Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing
Abstract
1. Introduction
2. Results
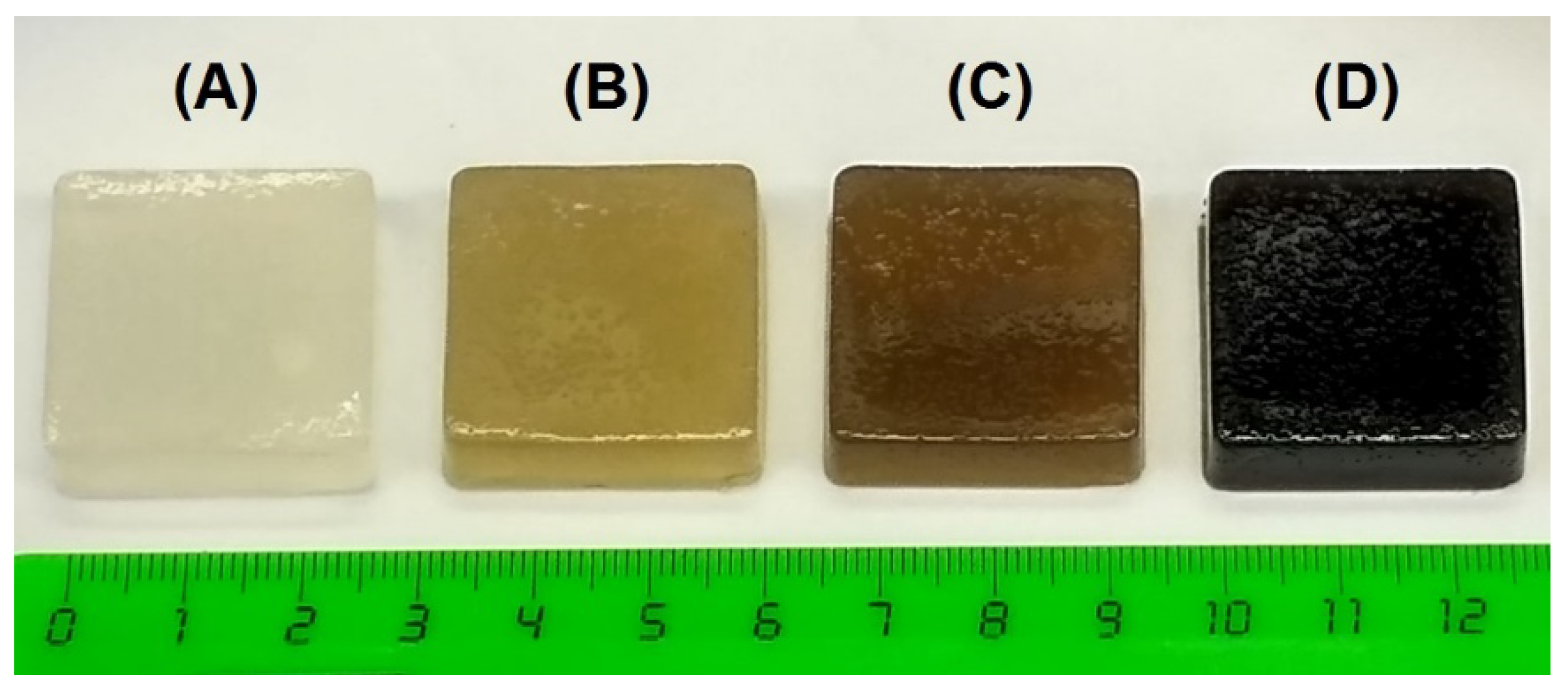
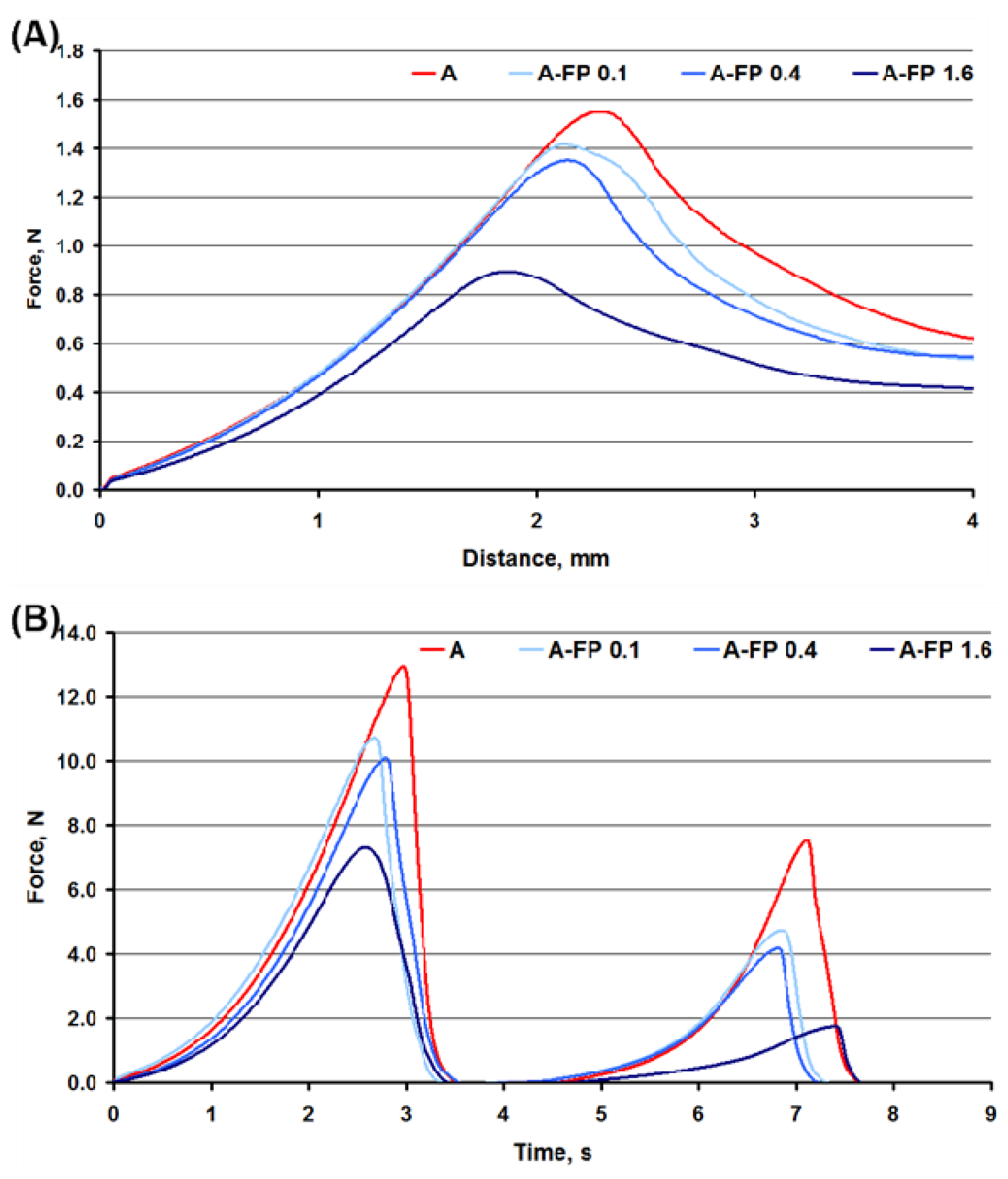
2.1. General Characterization and Mechanical Properties of Gels
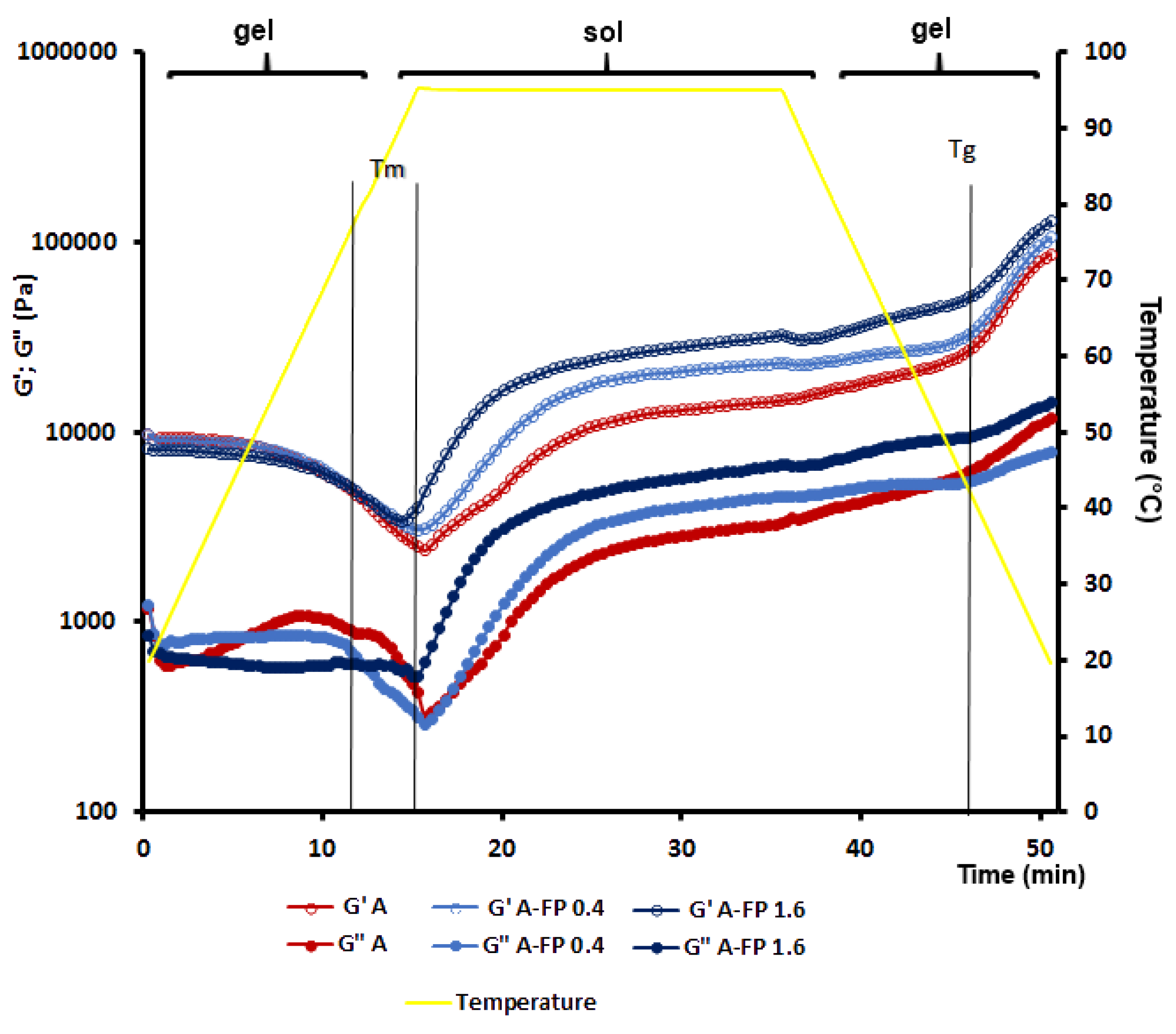
2.2. Rheological Properties of Gels
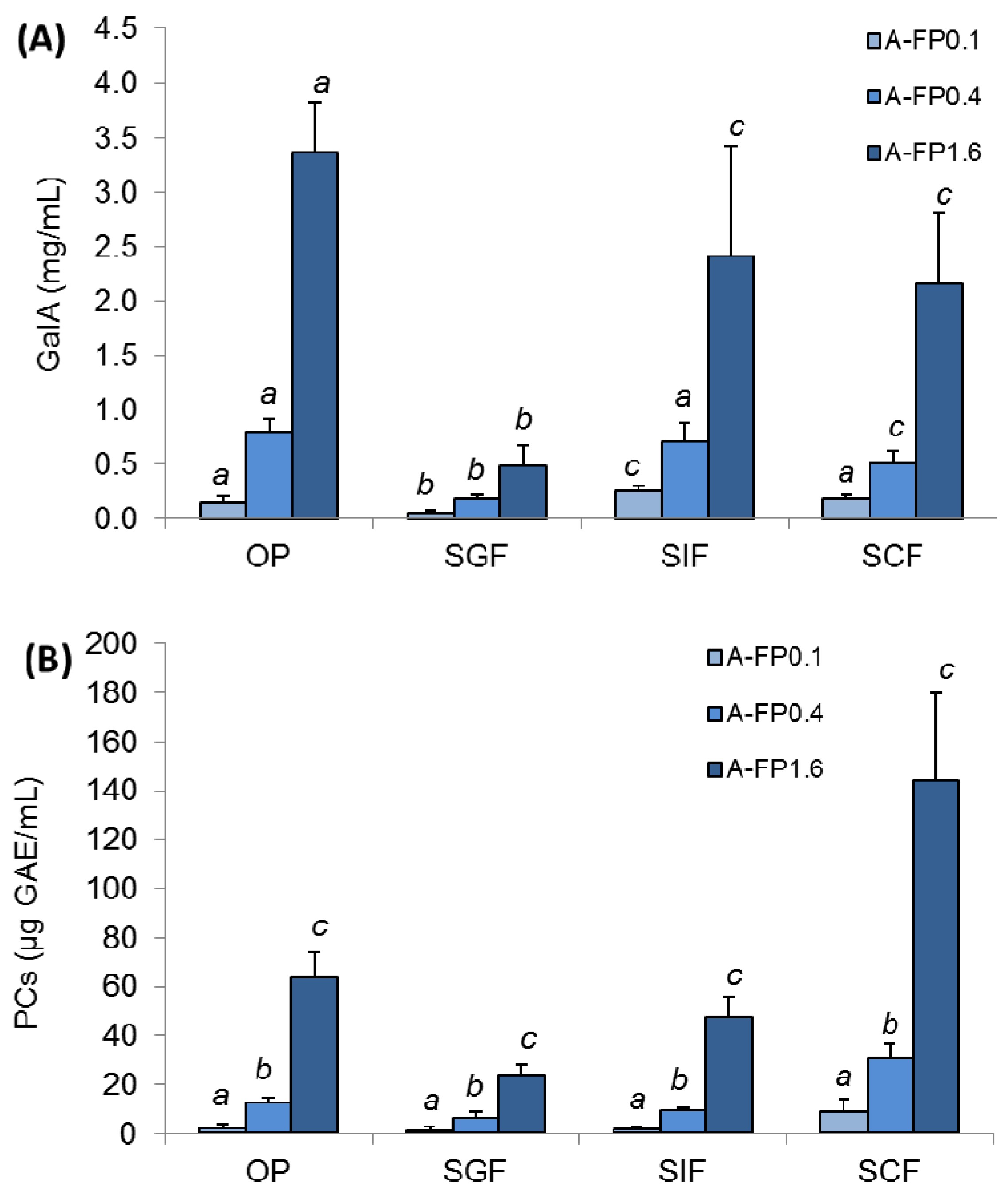
2.3. Pectin and PCs Release during Simulated Digestion of Gels
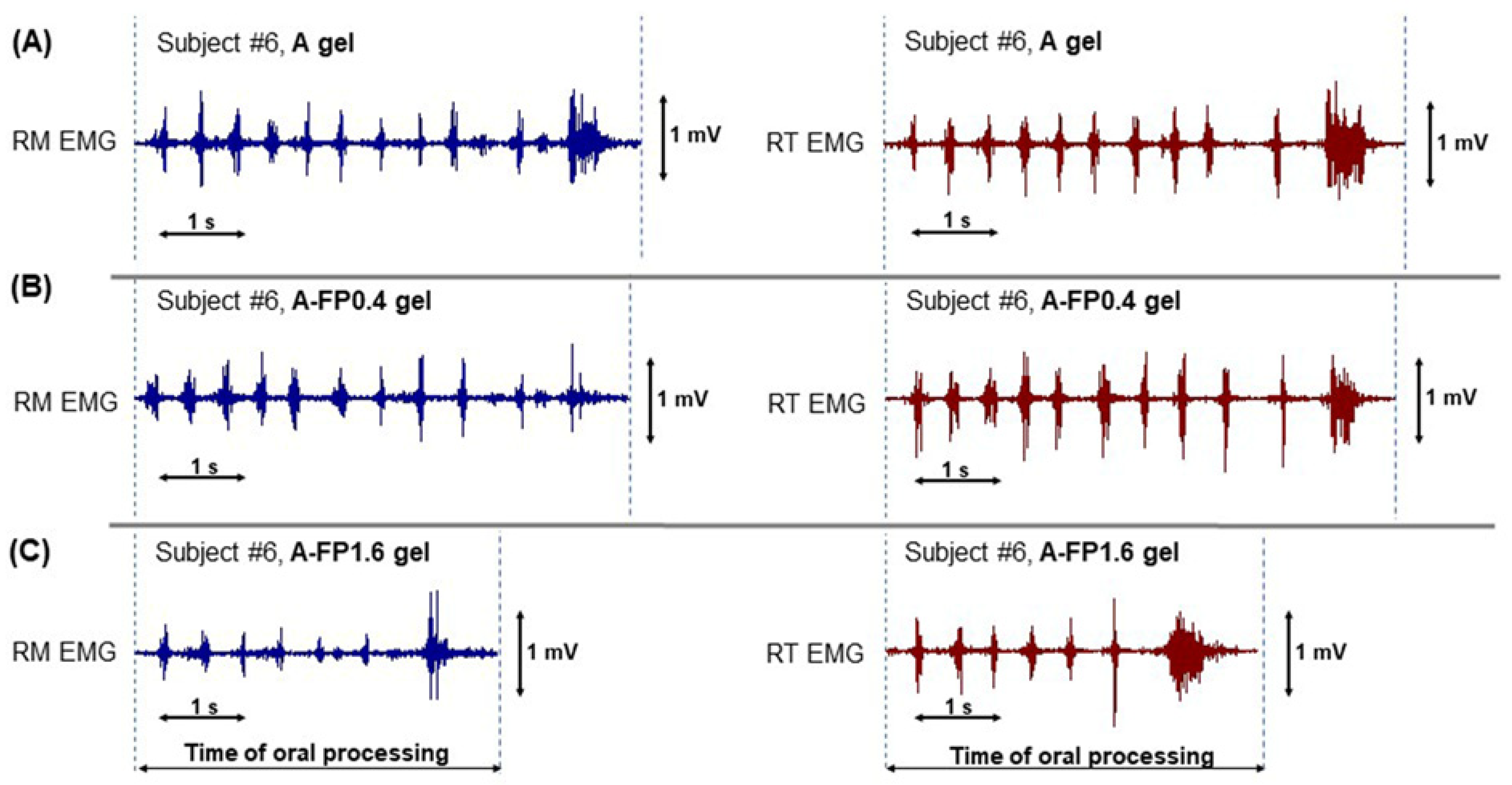
2.4. Oral Processing of Gels
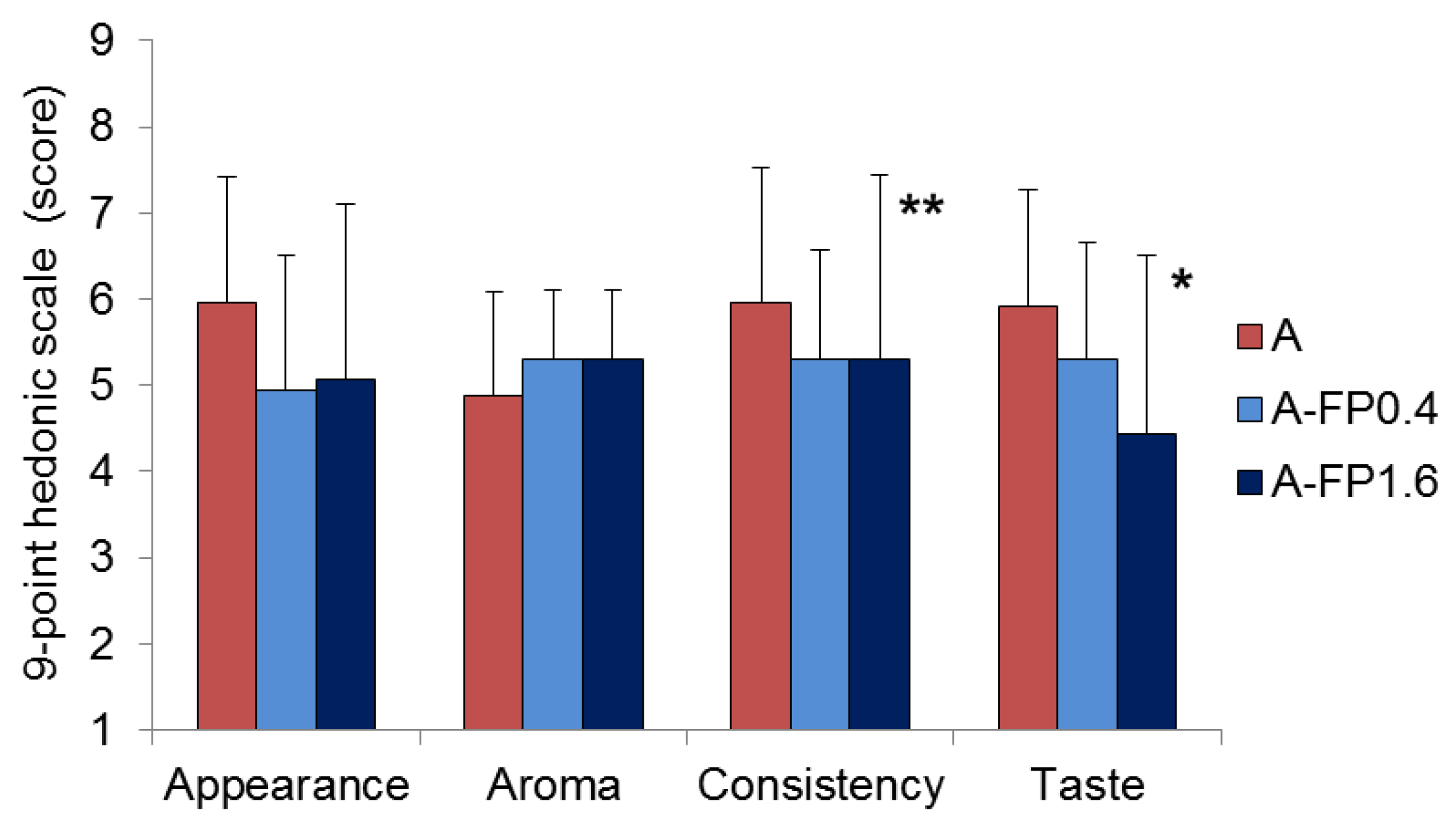
2.5. Acceptability of Gels
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and General Characterization of A and A-FP Gels
4.3. Measurement of Mechanical Properties
4.4. Measurement of Rheological Properties
4.5. In Vivo Oral Phase (OP) and Static In Vitro Gastrointestinal Digestion
4.6. EMG and Acceptability Test
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. Novel Technologies for Seaweed Polysaccharides Extraction and Their Use in Food with Therapeutically Applications—A Review. Foods 2022, 11, 2654. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.F. Agars. In Food Polysaccharides and Their Applications; Stephen, A.M., Phillips, G.J., Williams, P.A., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2006; pp. 217–238. [Google Scholar]
- Hehemann, J.-H.; Smyth, L.; Yadav, A.; Vocadlo, D.J.; Boraston, A.B. Analysis of keystone enzyme in agar hydrolysis provides insight into the degradation of a polysaccharide from red seaweeds. J. Biol. Chem. 2012, 287, 13985–13995. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Armisen, R.; Galatas, F. Agar. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2009; pp. 82–107. [Google Scholar]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowlan, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Yang, Y.; Anderson, C.T. Biosynthesis, localization, and function of pectins in plants. In Pectin: Technological and Physiological Properties; Springer: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Ropartz, D.; Ralet, M.