Tailoring Homogeneous Hydrogel Nanospheres by Facile Ultra-Sonication Assisted Cross-Linked Copolymerization for Rhodamine B Dye Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption of Rhodamine B onto Chitosan-cl-poly(MMA) Nanohydrogels Spheres
2.1.1. Adsorption Parameters
Effect of pH
Effect of Chitosan-cl-poly(MMA) Nanohydrogel Sphere Concentration
Effect of Contact Time and RhB Dye Concentration
2.1.2. Adsorption Kinetics and Isotherm Modeling
Adsorption Kinetics
Adsorption Isotherm
2.2. Adsorption Thermodynamics
2.3. Reusability
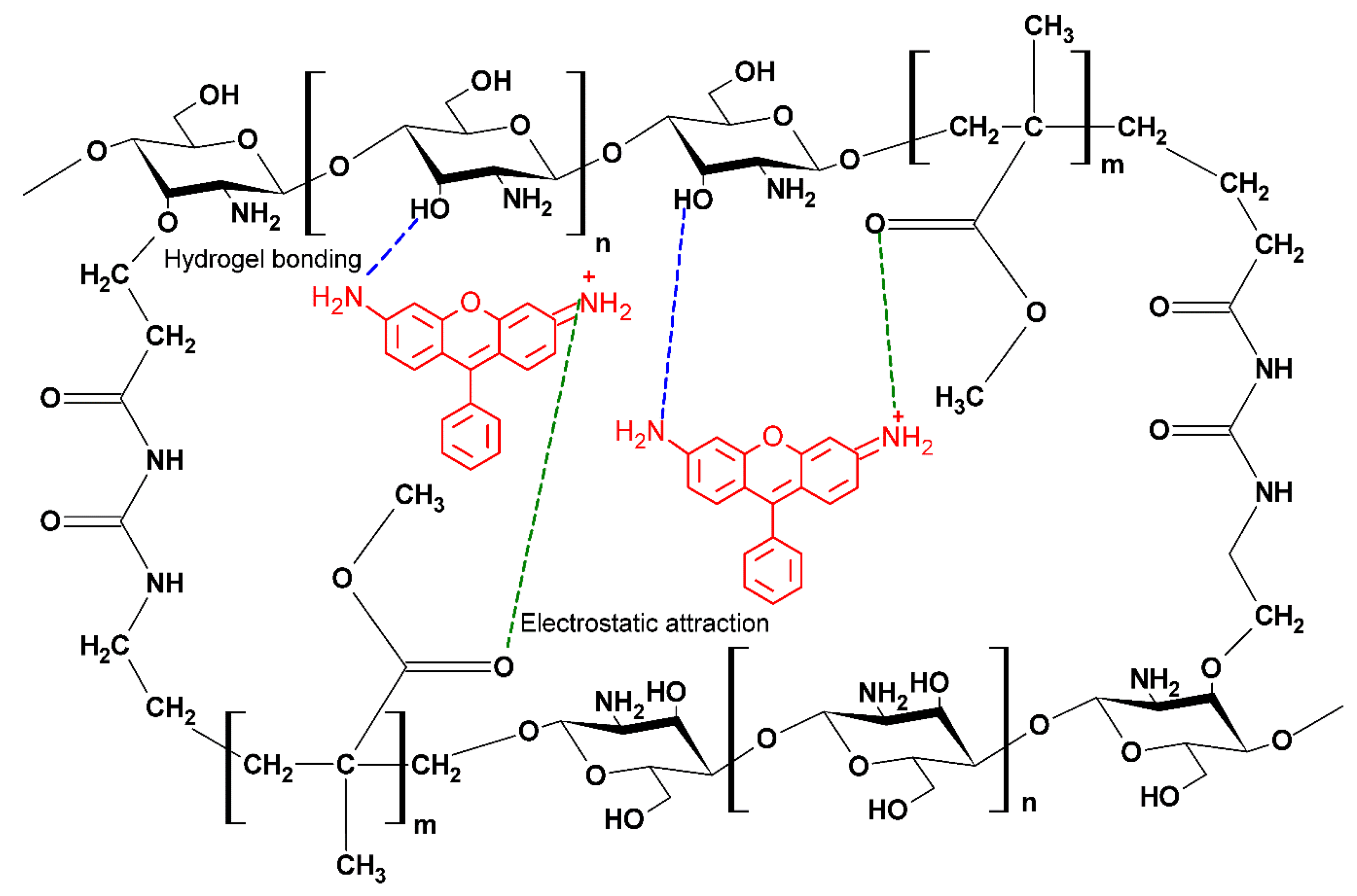
2.4. Possible Mechanism of Adsorption
3. Conclusions
4. Materials and Methods
4.1. Materials and Instruments
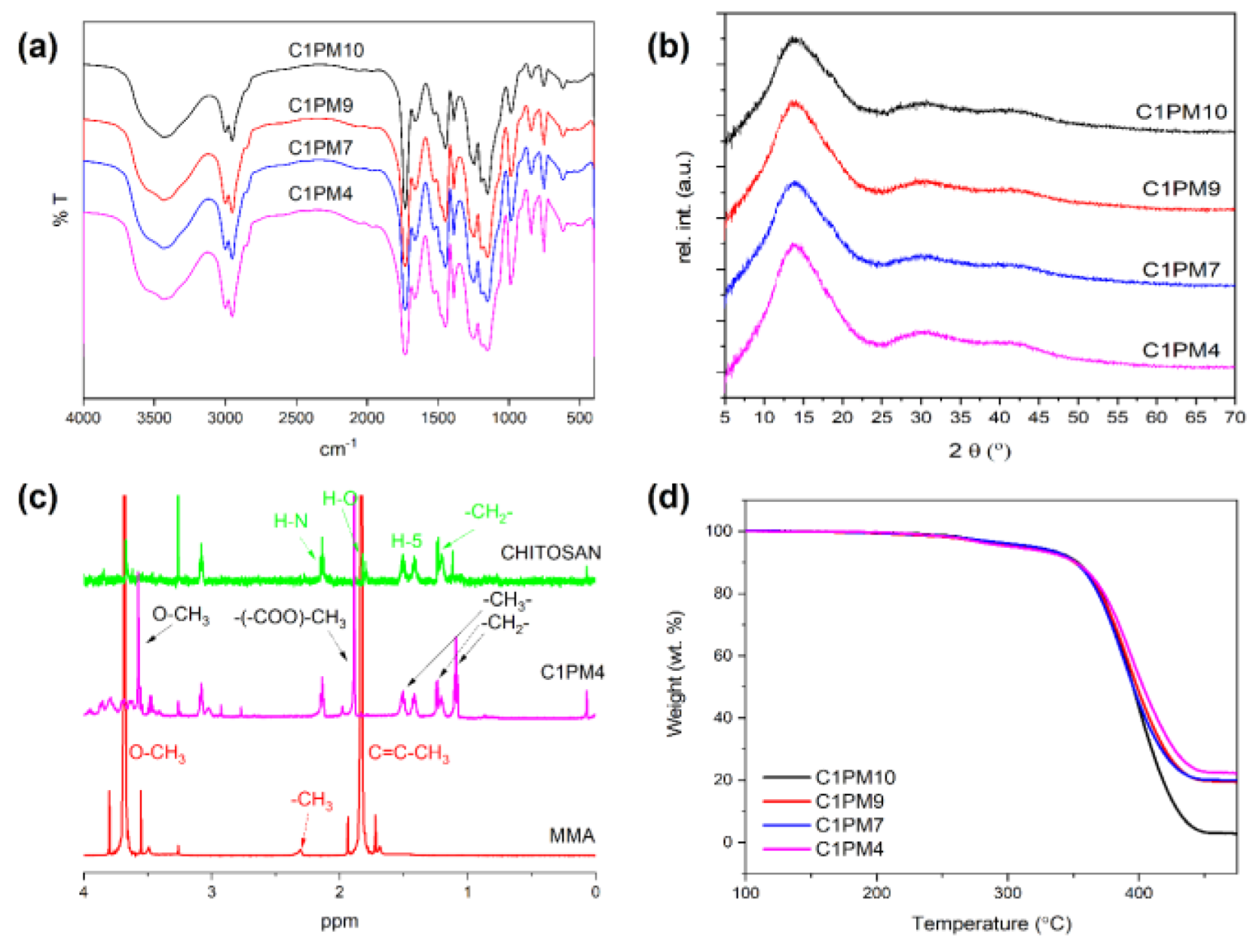
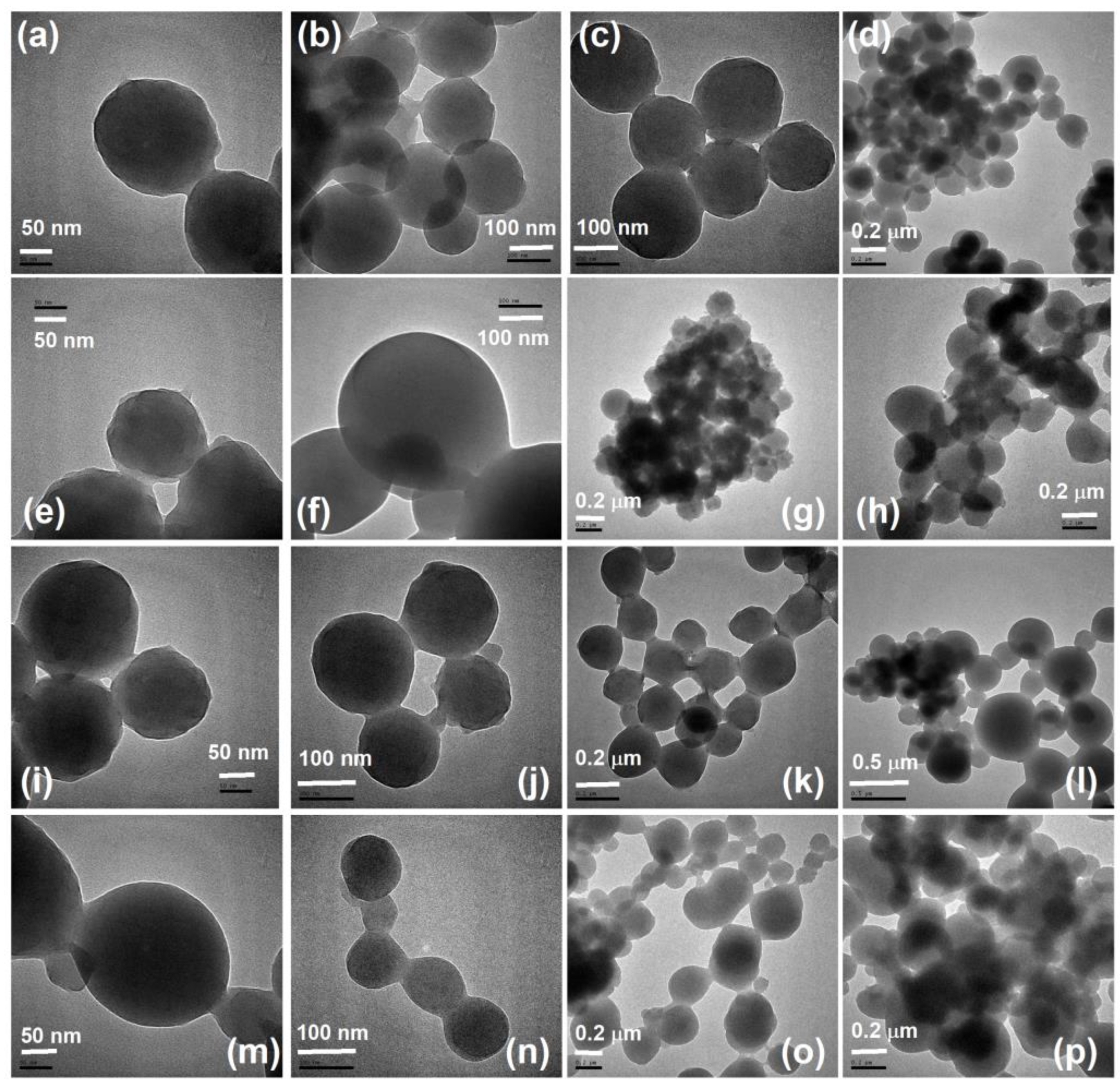
4.2. Preparation of Various Samples of Chitosan-cl-poly(MMA) Hydrogel Nanospheres
4.3. Characterization and Swelling Behavior
4.4. Adsorption of Rhodamine B onto Chitosan-cl-poly(MMA) Hydrogel Nanospheres
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhtar, S.; Khan, A.A.; Husain, Q. Potential of immobilized bitter gourd (Momordica charantia) peroxidases in the decolorization and removal of textile dyes from polluted wastewater and dyeing effluent. Chemosphere 2005, 60, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mittal, A.; Gajbe, V.; Mittal, J. Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: Bottom ash and de-oiled soya. Ind. Eng. Chem. Res. 2006, 45, 1446–1453. [Google Scholar] [CrossRef]
- Sharma, J.; Dhiman, P.; Kumar, A.; Dawi, E.A.; Rana, G.; Sharma, G. 2D–2D g-C3N5/Bi24O31Br10 S-scheme nanostructures with increased photocatalytic efficiency for crystal violet removal. Chem. Eng. Res. Des. 2023, 195, 432–446. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Sharifzade, G.; Asghari, A.; Rajabi, M. Highly effective adsorption of xanthene dyes (rhodamine B and erythrosine B) from aqueous solutions onto lemon citrus peel active carbon: Characterization, resolving analysis, optimization and mechanistic studies. RSC Adv. 2017, 7, 5362–5371. [Google Scholar] [CrossRef]
