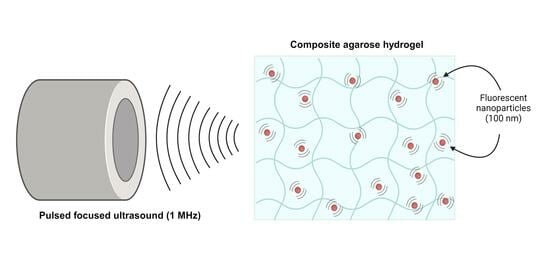
Nanoparticle Dynamics in Composite Hydrogels Exposed to Low-Frequency Focused Ultrasound
Abstract
1. Introduction
2. Results
2.1. Hydrogel Characterization
2.1.1. Increasing Acoustic Attenuation of Agarose Hydrogels with Additives
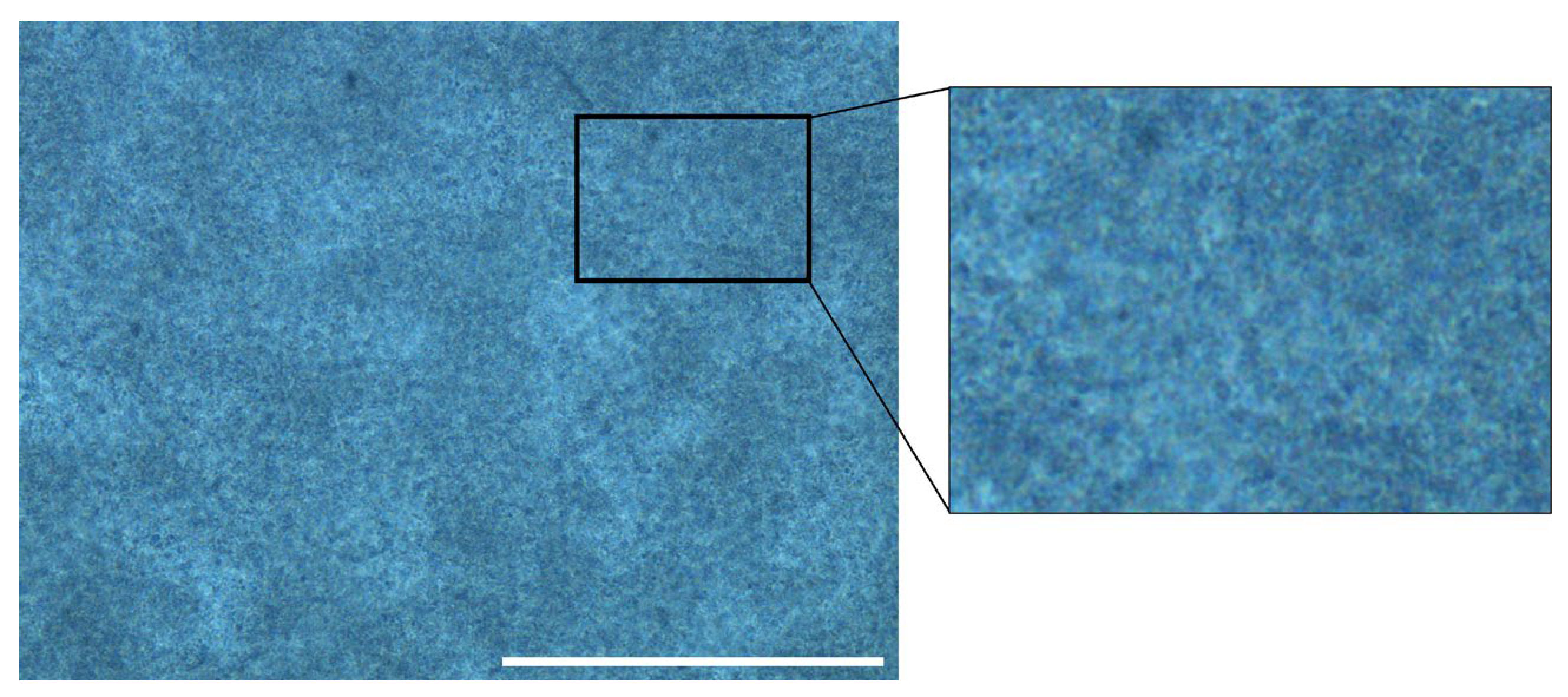
2.1.2. Distribution of Evaporated Milk and Hydrogel Water Content
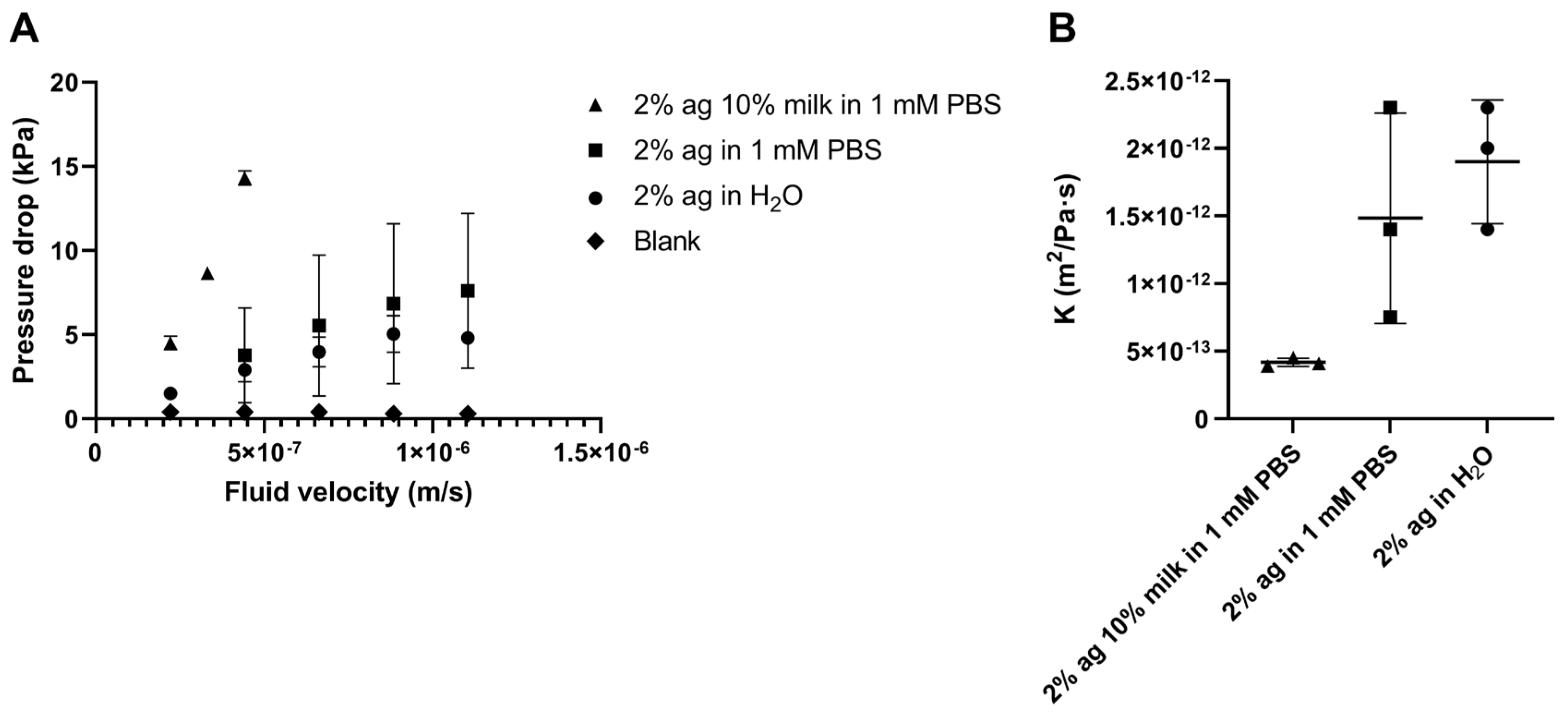
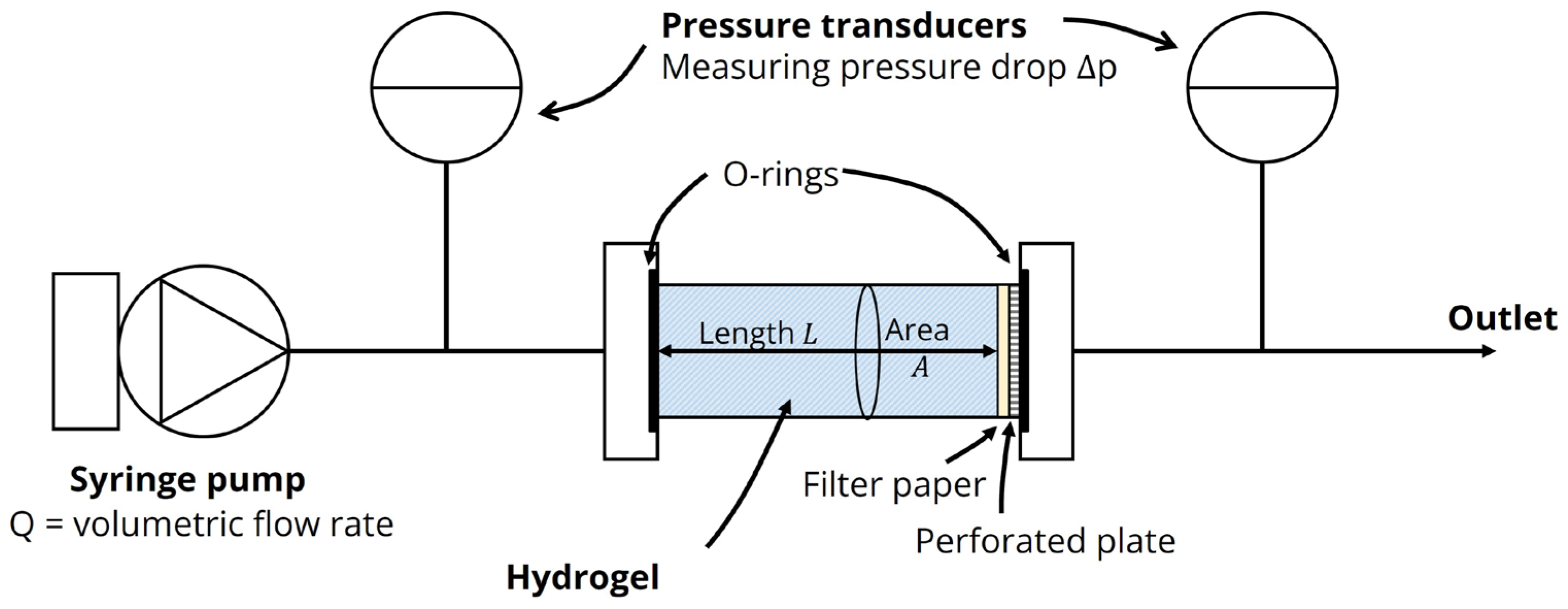
2.1.3. Hydraulic Conductivity of Agarose–Milk Hydrogels
2.2. Effect of Pulsed FUS on Nanoparticle Movement
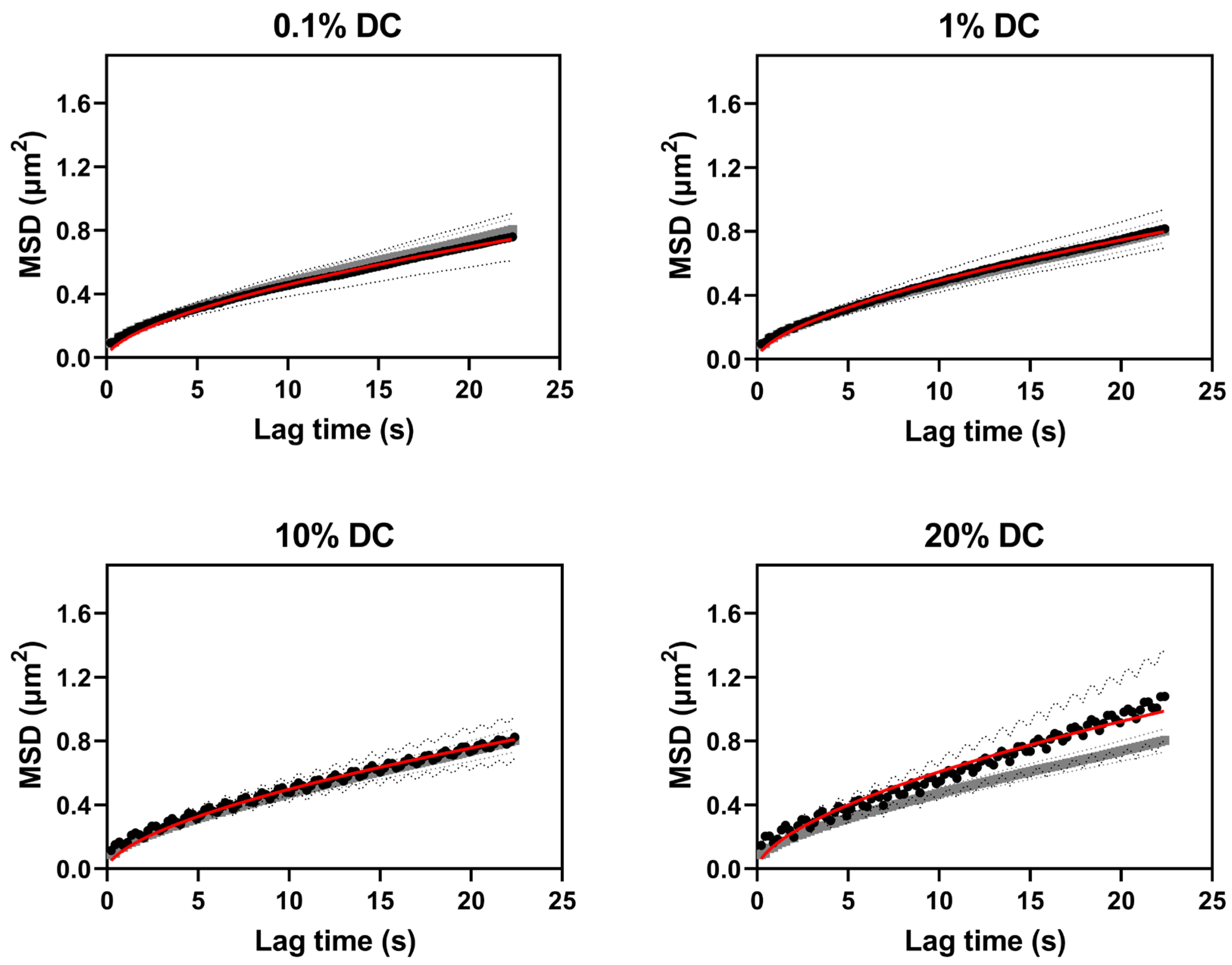
2.2.1. Nanoparticle Diffusion in Buffer and in Agarose–Milk Hydrogels
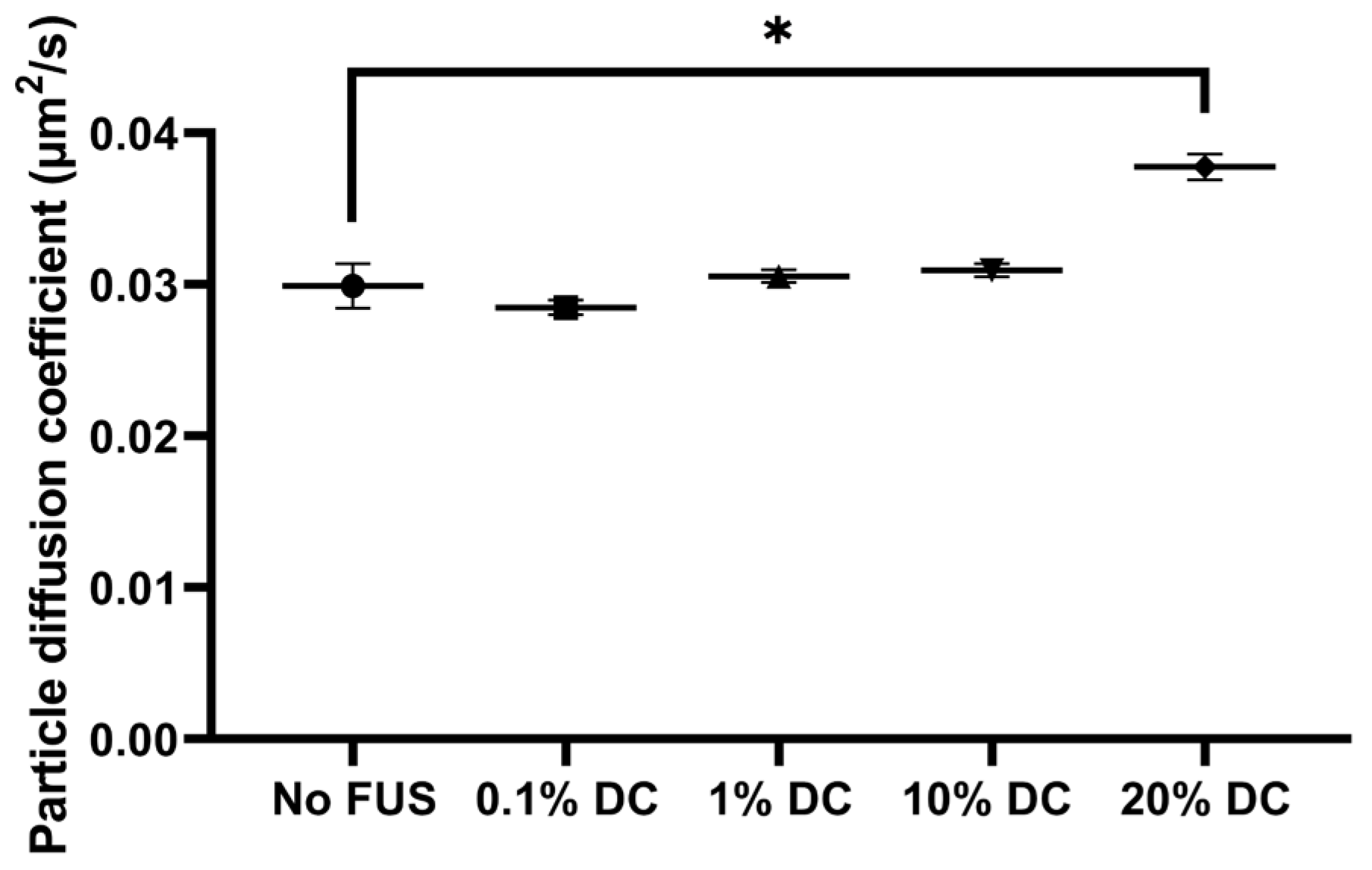
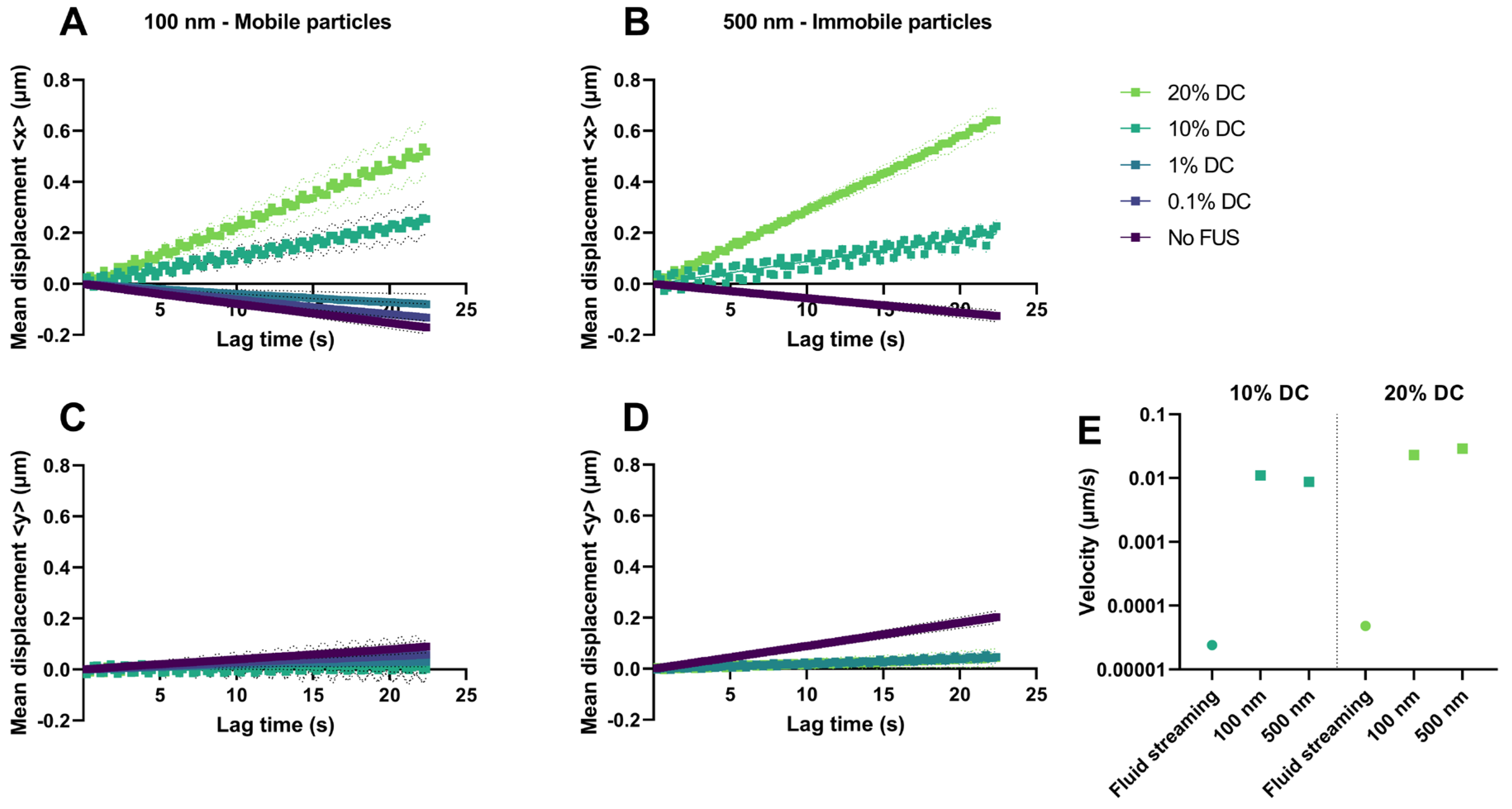
2.2.2. Pulsed FUS Increased Nanoparticle Diffusion
2.2.3. Acoustic Streaming vs. Hydrogel Displacement
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Nanoparticles
5.2. Preparation of Composite Agarose Hydrogels
5.3. Hydrogel Characterization
5.3.1. Acoustic Attenuation Measurements
5.3.2. Water Mass Fraction of Agarose–Milk Hydrogels
5.3.3. Phase Contrast Imaging of Agarose–Milk Hydrogels
5.3.4. Hydraulic Conductivity of Agarose Hydrogels
5.4. Nanoparticle Imaging and FUS Treatment
5.4.1. Confocal Imaging of Nanoparticles
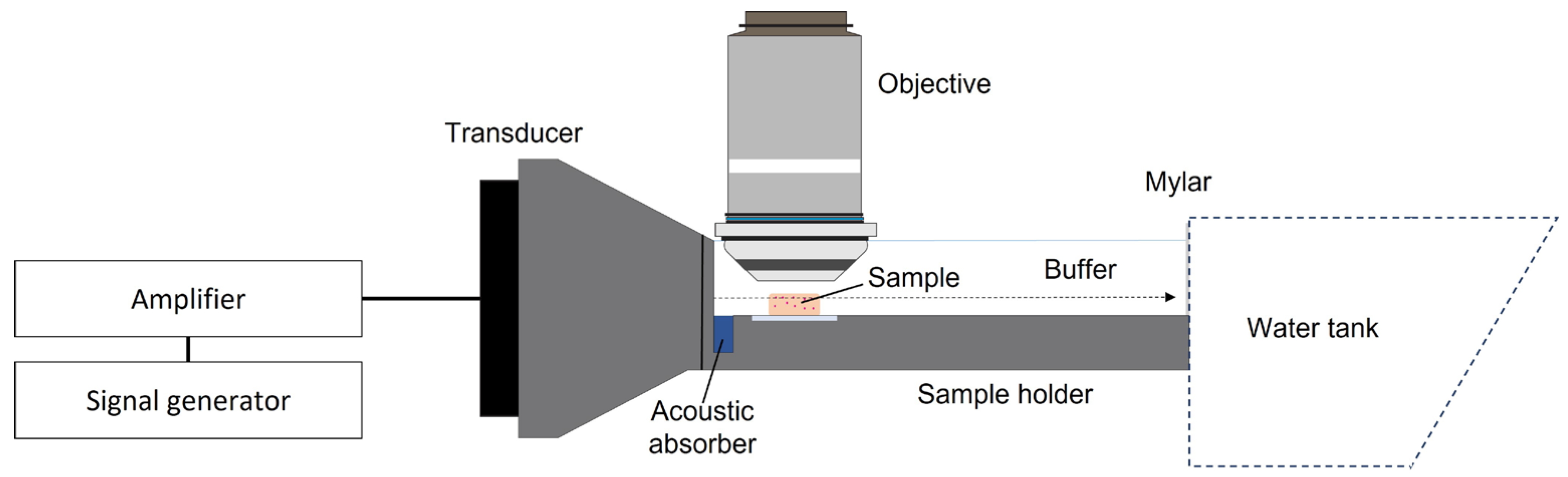
5.4.2. Ultrasound Setup
5.4.3. Ultrasound Treatment
5.4.4. Single-Particle Tracking and Estimation of Diffusion Coefficients
5.5. Volume Averaged Model of Acoustic Streaming
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Effect of Acoustic Streaming above the Hydrogel
References
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Wang, H.; Yu, J.; Lu, X.; He, X. Nanoparticle Systems Reduce Systemic Toxicity in Cancer Treatment. Nanomedicine 2016, 11, 103–106. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Boucher, Y.; Baxter, L.T.; Jain, R.K. Interstitial Pressure Gradients in Tissue-Isolated and Subcutaneous Tumors: Implications for Therapy. Cancer Res. 1990, 50, 4478–4484. [Google Scholar]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure—An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.d.L.; Lundstrøm, L.M.; Frengen, J.; Eikenes, L.; Bruland, Ø.S.; Kaalhus, O.; Hjelstuen, M.H.B.; Brekken, C. Radiation Improves the Distribution and Uptake of Liposomal Doxorubicin (Caelyx) in Human Osteosarcoma Xenografts. Cancer Res. 2004, 64, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Choe, J.W.; Pu, K.; Devulapally, R.; Bachawal, S.; Machtaler, S.; Chowdhury, S.M.; Luong, R.; Tian, L.; Khuri-Yakub, B.; et al. Ultrasound-Guided Delivery of MicroRNA Loaded Nanoparticles into Cancer. J. Control. Release 2015, 203, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S.K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation Enhances Liposome Accumulation and Penetration in Tumors with Low EPR. J. Control. Release 2016, 231, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Olsman, M.; Sereti, V.; Andreassen, K.; Snipstad, S.; van Wamel, A.; Eliasen, R.; Berg, S.; Urquhart, A.J.; Andresen, T.L.; Davies, C.d.L. Ultrasound-Mediated Delivery Enhances Therapeutic Efficacy of MMP Sensitive Liposomes. J. Control. Release 2020, 325, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ran, H.; Wang, Z.; Zhou, S.; Wang, Y. Targeted and PH-Facilitated Theranostic of Orthotopic Gastric Cancer via Phase-Transformation Doxorubicin-Encapsulated Nanoparticles Enhanced by Low-Intensity Focused Ultrasound (LIFU) with Reduced Side Effect. Int. J. Nanomed. 2019, 14, 7627–7642. [Google Scholar] [CrossRef] [PubMed]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; de Lange Davies, C. Ultrasound and Microbubbles to Beat Barriers in Tumors: Improving Delivery of Nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Jakhmola, A.; Hornsby, T.K.; Tavakkoli, J.J.; Kolios, M.C. Ultrasound-Mediated Nano Drug Delivery for Treating Cancer: Fundamental Physics to Future Directions. J. Control. Release 2023, 355, 552–578. [Google Scholar] [CrossRef]