-C. Pectin structure. In Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–36. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Tan, M.; Chang, S.; Liu, J.; Li, H.; Xu, P.; Wang, P.; Wang, X.; Zhao, M.; Zhao, B.; Wang, L.; et al. Physicochemical Properties, Antioxidant and Antidiabetic Activities of Polysaccharides from Quinoa (Chenopodium quinoa Willd.) Seeds. Molecules 2020, 25, 3840. [Google Scholar] [CrossRef]
- Wu, D.-T.; Liu, W.; Han, Q.-H.; Wang, P.; Xiang, X.-R.; Ding, Y.; Zhao, L.; Zhang, Q.; Li, S.-Q.; Qin, W. Extraction Optimization, Structural Characterization, and Antioxidant Activities of Polysaccharides from Cassia Seed (Cassia obtusifolia). Molecules 2019, 24, 2817. [Google Scholar] [CrossRef] [PubMed]
- Klosterhoff, R.R.; Bark, J.M.; Glänzel, N.M.; Iacomini, M.; Martinez, G.R.; Winnischofer, S.M.B.; Cordeiro, L.M.C. Structure and intracellular antioxidant activity of pectic polysaccharide from acerola (Malpighia emarginata). Int. J. Biol. Macromol. 2018, 106, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Patova, O.; Smirnov, V.; Golovchenko, V.; Vityazev, F.; Shashkov, A.; Popov, S. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Popov, S.; Smirnov, V.; Kvashninova, E.; Khlopin, V.; Vityazev, F.; Golovchenko, V. Isolation, chemical characterization and antioxidant activity of pectic polysaccharides of fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 7290. [Google Scholar] [CrossRef]