- Deng, F.; Xu, Z. Heteroatom-substituted rhodamine dyes: Structure and spectroscopic properties. Chin. Chem. Lett. 2019, 30, 1667–1681. [Google Scholar] [CrossRef]
- Aarthi, T.; Madras, G. Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind. Eng. Chem. Res. 2007, 46, 7–14. [Google Scholar] [CrossRef]
- Beaumont, P.C.; Johnson, D.G.; Parsons, B.J. Photophysical properties of laser dyes: Picosecond laser flash photolysis studies of Rhodamine 6G, Rhodamine B and Rhodamine 101. J. Chem. Soc. Faraday Trans. 1993, 89, 4185–4191. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef]
- Bera, K.K.; Majumdar, R.; Chakraborty, M.; Bhattacharya, S.K. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of toxic dye, Rhodamine-B under natural sunlight. J. Hazard. Mater. 2018, 352, 182–191. [Google Scholar] [CrossRef]
- Pirkarami, A.; Olya, M.E. Removal of dye from industrial wastewater with an emphasis on improving economic efficiency and degradation mechanism. J. Saudi Chem. Soc. 2017, 21, S179–S186. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A. Photocatalytic degradation of rhodamine B dye in wastewater using gelatin/CuS/PVA nanocomposites under solar light irradiation. J. Biomater. Nanobiotechnol. 2017, 8, 66. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gandhimathi, R. Removal of Rhodamine B from aqueous solution using graphite–graphite electro-Fenton system. Desalination Water Treat. 2014, 52, 1872–1877. [Google Scholar] [CrossRef]
- Dai, Q.; Jiang, L.; Luo, X. Electrochemical oxidation of rhodamine B: Optimization and degradation mechanism. Int. J. Electrochem. Sci. 2017, 12, 4265–4276. [Google Scholar] [CrossRef]