- Dayton, P.; Klibanov, A.; Brandenburger, G.; Ferrara, K. Acoustic Radiation Force in Vivo: A Mechanism to Assist Targeting of Microbubbles. Ultrasound Med. Biol. 1999, 25, 1195–1201. [Google Scholar] [CrossRef]
- Nightingale, K. Acoustic Radiation Force Impulse (ARFI) Imaging: A Review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef]
- Lighthill, J. Acoustic Streaming. J. Sound Vib. 1978, 63, 391–418. [Google Scholar] [CrossRef]
- El Ghamrawy, A.; de Comtes, F.; Koruk, H.; Mohammed, A.; Jones, J.R.; Choi, J.J. Acoustic Streaming in a Soft Tissue Microenvironment. Ultrasound Med. Biol. 2019, 45, 208–217. [Google Scholar] [CrossRef]
- Lea-Banks, H.; Teo, B.; Stride, E.; Coussios, C.C. The Effect of Particle Density on Ultrasound-Mediated Transport of Nanoparticles. Phys. Med. Biol. 2016, 61, 7906–7918. [Google Scholar] [CrossRef]
- Løvmo, M.K.; Yemane, P.T.; Bjørkøy, A.; Hansen, R.; Cleveland, R.O.; Angelsen, B.A.; de Lange Davies, C. Effect of Acoustic Radiation Force on Displacement of Nanoparticles in Collagen Gels. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, V.; Kimmel, E.; Iger, Y. Ultrasound-Facilitated Transport of Silver Chloride (AgCl) Particles in Fish Skin. J. Control. Release 2000, 68, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Afadzi, M.; Myhre, O.F.; Yemane, P.T.; Bjørkøy, A.; Torp, S.H.; van Wamel, A.; Lelu, S.; Angelsen, B.A.J.; de Lange Davies, C. Effect of Acoustic Radiation Force on the Distribution of Nanoparticles in Solid Tumors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 432–445. [Google Scholar] [CrossRef] [PubMed]
- El Ghamrawy, A. Acoustic Streaming in Soft Tissue-Mimicking Materials. PhD Thesis, Imperial College, London, UK, 2019. [Google Scholar]