- Akkaya, N.E.; Ergun, C.; Saygun, A.; Yesilcubuk, N.; Akel-Sadoglu, N.; Kavakli, I.H.; Turkmen, H.S.; Catalgil-Giz, Y. New biocompatible antibacterial wound dressing candidates; agar-locust bean gum and agar-salep films. Intern. J. Biomacromol. 2020, 155, 430–438. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Kakino, Y.; Tsukamoto, H.; Tahara, K.; Onodera, R.; Takeuchi, H. Control of drug diffusion behavior of xanthan and locust bean gum gel by agar gel. Chem. Pharm. Bull. 2016, 64, 1450–1457. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Goncalves, M.P. Strategies to improve the mechanical strength and water resistance of agar films for food packaging application. Carbohydr. Polym. 2015, 132, 196–204. [Google Scholar] [CrossRef]
- Rózek, A.; Achaerandio, I.; Güell, C.; López, F.; Ferrando, M. Direct formulation of a solid foodstuff with phenolic-rich multicomponent solutions from grape seed: Effects on composition and antioxidant properties. J. Agric. Food. Chem. 2008, 56, 4564–4576. [Google Scholar] [CrossRef]
- Banerjee, S.; Ravi, R.; Bhattacharya, S. Textural characterization of gellan and agar based fabricated gels with carrot juice. LWT-Food Sci. Technol. 2013, 53, 255–261. [Google Scholar] [CrossRef]
- Rahman, J.M.H.; Shiblee, N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Rheological and mechanical properties of edible gel materials for 3D food printing technology. Heliyon 2020, 6, e05859. [Google Scholar] [CrossRef] [PubMed]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Nie, S. Deciphering diet-gut microbiota-host interplay: Investigation of pectin. Trends Food Sci. Technol. 2020, 106, 171–181. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; Van de Velde, F.; Stieger, M. Bolus matters: The influence of food oral breakdown on dynamic texture perception. Food Funct. 2017, 8, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Pascua, Y.; Koc, H.; Foegeding, E.A. Food structure: Roles of mechanical properties and oral processing in determining sensory texture of soft materials. Curr. Opin. Colloid Interface Sci. 2013, 18, 324–333. [Google Scholar] [CrossRef]
