Polymeric Materials for Rare Earth Elements Recovery
Abstract
:1. Introduction
2. Polymeric Materials for REEs Recovery
2.1. Polymeric Resins
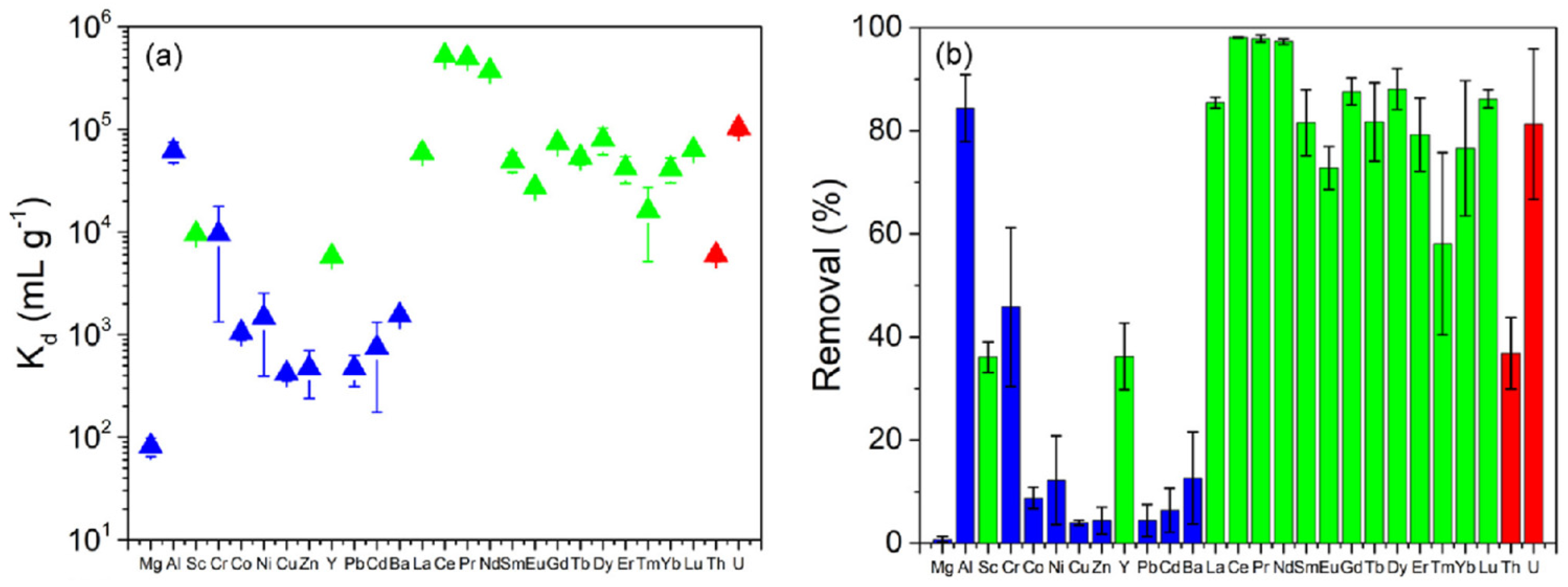
2.2. Polymer Membranes
2.3. Cross-Linked Polymer Network
2.4. Nanocomposite Polymers
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| REEs | Rare earth elements |
| DEHDGA | N,N-di(2-ethylhexyl)-diglycolamide |
| RE(III) | Rhenium(III) |
| Al(III) | Aluminum(III) |
| Fe(III) | Iron(III) |
| DGA | Diglycolamides |
| P(FPMAm-co-MAA) | Poly(ferrocenylpropyl methacrylamide-co-methacrylic acid) |
| Y | Yttrium |
| Nd | Neodymium |
| Eu | Europium |
| Gd | Gadolinium |
| Dy | Dysprosium |
| Ce | Cerium |
| CNT | Carbon nanotube |
| Fc | Ferrocene |
| NF | Nanofiltration |
| UF | Ultrafiltration |
| PDA | Polydopamine |
| PVDF | Polyvinylidene difluoride |
| PTFE | Polytetrafluorethylene |
| CTA | Cellulose triacetate |
| PEI | Polyethylenimine |
| pAAm | Polyacrylamide |
| PIM | Polymer inclusion membranes |
| MBAA | N,N-methylene-bis-acrylamide |
| PPN | Phosphate polymer nanogel |
| Kd | Distribution coefficients |
| NMs | Nanomaterials |
| GA | Graphite |
| GO-APTS | Graphene oxide-3-aminopropyltriethoxysilane |
| PVA | Polyvinyl alcohol |
| BPOP | Phosphate-functionalized porous organic polymers |
| AI | Artificial intelligence |
References
- Song, X.; Chang, M.-H.; Pecht, M. Rare-earth elements in lighting and optical applications and their recycling. JOM 2013, 65, 1276–1282. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; Makarava, I.; Kraslawski, A.; Repo, E. Global environmental cost of using rare earth elements in green energy technologies. Sci. Total Environ. 2022, 832, 155022. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, V.; Joshi, R.S. Investigation of rare earth particulate on tribological and mechanical properties of Al-6061 alloy composites for aerospace application. J. Mater. Res. Technol. 2019, 8, 3504–3516. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Chen, J.; Kallem, P.; Banat, F.; Qiu, H. Recent advances in selective separation technologies of rare earth elements: A review. J. Environ. Chem. Eng. 2022, 10, 107104. [Google Scholar] [CrossRef]
- Talan, D.; Huang, Q. A review of environmental aspect of rare earth element extraction processes and solution purification techniques. Miner. Eng. 2022, 179, 107430. [Google Scholar] [CrossRef]
- Egler, S.G.; Niemeyer, J.C.; Correia, F.V.; Saggioro, E.M. Effects of rare earth elements (REE) on terrestrial organisms: Current status and future directions. Ecotoxicology 2022, 31, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Pavón, S.; Haneklaus, N.; Meerbach, K.; Bertau, M. Iron (III) removal and rare earth element recovery from a synthetic wet phosphoric acid solution using solvent extraction. Miner. Eng. 2022, 182, 107569. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A comprehensive review of rare earth elements recovery from coal-related materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Romero, J. Solvent extraction of rare-earth elements with ionic liquids: Toward a selective and sustainable extraction of these valuable elements. Curr. Opin. Green Sustain. Chem. 2021, 27, 100428. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare earth elements recovery using selective membranes via extraction and rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Hermassi, M.; Granados, M.; Valderrama, C.; Skoglund, N.; Ayora, C.; Cortina, J. Impact of functional group types in ion exchange resins on rare earth element recovery from treated acid mine waters. J. Clean. Prod. 2022, 379, 134742. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q. Rare earth elements recovery using staged precipitation from a leachate generated from coarse coal refuse. Int. J. Coal Geol. 2018, 195, 189–199. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhao, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhang, L. Recovery of rare earth elements from phosphate rock by hydrometallurgical processes—A critical review. Chem. Eng. J. 2018, 335, 774–800. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Xie, X.; Tan, X.; Yu, Y.; Li, Y.; Wang, P.; Liang, Y.; Yan, Y. Effectively auto-regulated adsorption and recovery of rare earth elements via an engineered E. coli. J. Hazard. Mater. 2022, 424, 127642. [Google Scholar] [CrossRef]
- Jha, M.K.; Choubey, P.K.; Dinkar, O.S.; Panda, R.; Jyothi, R.K.; Yoo, K.; Park, I. Recovery of rare earth metals (REMs) from nickel metal hydride batteries of electric vehicles. Minerals 2021, 12, 34. [Google Scholar] [CrossRef]
- Mwewa, B.; Tadie, M.; Ndlovu, S.; Simate, G.S.; Matinde, E. Recovery of rare earth elements from acid mine drainage: A review of the extraction methods. J. Environ. Chem. Eng. 2022, 10, 107704. [Google Scholar] [CrossRef]
- Shin, W.-R.; Ahn, G.; Lee, J.-P.; Oh, I.-H.; Ahn, J.-Y.; Kim, Y.-H.; Chae, S. Recent Advances in Engineering Aptamer-based Sensing and Recovery of Heavy Metals and Rare Earth Elements for Environmental Sustainability. Chem. Eng. J. 2023, 472, 144742. [Google Scholar] [CrossRef]
- Hérès, X.; Blet, V.; Di Natale, P.; Ouaattou, A.; Mazouz, H.; Dhiba, D.; Cuer, F. Selective extraction of rare earth elements from phosphoric acid by ion exchange resins. Metals 2018, 8, 682. [Google Scholar] [CrossRef]
- Sim, G.; Hong, S.; Moon, S.; Noh, S.; Cho, J.; Triwigati, P.T.; Park, A.-H.A.; Park, Y. Simultaneous CO2 utilization and rare earth elements recovery by novel aqueous carbon mineralization of blast furnace slag. J. Environ. Chem. Eng. 2022, 10, 107327. [Google Scholar] [CrossRef]
- Xie, G.; Guan, Q.; Zhou, F.; Yu, W.; Yin, Z.; Tang, H.; Zhang, Z.; Chi, R.A. A Critical Review of the Enhanced Recovery of Rare Earth Elements from Phosphogypsum. Molecules 2023, 28, 6284. [Google Scholar] [CrossRef]