- Ma, D.; Marshall, J.S.; Wu, J. Measurement of Ultrasound-Enhanced Diffusion Coefficient of Nanoparticles in an Agarose Hydrogel. J. Acoust. Soc. Am. 2018, 144, 3496–3502. [Google Scholar] [CrossRef]
- Karki, A.; Marshall, J.S.; Wu, J. Effect of Ultrasound Amplitude and Frequency on Nanoparticle Diffusion in an Agarose Hydrogel. J. Acoust. Soc. Am. 2022, 152, 640–650. [Google Scholar] [CrossRef]
- Shen, H.; Tauzin, L.J.; Baiyasi, R.; Wang, W.; Moringo, N.; Shuang, B.; Landes, C.F. Single Particle Tracking: From Theory to Biophysical Applications. Chem. Rev. 2017, 117, 7331–7376. [Google Scholar] [CrossRef]
- Qian, H.; Sheetz, M.P.; Elson, E.L. Single Particle Tracking. Analysis of Diffusion and Flow in Two-Dimensional Systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef]
- Saxton, M.J. Single-Particle Tracking: Models of Directed Transport. Biophys. J. 1994, 67, 2110. [Google Scholar] [CrossRef]
- Saxton, M.J. Single Particle Tracking. In Fundamental Concepts in Biophysics; Humana Press: Totowa, NJ, USA, 2009; pp. 1–33. [Google Scholar]
- Burla, F.; Sentjabrskaja, T.; Pletikapic, G.; van Beugen, J.; Koenderink, G.H. Particle Diffusion in Extracellular Hydrogels. Soft Matter 2020, 16, 1366–1376. [Google Scholar] [CrossRef]
- Rose, K.A.; Lee, D.; Composto, R.J. PH-Mediated Nanoparticle Dynamics in Hydrogel Nanocomposites. Soft Matter 2021, 17, 2765–2774. [Google Scholar] [CrossRef]
- Coppens, B.; Belpaire, T.E.R.; Pešek, J.; Steenackers, H.P.; Ramon, H.; Smeets, B. Anomalous Diffusion of Nanoparticles in the Spatially Heterogeneous Biofilm Environment. iScience 2023, 26, 106861. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.L.; Lidke, K.A.; Pfeiffer, J.R.; Burns, A.R.; Wilson, B.S.; Oliver, J.M.; Lidke, D.S. Actin Restricts FcɛRI Diffusion and Facilitates Antigen-Induced Receptor Immobilization. Nat. Cell Biol. 2008, 10, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Price, S.E.N.; Hansen, R.; Gjennestad, M.A. A