Structure-Related Mechanical Properties and Bioactivity of Silica–Gelatin Hybrid Aerogels for Bone Regeneration
Abstract
1. Introduction
2. Results and Discussion
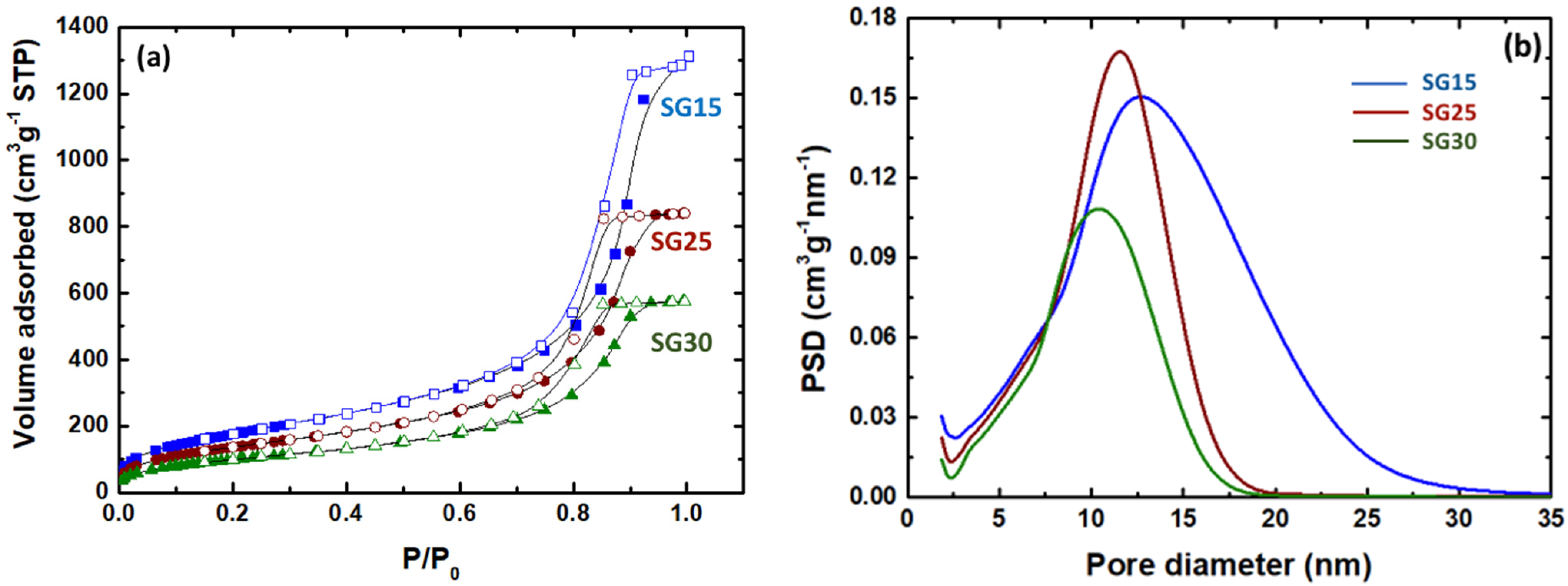
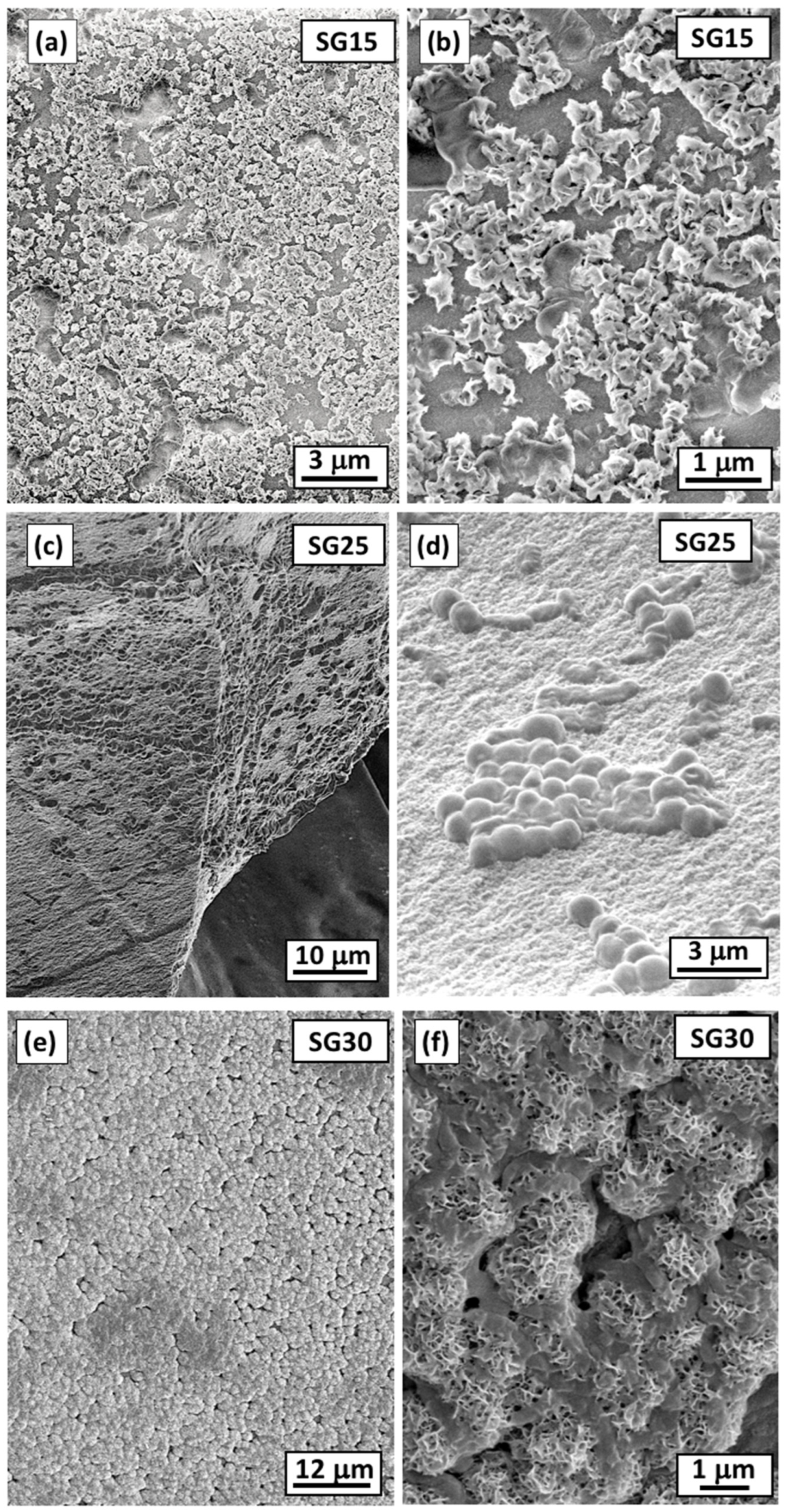
2.1. Physical and Textural Properties of the Hybrid Aerogels
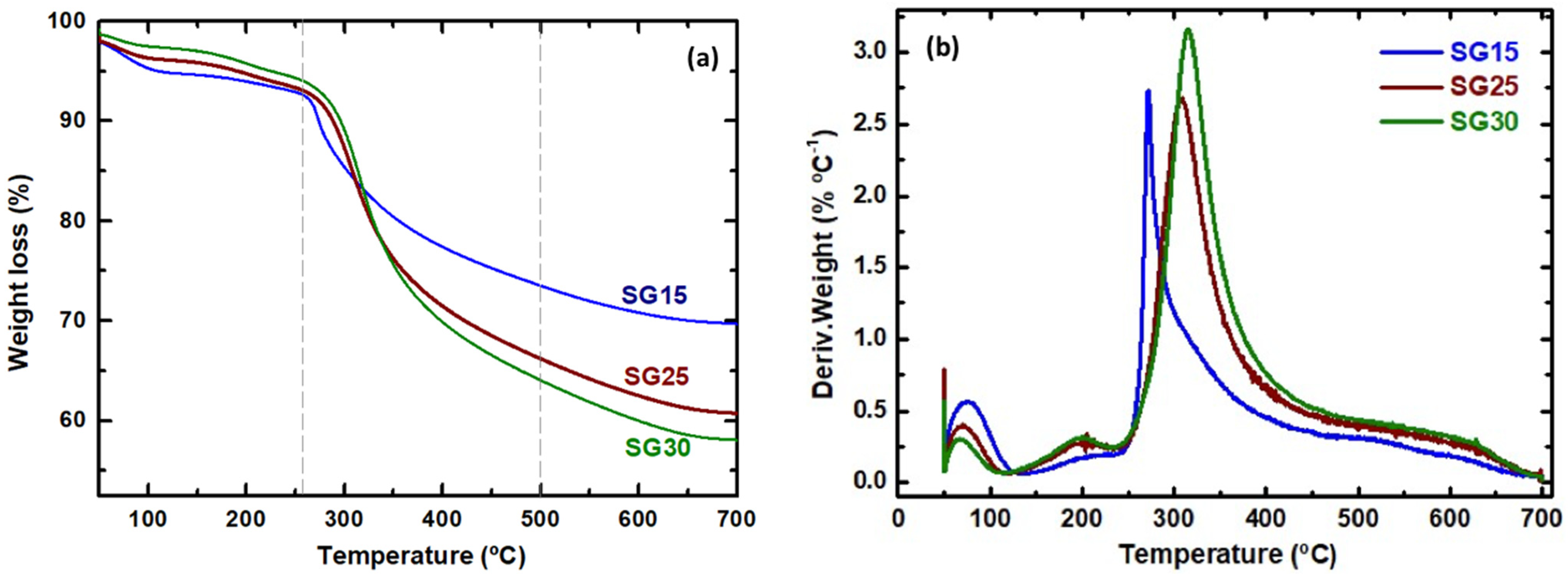
2.2. Thermogravimetric Analysis
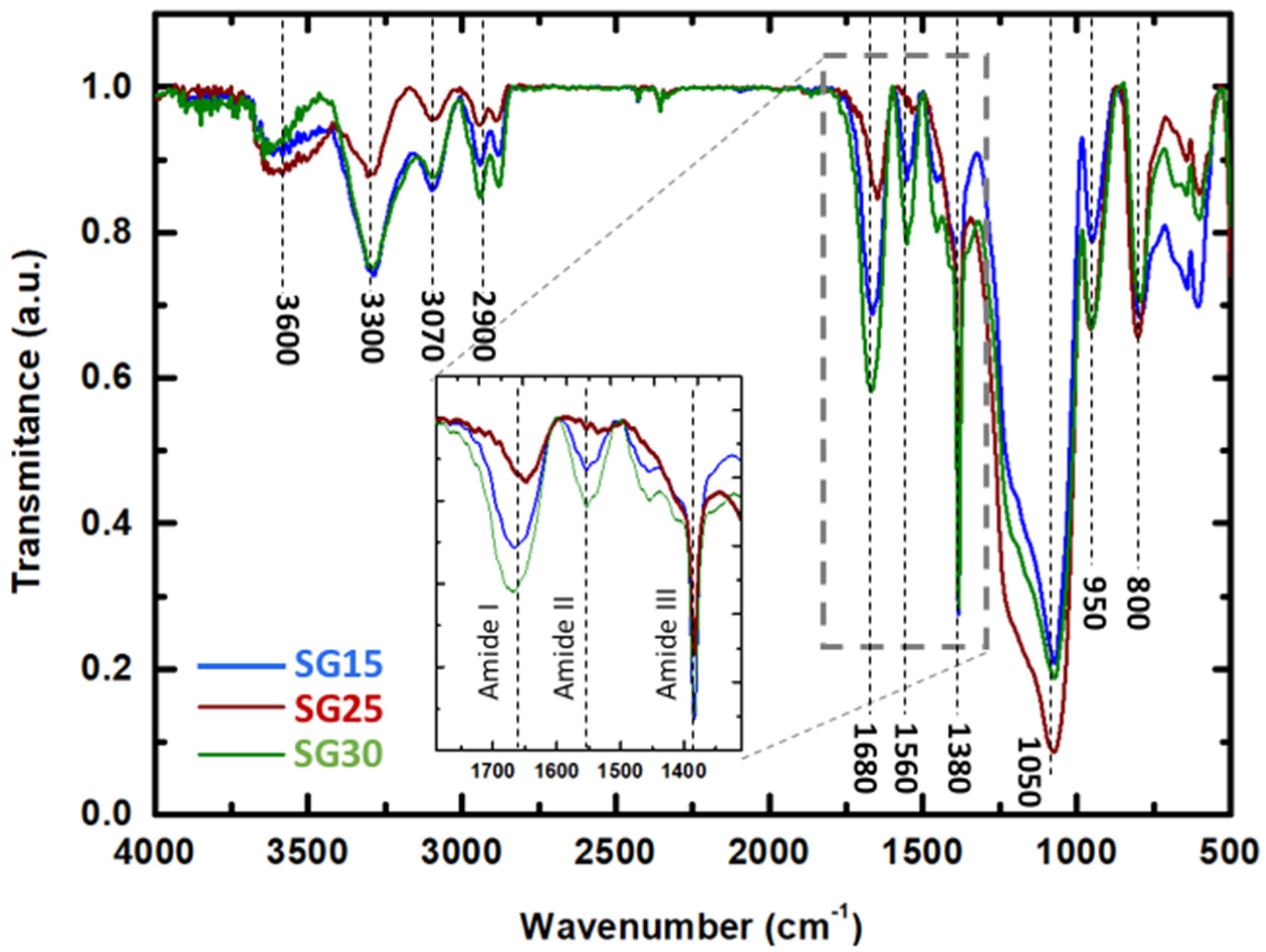
2.3. Fourier-transform Infrared Spectral Analysis
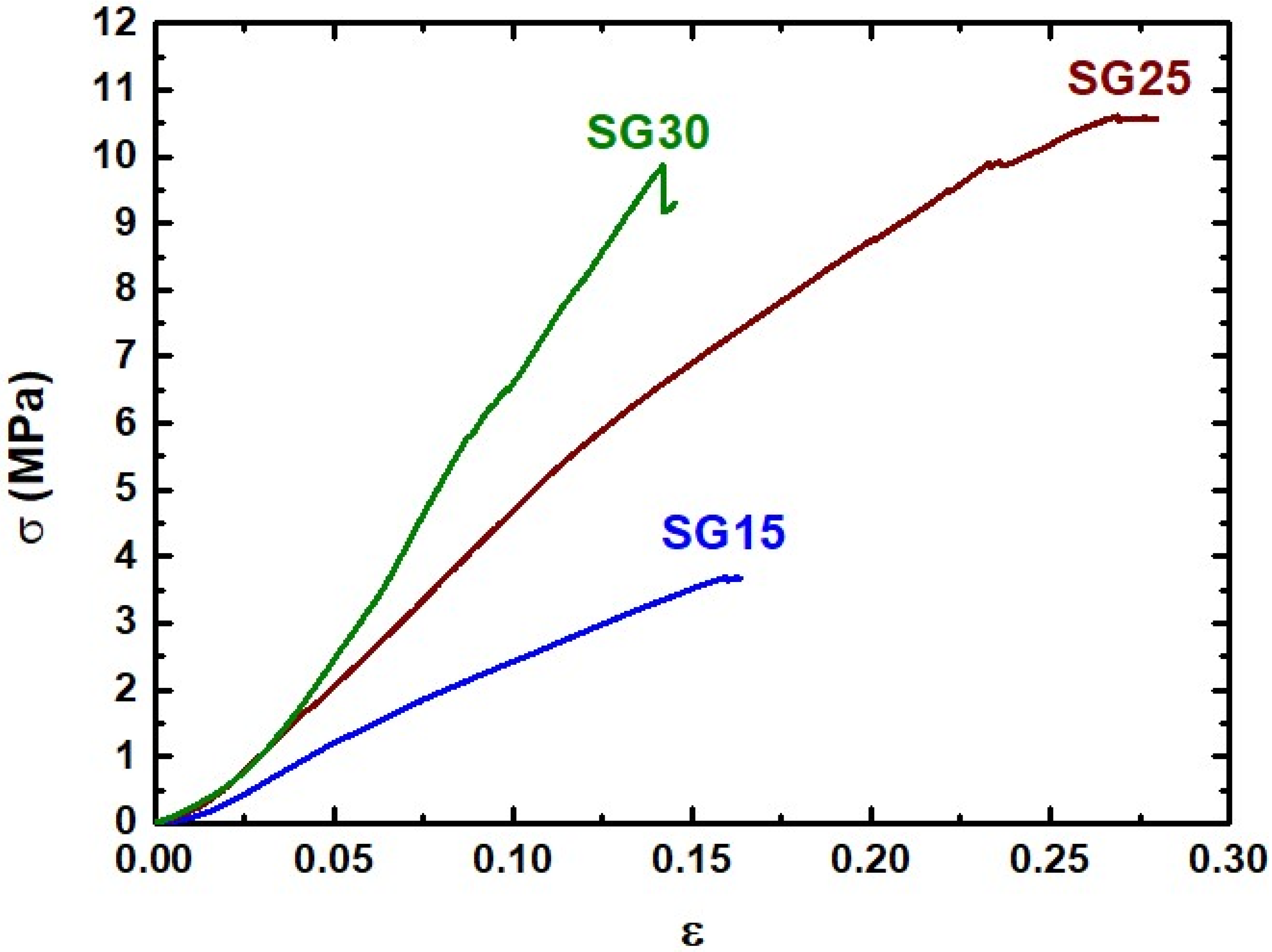
2.4. Mechanical Properties
2.5. Absorption Experiments
2.5.1. Swelling Ratio
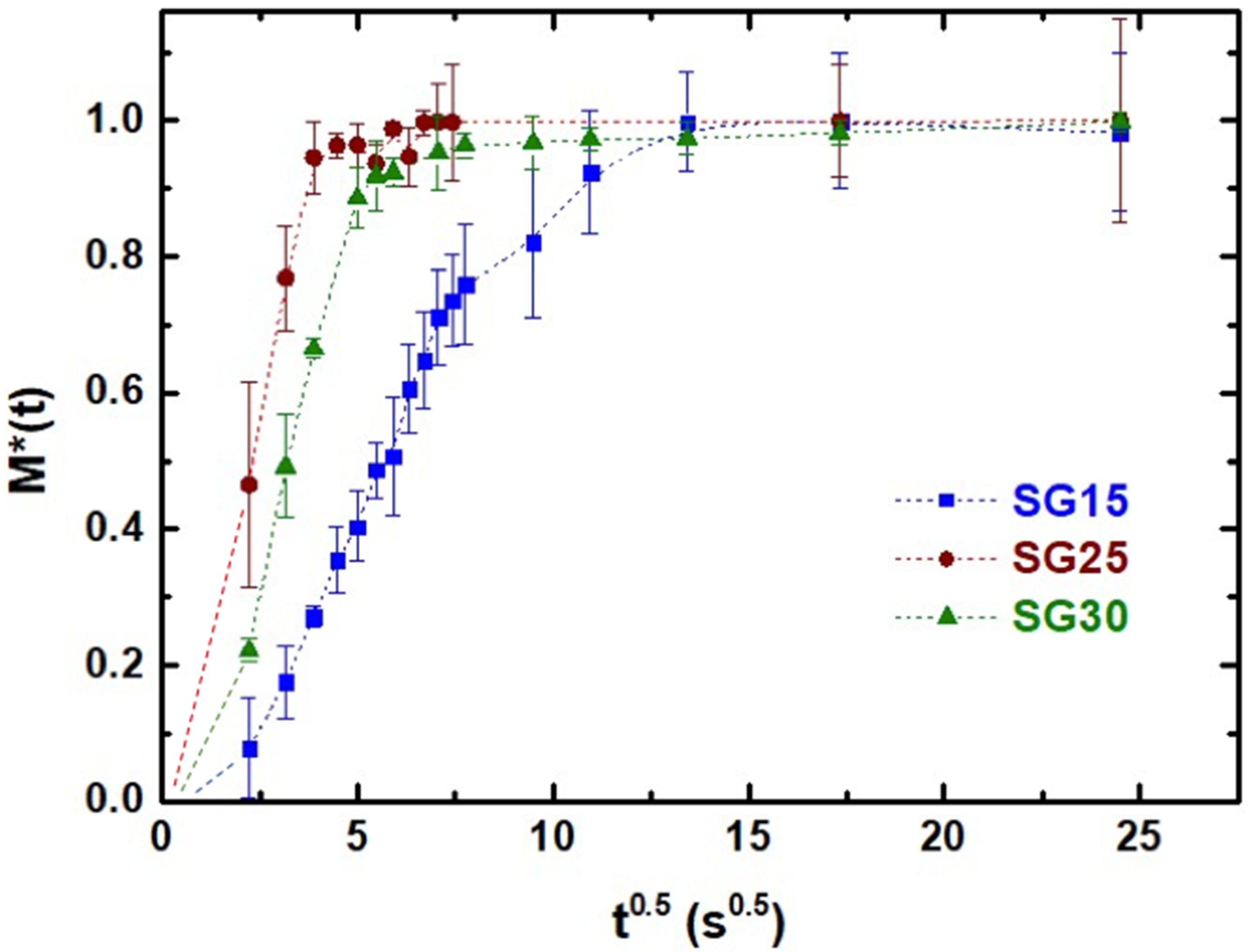
2.5.2. Swelling Kinetics
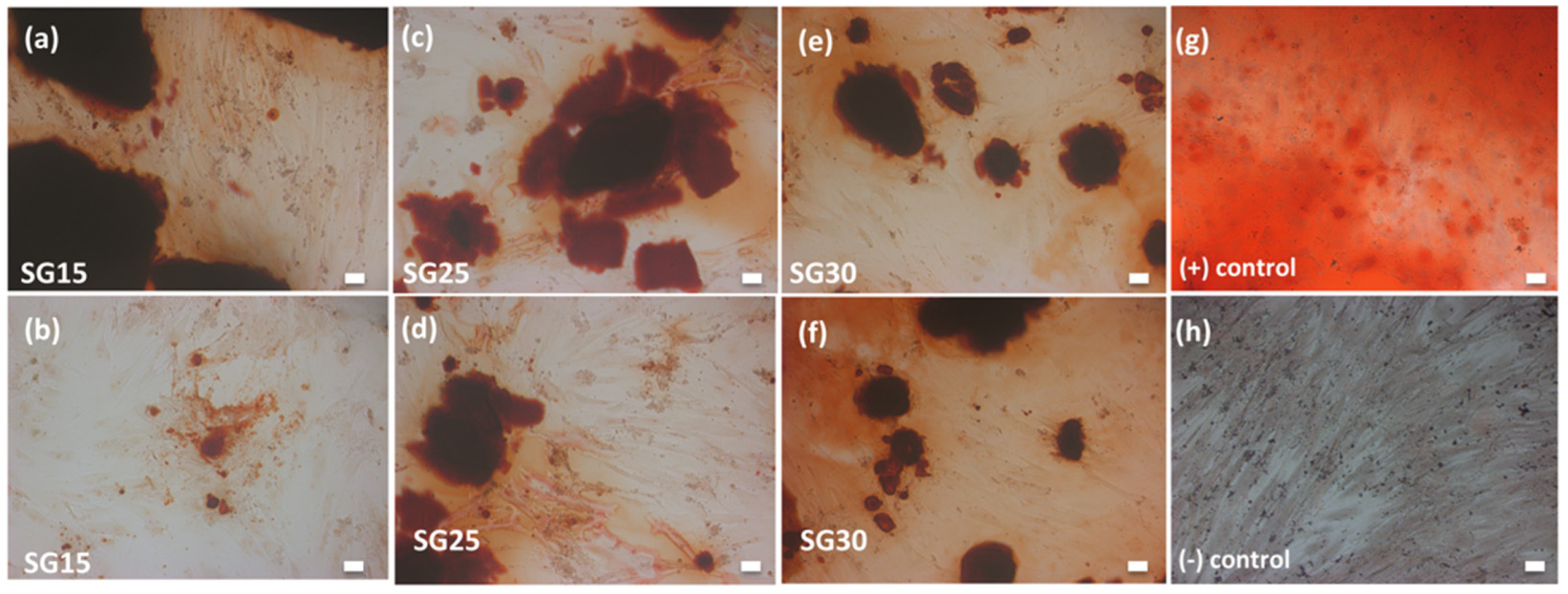
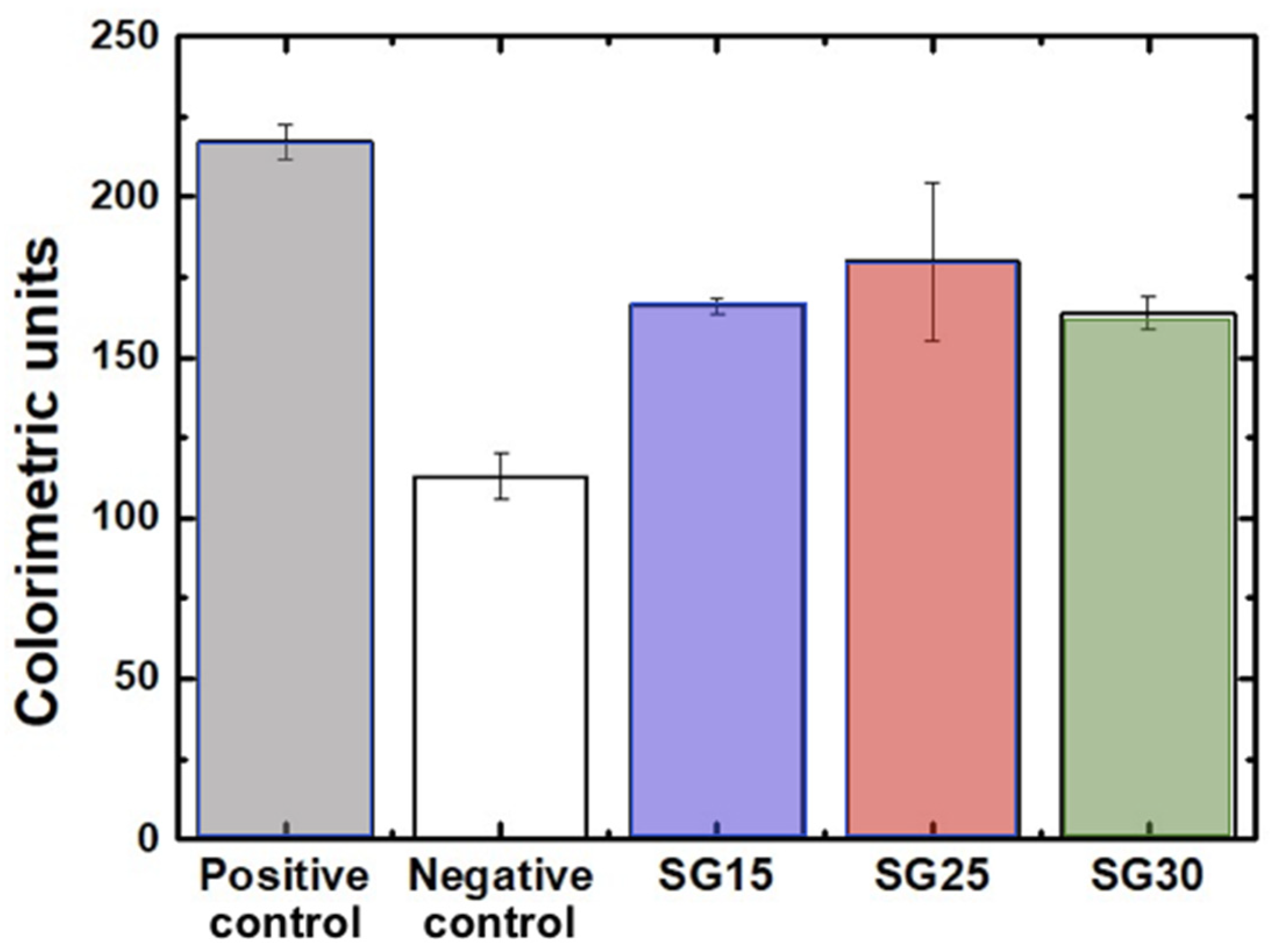
2.6. In Vitro Bioactivity in SBF
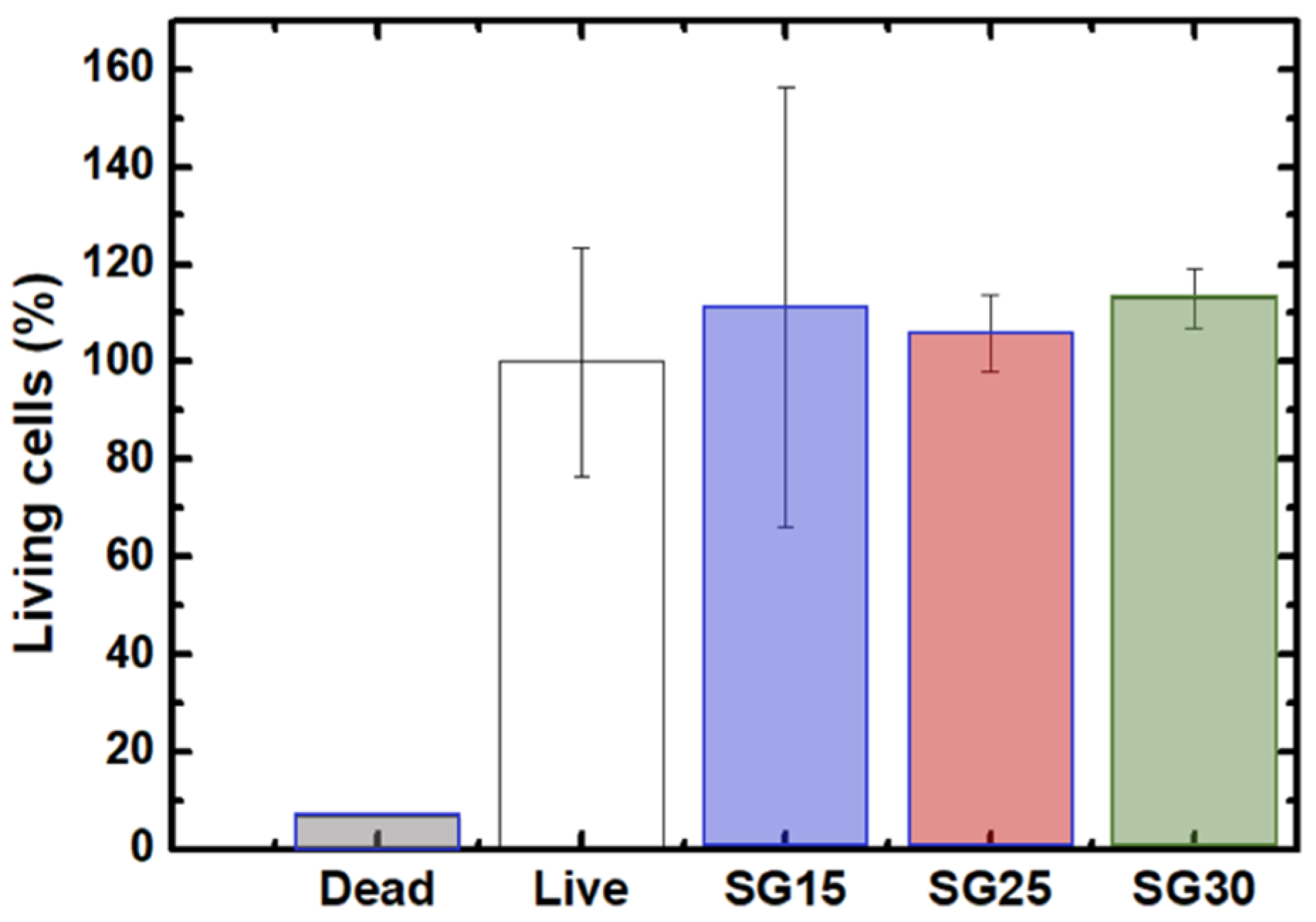
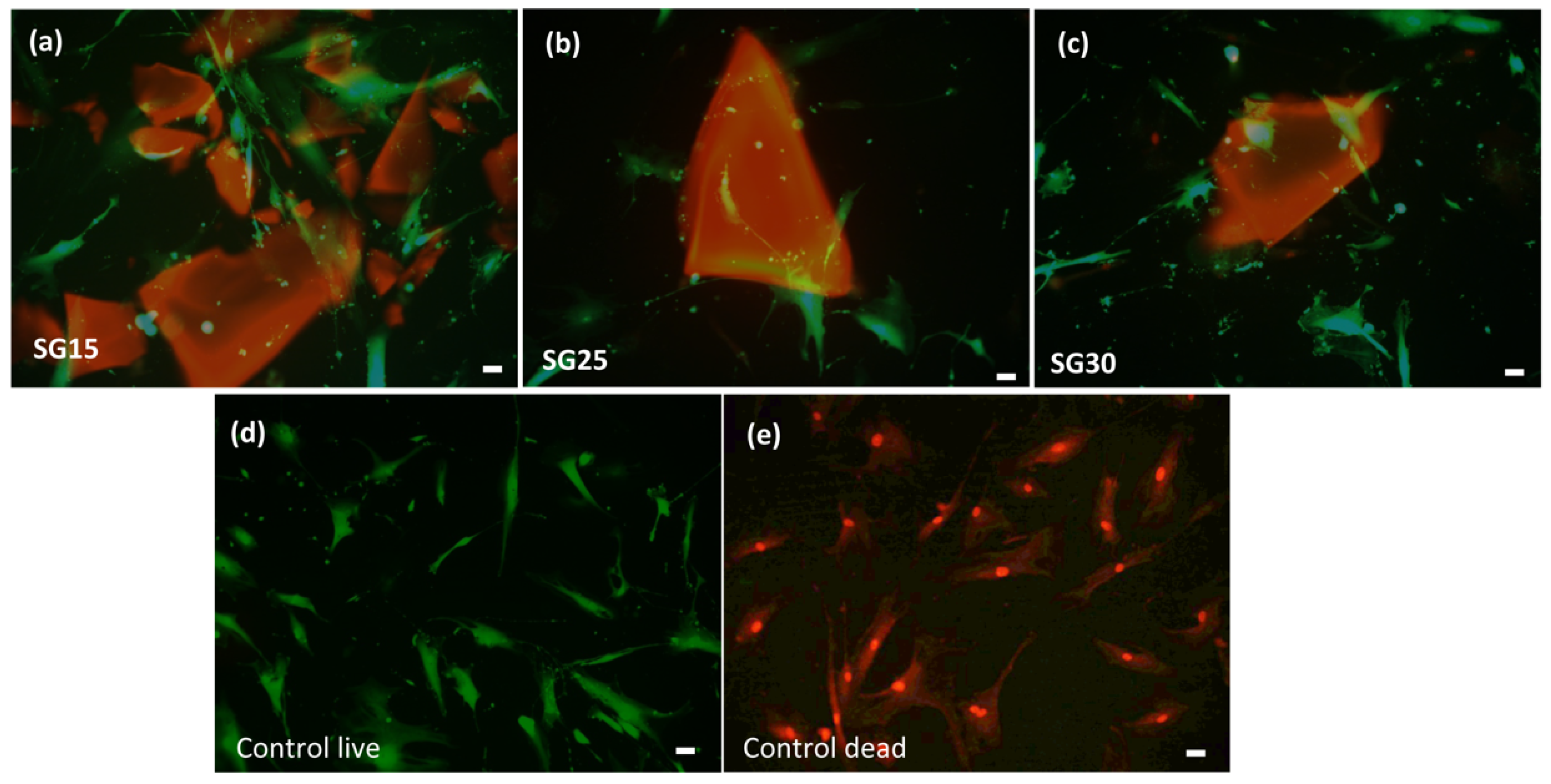
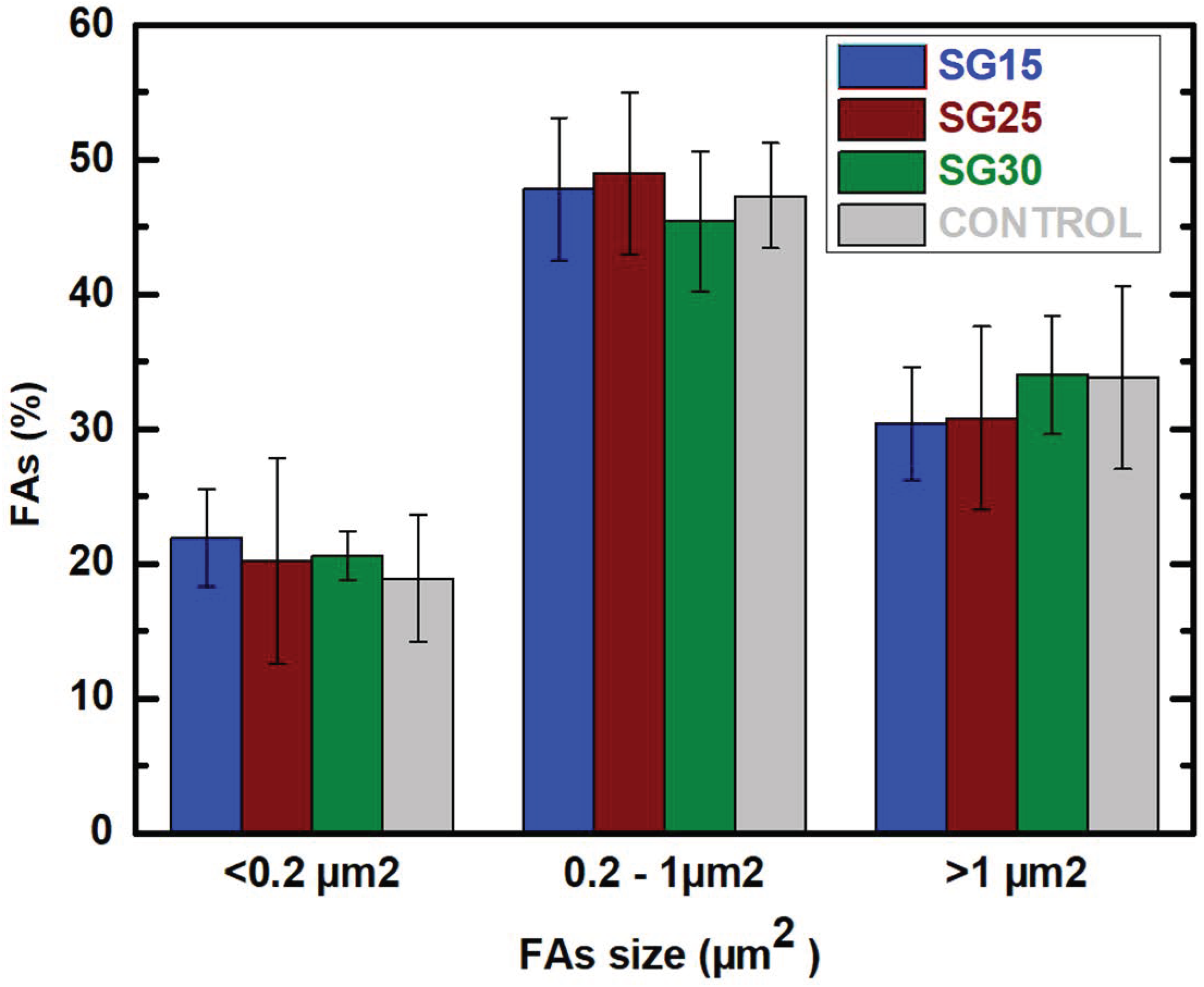
2.7. Live Dead Assays, Cell Morphology and Cytoskeletal Changes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Sols and Aerogels
4.3. Physical and Textural Characterization
4.4. Thermal Characterization
4.5. FTIR Spectroscopy
4.6. Mechanical Properties
4.7. Absorption Experiments in PBS
4.8. Biomineralization Experiments
4.9. Cell Culture
4.10. Live/Dead Cell Assay
4.11. Cell Morphology and Spreading
4.12. Actin Cytoskeletal Organization
4.13. Confocal Examination
4.14. Image Analysis
4.15. Mineralization
4.16. Detection of Mineralization
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herman, P.; Fábián, I.; Kalmár, J. Mesoporous silica-gelatin aerogels for the selective adsorption of aqueous Hg(II). ACS Appl. Nano Mater. 2020, 3, 195–206. [Google Scholar] [CrossRef]
- Yun, L.; Zhao, J.; Kang, X.; Du, Y.; Yuan, X.; Hou, X. Preparation and properties of monolithic and hydrophobic gelatin–silica composite aerogels for oil absorption. J. Sol-Gel Sci. Technol. 2017, 83, 197–206. [Google Scholar] [CrossRef]