- Mir, N.; Castano, C.E.; Rojas, J.V.; Norouzi, N.; Esmaeili, A.R.; Mohammadi, R. Self-separation of the adsorbent after recovery of rare-earth metals: Designing a novel non-wettable polymer. Sep. Purif. Technol. 2021, 259, 118152. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Honaker, R.Q. Association characteristic study and preliminary recovery investigation of rare earth elements from Fire Clay seam coal middlings. Fuel 2018, 215, 551–560. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current nature-based biological practices for rare earth elements extraction and recovery: Bioleaching and biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Nawab, A.; Honaker, R. Pilot Scale Testing of Lignite Adsorption Capability and the Benefits for the Recovery of Rare Earth Elements from Dilute Leach Solutions. Minerals 2023, 13, 921. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, S.; Xie, N.; Yang, L.; Wang, Q.; Wen, Y.; Chen, H.; Tang, Y. Green Approach for Rare Earth Element (REE) Recovery from Coal Fly Ash. Environ. Sci. Technol. 2023, 57, 5414–5423. [Google Scholar] [CrossRef]
- Salfate, G.; Sánchez, J. Rare Earth Elements Uptake by Synthetic Polymeric and Cellulose-Based Materials: A Review. Polymers 2022, 14, 4786. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Xu, J.; Tian, C.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Phosphate polymer nanogel for selective and efficient rare earth element recovery. Environ. Sci. Technol. 2021, 55, 12549–12560. [Google Scholar] [CrossRef] [PubMed]
- Nkinahamira, F.; Alsbaiee, A.; Zeng, Q.; Li, Y.; Zhang, Y.; Feng, M.; Yu, C.-P.; Sun, Q. Selective and fast recovery of rare earth elements from industrial wastewater by porous β-cyclodextrin and magnetic β-cyclodextrin polymers. Water Res. 2020, 181, 115857. [Google Scholar] [CrossRef] [PubMed]
- Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare earth element recovery from acidic extracts of Florida Phosphate mining materials using Chelating polymer 1-Octadecene, polymer with 2, 5-Furandione, Sodium salt. Minerals 2019, 9, 477. [Google Scholar] [CrossRef]
- Iftekhar, S.; Heidari, G.; Amanat, N.; Zare, E.N.; Asif, M.B.; Hassanpour, M.; Lehto, V.P.; Sillanpaa, M. Porous materials for the recovery of rare earth elements, platinum group metals, and other valuable metals: A review. Environ. Chem. Lett. 2022, 20, 3697–3746. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Mostapa, N.R.N.; Sarkar, M.S.; Biswas, T.K.; Rahman, M.L.; Arshad, S.E.; Sarjadi, M.S.; Kulkarni, A.D. Synthesis of ion imprinted polymers for selective recognition and separation of rare earth metals. J. Rare Earths 2017, 35, 177–186. [Google Scholar] [CrossRef]
- Di, T.; Tan, D.; Yu, Q.; Lin, J.; Zhu, T.; Li, T.; Li, L. Ultra-high performance of hyper-crosslinked phosphate-based polymer for uranium and rare earth element adsorption in aqueous solution. Langmuir 2019, 35, 13860–13871. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Uchida, E.; Kang, E.-T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Bousquet, A.; Awada, H.; Hiorns, R.C.; Dagron-Lartigau, C.; Billon, L. Conjugated-polymer grafting on inorganic and organic substrates: A new trend in organic electronic materials. Prog. Polym. Sci. 2014, 39, 1847–1877. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Rodrigues, D.G.; Monge, S.; Pellet-Rostaing, S.; Dacheux, N.; Bouyer, D.; Faur, C. A new carbamoylmethylphosphonic acid-based polymer for the selective sorption of rare earth elements. Chem. Eng. J. 2019, 371, 857–867. [Google Scholar] [CrossRef]