Volume-Averaged Model for Acoustic Streaming Induced by Focused Ultrasound in Soft Porous Media. J. Acoust. Soc. Am. 2023, 154, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Madsen, E.L.; Frank, G.R.; Dong, F. Liquid or Solid Ultrasonically Tissue-Mimicking Materials with Very Low Scatter. Ultrasound Med. Biol. 1998, 24, 535–542. [Google Scholar] [CrossRef]
- Cafarelli, A.; Verbeni, A.; Poliziani, A.; Dario, P.; Menciassi, A.; Ricotti, L. Tuning Acoustic and Mechanical Properties of Materials for Ultrasound Phantoms and Smart Substrates for Cell Cultures. Acta Biomater. 2017, 49, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Culjat, M.O.; Goldenberg, D.; Tewari, P.; Singh, R.S. A Review of Tissue Substitutes for Ultrasound Imaging. Ultrasound Med. Biol. 2010, 36, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Pishko, G.L.; Astary, G.W.; Mareci, T.H.; Sarntinoranont, M. Characterization of an Anisotropic Hydrogel Tissue Substrate for Infusion Testing. J. Appl. Polym. Sci. 2009, 114, 1992–2002. [Google Scholar] [CrossRef][Green Version]
- Johnson, E.M.; Deen, W.M. Hydraulic Permeability of Agarose Gels. AIChE J. 1996, 42, 1220–1224. [Google Scholar] [CrossRef]
- Mattern, K.J.; Nakornchai, C.; Deen, W.M. Darcy Permeability of Agarose-Glycosaminoglycan Gels Analyzed Using Fiber-Mixture and Donnan Models. Biophys. J. 2008, 95, 648–656. [Google Scholar] [CrossRef][Green Version]
- Baxter, L.T.; Jain, R.K. Transport of Fluid and Macromolecules in Tumors. I. Role of Interstitial Pressure and Convection. Microvasc. Res. 1989, 37, 77–104. [Google Scholar] [CrossRef]
- Boucher, Y.; Brekken, C.; Netti, P.; Baxter, L.; Jain, R. Intratumoral Infusion of Fluid: Estimation of Hydraulic Conductivity and Implications for the Delivery of Therapeutic Agents. Br. J. Cancer 1998, 78, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar] [PubMed]
