The Future Packaging of the Food Industry: The Development and Characterization of Innovative Biobased Materials with Essential Oils Added
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
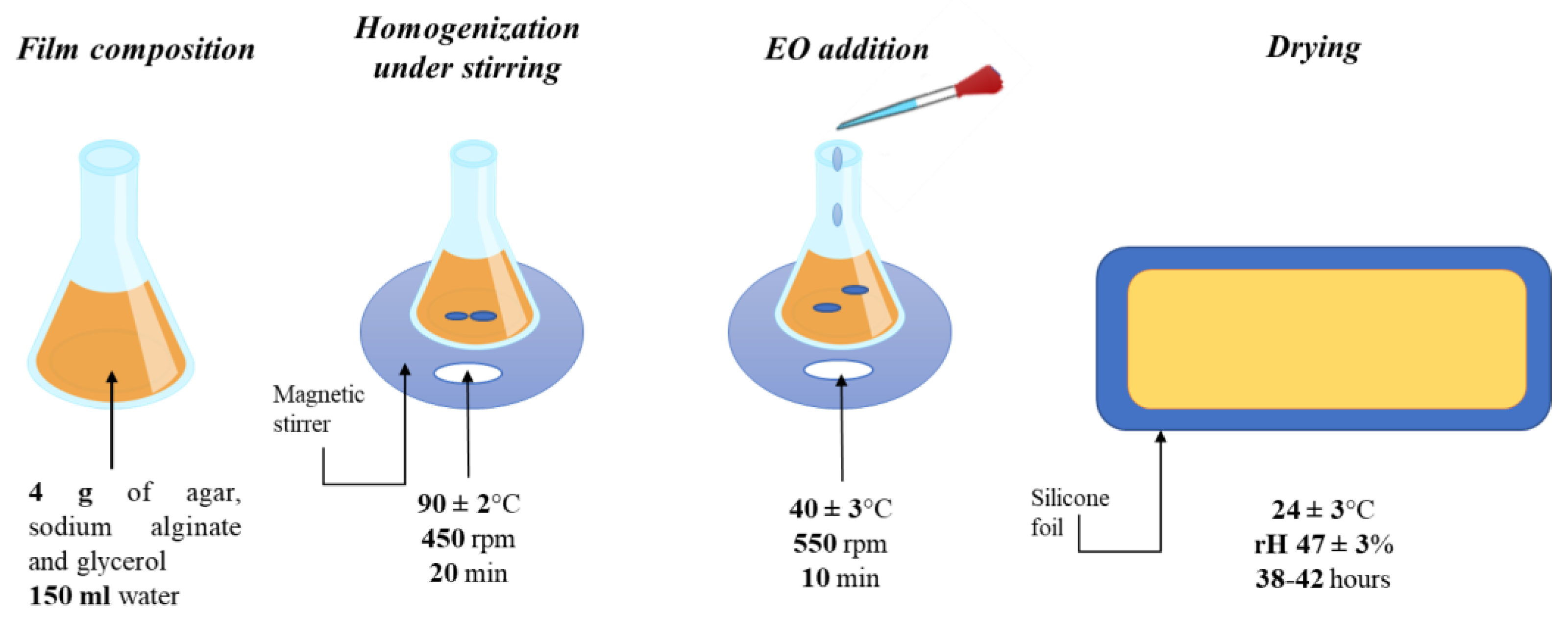
4. Materials and Methods
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, L.I.; Tan, H.L.; Pui, L.P. Development and characterization of alginate-based edible film incorporated with hawthorn berry (Crataegus pinnatifida) extract. J. Food Meas. Charact. 2021, 15, 2540–2548. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.; Mat Nor, M.S. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Maan, A.A.; Reiad Ahmed, Z.F.; Iqbal Khan, M.K.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K.H.; Roy, S.; Rhim, J.W. Advances in Functional Biopolymer-Based Nanocomposites for Active Food Packaging Applications. Polymers 2021, 13, 4198. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Rajesh Kumar Dora, T.; Ghosh, S.; Damodar, R. Synthesis and evaluation of physical properties of Agar biopolymer film coating; an alternative for food packaging industry. Mater. Res. Express 2020, 7, 095307. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2021, 20, 875–900. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Besliu, I.; Gutt, G. Edible Biopolymers-Based Materials for Food Applications—The Eco Alternative to Conventional Synthetic Packaging. Polymers 2021, 13, 3779. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine Polysaccharides for Wound Dressings Application: An Overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, V.; Cerbu, C.; Witkovski, L.; Potarniche, A.V.; Timar, M.C.; Zhyska, M.; Sabliov, C. Nanodelivery of essential oils as efficient tools against antimicrobial resistance: A review of the type and physical-chemical properties of the delivery systems and applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging; History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 32–58. [Google Scholar] [CrossRef]
- Vieira, T.M.; Moldão-Martins, M.; Alves, V.D. Design of Chitosan and Alginate Emulsion-Based Formulations for the Production of Monolayer Crosslinked Edible Films and Coatings. Foods 2021, 10, 1654. [Google Scholar] [CrossRef]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Jayakody, M.M.; Vanniarachchy, M.P.G.; Wijesekara, I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: A review. J. Food Meas. Charact. 2022, 16, 1195–1227. [Google Scholar] [CrossRef]
- EUR-Lex—32009R0450—EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R0450 (accessed on 23 July 2022).