- Vapnik, H.; Elbert, J.; Su, X. Redox-copolymers for the recovery of rare earth elements by electrochemically regenerated ion-exchange. J. Mater. Chem. A 2021, 9, 20068–20077. [Google Scholar] [CrossRef]
- Pereao, O.; Laatikainen, K.; Bode-Aluko, C.; Fatoba, O.; Omoniyi, E.; Kochnev, Y.; Nechaev, A.; Apel, P.; Petrik, L. Synthesis and characterisation of diglycolic acid functionalised polyethylene terephthalate nanofibers for rare earth elements recovery. J. Environ. Chem. Eng. 2021, 9, 105902. [Google Scholar] [CrossRef]
- Oye Auke, R.; Arrachart, G.; Tavernier, R.; David, G.; Pellet-Rostaing, S. Terephthalaldehyde–phenolic resins as a solid-phase extraction system for the recovery of rare-earth elements. Polymers 2022, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Callura, J.C.; Shi, Q.; Dzombak, D.A.; Karamalidis, A.K. Selective recovery of rare earth elements with ligand-functionalized polymers in fixed-bed adsorption columns. Sep. Purif. Technol. 2021, 265, 118472. [Google Scholar] [CrossRef]
- Rychkov, V.; Kirillov, E.; Kirillov, S.; Bunkov, G.; Botalov, M.; Semenishchev, V.; Smyshlyaev, D.; Malyshev, A.; Taukin, A.; Akcil, A. Rare earth element preconcentration from various primary and secondary sources by polymeric ion exchange resins. Sep. Purif. Rev. 2022, 51, 468–483. [Google Scholar] [CrossRef]
- Pinto, D.; Martins, A. Electrochemical elution of a cation-exchange polymeric resin for yttrium and rare earth recovery using a statistical approach. Hydrometallurgy 2001, 60, 99–104. [Google Scholar] [CrossRef]
- Li, X.-Z.; Sun, Y.-P. Evaluation of ionic imprinted polymers by electrochemical recognition of rare earth ions. Hydrometallurgy 2007, 87, 63–71. [Google Scholar] [CrossRef]
- Cui, H.; Feng, X.; Shi, J.; Liu, W.; Yan, N.; Rao, G.; Wang, W. A facile process for enhanced rare earth elements separation from dilute solutions using N, N-di (2-ethylhexyl)-diglycolamide grafted polymer resin. Sep. Purif. Technol. 2020, 234, 116096. [Google Scholar] [CrossRef]
- Archer, W.R.; Iftekhar, N.; Fiorito, A.; Winn, S.A.; Schulz, M.D. Synthesis of Phosphonated Polymer Resins for the Extraction of Rare-Earth Elements. ACS Appl. Polym. Mater. 2022, 4, 2506–2512. [Google Scholar] [CrossRef]
- Das, N.; Das, D. Recovery of rare earth metals through biosorption: An overview. J. Rare Earths 2013, 31, 933–943. [Google Scholar] [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Hanfi, M.Y.; Sayyed, M.; Al-Otaibi, J.S.; Cheira, M.F. Cetylpyridinium bromide/polyvinyl chloride for substantially efficient capture of rare earth elements from chloride solution. Polymers 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, M. Recovery of rare earth elements from electronic waste by diffusion dialysis. Sep. Purif. Technol. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Pereao, O.; Bode-Aluko, C.; Fatoba, O.; Laatikainen, K.; Petrik, L. Rare earth elements removal techniques from water/wastewater: A review. Desalin. Water Treat 2018, 130, 71–86. [Google Scholar] [CrossRef]