- Koc, H.; Vinyard, C.J.; Essick, G.K.; Foegeding, E.A. Food oral processing: Conversion of food structure to textural perception. Annu. Rev. Food Sci. Technol. 2013, 4, 237–266. [Google Scholar] [CrossRef]
- Wagoner, T.B.; Luck, P.J.; Foegeding, E.A. Caramel as a model system for evaluating the roles of mechanical properties and oral processing on sensory perception of texture. J. Food Sci. 2016, 81, S736–S744. [Google Scholar] [CrossRef]
- Kohyama, K.; Gao, Z.; Watanabe, T.; Ichihara, S.; Nakao, S.; Funami, T. Relationships between mechanical properties obtained from compression test and electromyography variables during natural oral processing of gellan gum gels. J. Text Stud. 2017, 48, 66–75. [Google Scholar] [CrossRef]
- Peyron, M.A.; Lassauzay, C.; Woda, A. Effects of increased hardness on jaw movement and muscle activity during chewing of visco-elastic model foods. Exp. Brain Res. 2002, 142, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Laureati, M.; Sandvik, P.; Almli, V.L.; Sandell, M.; Zeinstra, G.G.; Methven, L.; Wallner, M.; Jilani, H.; Alfaro, B.; Proserpio, C. Individual differences in texture preferences among European children: Development and validation of the Child Food Texture Preference Questionnaire (CFTPQ). Food Qual. Prefer. 2020, 80, 103828. [Google Scholar] [CrossRef]
- Duizer, L.M.; Field, K. Changes in sensory perception during aging. In Modifying Food Texture Volume 2: Sensory Analysis, Consumer Requirements and Preferences; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 19–44. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Ramos, F.; Saraiva, A.; Raposo, A. Texture-Modified Food for Dysphagic Patients: A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 5125. [Google Scholar] [CrossRef]