- Veres, P.; Kéri, M.; Bányai, I.; Lázár, I.; Fábián, I.; Domingo, C.; Kalmár, J. Mechanism of drug release from silica-gelatin aerogel—Relationship between matrix structure and release kinetics. Colloids Surf. B Biointerfaces 2017, 152, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Juhász, I.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, R.; Bosch-Rué, E.; Díez-Tercero, L.; Delgado, L.M.; Pérez, R.A. Tailorable low temperature silica-gelatin biomaterials for drug delivery. Ceram. Int. 2022, 48, 28659–28668. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-gelatin hybrids with tailorable degradation and mechanical properties for tissue regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Mahony, O.; Yue, S.; Hanna, J.V.; Smith, M.E.; Lee, P.D.; Jones, J.R. Silica—Gelatin hybrids for tissue regeneration: Inter-relationships between the process variables. J. Sol-Gel Sci. Technol. 2014, 69, 288–298. [Google Scholar] [CrossRef]
- Veres, P.; Király, G.; Nagy, G.; Lázár, I.; Fábián, I.; Kalmár, J. Biocompatible silica-gelatin hybrid aerogels covalently labeled with fluorescein. J. Non-Cryst. Solids 2017, 473, 17–25. [Google Scholar] [CrossRef]
- Ayers, M.R.; Hunt, A.J. Synthesis and properties of chitosan-silica hybrid aerogels. J. Non-Cryst. Solids 2001, 285, 123–127. [Google Scholar] [CrossRef]
- Reyes-Peces, M.V.; Pérez-Moreno, A.; De-los-Santos, D.M.; Mesa-Díaz, M.d.M.; Pinaglia-Tobaruela, G.; Vilches-Pérez, J.I.; Fernández-Montesinos, R.; Salido, M.; de la Rosa-Fox, N.; Piñero, M. Chitosan-GPTMS-silica hybrid mesoporous aerogels for bone tissue engineering. Polymer 2020, 12, 2723. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef] [PubMed]
- Horvat, G.; Pantić, M.; Knez, Ž.; Novak, Z. Preparation and characterization of polysaccharide—Silica hybrid aerogels. Sci. Rep. 2019, 9, 16492. [Google Scholar] [CrossRef] [PubMed]
- Demilecamps, A.; Beauger, C.; Hildenbrand, C.; Rigacci, A.; Budtova, T. Cellulose-silica aerogels. Carbohydr. Polym. 2015, 122, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Duraipandy, N.; Srivatsan, K.V.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Fabrication of hybrid collagen aerogels reinforced with wheat grass bioactives as instructive scaffolds for collagen turnover and angiogenesis for wound healing applications. ACS Appl. Mater. Interfaces 2017, 9, 16939–16950. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Stavinskaya, O.N.; Laguta, I.V. The properties of silica—Gelatin composites. Russ. J. Phys. Chem. A 2010, 84, 1045–1048. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Venkatesan, R.; Karuppiah, P.S.; Kumar, G.; Paliwal, P.; Krishnamurthy, S.; Kadalmani, B.; Mahto, S.K.; Misra, N. Silica release from silane cross-linked gelatin based hybrid scaffold affects cell proliferation. ACS Appl. Bio. Mater. 2020, 3, 197–207. [Google Scholar] [CrossRef]
- Kéri, M.; Forgács, A.; Papp, V.; Bányai, I.; Veres, P.; Len, A.; Dudás, Z.; Fábián, I.; Kalmár, J. Gelatin content governs hydration induced structural changes in silica-gelatin hybrid aerogels—Implications in drug delivery. Acta Biomater. 2020, 105, 131–145. [Google Scholar] [CrossRef]
- Mahesh, S.; Joshi, S.C. Thermal conductivity variations with composition of gelatin-silica aerogel-sodium dodecyl sulfate with functionalized multi-walled carbon nanotube doping in their composites. Int. J. Heat Mass Transf. 2015, 87, 606–615. [Google Scholar] [CrossRef]
- Gómez-Romero, P.; Sanchez, C. Hybrid materials, functional applications. An Introduction. In Functional Hybrid Materials; Gómez-Romero, P., Sanchez, C., Eds.; Wiley-VCH Verlag: Hoboken, NJ, USA, 2005; pp. 1–14. ISBN 3527304843. [Google Scholar]
- Connell, L.S.; Gabrielli, L.; Mahony, O.; Russo, L.; Cipolla, L.; Jones, J.R. Functionalizing natural polymers with alkoxysilane coupling agents: Reacting 3-glycidoxypropyl trimethoxysilane with poly(γ-glutamic acid) and gelatin. Polym. Chem. 2017, 8, 1095–1103. [Google Scholar] [CrossRef]
- Martinez-Ibañez, M.; Aldalur, I.; Romero-Gavilán, F.J.; Suay, J.; Goñi, I.; Gurruchaga, M. Design of nanostructured siloxane-gelatin coatings: Immobilization strategies and dissolution properties. J. Non-Cryst. Solids 2018, 481, 368–374. [Google Scholar] [CrossRef]
- Houaoui, A.; Szczodra, A.; Lallukka, M.; El-Guermah, L.; Agniel, R.; Pauthe, E.; Massera, J.; Boissiere, M. New generation of hybrid materials based on gelatin and bioactive glass particles for bone tissue regeneration. Biomolecules 2021, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New trends in bio-based aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Veres, P.; López-Periago, A.M.; Lázár, I.; Saurina, J.; Domingo, C. Hybrid aerogel preparations as drug delivery matrices for low water-solubility drugs. Int. J. Pharm. 