- Valappil, R.S.K.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Jha, R.; Rao, M.D.; Meshram, A.; Verma, H.R.; Singh, K.K. Potential of polymer inclusion membrane process for selective recovery of metal values from waste printed circuit boards: A review. J. Clean. Prod. 2020, 265, 121621. [Google Scholar] [CrossRef]
- Marjani, A.; Taghvaie Nakhjiri, A.; Adimi, M.; Fathinejad Jirandehi, H.; Shirazian, S. Modification of polyethersulfone membrane using MWCNT-NH2 nanoparticles and its application in the separation of azeotropic solutions by means of pervaporation. PLoS ONE 2020, 15, e0236529. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Reig, M.; Gibert, O.; Torres, E.; Ayora, C.; Cortina, J.-L. Application of nanofiltration for acidic waters containing rare earth elements: Influence of transition elements, acidity and membrane stability. Desalination 2018, 430, 33–44. [Google Scholar] [CrossRef]
- López, J.; Reig, M.; Vecino, X.; Gibert, O.; Cortina, J. Comparison of acid-resistant ceramic and polymeric nanofiltration membranes for acid mine waters treatment. Chem. Eng. J. 2020, 382, 122786. [Google Scholar] [CrossRef]
- Lai, W.; Li, Z.; Bai, J.; Xiao, L.; Zheng, P.; Bai, L.; Yang, Y.; Liao, C.; Shan, L.; Luo, S. Acid-Resistant Nanofiltration Facilitated Nonsaponified Extraction of Rare-Earth Elements. Ind. Eng. Chem. Res. 2023, 62, 11930–11938. [Google Scholar] [CrossRef]
- Chen, G.E.; Sun, D.; Xu, Z.L. Rare earth ion from aqueous solution removed by polymer enhanced ultrafiltration process. Adv. Mater. Res. 2011, 233, 959–964. [Google Scholar] [CrossRef]
- Kose-Mutlu, B.; Hsu-Kim, H.; Wiesner, M.R. Separation of rare earth elements from mixed-metal feedstocks by micelle enhanced ultrafiltration with sodium dodecyl sulfate. Environ. Technol. 2022, 43, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Hammami, M.; Ennigrou, D.J.; Naifer, K.H.; Ferid, M. Retention of samarium ions from aqueous solutions by poly (acrylic acid)-enhanced ultrafiltration. Desalination Water Treat. 2015, 56, 2715–2722. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, J.; Shah, K.; Haque, N.; Bhuiyan, M.A. Rejection of rare earth elements from a simulated acid mine drainage using forward osmosis: The role of membrane orientation, solution pH, and temperature variation. Process Saf. Environ. Prot. 2019, 126, 53–59. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, R.; Ma, F.; Xing, W.; Pan, J. Three-dimensional macroporous wood-based selective separation membranes decorated with well-designed Nd (III)-imprinted domains: A high-efficiency recovery system for rare earth element. J. Colloid Interface Sci. 2021, 587, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Chen, L.; Wang, Y.; Yan, Y. Selective separation of low concentration rare earths via coordination-induced ion imprinted electrospun membranes. J. Membr. Sci. 2022, 658, 120759. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Yan, Y.; Chen, J.; Dai, J.; Dai, X. Separation of adjacent heavy rare earth Lutetium (III) and Ytterbium (III) by task-specific ionic liquid Cyphos IL 104 embedded polymer inclusion membrane. J. Membr. Sci. 2020, 610, 118263. [Google Scholar] [CrossRef]
