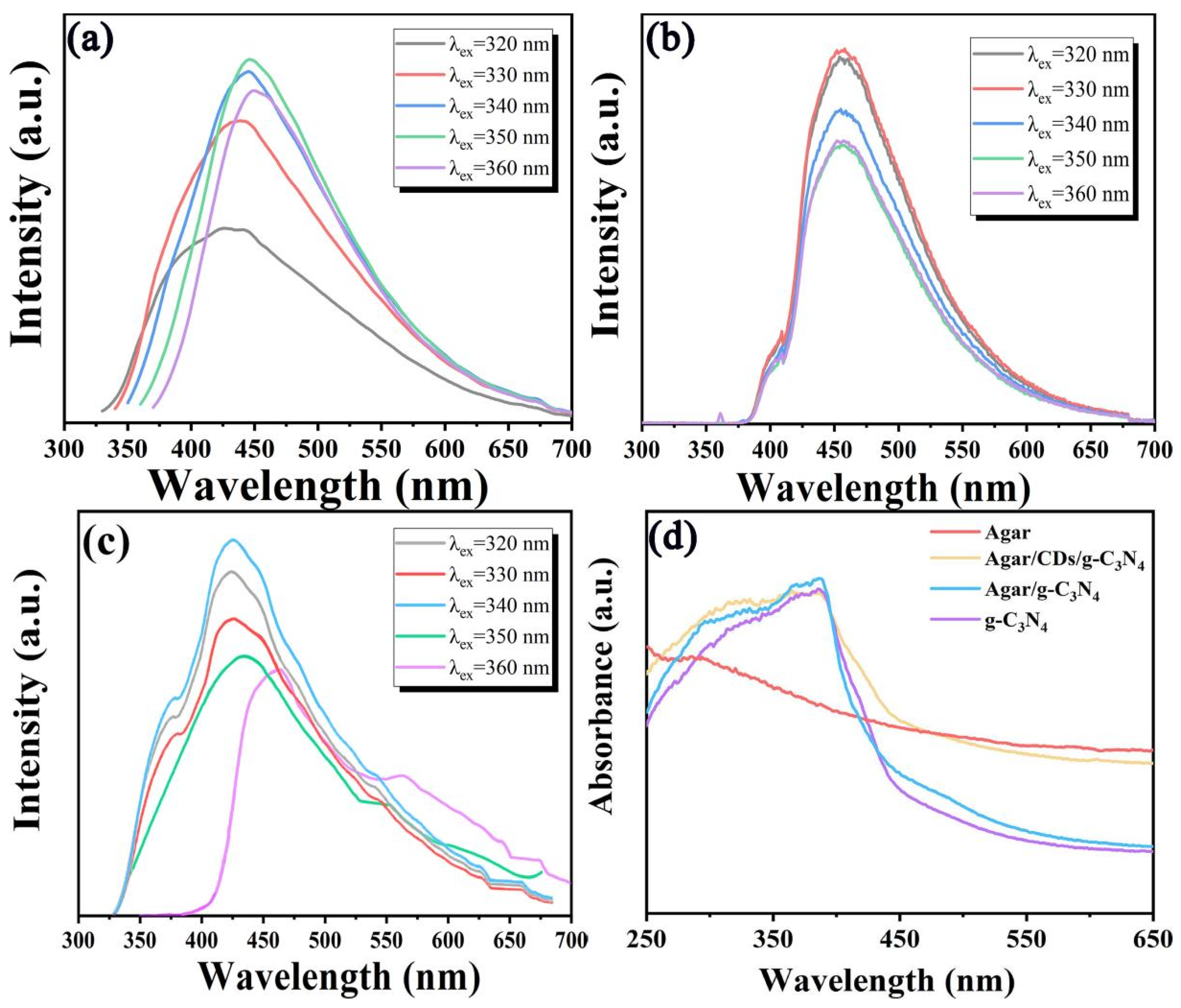
In order to explore the optical properties of CDs, 3-g-C
3N
4, Agar/g-C
3N
4 aerogel and Agar/CDs/g-C
3N
4 aerogel, the PL and UV-Vis absorption spectra were recorded. In
Figure 4a, it can be observed that CDs have the typical excitation wavelength dependence. The emission peak of CDs is about 410 nm excitated by 320 nm. With the increase in excitation wavelength, the emission peak of CDs gradually redshifts. Meanwhile, the emission intensity of CDs shows first increasing and then decreasing. When the excitation wavelength is 350 nm, the emission peak of CDs is fixed at about 450 nm, and the fluorescence emission intensity of CDs is the highest. In
Figure 4b, the emission peak of 3-g-C
3N
4 is not affected by the excitation wavelength. Its maximum emission wavelength is 455 nm under 330 nm. Due to the addition of CDs, the emission spectra of Agar/CDs/g-C
3N
4 aerogel were significantly enhanced at 330–400 nm and 500–700 nm compared with pure 3-g-C
3N
4 (
Figure 4c). Moreover, the Agar/CDs/g-C
3N
4 aerogel has so wide spectral range that covers the whole visible-light wavelength range (350–700 nm), indicating that visible light can be well utilized by the Agar/CDs/g-C
3N
4 aerogel. Generally, the efficiency of light absorption and carrier separation will affect the photocatalytic performance of catalysts [
28,
29]. Thus the absorption spectra of 3-g-C
3N
4, Agar/g-C
3N
4 aerogel and Agar/CDs/g-C
3N
4 aerogel were explored. As shown in
Figure 4d, due to the successful combination of CDs, the absorption intensity of Agar/CDs/g-C
3N
4 aerogel was significantly improved after 400 nm. Furthermore, compared with 3-g-C
3N
4, the absorption edge of Agar/CDs/g-C