- Puleo, S.; Valentino, M.; Masi, P.; DiMonaco, R. Hardness sensitivity: Are old, young, female and male subjects all equally sensitive? Food Qual. Prefer. 2021, 90, 104118. [Google Scholar] [CrossRef]
- Mendoza, M.A.; Santagiuliana, M.; Ong, X.; Piqueras-Fiszman, B.; Scholten, E.; Stieger, M. How addition of peach gel particles to yogurt affects oral behavior, sensory perception and liking of consumers differing in age. Food Res. Intern. 2020, 134, 109213. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Yaich, H.; Hidouri, H.; Attia, H.; Ayadi, M.A. Effect of substituted gelling agents from pomegranate peel on colour, textural and sensory properties of pomegranate jam. Food Chem. 2018, 239, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Shivhare, U.S. Rheological, textural, micro-structural and sensory properties of mango jam. J. Food Eng. 2010, 100, 357–365. [Google Scholar] [CrossRef]
- Khin, M.N.; Goff, H.D.; Nsor-Atindana, J.; Ahammed, S.; Liu, F.; Zhong, F. Effect of texture and structure of polysaccharide hydrogels containing maltose on release and hydrolysis of maltose during digestion: In vitro study. Food Hydrocoll. 2021, 112, 106326. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.D.P.M.; Martins, I.M.; de Ávila, A.R.A.; Nakajima, V.M.; Dos Prazeres, A.R.; Macedo, J.A.; Macedo, G.A. Flavanones biotransformation of citrus by-products improves antioxidant and ACE inhibitory activities in vitro. Food Biosci. 2020, 38, 100787. [Google Scholar] [CrossRef]