2015, 496, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Sachithanadam, M.; Joshi, S.C. High strain recovery with improved mechanical properties of gelatin-silica aerogel composites post-binding treatment. J. Mater. Sci. 2014, 49, 163–179. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Sol-gel preparation and in vitro deposition of apatite on porous gelatin-siloxane hybrids. J. Non-Cryst. Solids 2001, 285, 116–122. [Google Scholar] [CrossRef]
- Ren, L.; Tsuru, K.; Hayakawa, S.; Osaka, A. Novel approach to fabricate porous gelatin—Siloxane hybrids for bone tissue engineering. Biomaterials 2002, 23, 4765–4773. [Google Scholar] [CrossRef]
- Pietras, P.; Przekop, R.; Maciejewski, H. New approach to preparation of gelatine / SiO2 hybrid systems by the sol-gel process. Ceramics-Silikáty 2013, 57, 58–65. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1052. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Gregg, S.J., Ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Retuert, J.; Quijada, R.; Yazdani-pedram, M.; Martı, Y. Highly porous silica networks derived from gelatin / siloxane hybrids prepared starting from sodium metasilicate. J. Non-Cryst. Solids 2004, 347, 273–278. [Google Scholar] [CrossRef]
- Hu, Y.; Mackenzie, J.D. Structure-related mechanical properties of ormosils by sol-gel process. Mater. Res. Soc. Symp. Proc. 1992, 271, 681. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Huang, Q.; Iwamoto, T. Mechanical properties of ormosils. J. Sol-Gel Sci. Technol. 1996, 7, 151–161. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Toledo-Fernández, J.A.; De La Rosa-Fox, N.; Piñero, M.; Esquivias, L. Percolation of the organic phase in hybrid organic-inorganic aerogels. J. Sol-Gel Sci. Technol. 2009, 50, 170–175. [Google Scholar] [CrossRef]
- Morales-Florez, V.; Piñero, M.; Braza, V.; del Mar Mesa, M.; Esquivias, L.; de la Rosa-Fox, N. Absorption capacity, kinetics and mechanical behaviour in dry and wet states of hydrophobic DEDMS/TEOS-based silica aerogels. J. Sol-Gel Sci. Technol. 2017, 81, 600–610. [Google Scholar] [CrossRef]