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Santhosh, R.; Nath, D.; Sarkar, P. Novel food packaging materials including plant-based byproducts: A review. Trends Food Sci. Technol. 2021, 118, 471–489. [Google Scholar] [CrossRef]
- Dash, K.K.; Kumar, A.; Kumari, S.; Malik, M.A. Silver Nanoparticle Incorporated Flaxseed Protein-Alginate Composite Films: Effect on Physicochemical, Mechanical, and Thermal Properties. J. Polym. Environ. 2021, 29, 3649–3659. [Google Scholar] [CrossRef]
- Syafiq, R.M.O.; Sapuan, S.M.; Zuhri, M.R.M. Effect of cinnamon essential oil on morphological, flammability and thermal properties of nanocellulose fibre-reinforced starch biopolymer composites. Nanotechnol. Rev. 2020, 9, 1147–1159. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, M.; Feng, X.; Yao, Z.; Wang, T.; Xu, C. Effect of lemon essential oil-enriched coating on the postharvest storage quality of citrus fruits. Food Sci. Technol. 2022, 42, e125421. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sarmeen, D.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 60, 340–351. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.C.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Kavas, G.; Kavas, N.; Saygili, D. The Effects of Thyme and Clove Essential Oil Fortified Edible Films on the Physical, Chemical and Microbiological Characteristics of Kashar Cheese. J. Food Qual. 2015, 38, 405–412. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Utomo, D.P.; Raemas, A.F.; Kusworo, T.D.; Jos, B.; Djaeni, M. Alginate/κ-Carrageenan-Based Edible Films Incorporated with Clove Essential Oil: Physico-Chemical Characterization and Antioxidant-Antimicrobial Activity. Polymers 2021, 13, 354. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Antimicrobial activity of alginate/clay nanocomposite films enriched with essential oils against three common foodborne pathogens. Food Control 2014, 36, 1–7. [Google Scholar] [CrossRef]
- Jalali, N.; Ariiai, P.; Fattahi, E. Effect of alginate/carboxyl methyl cellulose composite coating incorporated with clove essential oil on the quality of silver carp fillet and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Food Sci. Technol. 2016, 53, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Akhter, R.; Masoodi, F.A.; Wani, T.A.; Rather, S.A. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int. J. Biol. Macromol. 2019, 137, 1245–1255. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef]
- Azadbakht, E.; Maghsoudlou, Y.; Khomiri, M.; Kashiri, M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packag. Shelf Life 2018, 7, 65–72. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Lan, X.; Huang, D.; Luo, T.; Ji, J.; Mafang, Z.; Maio, X.; Wang, H.; Wang, W. Electrospun Gelatin Nanofibers Encapsulated with Peppermint and Chamomile Essential Oils as Potential Edible Packaging. J. Agric. Food Chem. 2019, 67, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, Y.; Pajohi-Alamoti, M.; Bazargani-Gilani, B. Development of cellulose nanofibers coating incorporated with ginger essential oil and citric acid to extend the shelf life of ready-to-cook barbecue chicken. J. Food Process. Preserv. 2019, 43, e14114. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, E.