- Sharma, B.; Thakur, S.; Trache, D.; Yazdani Nezhad, H.; Thakur, V.K. Microwave-assisted rapid synthesis of reduced graphene oxide-based gum tragacanth hydrogel nanocomposite for heavy metal ions adsorption. Nanomaterials 2020, 10, 1616. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Chaudhary, J.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, V.K. Hydrogel of gelatin in the presence of graphite for the adsorption of dye: Towards the concept for water purification. J. Environ. Chem. Eng. 2021, 9, 104762. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O.A. Synthesis, swelling and adsorption studies of a pH-responsive sodium alginate–poly (acrylic acid) superabsorbent hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Shirsath, S.R.; Patil, A.P.; Patil, R.; Naik, J.B.; Gogate, P.R.; Sonawane, S.H. Removal of Brilliant Green from wastewater using conventional and ultrasonically prepared poly (acrylic acid) hydrogel loaded with kaolin clay: A comparative study. Ultrason. Sonochemistry 2013, 20, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.; Chakma, S. Advances in the preparation of hydrogel for wastewater treatment: A concise review. J. Environ. Chem. Eng. 2019, 7, 103295. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Du, J.; Mu, T.; Liu, Z.; Chen, J.; Han, B. Preparation of cadmium sulfide/poly(methyl methacrylate) composites by precipitation with compressed CO2. J. Appl. Polym. Sci. 2004, 94, 1643–1648. [Google Scholar] [CrossRef]
- Tözüm, M.S.; Aksoy, S.A.; Alkan, C. Microencapsulation of Three-Component Thermochromic System for Reversible Color Change and Thermal Energy Storage. Fibers Polym. 2018, 19, 660–669. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Wang, G.; Wang, C.; Chu, F. Synthesis of Lignin-Based MMA-co-BA Hybrid Resins from Cornstalk Residue via RAFT Miniemulsion Polymerization and Their Characteristics. Polymers 2021, 13, 968. [Google Scholar] [CrossRef] [PubMed]