3N
4 aerogel is red shifted and its optical band gap is reduced. The narrower optical gap also means that more visible light can be used for photocatalytic activities.
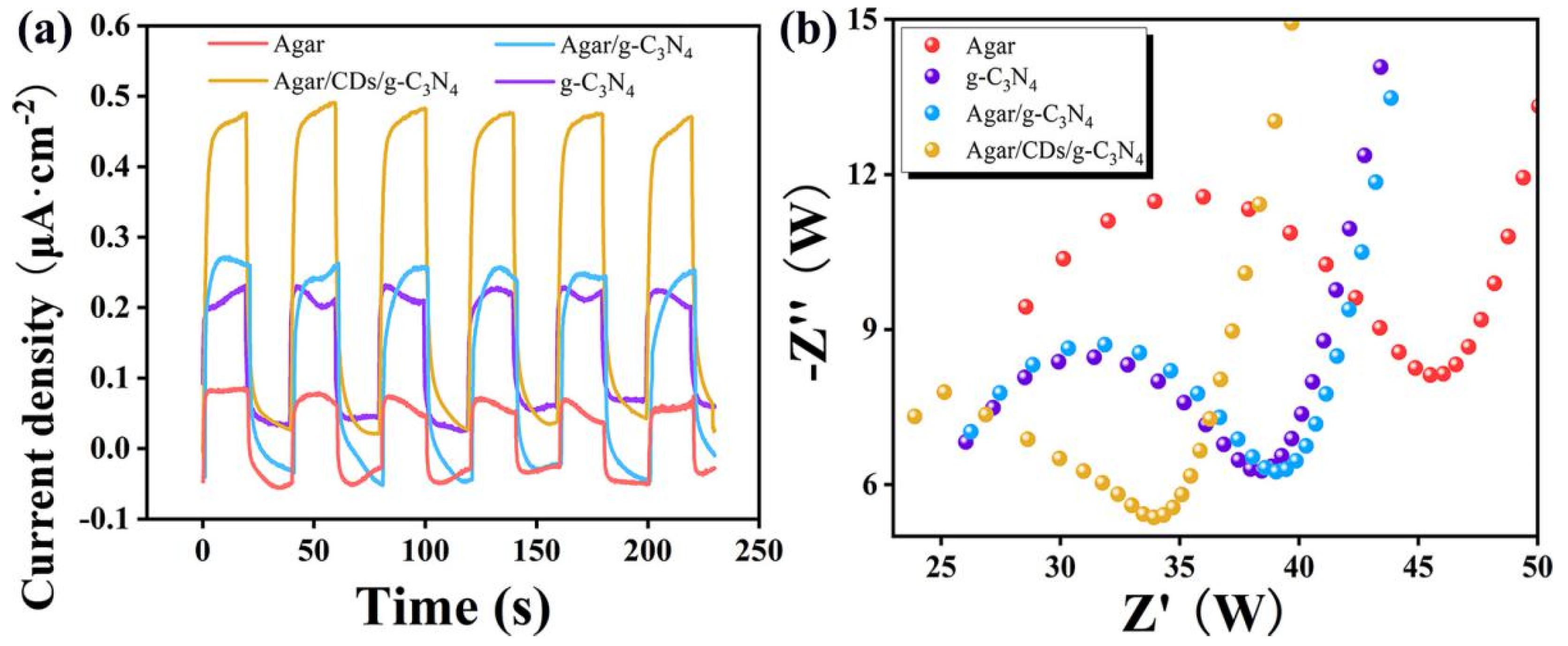
Furthermore, the differences in the photo-generated carriers in a series of samples were studied through photocurrent and impedance tests. As shown in
Figure 5a, at the beginning of light illumination, the photocurrent density of each sample rapidly increases. After the end of light illumination, the photocurrent value rapidly decreases again. It indicates that all samples have good light response ability. By switching the laser light six times in a row, all samples show six consecutive stable responses, suggesting the stable output of abundant photo-generated carriers. In fact, high photocurrent density represents a high concentration of photo-generated carriers, indicating good photoelectric conversion ability of samples. Obviously, the photocurrent density of Agar/CDs/g-C
3N
4 aerogel is the highest, which is 3.7 times that of agar, 2.4 times that of 3-g-C
3N
4, and 1.6 times that of Agar/g-C
3N
4 aerogel. What’s more, the EIS measurement is used for further quantitative analysis of the resistance characteristics of Agar, 3-g-C
3N
4, Agar/g-C
3N
4, and Agar/CDs/g-C
3N
4 aerogel in
Figure 5b. Generally, the smaller radius of the Nyquist plots corresponds to the faster electron transfer kinetics of the redox reaction and lower charge transfer resistance. The diameter of the Nyquist plot of Agar/CDs/g-C
3N
4 aerogel is the smallest. It is proved that, compared to the other three samples, Agar/CDs/g-C
3N
