Optimising Soy and Pea Protein Gelation to Obtain Hydrogels Intended as Precursors of Food-Grade Dried Porous Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Protein Type and Concentration
2.2. Effect of pH
2.3. Effect of Ionic Strength
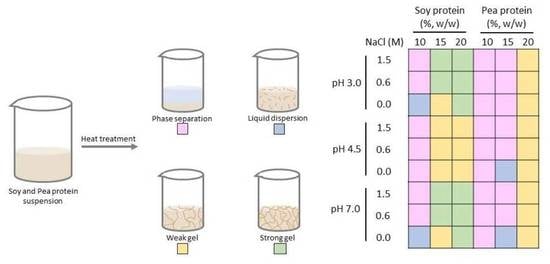
2.4. Gelation Map
3. Conclusions
4. Materials and Methods
4.1. Soy and Pea Protein Solution Preparation
4.2. Heat Treatment
4.3. Image Acquisition
4.4. Rheological Properties
4.5. Physical Stability
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoshnevis, H.; Mint, S.M.; Yedinak, E.; Tran, T.Q.; Zadhoush, A.; Youssefi, M.; Pasquali, M.; Duong, H.M. Super High-Rate Fabrication of High-Purity Carbon Nanotube Aerogels from Floating Catalyst Method for Oil Spill Cleaning. Chem. Phys. Lett. 2018, 693, 146–151. [Google Scholar] [CrossRef]
- Di Luigi, M.; Guo, Z.; An, L.; Armstrong, J.N.; Zhou, C.; Ren, S. Manufacturing Silica Aerogel and Cryogel through Ambient Pressure and Freeze Drying. RSC Adv. 2022, 12, 21213–21222. [Google Scholar] [CrossRef] [PubMed]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Bazan-Wozniak, A.; Pietrzak, R. Amine-Modified Carbon Xerogels as Effective Carbon-Based Adsorbents of Anionic Dye from Aqueous Solutions. Materials 2022, 15, 5736. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, L.; Cao, H.; Chang, Y.; Tang, K.; Cao, Z.; Chang, J.; Cao, Y.; Wang, W.; Gao, M.; et al. Carbon Materials Derived from Chitosan/Cellulose Cryogel-Supported Zeolite Imidazole Frameworks for Potential Supercapacitor Application. Carbohydr. Polym. 2017, 175, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, W.; Yamasaki, S.; Fujisawa, S.; Kodama, T.; Shiomi, J.; Kanamori, K.; Saito, T. Mechanically Strong, Scalable, Mesoporous Xerogels of Nanocellulose Featuring Light Permeability, Thermal Insulation, and Flame Self-Extinction. ACS Nano 2021, 15, 1436–1444. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E. Physicochemical, Complexation and Catalytic Properties of Polyampholyte Cryogels. Gels 2019, 5, 8. [Google Scholar] [CrossRef]
- Pham, T.H.; Jung, S.H.; Kim, Y.J.; Kim, T.Y. Adsorptive Removal and Recovery of Organic Pollutants from Wastewater Using Waste Paper-Derived Carbon-Based Aerogel. Chemosphere 2021, 268, 129319. [Google Scholar] [CrossRef]
- Križman, K.; Novak, S.; Kristl, J.; Majdič, G.; Drnovšek, N. Long-Acting Silk Fibroin Xerogel Delivery Systems for Controlled Release of Estradiol. J. Drug. Deliv. Sci. Technol. 2021, 65, 102701. [Google Scholar] [CrossRef]
- Sharma, M.; Tavares, A.P.M.; Nunes, J.C.F.; Singh, N.; Mondal, D.; Neves, M.C.; Prasad, K.; Freire, M.G. Hybrid Alginate–Protein Cryogel Beads: Efficient and Sustainable Bio-Based Materials to Purify Immunoglobulin G Antibodies. Green Chem. 2020, 22, 2225–2233. [Google Scholar] [CrossRef]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. ScCO2-Foamed Silk Fibroin Aerogel/Poly(ε-Caprolactone) Scaffolds Containing Dexamethasone for Bone Regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Varganici, C.D.; Dinu, M.V. Development of Antioxidant and Antimicrobial Xanthan-Based Cryogels with Tuned Porous Morphology and Controlled Swelling Features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, C.; Schuster, R.; Rosenecker, E.; Selmer, I.; Smirnova, I.; Kulozik, U. In-Vitro-Digestion and Swelling Kinetics of Whey Protein, Egg White Protein and Sodium Caseinate Aerogels. Food Hydrocoll. 2020, 101, 105534. [Google Scholar] [CrossRef]
- Selmer, I.; Karnetzke, J.; Kleemann, C.; Lehtonen, M.; Mikkonen, K.S.; Kulozik, U.; Smirnova, I. Encapsulation of Fish Oil in Protein Aerogel Micro-Particles. J. Food Eng. 2019, 260, 1–11. [Google Scholar] [CrossRef]