- Valentini, M.; Vaccaro, A.; Rehor, A.; Napoli, A.; Hubbell, J.A.; Tirelli, N. Diffusion NMR Spectroscopy for the Characterization of the Size and Interactions of Colloidal Matter: The Case of Vesicles and Nanoparticles. J. Am. Chem. Soc. 2004, 126, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Zhu, L.; Yang, K.; Zhuang, R.; Xie, J.; Zhang, F. Diffusion-Weighted Magnetic Resonance Imaging for Therapy Response Monitoring and Early Treatment Prediction of Photothermal Therapy. ACS Appl. Mater. Interfaces 2016, 8, 5137–5147. [Google Scholar] [CrossRef]
- Labouta, H.I.; Gomez-Garcia, M.J.; Sarsons, C.D.; Nguyen, T.; Kennard, J.; Ngo, W.; Terefe, K.; Iragorri, N.; Lai, P.; Rinker, K.D.; et al. Surface-Grafted Polyethylene Glycol Conformation Impacts the Transport of PEG-Functionalized Liposomes through a Tumour Extracellular Matrix Model. RSC Adv. 2018, 8, 7697–7708. [Google Scholar] [CrossRef]
- Pluen, A.; Netti, P.A.; Jain, R.K.; Berk, D.A. Diffusion of Macromolecules in Agarose Gels: Comparison of Linear and Globular Configurations. Biophys. J. 1999, 77, 542–552. [Google Scholar] [CrossRef]
- Fatin-Rouge, N.; Starchev, K.; Buffle, J. Size Effects on Diffusion Processes within Agarose Gels. Biophys. J. 2004, 86, 2710–2719. [Google Scholar] [CrossRef]
- Pernodet, N.; Maaloum, M.; Tinland, B. Pore Size of Agarose Gels by Atomic Force Microscopy. Electrophoresis 1997, 18, 55–58. [Google Scholar] [CrossRef]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose Gel Structure Using Atomic Force Microscopy: Gel Concentration and Ionic Strength Effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef]
- Hynynen, K.; Edwards, D.K. Temperature Measurements during Ultrasound Hyperthermia. Med. Phys. 1989, 16, 618–626. [Google Scholar] [CrossRef]
- Balakrishnan, V.; Venkataraman, G. Two-State Random Walk Model of Lattice Diffusion. 1. Self-Correlation Function. Pramana 1981, 16, 109–130. [Google Scholar] [CrossRef]