4 aerogel has more outstanding charge transfer ability, and excellent charge transfer and separation rates are crucial for improving photocatalytic performance [
19].
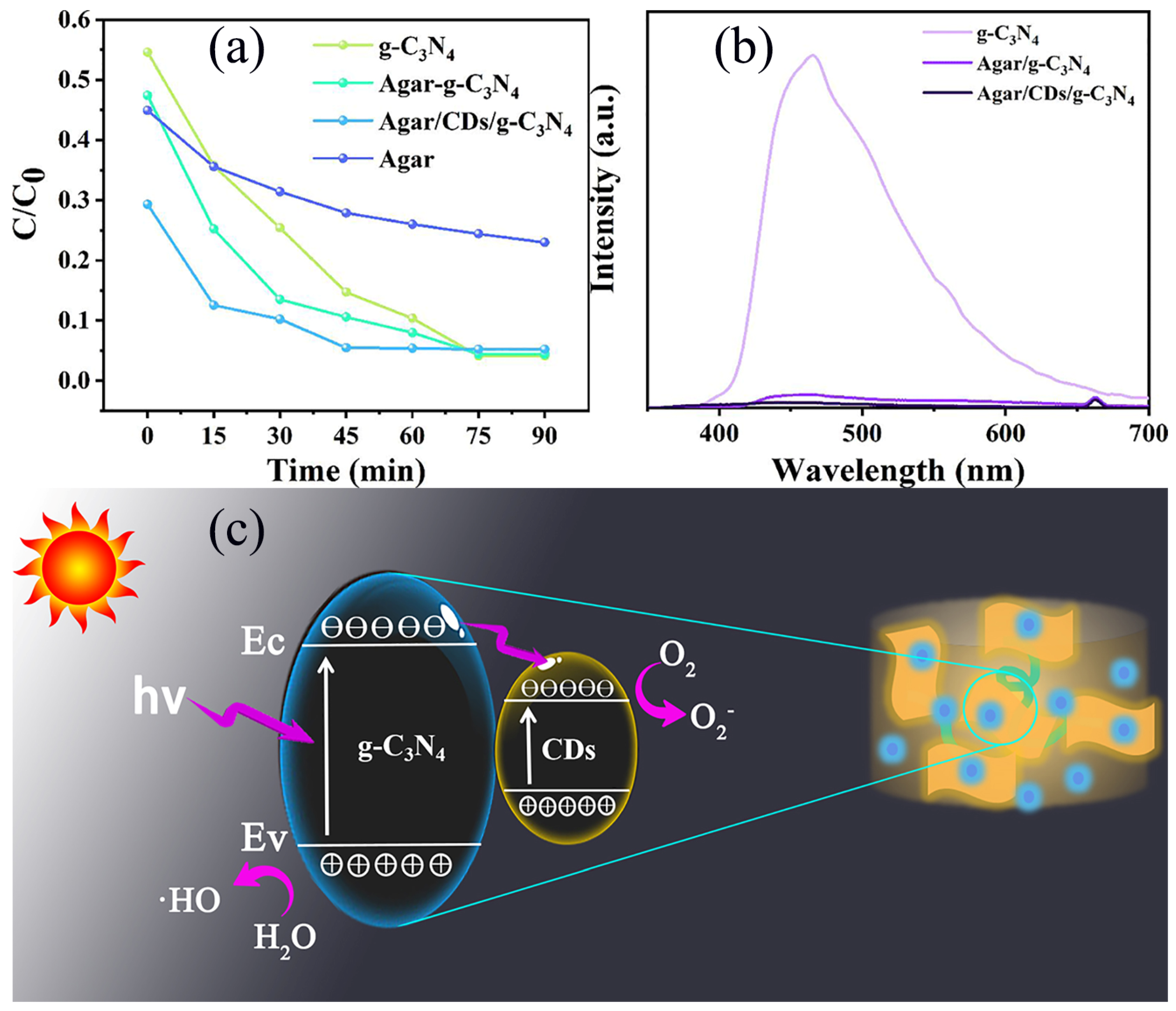
The degradation of AMX by Agar, g-C
3N
4, Agar/g-C
3N
4 aerogel and Agar/CDs/g-C
3N
4 aerogel under visible light irradiation was studied. Before turning on the xenon lamp for photocatalytic experiments, the sample was added to the AMX solution and stirred for 30 minutes in the dark to achieve adsorption–desorption equilibrium. As shown in
Figure 6a, when the adsorption equilibrium is reached at 0 min, the removal performance of all aerogel samples for AMX is higher than that of pure g-C
3N
4 due to the fluffy porous structure formed by agar. On the one hand, the 3D structure of aerogel can provide a convenient transfer channel for the adsorption and in situ degradation of AMX. On the other hand, it can avoid the aggregation of g-C
3N
4 and prevent them from dispersing in water, greatly simplifying the collection and separation of g-C
3N
4 from water. After light irradiation, the degradation efficiency of agar is very low, because pure agar has little photocatalytic performance. Agar/g-C
3N
4 aerogel shows a stronger removal efficiency compared with g-C
3N
4. The reason is the effect of adsorption from agar. More importantly, after the successful recombination of CDs, the photocatalytic ability of Agar/CDs/g-C
3N
4 was further improved. AMX was almost completely degraded by Agar/CDs/g-C
3N
4 after 45 min of illumination, while it takes 75 min for Agar/g-C
3N
4 aerogel and g-C
3N
4 to completely remove AMX. It is not only due to the high adsorption ability brought by agar, but also thanks to the introduction of CDs. Compared with g-C
3N