- Rahim, S.; Rauf, A.; Rauf, S.; Shah, M.R.; Malik, M.I. Enhanced electrochemical response of a modified glassy carbon electrode by poly(2-vinlypyridine-b-methyl methacrylate) conjugated gold nanoparticles for detection of nicotine. RSC Adv. 2018, 8, 35776–35786. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Sain, S.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ennis, C.J.; Rahman, P.K.S.M. Synthesis and characterization of PMMA-cellulose nanocomposites by in situ polymerization technique. J. Appl. Polym. Sci. 2012, 126, E127–E134. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Lian, Q.; Yang, H.; Wang, X. Surface Molecularly Imprinted Polymer of Chitosan Grafted Poly(methyl methacrylate) for 5-Fluorouracil and Controlled Release. Sci. Rep. 2016, 6, 21409. [Google Scholar] [CrossRef]
- Bhat, A.H.; Bhat, I.-U.-H.; Khalil, H.P.S.A.; Mishra, R.K.; Datt, M.; Banthia, A.K. Development and Material Properties of Chitosan and Phosphomolybdic Acid-based Composites. J. Compos. Mater. 2010, 45, 39–49. [Google Scholar] [CrossRef]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-graft-Poly(l-lactide) Copolymers for Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, T.; Yadav, S.; Krithiga, G.; Sastry, T.P. Chitosan as a matrix for grafting methyl methacrylate: Synthesis, characterization and evaluation of grafts for biomedical applications. Polym. Bull. 2016, 73, 3105–3117. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Pourjavadi, A.; Zohuriaan-Mehr, M.J. Synthesis and Properties of Highly Swelling PAAm/Chitosan Semi-IPN Hydrogels. In Macromolecular Simposia; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 274, pp. 171–176. [Google Scholar]