- Curran, K.; Marshall, J.S. Stochastic Model of Oscillatory Diffusion for Colloidal Particles in a Fixed Porous Bed. Chem. Eng. Sci. 2021, 246, 116993. [Google Scholar] [CrossRef]
- Marshall, J.S.; Arnold, C.; Curran, K.; Chivers, T. Statistics of Particle Diffusion Subject to Oscillatory Flow in a Porous Bed. Chem. Eng. Sci. 2021, 231, 116239. [Google Scholar] [CrossRef]
- Lee, S.; Han, H.; Koo, H.; Na, J.H.; Yoon, H.Y.; Lee, K.E.; Lee, H.; Kim, H.; Kwon, I.C.; Kim, K. Extracellular Matrix Remodeling in Vivo for Enhancing Tumor-Targeting Efficiency of Nanoparticle Drug Carriers Using the Pulsed High Intensity Focused Ultrasound. J. Control. Release 2017, 263, 68–78. [Google Scholar] [CrossRef]
- Mohammadabadi, A.; Huynh, R.N.; Wadajkar, A.S.; Lapidus, R.G.; Kim, A.J.; Raub, C.B.; Frenkel, V. Pulsed Focused Ultrasound Lowers Interstitial Fluid Pressure and Increases Nanoparticle Delivery and Penetration in Head and Neck Squamous Cell Carcinoma Xenograft Tumors. Phys. Med. Biol. 2020, 65, 125017. [Google Scholar] [CrossRef]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New Insight into Agarose Gel Mechanical Properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Allan, D.B.; Caswell, T.; Keim, N.C.; van der Wel, C.M.; Verweij, R.W. Soft-Matter/Trackpy: Trackpy v0.5.0. version 0.5.0; Zenodo: Geneva, Switzerland, 2021. [CrossRef]
- Ruthardt, N.; Lamb, D.C.; Bräuchle, C. Single-Particle Tracking as a Quantitative Microscopy-Based Approach to Unravel Cell Entry Mechanisms of Viruses and Pharmaceutical Nanoparticles. Mol. Ther. 2011, 19, 1199–1211. [Google Scholar] [CrossRef]
- Nyborg, W.L. Acoustic Streaming Due to Attenuated Plane Waves. J. Acoust. Soc. Am. 1953, 25, 68–75. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in Porous Media I: A Theoretical Derivation of Darcy’s Law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Brinkman, H.C. A Calculation of the Viscous Force Exerted by a Flowing Fluid on a Dense Swarm of Particles. Flow Turbul. Combust. 1949, 1, 27. [Google Scholar] [CrossRef]