- Ge, S.; Liu, Q.; Li, M.; Liu, J.; Lu, H.; Li, F.; Zhang, S.; Sun, Q.; Xiong, L. Enhanced mechanical properties and gelling ability of gelatin hydrogels reinforced with chitin whiskers. Food Hydrocoll. 2018, 75, 1–12. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Rustad, T.; Guerreroa, A. Rheological properties and antioxidant activity of protein gels-like systems made from crayfish concentrate and hydrolysates. Food Bioprod. Process. 2017, 102, 167–176. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.C.; Shangguan, X.; Wang, H.; Sha, X.; Bansal, N. Rheological behavior, emulsifying properties and structural characterization of phosphorylated fish gelatin. Food Chem. 2018, 246, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, S.D. Applicability of rheological models to the interpretation of flow and processing behavior of fluid food products. J. Texture Stud. 1971, 2, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, D.H.S.; Bhattacharya, M. Relaxation behavior and the application of integral constitutive equations to wheat dough. J. Texture Stud. 1996, 27, 517–544. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Fedyuneva, M.I.; Golovchenko, V.V.; Patova, O.A.; Ipatova, E.U.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G. Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr. Polym. 2017, 157, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Usov, A.I.; Bilan, M.I.; Klochkova, N.G. Polysaccharides of algae. 48. Polysaccharide composition of several Calcareous red algae: Isolation of alginate from Corallina Pilulifera P. et R. (Rhodophyta, Corallinaceae). Bot. Mar. 1995, 38, 43–51. [Google Scholar] [CrossRef]
- Peng, J.; Lu, L.; Zhu, K.X.; Guo, X.N.; Chen, Y.; Zhou, H.M. Effect of rehydration on textural properties, oral behavior, kinetics and water state of textured wheat gluten. Food Chem. 2022, 376, 131934. [Google Scholar] [CrossRef]
- Kohyama, K.; Gao, Z.; Ishihara, S.; Funami, T.; Nishinari, K. Electromyography analysis of naturalmastication behavior using varying mouthful quantities of two types of gels. Physiol. Behav. 2016, 161, 174–182. [Google Scholar] [CrossRef]