- Schroeter, B.; Yonkova, V.P.; Goslinska, M.; Orth, M.; Pietsch, S.; Gurikov, P.; Smirnova, I.; Heinrich, S. Spray Coating of Cellulose Aerogel Particles in a Miniaturized Spouted Bed. Cellulose 2021, 28, 7795–7812. [Google Scholar] [CrossRef]
- Koshy, S.T.; Zhang, D.K.Y.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable Nanocomposite Cryogels for Versatile Protein Drug Delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef]
- Plazzotta, S.; Alongi, M.; De Berardinis, L.; Melchior, S.; Calligaris, S.; Manzocco, L. Steering Protein and Lipid Digestibility by Oleogelation with Protein Aerogels. Food Funct. 2022, 13, 10601–10609. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; McHugh, M.A. Superabsorbent Alginate Aerogels. J. Supercrit. Fluids 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Manzocco, L.; Plazzotta, S.; Powell, J.; de Vries, A.; Rousseau, D.; Calligaris, S. Structural Characterisation and Sorption Capability of Whey Protein Aerogels Obtained by Freeze-Drying or Supercritical Drying. Food Hydrocoll. 2022, 122, 107117. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structure of Oleogels from κ-Carrageenan Templates as Affected by Supercritical-CO2-Drying, Freeze-Drying and Lettuce-Filler Addition. Food Hydrocoll. 2019, 96, 1–10. [Google Scholar] [CrossRef]
- Plazzotta, S.; Jung, I.; Schroeter, B.; Subrahmanyam, R.P.; Smirnova, I.; Calligaris, S.; Gurikov, P.; Manzocco, L. Conversion of Whey Protein Aerogel Particles into Oleogels: Effect of Oil Type on Structural Features. Polymers 2021, 13, 4063. [Google Scholar] [CrossRef]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structural Characterization of Oleogels from Whey Protein Aerogel Particles. Int. Food Res. J. 2020, 132, 109099. [Google Scholar] [CrossRef]
- Brodkorb, A.; Croguennec, T.; Bouhallab, S.; Kehoe, J.J. Heat-Induced Denaturation, Aggregation and Gelation of Whey Proteins. In Advanced Dairy Chemistry, 4th ed.; Springer: Manhattan, NY, USA, 2016; Volume 1B, pp. 155–178. [Google Scholar] [CrossRef]
- Manzocco, L.; Valoppi, F.; Calligaris, S.; Andreatta, F.; Spilimbergo, S.; Nicoli, M.C. Exploitation of κ-Carrageenan Aerogels as Template for Edible Oleogel Preparation. Food Hydrocoll. 2017, 71, 68–75. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakuma, W.; Yasui, H.; Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A.; Kanamori, K. Nanocellulose Xerogels with High Porosities and Large Specific Surface Areas. Front. Chem. 2019, 7, 316. [Google Scholar] [CrossRef] [PubMed]
- Buchtová, N.; Budtova, T. Cellulose Aero-, Cryo- and Xerogels: Towards Understanding of Morphology Control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous Starch Materials via Supercritical- and Freeze-Drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-Based Aerogels—Promising Biodegradable Carriers for Drug Delivery Systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of Novel Whey Protein-Based Aerogels as Drug Carriers for Life Science Applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of Egg White Protein Aerogels as New Matrix Material for Microencapsulation in Food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- Kleemann, C.; Selmer, I.; Smirnova, I.; Kulozik, U. Tailor Made Protein Based Aerogel Particles from Egg White Protein, Whey Protein Isolate and Sodium Caseinate: Influence of the Preceding Hydrogel Characteristics. Food Hydrocoll. 2018, 83, 365–374. [Google Scholar] [CrossRef]