- Makowka, A.; Pospiech, B. Synthesis of polymer inclusion membranes based on cellulose triacetate for recovery of lanthanum (III) from aqueous solutions. Autex Res. J. 2019, 19, 288–292. [Google Scholar] [CrossRef]
- Nielsen, L.E. Cross-linking–effect on physical properties of polymers. J. Macromol. Sci. Part C 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J., Jr. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Nouri, N.; Ziaei-Rad, S. A molecular dynamics investigation on mechanical properties of cross-linked polymer networks. Macromolecules 2011, 44, 5481–5489. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Yang, H. Ultrafast and stable adsorption–desorption performance for recovery of valuable rare-earth ions using high-density polyacrylic acid brush-grafted polypropylene fibers optimized by RSM models. Ind. Eng. Chem. Res. 2020, 59, 7746–7754. [Google Scholar] [CrossRef]
- Ye, Q.; Jin, X.; Zhu, B.; Gao, H.; Wei, N. Lanmodulin-Functionalized Magnetic Nanoparticles as a Highly Selective Biosorbent for Recovery of Rare Earth Elements. Environ. Sci. Technol. 2023, 57, 4276–4285. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Liang, J.; Niu, C.; Wang, H.; Luo, Y.; Xing, W.; Ye, S.; Liang, C.; Guo, H.; Guo, J. Amidoxime-based materials for uranium recovery and removal. J. Mater. Chem. A 2020, 8, 7588–7625. [Google Scholar] [CrossRef]
- He, C.; Salih, K.A.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.-H.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient recovery of rare earth elements (Pr (III) and Tm (III)) from mining residues using a new phosphorylated hydrogel (Algal Biomass/PEI). Metals 2021, 11, 294. [Google Scholar] [CrossRef]
- Wang, Q.; Wilfong, W.C.; Kail, B.W.; Yu, Y.; Gray, M.L. Novel polyethylenimine–acrylamide/SiO2 hybrid hydrogel sorbent for rare-earth-element recycling from aqueous sources. ACS Sustain. Chem. Eng. 2017, 5, 10947–10958. [Google Scholar] [CrossRef]
- Ren, S.; Yang, X.; Tang, L.; Du, X.; Li, M.; Yin, X. A chitosan-based Y3+-imprinted hydrogel with reversible thermo-responsibility for the recovery of rare earth metal. Appl. Surf. Sci. 2023, 611, 155602. [Google Scholar] [CrossRef]
- Romal, J.R.A.; Ong, S.K. Marine polysaccharide-based hydrogels for critical materials selective removal and recovery: A review. Coord. Chem. Rev. 2023, 482, 215054. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Chirani, N.; Yahia, L.; Gritsch, L.; Motta, F.L.; Chirani, S.; Farè, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar]
- Ninciuleanu, C.M.; Ianchiș, R.; Alexandrescu, E.; Mihăescu, C.I.; Burlacu, S.; Trică, B.; Nistor, C.L.; Preda, S.; Scomoroscenco, C.; Gîfu, C. Adjusting some properties of poly (methacrylic acid)(nano) composite hydrogels by means of silicon-containing inorganic fillers. Int. J. Mol. Sci. 2022, 23, 10320. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Dakroury, G.A.R.S.; Borai, E.H. Efficient sorption and group separation of rare earth elements using modified CuO nanocomposite. Surf. Interfaces 2022, 33, 102233. [Google Scholar] [CrossRef]