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf life assessment of fresh poultry meat packaged in novel bionanocomposite of chitosan/montmorillonite incorporated with ginger essential oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Al-Hilifi, S.A.; Al-Ali, R.M.; Petkoska, A.T. Ginger Essential Oil as an Active Addition to Composite Chitosan Films: Development and Characterization. Gels 2022, 8, 327. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y.; Cao, A. The physiochemical and preservation properties of fish sarcoplasmic protein/chitosan composite films containing ginger essential oil emulsions. J. Food Process Eng. 2020, 43, e13495. [Google Scholar] [CrossRef]
- Mostaghimi, M.; Majdinasab, M.; Hosseini, S.M. Characterization of Alginate Hydrogel Beads Loaded with Thyme and Clove Essential Oils Nanoemulsions. J. Polym. Environ. 2022, 30, 1647–1661. [Google Scholar] [CrossRef]
- Valizadeh, A.; Shirzad, M.; Esmaeili, F.; Amani, A. Increased antibacterial activity of Cinnamon Oil Microemulsionin Comparison with Cinnamon Oil Bulk and Nanoemulsion. Nanomed. Res. J. 2018, 3, 37–43. [Google Scholar]
- Lim, Z.Q.; Tong, S.Y.; Wang, K.; Lim, P.N.; Thian, E.S. Cinnamon oil incorporated polymeric films for active food packaging. Mater. Lett. 2022, 313, 131744. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. Antibacterial activity of agar-based films containing nisin, cinnamon EO, and ZnO nanoparticles. J. Food Saf. 2018, 38, e12440. [Google Scholar] [CrossRef]