- Arboleda, J.C.; Hughes, M.; Lucia, L.A.; Laine, J.; Ekman, K.; Rojas, O.J. Soy Protein-Nanocellulose Composite Aerogels. Cellulose 2013, 20, 2417–2426. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Bornkeßel, A.C.; Jung, I.; Schröter, B.; Smirnova, I.; Kulozik, U. Microstructures of Potato Protein Hydrogels and Aerogels Produced by Thermal Crosslinking and Supercritical Drying. Food Hydrocoll. 2021, 112, 106305. [Google Scholar] [CrossRef]
- Yetiskin, B.; Okay, O. High-Strength and Self-Recoverable Silk Fibroin Cryogels with Anisotropic Swelling and Mechanical Properties. Int. J. Biol. Macromol. 2019, 122, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk Fibroin Aerogels for Drug Delivery Applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, G.; Liu, X.; Liu, H.; He, Y.; Zhu, D. Morphological and Structural Changes in Thermally-Induced Soybean Protein Isolate Xerogels Modulated by Soybean Polysaccharide Concentration. Food Hydrocoll. 2022, 133, 107967. [Google Scholar] [CrossRef]
- Andlinger, D.J.; Schlemmer, L.; Jung, I.; Schroeter, B.; Smirnova, I.; Kulozik, U. Hydro- and Aerogels from Ethanolic Potato and Whey Protein Solutions: Influence of Temperature and Ethanol Concentration on Viscoelastic Properties, Protein Interactions, and Microstructure. Food Hydrocoll. 2022, 125, 107424. [Google Scholar] [CrossRef]
- Cheng, K.; Tao, X.; Qi, Z.; Yin, Z.; Kundu, S.C.; Lu, S. Highly Absorbent Silk Fibroin Protein Xerogel. ACS Biomater. Sci. Eng. 2021, 7, 3594–3607. [Google Scholar] [CrossRef]
- de Boer, J.; Aiking, H. On the Merits of Plant-Based Proteins for Global Food Security: Marrying Macro and Micro Perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Prag, A.A.; Henriksen, C.B. Transition from Animal-Based to Plant-Based Food Production to Reduce Greenhouse Gas Emissions from Agriculture—The Case of Denmark. Sustainability 2020, 12, 8228. [Google Scholar] [CrossRef]
- Machovina, B.; Feeley, K.J.; Ripple, W.J. Biodiversity Conservation: The Key Is Reducing Meat Consumption. Sci. Total Environ. 2015, 536, 419–431. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Induced by Heat Treatment. Int. Food Res. J. 2010, 43, 509–515. [Google Scholar] [CrossRef]
- Yin, X.; Cheng, H.; Wusigale; Dong, H.; Huang, W.; Liang, L. Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants 2022, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Chassenieux, C. Heat-Induced Gelation of Plant Globulins. Curr. Opin. Food Sci. 2019, 27, 18–22. [Google Scholar] [CrossRef]
- Chen, N.; Lin, L.; Sun, W.; Zhao, M. Stable and pH-Sensitive Protein Nanogels Made by Self-Assembly of Heat Denatured Soy Protein. J. Agric. Food Chem. 2014, 62, 9553–9561. [Google Scholar] [CrossRef] [PubMed]
- Ako, K.; Nicolai, T.; Durand, D.; Brotons, G. Micro-Phase Separation Explains the Abrupt Structural Change of Denatured Globular Protein Gels on Varying the Ionic Strength or the pH. Soft Matter 2009, 5, 4033–4041. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Gruppen, H.; van Vliet, T. Influence of pH and Ionic Strength on Heat-Induced Formation and Rheological Properties of Soy Protein Gels in Relation to Denaturation and Their Protein Compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef]
- Fitzpatrick, S.E.; Staiger, M.P.; Deb-Choudhury, S.; Ranford, S. Protein-Based Aerogels: Processing and Morphology. RSC Green Chem. 2018, 201, 67–102. [Google Scholar] [CrossRef]
- Hermansson, A.M. Aggregation and Denaturation Involved In Gel Formation. ACS Symp. Ser. 1979, 5, 81–103. [Google Scholar] [CrossRef]
- Hermansson, A.M. Soy Protein Gelation. J. Am. Oil Chem. Soc. 1986, 63, 658–666. [Google Scholar] [CrossRef]