- Goyal, M.; Singh, S.; Bansal, R.C. Equilibrium and dynamic adsorption of methylene blue from aqueous solutions by surface modified activated carbons. Carbon Lett. (Carbon Lett.) 2004, 5, 170–179. [Google Scholar]
- Mousavi, S.A.; Kamarehie, B.; Almasi, A.; Darvishmotevalli, M.; Salari, M.; Moradnia, M.; Azimi, F.; Ghaderpoori, M.; Neyazi, Z.; Karami, M.A. Removal of Rhodamine B from aqueous solution by stalk corn activated carbon: Adsorption and kinetic study. Biomass Convers. Biorefinery 2023, 13, 7927–7936. [Google Scholar] [CrossRef]
- Dada, A.O.; Adekola, F.; Odebunmi, E. A novel zerovalent manganese for removal of copper ions: Synthesis, characterization and adsorption studies. Appl. Water Sci. 2017, 7, 1409–1427. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Akinlabi, A.K. Congo red biosorption on palm kernel seed coat. Ind. Eng. Chem. Res. 2009, 48, 6188–6196. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Azadirachta indica leaf powder as an effective biosorbent for dyes: A case study with aqueous Congo Red solutions. J. Environ. Manag. 2004, 71, 217–229. [Google Scholar] [CrossRef]
- Bhowmik, S.; Chakraborty, V.; Das, P. Batch adsorption of indigo carmine on activated carbon prepared from sawdust: A comparative study and optimization of operating conditions using Response Surface Methodology. Results Surf. Interfaces 2021, 3, 100011. [Google Scholar] [CrossRef]
- Harrache, Z.; Abbas, M.; Aksil, T.; Trari, M. Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem. J. 2019, 144, 180–189. [Google Scholar] [CrossRef]
- Ganguly, P.; Sarkhel, R.; Das, P. Synthesis of pyrolyzed biochar and its application for dye removal: Batch, kinetic and isotherm with linear and non-linear mathematical analysis. Surf. Interfaces 2020, 20, 100616. [Google Scholar] [CrossRef]