- Ramadan, H.; Coradin, T.; Masse, S. Synthesis and characterization of mesoporous hybrid silica-polyacrylamide aerogels. Silicon 2020, 3, 63–75. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef]
- Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Voron, N.G. Polyelectrolyte polysaccharide-gelatin complexes: Rheology and structure. Polymers 2020, 12, 266. [Google Scholar] [CrossRef]
- Tengroth, C.; Gasslander, U.; Andersson, F.O.; Jacobsson, S.P. Cross-linking of gelatin capsules with formaldehyde and other aldehydes: An FTIR spectroscopy study. Pharm. Dev. Technol. 2005, 10, 405–412. [Google Scholar] [CrossRef]
- Masaro, L.; Zhu, X.X. Physical models of diffusion for polymer solutions, gels and solids. Prog. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Tiǧli Aydin, R.S.; Pulat, M. 5-fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 313961. [Google Scholar] [CrossRef]
- Magdalena, G.; Ostrowska-Czubenko, J. Mechanism of water diffusion into noncrosslinked and ionically crosslinked chitosan membranes. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 59–66. [Google Scholar]
- Xia, W.; Lin, K.; Gou, Z.; Engqvist, H. Morphology control of hydroxyapatite crystal and its aggregates. In Hydroxyapatite Synthesis, Properties and Applications; Gshalaev, V.S., Demirchan, A.C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 243–265. ISBN 9781620819340. [Google Scholar]
- Raucci, M.G.; Amora, U.D.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation routes of gelatin-based scaffolds to enhance at nanoscale level bone tissue regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef]
- Bittig, A.T.; Matschegewski, C.; Nebe, J.B.; Stählke, S.; Uhrmacher, A.M. Membrane related dynamics and the formation of actin in cells growing on micro-topographies: A spatial computational model. BNC Syst. Biol. 2014, 8, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Coyer, S.R.; Singh, A.; Dumbauld, D.W.; Calderwood, D.A.; Craig, S.W.; Garcı, J. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell Sci. 2010, 125, 5110–5123. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2011, 466, 263–266. [Google Scholar] [CrossRef]