- KangII, J.; Matsumura, Y.; Mori, T. Characterization of Texture and Mechanical Properties of Heat-Induced Soy Protein Gels. J. Am. Oil Chem. Soc. 1991, 68, 339–345. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. The Effect of Adding NaCl on Thermal Aggregation and Gelation of Soy Protein Isolate. Food Hydrocoll. 2017, 70, 88–95. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High Moisture Extrusion Cooking of Pea Protein Isolates: Raw Material Characteristics, Extruder Responses, and Texture Properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Shand, P.J.; Ya, H.; Pietrasik, Z.; Wanasundara, P.K.J.P.D. Physicochemical and Textural Properties of Heat-Induced Pea Protein Isolate Gels. Food Chem. 2007, 102, 1119–1130. [Google Scholar] [CrossRef]
- Hua, Y.; Cui, S.W.; Wang, Q.; Mine, Y.; Poysa, V. Heat Induced Gelling Properties of Soy Protein Isolates Prepared from Different Defatted Soybean Flours. Int. Food Res. J. 2005, 38, 377–385. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A.J.S. Heat-Induced Gelation of Pea Legumin: Comparison with Soybean Glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, K.R.; Cavallieri, Â.L.F.; da Cunha, R.L. Cold-Set Whey Protein Gels Induced by Calcium or Sodium Salt Addition. Int. J. Food Sci. Technol. 2010, 45, 348–357. [Google Scholar] [CrossRef]
- Nicolai, T. Formation and Functionality of Self-Assembled Whey Protein Microgels. Colloids Surf. B. 2016, 137, 32–38. [Google Scholar] [CrossRef]
- Nicolai, T.; Durand, D. Controlled Food Protein Aggregation for New Functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- van der Linden, E.; Venema, P. Self-Assembly and Aggregation of Proteins. Curr. Opin. Colloid Interface Sci. 2007, 12, 158–165. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Chassenieux, C.; Nicolai, T. Structure of Self-Assembled Native Soy Globulin in Aqueous Solution as a Function of the Concentration and the pH. Food Hydrocoll. 2016, 56, 417–424. [Google Scholar] [CrossRef]
- Kumar, S.; Nussinov, R. Relationship between Ion Pair Geometries and Electrostatic Strengths in Proteins. Biophys. J. 2002, 83, 1595–1612. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L.; Chen, J. pH Shifting Alters Solubility Characteristics and Thermal Stability of Soy Protein Isolate and Its Globulin Fractions in Different pH, Salt Concentration, and Temperature Conditions. J. Agric. Food Chem. 2010, 58, 8035–8042. [Google Scholar] [CrossRef] [PubMed]
- Puppo, M.C.; Lupano, C.E.; Añón, M.C. Gelation of Soybean Protein Isolates in Acidic Conditions. Effect of pH and Protein Concentration. J. Agric. Food Chem. 1995, 43, 2356–2361. [Google Scholar] [CrossRef]
- Puppo, M.C.; Añón, M.C. Structural Properties of Heat-Induced Soy Protein Gels As Affected by Ionic Strength and pH. J. Agric. Food Chem. 1998, 46, 3583–3589. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, C.; Zhang, M.; Xu, Y.; Zhou, B.; Su, Y.; Yang, Y. Characteristics of Gelling and Water Holding Properties of Hen Egg White/Yolk Gel with NaCl Addition. Food Hydrocoll. 2018, 77, 887–893. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhang, M.; Zhai, Y.; Zhou, B.; Su, Y.; Yang, Y. Effects of Selected Phosphate Salts on Gelling Properties and Water State of Whole Egg Gel. Food Hydrocoll. 2018, 77, 1–7. [Google Scholar] [CrossRef]
- Croguennec, T.; Nau, F.; Brulé, G. Influence of pH and Salts on Egg White Gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Khemakhem, M.; Attia, H.; Ayadi, M.A. The Effect of pH, Sucrose, Salt and Hydrocolloid Gums on the Gelling Properties and Water Holding Capacity of Egg White Gel. Food Hydrocoll. 2019, 87, 11–19. [Google Scholar] [CrossRef]