- Nakhjiri, M.T.; Marandi, G.B.; Kurdtabar, M. Poly (AA-co-VPA) hydrogel cross-linked with N-maleyl chitosan as dye adsorbent: Isotherms, kinetics and thermodynamic investigation. Int. J. Biol. Macromol. 2018, 117, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly (acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Ohemeng-Boahen, G.; Sewu, D.D.; Tran, H.N.; Woo, S.H. Enhanced adsorption of congo red from aqueous solution using chitosan/hematite nanocomposite hydrogel capsule fabricated via anionic surfactant gelation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126911. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin, D.F.A. Enhanced sorption of methylene blue from aqueous solutions by semi-IPN composite cryogels with anionically modified potato starch entrapped in PAAm matrix. Chem. Eng. J. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Belhachemi, M.; Addoun, F. Comparative adsorption isotherms and modeling of methylene blue onto activated carbons. Appl. Water Sci. 2011, 1, 111–117. [Google Scholar] [CrossRef]
- Nanta, P.; Kasemwong, K.; Skolpap, W. Isotherm and kinetic modeling on superparamagnetic nanoparticles adsorption of polysaccharide. J. Environ. Chem. Eng. 2018, 6, 794–802. [Google Scholar] [CrossRef]
- Tran, H.N.; Lima, E.C.; Juang, R.-S.; Bollinger, J.-C.; Chao, H.-P. Thermodynamic parameters of liquid–phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J. Environ. Chem. Eng. 2021, 9, 106674. [Google Scholar] [CrossRef]
- Khan, T.A.; Rahman, R.; Khan, E.A. Decolorization of bismarck brown R and crystal violet in liquid phase using modified pea peels: Non-linear isotherm and kinetics modeling. Model. Earth Syst. Environ. 2016, 2, 1–11. [Google Scholar] [CrossRef]