- Kunová, S.; Sendra, E.; Haščík, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Influence of Essential Oils on the Microbiological Quality of Fish Meat during Storage. Animals 2021, 11, 3145. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Kulawik, P. The antimicrobial effect of grapefruit peel essential oil and its nanoemulsion on fish spoilage bacteria and food-borne pathogens. LWT 2021, 136, 110362. [Google Scholar] [CrossRef]
- Khalid, K.A.; Darwesh, O.M.; Ahmed, A.M. Peel Essential Oils of Citrus Types and Their Antimicrobial Activities in Response to Various Growth Locations. J. Essent. Oil-Bear. Plants 2021, 24, 480–499. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; Albiero, B.; Cipriano, L.; de Oliviera, C.C.; Bezerra, N.; Oldoni, F.; Egea, M.; de Azeredo, H.M.C.; Ferreira, M.D. Arrowroot starch-based films incorporated with a carnauba wax nanoemulsion, cellulose nanocrystals, and essential oils: A new functional material for food packaging applications. Cellulose 2021, 28, 6499–6511. [Google Scholar] [CrossRef]
- Bag, A.; aChattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, 131321. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita Puscaselu, R.; Amariei, S.; Norocel, L.; Gutt, G. New edible packaging material with function in shelf life extension: Applications for the meat and cheese industries. Foods 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- “Tensile Testing of Thin Plastic Sheeting (Film) ASTM D882”. Available online: https://www.intertek.com/polymers/tensile-testing/astm-d882/ (accessed on 8 August 2022).
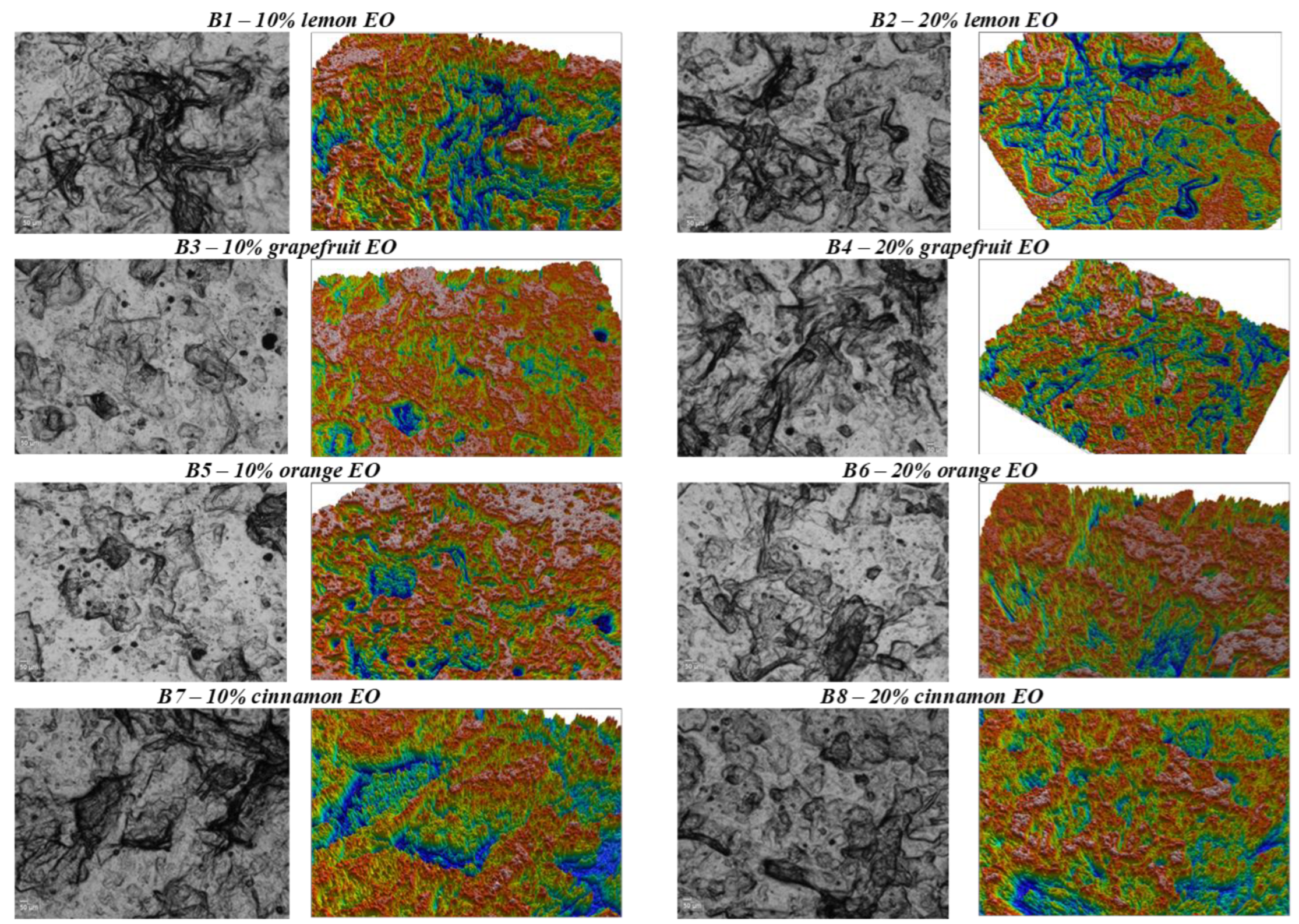
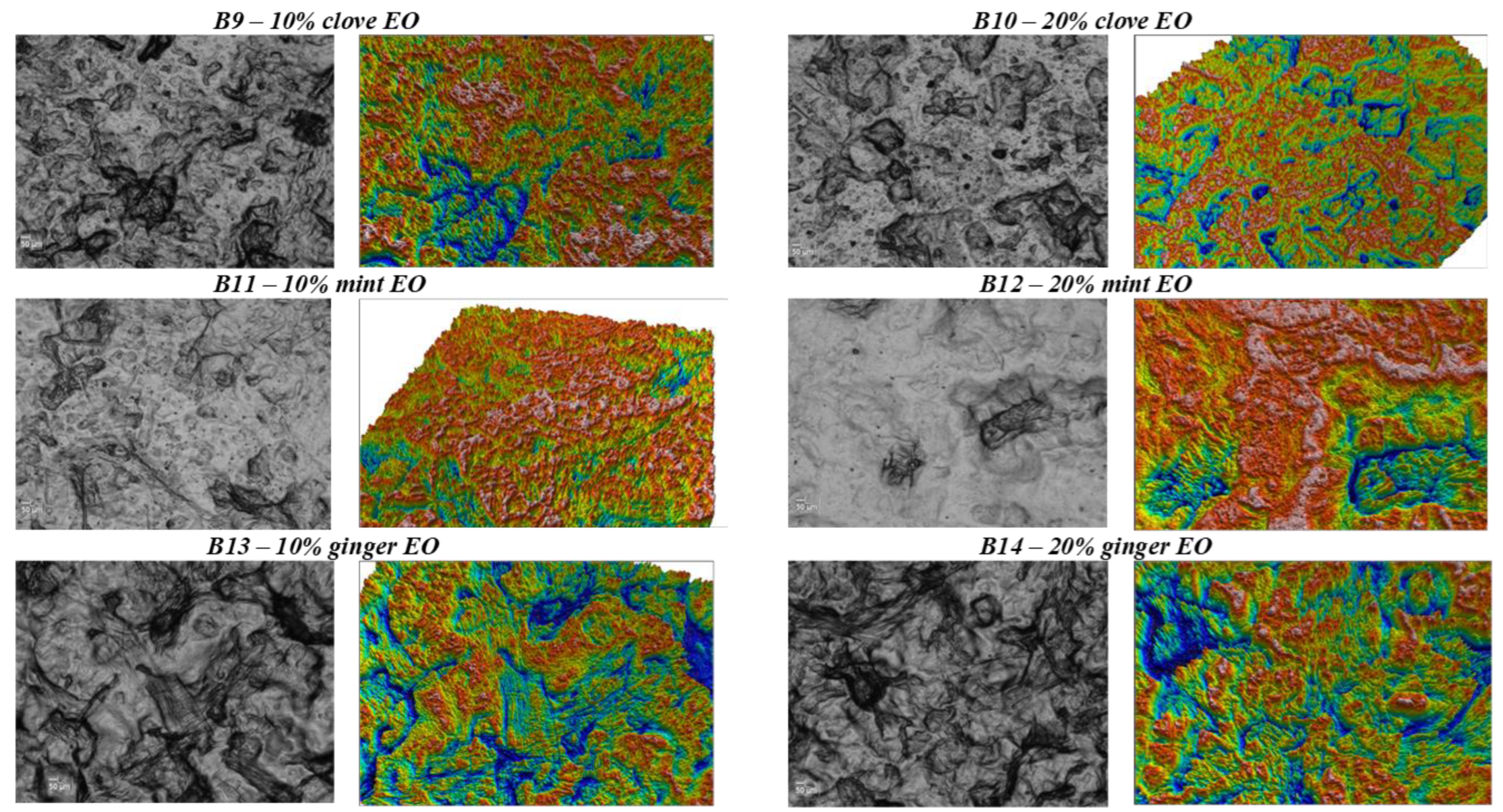
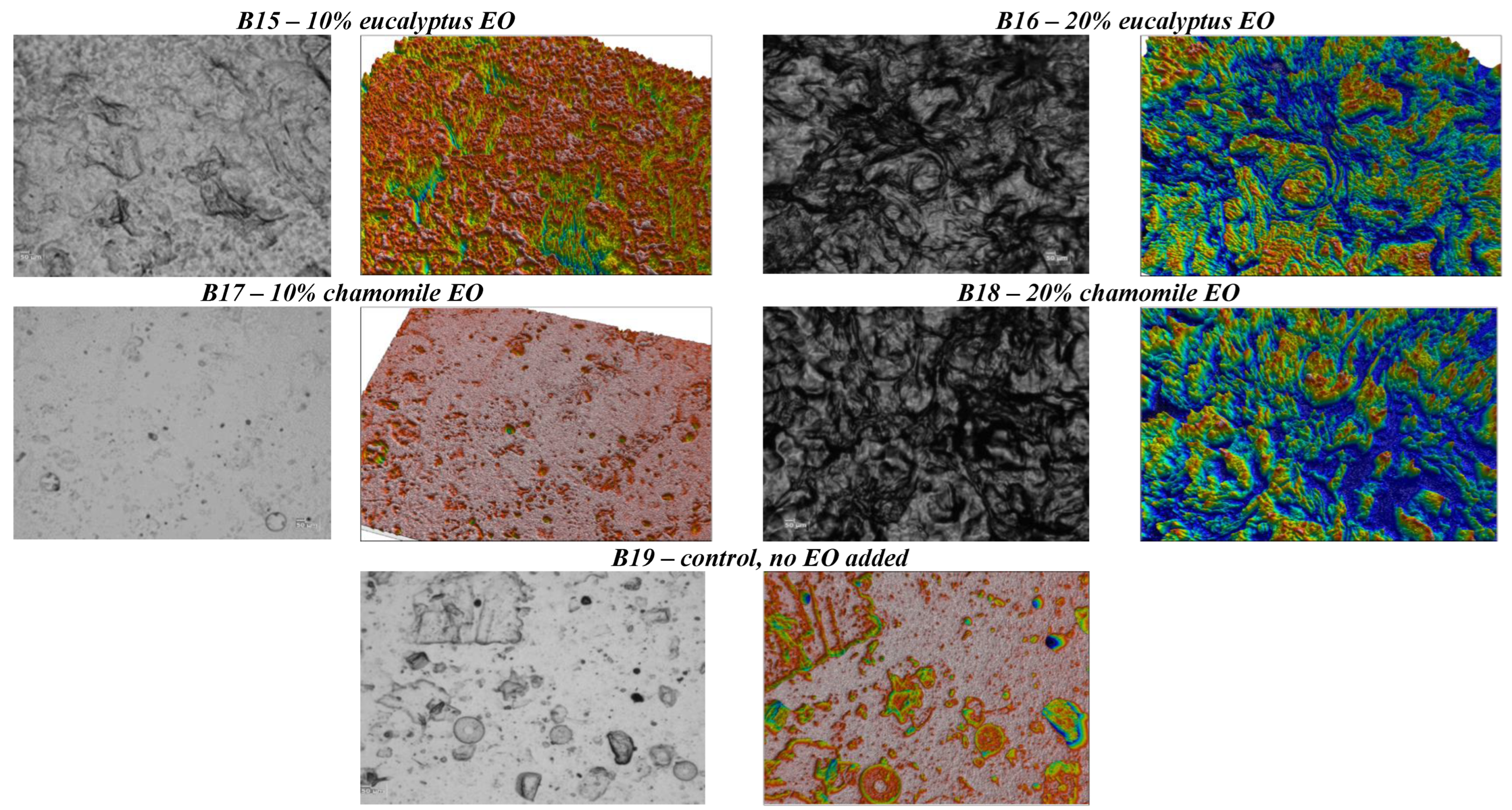

| Biopolymer | EO | Food Product | Beneficial Effect | References |
|---|---|---|---|---|
| Starch | Cinnamon | Active packaging | Thermal stability, but porous microstructure | [26] |