- Munialo, C.D.; van der Linden, E.; de Jongh, H.H.J. The Ability to Store Energy in Pea Protein Gels Is Set by Network Dimensions Smaller than 50nm. Food Res. Int. 2014, 64, 482–491. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Gonzalez, R.; Subirade, M. Formation of Soy Protein Isolate Cold-Set Gels: Protein and Salt Effects. J. Food Sci. 2005, 70, C67–C73. [Google Scholar] [CrossRef]
- Urbonaite, V.; de Jongh, H.H.J.; van der Linden, E.; Pouvreau, L. Origin of water loss from soy protein gels. J. Agric. Food Chem. 2014, 62, 7550–7558. [Google Scholar] [CrossRef]
- Urbonaite, V.; de Jongh, H.H.J.; van der Linden, E.; Pouvreau, L. Water holding of soy protein gels is set by coarseness, modulated by calcium binding, rather than gel stiffness. Food Hydrocoll. 2015, 46, 103–111. [Google Scholar] [CrossRef]

| Protein | Concentration (%, w/w) | Appearance | G′ × 102 (Pa) | G″ × 102 (Pa) | Tan δ | WHC |
|---|---|---|---|---|---|---|
| SPI | 10 |  | N.D. | N.D. | N.D. | N.D. |
| 15 | 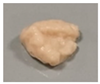 | 3.94 ± 0.24 c | 0.77 ± 0.04 c | 0.19 ± 0.01 b | 89.51 ± 3.56 a | |
| 20 |  | 47.57 ± 1.61 a | 6.60 ± 0.24 a | 0.14 ± 0.01 c | 99.60 ± 0.44 a | |
| PPI | 10 |  | N.D. | N.D. | N.D. | N.D. |
| 15 |  | N.D. | N.D. | N.D. | N.D. | |
| 20 | 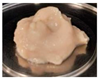 | 13.64 ± 0.24 b | 3.13 ± 0.05 b | 0.23 ± 0.01 a | 75.19 ± 0.03 b |
| Protein | pH | Appearance | G′ × 102 (Pa) | G″ × 102 (Pa) | Tan δ | WHC |
|---|---|---|---|---|---|---|
| SPI | 3.0 |  | 30.14 ± 2.78 a | 4.10 ± 0.46 a | 0.14 ± 0.01 c | 99.80 ± 0.09 a |
| 4.5 |  | 24.32 ± 0.66 b | 3.36 ± 0.85 b | 0.14 ± 0.01 c | 99.68 ± 0.07 a | |
| PPI | 3.0 | 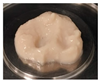 | 8.52 ± 1.26 c | 2.38 ± 0.31 c | 0.28 ± 0.01 a | 67.07 ± 0.73 b |
| 4.5 | 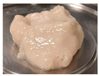 | 7.89 ± 0.59 d | 1.99 ± 0.73 d | 0.25 ± 0.01 b | 75.00 ± 2.99 b |
| Protein | Concentration (%, w/w) | Ionic Strength (M) | Appearance | G′ × 102 (Pa) | G″ × 102 (Pa) | Tan δ | WHC |
|---|---|---|---|---|---|---|---|
| SPI | 10 | 0.6 |  | N.D. | N.D. | N.D. | N.D. |
| 1.5 | 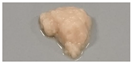 | N.D. | N.D. | N.D. | N.D. | ||
| 20 | 0.6 |  | 58.21 ± 7.10 b | 9.97 ± 1.19 b | 0.17 ± 0.01 b | 97.95 ± 0.70 a | |
| 1.5 |  | 115.51 ± 46.08 a | 22.40 ± 8.52 a | 0.19 ± 0.01 a | 89.24 ± 3.17 b | ||
| PPI | 15 | 0.6 |  | N.D. | N.D. | N.D. | N.D. |
| 1.5 |  | N.D. | N.D. | N.D. | N.D. | ||
| 20 | 0.6 |  | 48.48 ± 0.29 b | 11.52 ± 0.07 b | 0.24 ± 0.01 c | 84.76 ± 3.56 b | |
| 1.5 | 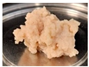 | 51.57 ± 6.38 b | 12.56 ± 1.64 b | 0.24 ± 0.01 c | 91.96 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Berardinis, L.; Plazzotta, S.; Manzocco, L. Optimising Soy and Pea Protein Gelation to Obtain Hydrogels Intended as Precursors of Food-Grade Dried Porous Materials. Gels 2023, 9, 62. https://doi.org/10.3390/gels9010062
De Berardinis L, Plazzotta S, Manzocco L. Optimising Soy and Pea Protein Gelation to Obtain Hydrogels Intended as Precursors of Food-Grade Dried Porous Materials. Gels. 2023; 9(1):62. https://doi.org/10.3390/gels9010062
Chicago/Turabian StyleDe Berardinis, Lorenzo, Stella Plazzotta, and Lara Manzocco. 2023. "Optimising Soy and Pea Protein Gelation to Obtain Hydrogels Intended as Precursors of Food-Grade Dried Porous Materials" Gels 9, no. 1: 62. https://doi.org/10.3390/gels9010062
APA StyleDe Berardinis, L., Plazzotta, S., & Manzocco, L. (2023). Optimising Soy and Pea Protein Gelation to Obtain Hydrogels Intended as Precursors of Food-Grade Dried Porous Materials. Gels, 9(1), 62. https://doi.org/10.3390/gels9010062