- Qi, X.-H.; Du, K.-Z.; Feng, M.-L.; Gao, Y.-J.; Huang, X.-Y.; Kanatzidis, M.G. Layered A 2Sn3S7· 1.25 H2O (A= Organic Cation) as efficient ion-exchanger for rare earth element recovery. J. Am. Chem. Soc. 2017, 139, 4314–4317. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Lin, Z. Fabrication of titanium phosphate@ graphene oxide nanocomposite and its super performance on Eu 3+ recycling. J. Mater. Chem. A 2014, 2, 14979–14985. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Yu, K.; Ravi, S.; Ahn, W.-S. Selective adsorption of rare earth elements over functionalized Cr-MIL-101. ACS Appl. Mater. Interfaces 2018, 10, 23918–23927. [Google Scholar] [CrossRef]
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 2014, 2, 19415–19426. [Google Scholar] [CrossRef]
- Hu, Y.; Drouin, E.; Larivière, D.; Kleitz, F.; Fontaine, F.-G. Highly efficient and selective recovery of rare earth elements using mesoporous silica functionalized by preorganized chelating ligands. ACS Appl. Mater. Interfaces 2017, 9, 38584–38593. [Google Scholar] [CrossRef]
- Noack, C.W.; Perkins, K.M.; Callura, J.C.; Washburn, N.R.; Dzombak, D.A.; Karamalidis, A.K. Effects of ligand chemistry and geometry on rare earth element partitioning from saline solutions to functionalized adsorbents. ACS Sustain. Chem. Eng. 2016, 4, 6115–6124. [Google Scholar] [CrossRef]
- Callura, J.C.; Perkins, K.M.; Baltrus, J.P.; Washburn, N.R.; Dzombak, D.A.; Karamalidis, A.K. Adsorption kinetics, thermodynamics, and isotherm studies for functionalized lanthanide-chelating resins. J. Colloid Interface Sci. 2019, 557, 465–477. [Google Scholar] [CrossRef]
- Rahman, M.M.; Awual, M.R.; Asiri, A.M. Preparation and evaluation of composite hybrid nanomaterials for rare-earth elements separation and recovery. Sep. Purif. Technol. 2020, 253, 117515. [Google Scholar] [CrossRef]
- Cardoso, C.E.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of rare earth elements by carbon-based nanomaterials—A review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, Y.; Chen, W.; Weng, X.; Owens, G.; Chen, Z. Recovery and removal of rare earth elements from mine wastewater using synthesized bio-nanoparticles derived from Bacillus cereus. Chem. Eng. J. 2023, 459, 141585. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Z.; Chen, Z. Microbial synthesized iron nanoparticles after recovering rare earth elements used for removing arsenic in mine groundwater. Sep. Purif. Technol. 2023, 327, 124938. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Casas, A.; Sillanpää, M. Synthesis of novel GA-g-PAM/SiO2 nanocomposite for the recovery of rare earth elements (REE) ions from aqueous solution. J. Clean. Prod. 2018, 170, 251–259. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Wei, Z.; Yang, W.; Yu, Y. Highly efficient recovery of heavy rare earth elements by using an amino-functionalized magnetic graphene oxide with acid and base resistance. J. Hazard. Mater. 2022, 424, 127370. [Google Scholar] [CrossRef] [PubMed]