- Legerstee, K.; Houtsmuller, A.B. A layered view on focal adhesions. Biology 2021, 10, 1189. [Google Scholar] [CrossRef]
- Cohen, D.M.; Kutscher, B.; Chen, H.; Murphy, D.B.; Craig, S.W. A conformational switch in vinculin drives formation and dynamics of a talin-vinculin complex at focal adhesions. J. Biol. Chem. 2006, 281, 16006–16015. [Google Scholar] [CrossRef]
- Humphries, J.D.; Wang, P.; Streuli, C.; Geiger, B.; Humphries, M.J.; Ballestrem, C. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J. Cell Biol. 2007, 179, 1043–1057. [Google Scholar] [CrossRef]
- Natale, C.F.; Ventre, M.; Netti, P.A. Biomaterials Tuning the material-cytoskeleton crosstalk via nanocon fi nement of focal adhesions. Biomaterials 2014, 35, 2743–2751. [Google Scholar] [CrossRef]
- Bays, J.L.; Demali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Zonderland, J.; Wieringa, P.; Moroni, L. A quantitative method to analyse F-actin distribution in cells. MethodsX 2019, 6, 2562–2569. [Google Scholar] [CrossRef]
- Salido, M.; Vilches, J.I.; Gutiérrez, J.L.; Vilches, J. Actin cytoskeletal organization in human osteoblasts grown on different dental titanium implant surfaces. Histol Histopathol. 2007, 22, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Salido, M.; Vilches-perez, J.I.; Gonzalez, J.L.; Vilches, J. Mitochondrial bioenergetics and distribution in living human osteoblasts grown on implant surfaces. Histol Histopathol. 2009, 24, 1275–1286. [Google Scholar] [PubMed]
- Lamers, E.; van Horssen, R.; te Riet, J.; van Delft, F.C.M.J.M.; Luttge, R.; Walboomers, X.F.; Jansen, J.A. The influence of nanoscale topographical cues on initial osteoblast morphology and migration. Eur. Cells Mater. 2010, 20, 329–343. [Google Scholar] [CrossRef]
- Iv, O.J.J.; Merife, A.; Zhang, Y.; Lemmon, C.A. Hydroxyapatite particle density regulates osteoblastic differentiation through β -catenin translocation. Front. Bioeng. Biotechnol. 2021, 8, 591084. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Faivre, B.; Clainche, C. Le Integrating actin dynamics, mechanotransduction and integrin activation: The multiple functions of actin binding proteins in focal adhesions. Eur. J. Cell Biol. 2013, 92, 339–348. [Google Scholar] [CrossRef]