| Chitosan | Lemon | Citrus | Extended shelf -life, improved food storage quality. antimicrobial effect | [27] |
| Collagen/chitosan | Lemon | Pork meat | Prolonged the shelf life for 21 days, inhibited lipid oxidation, and prevent microbial proliferation | [28] |
| Pectin | Clove | Fish (bream) | Inhibited the growth of Gram-negative bacteria; the level of lactic acid bacteria remained constant | [29] |
| Whey protein isolate | Clove | Cheese | Positive effects of the physical-chemical properties of cheese. E. coli, S. aureus, and L. monocytogenes decreased during 60 days of testing | [30] |
| Alginate/k-carrageenan | Clove | Food packaging | Antimicrobial and antioxidant effect; the addition of EO reduced the mechanical properties of film, but improved the flexibility | [31] |
| Alginate/clay | Clove, coriander, cinnamon, cumin, caraway, marjoram | Food packaging | Inhibited the growth of Gram-negative bacteria | [32] |
| Alginate/CMC | Clove | Fish | Without loss of color, odor, texture during storage (16 days) | [33] |
| Chitosan/pectin/starch | Mint | Food packaging | Antioxidant and antimicrobial effect; improved barrier properties, tensile strength, and thermal stability | [34] |
| Sodium alginate | Carvacrol | White mushrooms | Improved mechanical properties, water resistance, light barrier property, and antiviral properties | [35] |
| Chitosan | Eucalyptus | Sliced sausages | Good antimicrobial activity | [36] |
| Gelatin | Chamomile and peppermint | Edible packaging | The antioxidant activity and bioactivity were improved | [37] |