- Comandella, D.; Bonani, W.; Ciscar, J.B.; Ponti, J.; Cologna, M.; Popa, K.; Gilliland, D. Recovery of rare earth elements by nanometric CeO2 embedded into electrospun PVA nanofibres. RSC Adv. 2021, 11, 19351–19362. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Kim, S.-Y.; Bae, Y.-S. Novel benzylphosphate-based covalent porous organic polymers for the effective capture of rare earth elements from aqueous solutions. J. Hazard. Mater. 2022, 424, 127356. [Google Scholar] [CrossRef]
- Filho, W.L.; Kotter, R.; Özuyar, P.G.; Abubakar, I.R.; Eustachio, J.H.P.P.; Matandirotya, N.R. Understanding Rare Earth Elements as Critical Raw Materials. Sustainability 2023, 15, 1919. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mastalerz, M.; Drobniak, A.; Karacan, C.Ö. Machine learning and data augmentation approach for identification of rare earth element potential in Indiana Coals, USA. Int. J. Coal Geol. 2022, 259, 104054. [Google Scholar] [CrossRef]
- Iqbal, J.; Tyagi, A.; Jain, M. Artificial neural network based modeling of liquid membranes for separation of dysprosium. J. Rare Earths 2021, 41, 440–445. [Google Scholar] [CrossRef]
- de Vargas Brião, G.; Franco, D.S.P.; da Silva, F.V.; da Silva, M.G.C.; Vieira, M.G.A. Critical rare earth metal adsorption onto expanded vermiculite: Accurate modeling through response surface methodology and machine learning techniques. Sustain. Chem. Pharm. 2023, 31, 100938. [Google Scholar] [CrossRef]

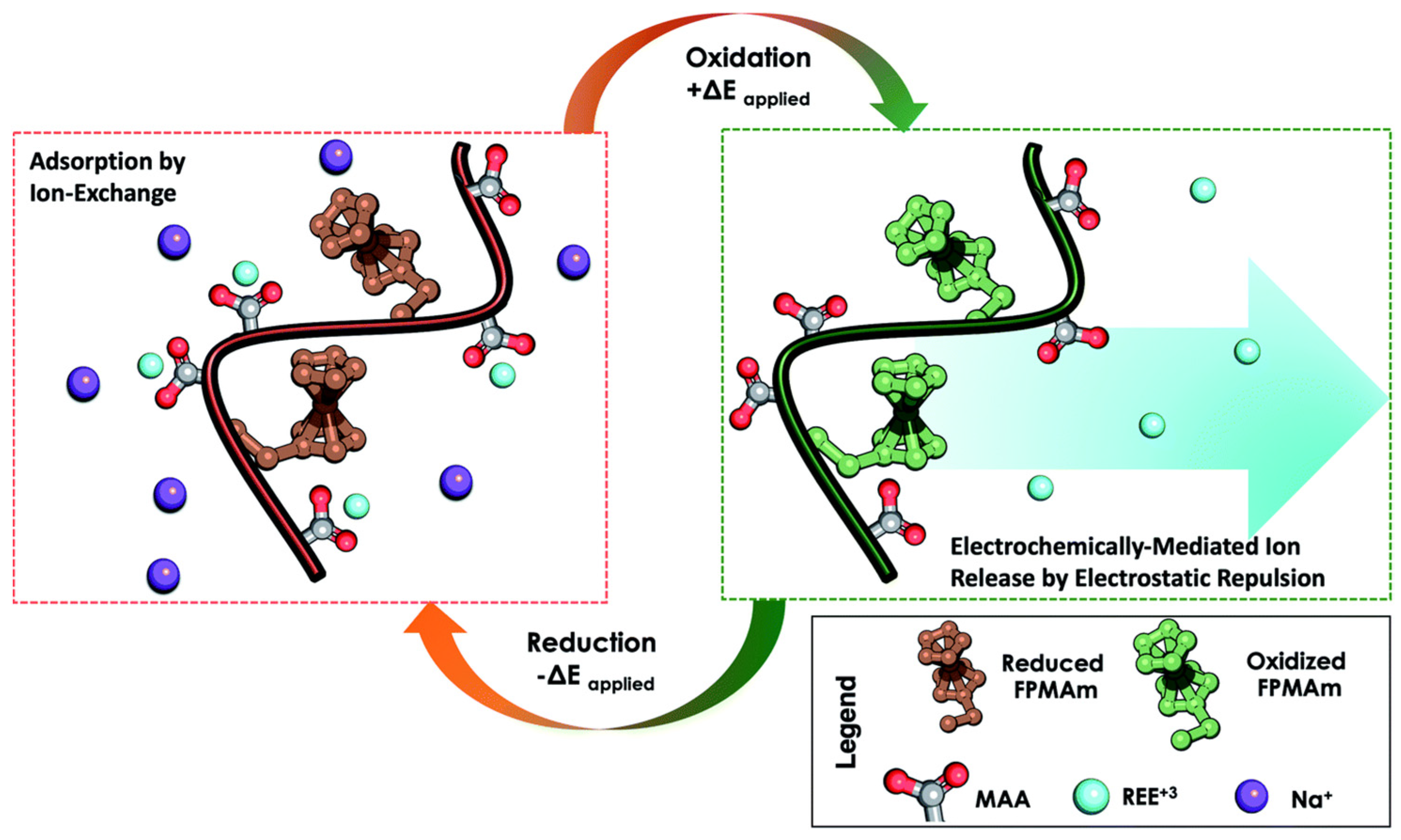
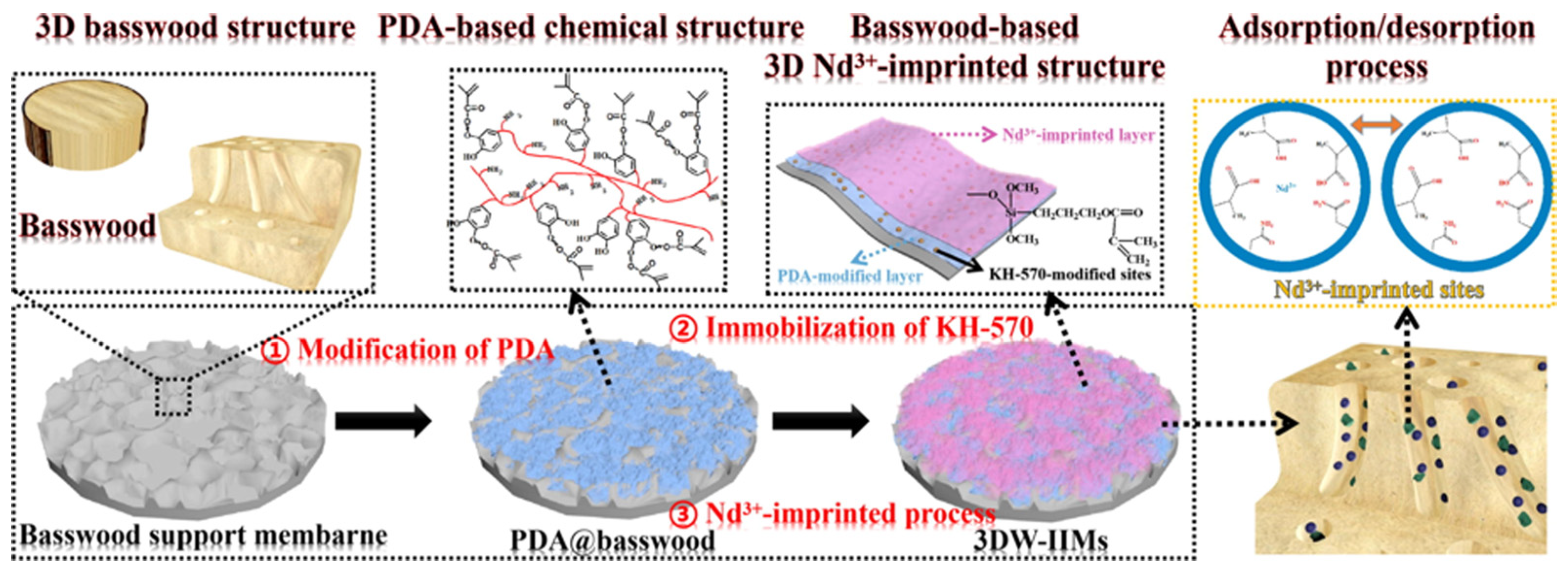
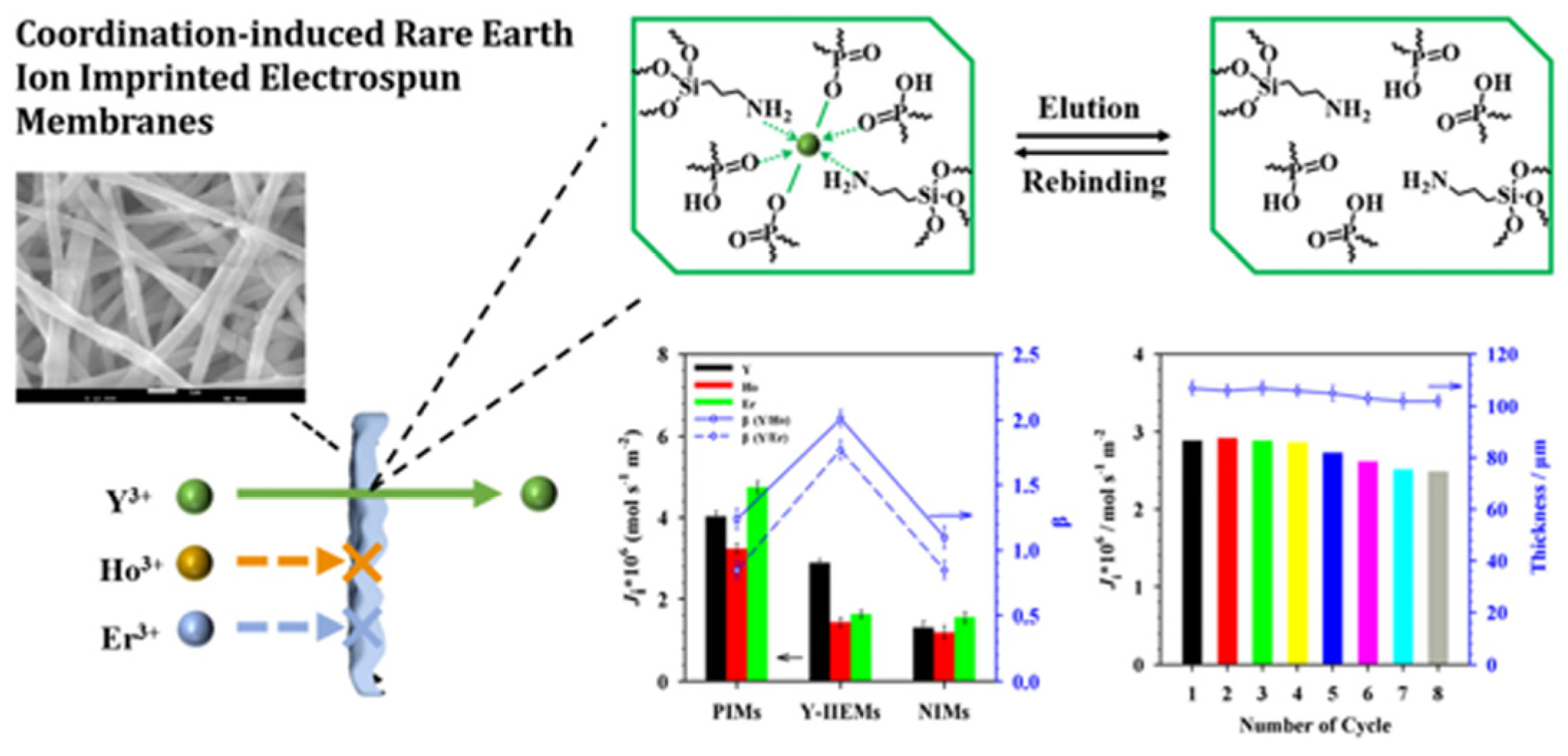
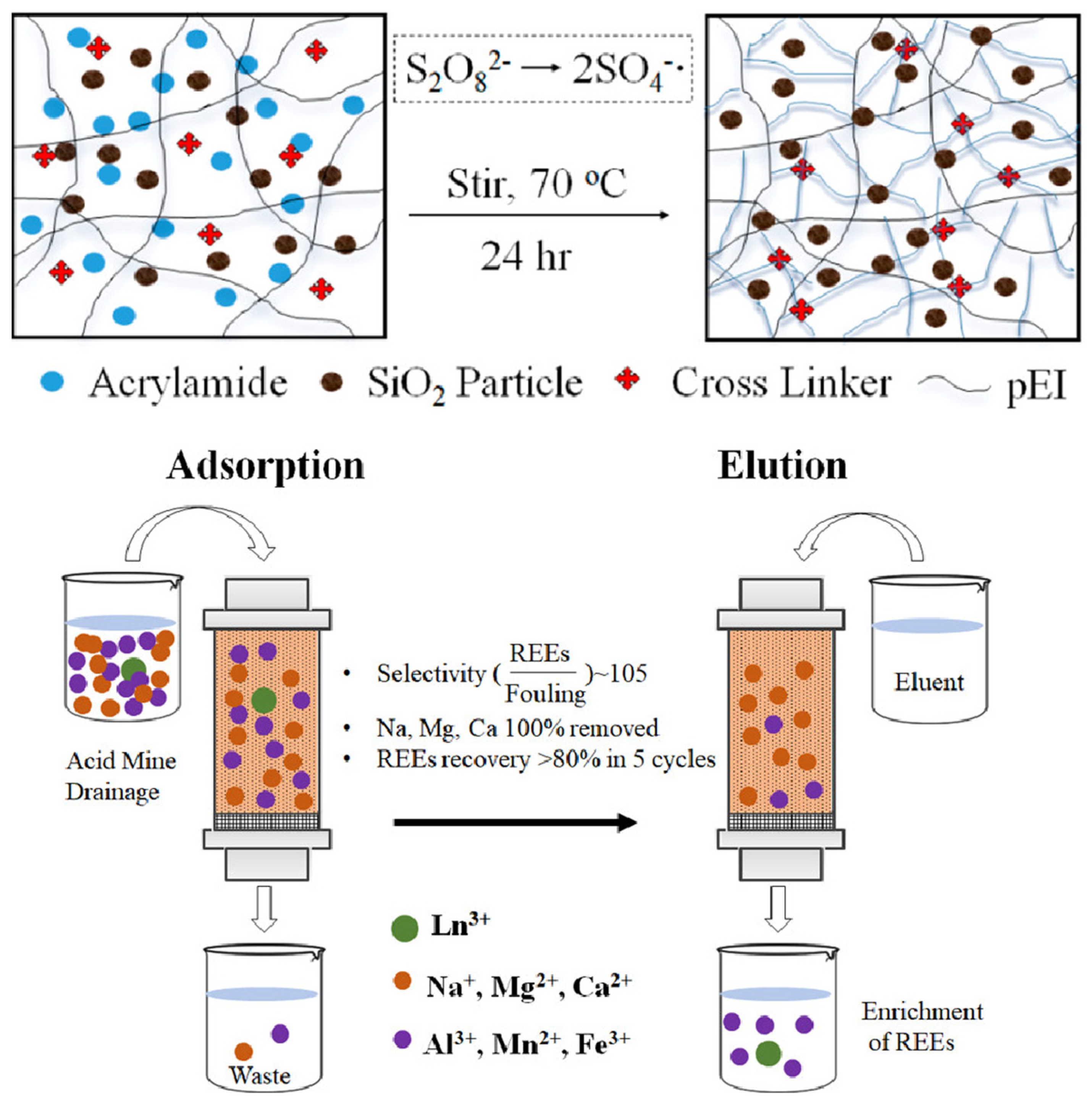



| Polymeric Membranes | Composition | Advantages | Applilcation |
|---|---|---|---|
| Polymeric Resin Membranes | Cross-linked polymeric resins with functional groups | High REE selectivity, regenerate ability | Solution, hydrometallurgical processes |
| Polymer Inclusion Membranes (PIMs) | 3D-polymer network | Highly versatile, high stability | Continuous membrane-based extraction and separation |
| Phosphate-Functionalized Polymeric Membranes | membranes incorporate phosphate groups | Strong affinity, Excellent selectivity | Complex solutions, such as acid mine drainage |
| Amino-Functionalized Polymeric Membranes | membranes contain amino groups | High selectivity | Recovery of specific REEs, wastewater treatment |
| Magnetic Polymer Membranes | Magnetic nanoparticles embedded within polymer matrices | Easy separation | Selective extraction of specific magnetic REEs |
| Composite Polymeric Membranes | Combinations of various polymers, fillers, and additives | Customization | Versatile for use in various extraction and separation processes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Gao, Y. Polymeric Materials for Rare Earth Elements Recovery. Gels 2023, 9, 775. https://doi.org/10.3390/gels9100775
Zhang H, Gao Y. Polymeric Materials for Rare Earth Elements Recovery. Gels. 2023; 9(10):775. https://doi.org/10.3390/gels9100775
Chicago/Turabian StyleZhang, Hongtao, and Yongfeng Gao. 2023. "Polymeric Materials for Rare Earth Elements Recovery" Gels 9, no. 10: 775. https://doi.org/10.3390/gels9100775
APA StyleZhang, H., & Gao, Y. (2023). Polymeric Materials for Rare Earth Elements Recovery. Gels, 9(10), 775. https://doi.org/10.3390/gels9100775