4, the fluorescence emission intensity of Agar/g-C
3N
4 aerogel decreases sharply, indicating that it has higher carrier separation efficiency (
Figure 6b). The reason is the unique 3D interconnected porous structure of aerogel hinders the recombination of electrons and holes. With the successful combination of CDs, the fluorescence emission intensity of Agar/CDs/g-C
3N
4 aerogel further decreased, indicating that CDs inhibited the rapid recombination of photo-generated electrons and holes. In a word, the Agar/CDs/g-C
3N
4 aerogel has the best carrier transport efficiency, which is consistent with the analysis results of photocurrent and impedance. It is because photo-generated electrons and holes are transferred from the g-C
3N
4 surface to the surface of CDs under visible light irradiation. Then, free groups (such as O
2− and ·HO) are generated for the degradation of AMX in the water and oxygen environment (
Figure 6c). AMX molecules and intermediates might be oxidized into other small molecules and ultimately mineralized into H
2O and CO
2. Most of the photodegradation intermediates of AMX are harmless and avirulent [
27,
28]. After considering the materials (including glucose, agar, melamine, and deionized water), consumables (including dialysis bags and test tubes), and energy costs, the cost of preparing 10 g Agar/CDs/g-C
3N
4 aerogel was calculated at about 17.55 RMB. The commercial P25 photocatalyst is priced at 28 RMB per 10 g. More importantly, Agar/CDs/g-C
3N
4 aerogel can degrade AMX in 45 minutes, while the degradation of commercial P25 for AMX takes 180 min [
30]. Thus, Agar/CDs/g-C
3N
4 aerogel has been proven to have good application prospects and commercial value.