| Cellulose | Ginger | Barbecue chicken | Improved spoilage control; the addition of essential oils prolonged the meat shelf life by more than 6 days. | [38] |
| Chitosan | Ginger | Fresh poultry meat | Reduced coliforms proliferation; the addition of EO showed minimum changes than the product uncoated | [39] |
| Chitosan | Ginger | Active packaging | Strong antimicrobial activity | [40] |
| Chitosan/protein | Ginger | Fish | Stored at 4 °C, the shelf life has been extended | [41] |
| EO | M | TC | LM | CF | SA | EC | References |
|---|---|---|---|---|---|---|---|
| Cinnamon | √ | √ | √ | √ | √ | √ | [43,44,45,46] |
| Lemon | √ | √ | √ | √ | √ | [27,46,47,48] | |
| Grapefruit | √ | √ | √ | √ | [48,49,50] | ||
| Orange | √ | √ | √ | √ | √ | [48,50] | |
| Clove | √ | √ | √ | √ | √ | [29,30,31,32] | |
| Peppermint | √ | √ | √ | √ | [34,51] | ||
| Eucalyptus | √ | √ | √ | [36] | |||
| Chamomile | √ | √ | √ | [37] | |||
| Ginger | √ | √ | √ | √ | √ | [39,52] |
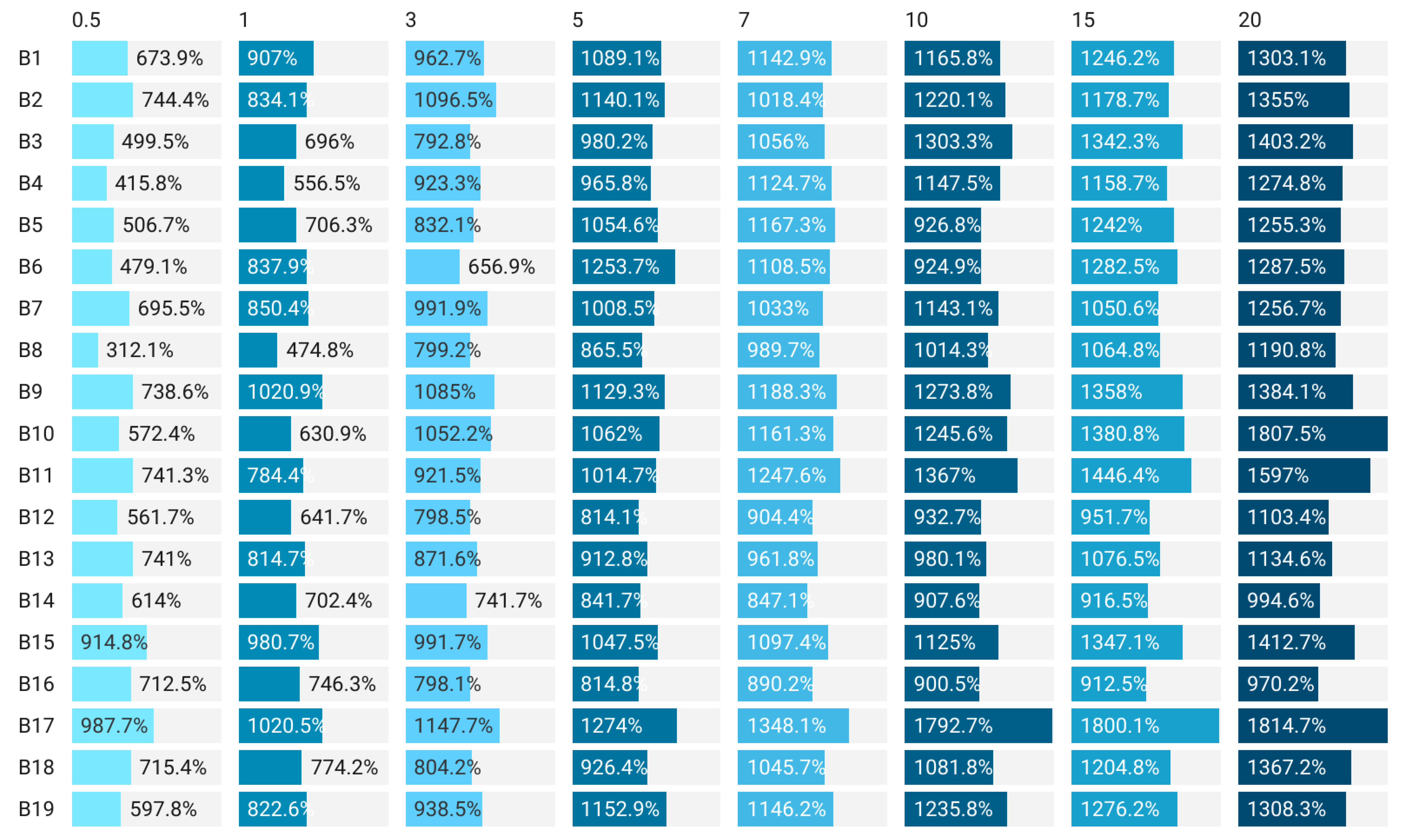
| Sample | Thickness, µm | Retraction Ratio, % | Roughness, nm | Tensile Strength, MPa | Elongation, % | Moisture Content, % | Water Activity Index |
|---|---|---|---|---|---|---|---|
| B1 | 99.80 b,c ± 2.40 | 4.95 i,j ± 2.28 | 253.10 b,c,d,e,f ± 2.99 | 0.101 l ± 0.10 | 1.51 r ± 0.01 | 11.95 e ± 0.03 | 0.33 c ± 0.01 |
| B2 | 100.60 b ± 1.85 | 4.19 j ± 1.76 | 333.20 a,b ± 3.55 | 0.098 l ± 0.05 | 1.91 q ± 0.05 | 11.24 f ± 0.01 | 0.29 d ± 0.04 |
| B3 | 88.80 f,g ± 1.47 | 15.43 e,f ± 1.40 | 366.20 a ± 3.03 | 0.231 e ± 0.05 | 14.97 d ± 0.20 | 13.24 c ± 0.22 | 0.30 c,d ± 0.01 |
| B4 | 90.80 e,f ± 1.17 | 13.52 f,g ± 1.11 | 243.70 b,c,d,e,f,g ± 4.16 | 0.172 f ± 0.01 | 11.04 g ± 0.33 | 11.27 f ± 0.61 | 0.30 c,d ± 0.01 |