- Riegert, J.; Töpel, A.; Schieren, J.; Coryn, R.; Dibenedetto, S.; Braunmiller, D.; Zajt, K.; Schalla, C.; Rütten, S.; Zenke, M.; et al. Guiding cell adhesion and motility by modulating cross-linking and topographic properties of microgel arrays. PLoS ONE 2021, 16, e0257495. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Siegel, R.W.; Bizios, R. Enhanced osteoclast like-cell functions on nanophase ceramics. Ann. Biomed. Eng. 2000, 28, 1803–1810. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; González-Calbet, J.M.; Vallet-Regí, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Jones, J.R. Observing cell response to biomaterials. Mater. Today 2006, 9, 34–43. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Bocaccini, A.R. Biodegradable and bioactive porous polymer / inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. Part A 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]


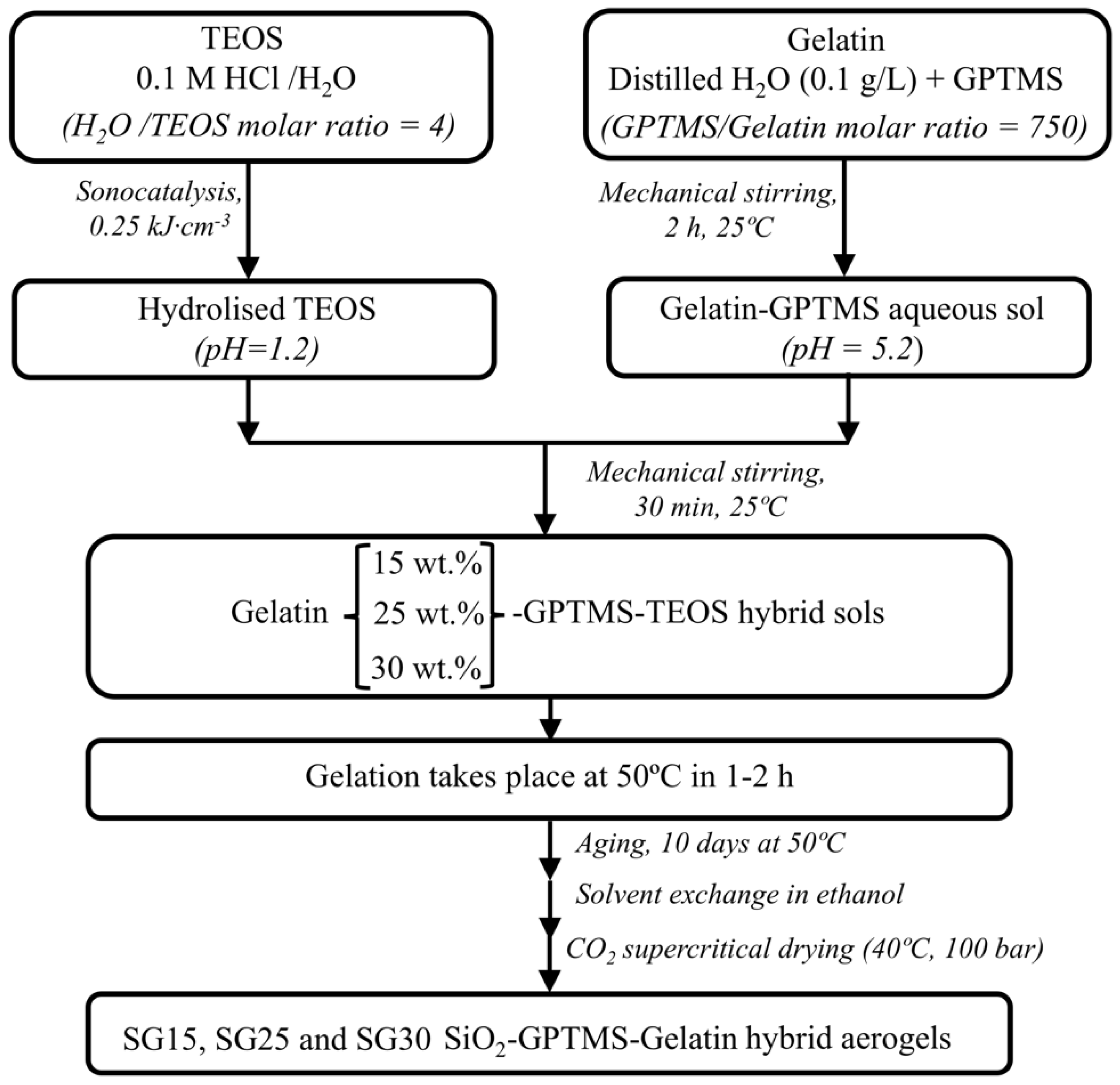
| Sample Code | Gelatin (g) | SiO2 from TEOS (g) | SiO2 from GPTMS 1 (g) | Gelatin Content (wt. %) |
|---|---|---|---|---|
| SG15 | 0.8 | 4.1 | 0.4 | 15.1 |
| SG25 | 1.6 | 4.1 | 0.6 | 25.4 |
| SG30 | 2.4 | 4.1 | 1.3 | 30.7 |
| Sample Code | Bulk Density, ρa (g cm−3) | Volume Shrinkage (%) | SBET (m2g−1) | Pore Volume, Vp (cm3g−1) | Pore Size 1, dpore (nm) |
|---|---|---|---|---|---|
| SG15 | 0.41 ± 0.01 | 3.3 ± 1.0 | 651 ± 11 | 1.98 | 10.8 |
| SG25 | 0.56 ± 0.01 | 28.8 ± 2.7 | 482 ± 15 | 1.30 | 9.20 |
| SG30 | 0.69 ± 0.03 | 44.6 ± 1.0 | 361 ± 10 | 0.89 | 8.60 |
| Sample | Young’s Modulus (MPa) | Compressive Strength (Mpa) | Maximum Compressive Strain (%) | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| SG15 | 30.81 ± 1.72 | 3.71 ± 0.33 | 3.69 ± 0.25 | 0.33 ± 0.01 | 16.29 ± 0.21 | 4.55 ± 0.44 |
| SG25 | 45.74 ± 3.26 | -- | 9.67 ± 1.66 | -- | 27.67 ± 0.47 | -- |
| SG30 | 78.55 ± 5.11 | 1.65 ± 0.36 | 9.90 ± 2.35 | 0.10 ± 0.01 | 14.06 ± 1.35 | 4.08 ± 0.53 |