- Lawless, H.; Heymann, H. Introduction. In Sensory Evaluation of Food; Food Science Text Series; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]


| A | A-FP0.1 | A-FP0.4 | A-FP1.6 | |
|---|---|---|---|---|
| Total PC, μgGAE/g (n = 3) | - | 23 ± 5 a | 84 ± 17 b | 418 ± 101 c |
| Loading efficiency, % (n = 3) | - | 86 ± 18 a | 78 ± 15 a | 97 ± 23 a |
| Puncture test: Hardness, N (n = 12) Consistency, mJ (n = 12) | 1.57 ± 0.09 a 3.18 ± 0.10 a | 1.43 ± 0.33 a,b 2.74 ± 0.55 b | 1.35 ± 0.26 b 2.66 ± 0.45 b | 0.91 ± 0.14 c 1.99 ± 0.29 c |
| pH (n = 3) | 6.22 ± 0.17 a | 5.45 ± 0.07 b | 5.16 ± 0.03 c | 4.77 ± 0.02 d |
| WL, % (n = 8) | 92.2 ± 0.1 a | 92.0 ± 0.1 b | 91.6 ± 0.1 c | 90.3 ± 0.2 d |
| Serum release, % (n = 12) | 0.68 ± 0.08 a | 0.67 ± 0.27 a | 0.46 ± 0.19 b | 0.33 ± 0.09 c |
| Tg, °C (n = 8) | 39.8 ± 1.2 a | 40.6 ± 1.6 a | 40.1 ± 0.9 a | 41.0 ± 0.8 b |
| Tm, °C (n = 8) | 69.4 ± 4.3 a | 69.4 ± 4.4 a | 70.2 ± 2.6 a | 71.0 ± 2.6 a |
| A | A-FP0.1 | A-FP0.4 | A-FP1.6 | |
|---|---|---|---|---|
| Hardness, N | 12.3 ± 0.3 a | 10.7 ± 2.8 a,b | 10.1 ± 2.0 b | 7.3 ± 1.0 c |
| Cohesiveness | 0.43 ± 0.02 a | 0.33 ± 0.14 b | 0.27 ± 0.12 b | 0.20 ± 0.02 c |
| Chewiness | 4.5 ± 0.4 a | 3.5 ± 2.2 a,b | 3.1 ± 1.8 b | 1.5 ± 0.3 c |
| Springiness | 0.83 ± 0.01 a | 0.90 ± 0.06 b | 0.91 ± 0.06 b | 1.03 ± 0.5 c |
| Resilience | 0.16 ± 0.02 a | 0.27 ± 0.11 b | 0.29 ± 0.12 b | 0.45 ± 0.05 c |
| Gels | Storage Modulus | Viscosity | |||
|---|---|---|---|---|---|
| A (Pa) | B (Slope) | R2 | ηapp 0.045, Hz | ηapp 10.500, Hz | |
| A | 8550.0 | 0.054 | 0.951 | 12939.6 ± 3048.5 a | 152.1 ± 21.9 a |
| A-FP0.1 | 9110.6 | 0.032 | 0.998 | 14143.6 ± 3235.0 a | 149.8 ± 29.4 a |
| A-FP0.4 | 9478.9 | 0.037 | 0.996 | 14574.9 ± 3323.2 a | 149.2 ± 29.3 a |