- Arthur, J.K. Experimental Investigation of Porous Medium Structural Effects on a Coupled Porous Media-Free Zone Laminar Flow. SN Appl. Sci. 2019, 1, 1062. [Google Scholar] [CrossRef]


| Agarose Concentration | Additive | Additive Concentration | Acoustic Attenuation (dB/cm) | ||
|---|---|---|---|---|---|
| 1 MHz | 3.5 MHz | 5 MHz | |||
| 2 % w/v (n = 3) | - | - | 0.02 | 0.03 | 0.07 |
| BSA | 10% w/v | 0.03 | 0.02 | 0.05 | |
| 20% w/v | 0.03 | 0.03 | 0.12 | ||
| Evaporated milk | 5% v/v | 0.01 | 0.06 | 0.12 | |
| 10% v/v | 0.02 | 0.02 | 0.09 | ||
| 5 % w/v (n = 5) | - | - | 0.01 | 0.03 | 0.09 |
| Evaporated milk | 10% v/v | 0.04 | 0.29 | 0.12 | |
| 20% v/v | 0.04 | 0.24 | 0.07 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Einen, C.; Price, S.E.N.; Ulvik, K.; Gjennestad, M.A.; Hansen, R.; Kjelstrup, S.; Davies, C.d.L. Nanoparticle Dynamics in Composite Hydrogels Exposed to Low-Frequency Focused Ultrasound. Gels 2023, 9, 771. https://doi.org/10.3390/gels9100771
Einen C, Price SEN, Ulvik K, Gjennestad MA, Hansen R, Kjelstrup S, Davies CdL. Nanoparticle Dynamics in Composite Hydrogels Exposed to Low-Frequency Focused Ultrasound. Gels. 2023; 9(10):771. https://doi.org/10.3390/gels9100771
Chicago/Turabian StyleEinen, Caroline, Sebastian E. N. Price, Kim Ulvik, Magnus Aa. Gjennestad, Rune Hansen, Signe Kjelstrup, and Catharina de Lange Davies. 2023. "Nanoparticle Dynamics in Composite Hydrogels Exposed to Low-Frequency Focused Ultrasound" Gels 9, no. 10: 771. https://doi.org/10.3390/gels9100771
APA StyleEinen, C., Price, S. E. N., Ulvik, K., Gjennestad, M. A., Hansen, R., Kjelstrup, S., & Davies, C. d. L. (2023). Nanoparticle Dynamics in Composite Hydrogels Exposed to Low-Frequency Focused Ultrasound. Gels, 9(10), 771. https://doi.org/10.3390/gels9100771