| Samples | Swelling Ratio | Linear Fit: (M(t)/M∞) = a + bt0.5 | ||
|---|---|---|---|---|
| a | b | R2 | ||
| SG15 | 2.32 ± 0.11 | −0.22 ± 0.03 | 0.13 ± 0.02 | 0.9948 |
| SG25 | 3.42 ± 0.21 | −0.18 ± 0.07 | 0.29 ± 0.02 | 0.9893 |
| SG30 | 3.04 ± 0.14 | −0.29 ± 0.06 | 0.24 ± 0.02 | 0.9847 |
| Sample | Area (μm2) | Perimeter (μm) | Circularity | Aspect Ratio | Roundness | Solidity |
|---|---|---|---|---|---|---|
| SG15 | 16271 ± 21547 | 958 ± 786 | 0.189 ± 0.089 | 3.63 ± 1.74 | 0.33 ± 0.13 * | 0.64 ± 0.21 |
| SG25 | 6229 ± 7087 * | 459 ± 349 * | 0.360 ± 0.178 | 2.26 ± 0.96 | 0.50 ± 0.18 | 0.71 ± 0.08 |
| SG30 | 29,077 ± 44,946 * | 1231 ± 1166 * | 0.207 ± 0.157 | 3.13 ± 2.03 | 0.42 ± 0.23 * | 0.65 ± 0.16 |
| Control | 5302 ± 1268 | 630 ± 21 * | 0.166 ± 0.028 | 3.02 ± 1.87 | 0.87 ± 0.08 * | 0.68 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Peces, M.V.; Fernández-Montesinos, R.; Mesa-Díaz, M.d.M.; Vilches-Pérez, J.I.; Cárdenas-Leal, J.L.; de la Rosa-Fox, N.; Salido, M.; Piñero, M. Structure-Related Mechanical Properties and Bioactivity of Silica–Gelatin Hybrid Aerogels for Bone Regeneration. Gels 2023, 9, 67. https://doi.org/10.3390/gels9010067
Reyes-Peces MV, Fernández-Montesinos R, Mesa-Díaz MdM, Vilches-Pérez JI, Cárdenas-Leal JL, de la Rosa-Fox N, Salido M, Piñero M. Structure-Related Mechanical Properties and Bioactivity of Silica–Gelatin Hybrid Aerogels for Bone Regeneration. Gels. 2023; 9(1):67. https://doi.org/10.3390/gels9010067
Chicago/Turabian StyleReyes-Peces, María V., Rafael Fernández-Montesinos, María del Mar Mesa-Díaz, José Ignacio Vilches-Pérez, Jose Luis Cárdenas-Leal, Nicolás de la Rosa-Fox, Mercedes Salido, and Manuel Piñero. 2023. "Structure-Related Mechanical Properties and Bioactivity of Silica–Gelatin Hybrid Aerogels for Bone Regeneration" Gels 9, no. 1: 67. https://doi.org/10.3390/gels9010067
APA StyleReyes-Peces, M. V., Fernández-Montesinos, R., Mesa-Díaz, M. d. M., Vilches-Pérez, J. I., Cárdenas-Leal, J. L., de la Rosa-Fox, N., Salido, M., & Piñero, M. (2023). Structure-Related Mechanical Properties and Bioactivity of Silica–Gelatin Hybrid Aerogels for Bone Regeneration. Gels, 9(1), 67. https://doi.org/10.3390/gels9010067