- Chen, T.; Da, T.; Ma, Y. Reasonable calculation of the thermodynamic parameters from adsorption equilibrium constant. J. Mol. Liq. 2021, 322, 114980. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Çelikçapa, S.; Doğan, M. Sorption of acid red 57 from aqueous solution onto sepiolite. J. Hazard. Mater. 2004, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Sadik, W.A.A.; El-Demerdash, A.G.M.; Tamer, T.M.; Khalifa, R.E.; Mohyeldin, M.S.; Abdelwahed, N.A. Fabrication of semi-interpenetrated PVA/PAMPS hydrogel as a reusable adsorbent for cationic methylene blue dye: Isotherms, kinetics and thermodynamics studies. Polym. Bull. 2021, 78, 6649–6673. [Google Scholar] [CrossRef]
- Du, J.; Yang, X.; Xiong, H.; Dong, Z.; Wang, Z.; Chen, Z.; Zhao, L. Ultrahigh adsorption capacity of acrylic acid-grafted xanthan gum hydrogels for rhodamine B from aqueous solution. J. Chem. Eng. Data 2021, 66, 1264–1272. [Google Scholar] [CrossRef]
- Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M. Adsorption of Rhodamine B dye from aqueous solution onto acid treated banana peel: Response surface methodology, kinetics and isotherm studies. PLoS ONE 2019, 14, e0216878. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, X.; Zhong, H.; Wang, H.; Jiang, L.; Zeng, G.; Wang, H.; Liu, Z.; Li, Y. Highly efficient adsorption of Congo red in single and binary water with cationic dyes by reduced graphene oxide decorated NH2-MIL-68 (Al). J. Mol. Liq. 2017, 247, 215–229. [Google Scholar] [CrossRef]
- Soltani, R.; Marjani, A.; Shirazian, S. Facile one-pot synthesis of thiol-functionalized mesoporous silica submicrospheres for Tl (I) adsorption: Isotherm, kinetic and thermodynamic studies. J. Hazard. Mater. 2019, 371, 146–155. [Google Scholar] [CrossRef]



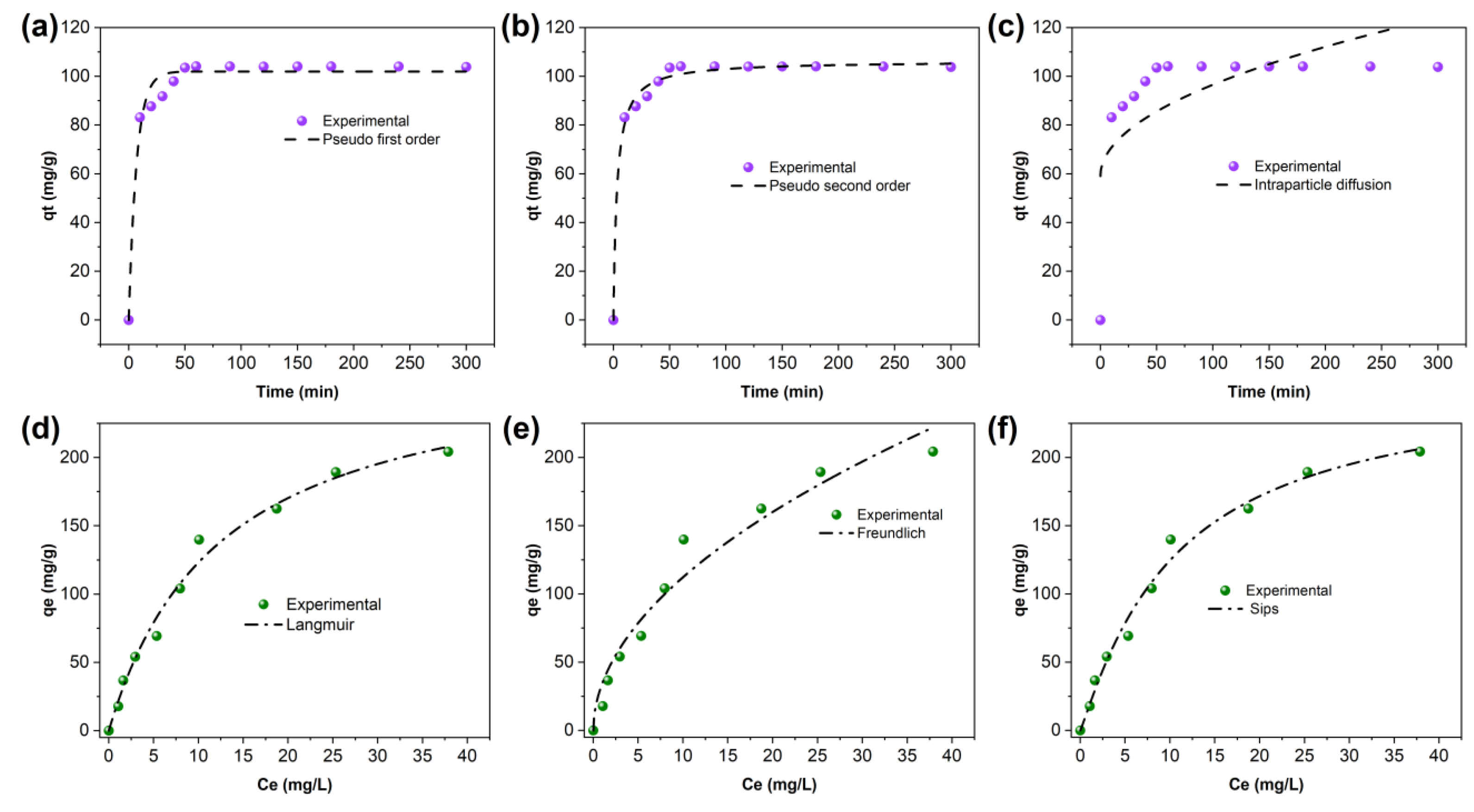

| Pseudo-first-order | qe(mg g−1) | K1(min−1) | R2 |
| 101.98 | 0.14247 | 0.9747 | |
| Pseudo-second-order | qe(mg g−1) | K2(g mg−1 min−1) | R2 |
| 106.59707 | 0.00294 | 0.9922 | |
| Intra-particle diffusion | Kd | C | R2 |