| A-FP1.6 | 7646.3 | 0.047 | 0.997 | 11439.0 ± 1918.9 a | 129.3 ± 18.7 b |
| A | A-FP0.4 | A-FP1.6 | |
|---|---|---|---|
| Chewing time, s | 13.2 ± 8.2 a | 12.6 ± 8.7 a | 9.3 ± 5.1 b |
| Number of chews, times | 18.3 ± 6.9 a | 18.7 ± 12.0 a | 13.3 ± 5.9 b |
| RM activity per sequence, mV × s | 0.26 ± 0.08 a | 0.25 ± 0.14 a | 0.18 ± 0.93 b |
| RT activity per sequence, mV × s | 0.37 ± 0.20 a | 0.35 ± 0.20 a | 0.19 ± 0.12 b |
| Total muscle activities per sequence, mV × s | 0.63 ± 0.26 a | 0.59 ± 0.29 a | 0.40 ± 0.21 b |
| Total muscle activities per chew, mV × s | 0.04 ± 0.01 a | 0.03 ± 0.02 a | 0.03 ± 0.01 b |
| Sample | UA a | Gal a | Xyl a | Glc a | Rha a | Ara a | Mw, kDa |
|---|---|---|---|---|---|---|---|
| A | 3.9 | 20.0 | 3.0 | 0.9 | 0.2 | 0.7 | 280 |
| FP | 66.1 | 1.7 | 0.9 | 0.5 | 1.2 | 0.9 | 232 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, S.; Smirnov, V.; Paderin, N.; Khramova, D.; Chistiakova, E.; Vityazev, F.; Golovchenko, V. Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels 2022, 8, 708. https://doi.org/10.3390/gels8110708
Popov S, Smirnov V, Paderin N, Khramova D, Chistiakova E, Vityazev F, Golovchenko V. Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels. 2022; 8(11):708. https://doi.org/10.3390/gels8110708
Chicago/Turabian StylePopov, Sergey, Vasily Smirnov, Nikita Paderin, Daria Khramova, Elizaveta Chistiakova, Fedor Vityazev, and Victoria Golovchenko. 2022. "Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing" Gels 8, no. 11: 708. https://doi.org/10.3390/gels8110708
APA StylePopov, S., Smirnov, V., Paderin, N., Khramova, D., Chistiakova, E., Vityazev, F., & Golovchenko, V. (2022). Enrichment of Agar Gel with Antioxidant Pectin from Fireweed: Mechanical and Rheological Properties, Simulated Digestibility, and Oral Processing. Gels, 8(11), 708. https://doi.org/10.3390/gels8110708