| 3.75 | 59.05 | 0.39973 |
| Langmuir | Freundlich | Sips |
|---|---|---|
| Parameters | Parameters | Parameters |
| qmax (mg/g): 276.26 | KF (mg/g): 34.54 | qs (mg/g): 257.95 |
| KL (L/mg): 0.08 | n: 0.511 | Ks (L/mg): 0.076 |
| R2: 0.987 | R2: 0.959 | ns: 1.089 |
| R2: 0.986 |
| ΔH° (KJ mol−1) | ΔS° (KJ mol−1 K−1) | ΔG° (KJ mol−1) | ||||
|---|---|---|---|---|---|---|
| 298.15K | 308.15K | 318.15 K | 328.15 K | 338.15 K | ||
| −16.13 | 71.00 | −5.02 | −5.83 | −6.47 | −6.95 | −8.02 |
| Sample Name | Chitosan (10%):MMA (Weight by Weight) |
|---|---|
| C1PM4 | 1:4 |
| C1PM7 | 1:7 |
| C1PM9 | 1:9 |
| C1PM10 | 1:10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, G.; García-Peñas, A.; Verma, Y.; Kumar, A.; Dhiman, P.; Stadler, F.J. Tailoring Homogeneous Hydrogel Nanospheres by Facile Ultra-Sonication Assisted Cross-Linked Copolymerization for Rhodamine B Dye Adsorption. Gels 2023, 9, 770. https://doi.org/10.3390/gels9100770
Sharma G, García-Peñas A, Verma Y, Kumar A, Dhiman P, Stadler FJ. Tailoring Homogeneous Hydrogel Nanospheres by Facile Ultra-Sonication Assisted Cross-Linked Copolymerization for Rhodamine B Dye Adsorption. Gels. 2023; 9(10):770. https://doi.org/10.3390/gels9100770
Chicago/Turabian StyleSharma, Gaurav, Alberto García-Peñas, Yaksha Verma, Amit Kumar, Pooja Dhiman, and Florian J. Stadler. 2023. "Tailoring Homogeneous Hydrogel Nanospheres by Facile Ultra-Sonication Assisted Cross-Linked Copolymerization for Rhodamine B Dye Adsorption" Gels 9, no. 10: 770. https://doi.org/10.3390/gels9100770
APA StyleSharma, G., García-Peñas, A., Verma, Y., Kumar, A., Dhiman, P., & Stadler, F. J. (2023). Tailoring Homogeneous Hydrogel Nanospheres by Facile Ultra-Sonication Assisted Cross-Linked Copolymerization for Rhodamine B Dye Adsorption. Gels, 9(10), 770. https://doi.org/10.3390/gels9100770