| B5 | 81.00 h,i ± 2.68 | 22.86 c,d ± 2.55 | 255.00 b,c,d,e,f ± 1.03 | 0.133 l ± 0.01 | 7.10 j ± 0.15 | 13.73 b ±0.13 | 0.30 c,d ± 0.08 |
| B6 | 93.60 d,e ± 1.85 | 10.86 g,h ± 1.76 | 304.87 a,b,c,d ± 0.85 | 0.146 g ± 0.01 | 5.37 n ± 0.11 | 13.53 b ±0.29 | 0.30 c,d ± 0.03 |
| B7 | 98.80 b,c ± 1.94 | 5.90 i,j ± 1.84 | 307.47 a,b,c,d ± 1.96 | 0.137 i ± 0.01 | 6.92 k ± 0.10 | 13.13 c ± 0.14 | 0.33 c ± 0.01 |
| B8 | 101.00 b ± 0.89 | 3.81 j ± 0.85 | 308.97 a,b,c,d ± 0.47 | 0.130 k ± 0.05 | 8.33 i ± 0.21 | 12.74 d ±0.68 | 0.30 c,d ± 0.02 |
| B9 | 91.60 e,f ± 1.50 | 12.76 f,g ± 1.42 | 293.67 a,b,c,d ± 0.59 | 0.093 m ± 0.01 | 3.74 p ±0.07 | 13.24 c ± 0.76 | 0.29 c,d ± 0.01 |
| B10 | 98.80 a,b ± 1.67 | 4.76 i,j ± 1.59 | 314.33 a,b,c ± 0.51 | 0.142 h ± 0.01 | 13.87 e ± 0.07 | 13.04 c ± 0.75 | 0.27 d ± 0.01 |
| B11 | 59.40 j ± 1.36 | 43.43 b ± 1.29 | 216.17 d,e,f,g ± 0.68 | 0.274 c ± 0.05 | 17.45 b ± 0.18 | 11.26 f ± 0.25 | 0.52 b ± 0.02 |
| B12 | 76.80 i ± 1.60 | 26.86 c ± 1.52 | 169.23 f,g ± 0.57 | 0.288 a ± 0.15 | 16.42 c ± 0.02 | 10.34 g ±0.20 | 0.52 a,b ± 0.02 |
| B13 | 101.20 b ± 1.33 | 3.62 j ± 1.26 | 248.37 b,c,d,e,f ± 4.46 | 0.261 d ± 0.10 | 26.11 a ± 0.03 | 11.73 e ± 0.19 | 0.56 a ± 0.03 |
| B14 | 102.00 a,b ±1.10 | 2.86 j ± 1.04 | 288.27 a,b,c,d,e ± 4.02 | * | * | 8.73 h ± 0.20 | 0.56 a ± 0.02 |
| B15 | 92.00 d,e,f ± 0.89 | 12.38 f,g,h ± 0.85 | 230.60 c,d,e,f,g ± 3.04 | 0.175 g ± 0.10 | 8.93 h ± 0.02 | 8.51 h ± 0.43 | 0.56 a ± 0.02 |
| B16 | 98.80 b,c ± 1.33 | 5.90 i,j ± 1.26 | 192.03 e,f,g ± 3.61 | 0.148 g ± 0.05 | 6.71 l ± 0.06 | 19.34 a ±0.11 | 0.55 a,b ± 0.01 |
| B17 | 53.00 k ± 1.67 | 49.52 a ± 1.59 | 146.90 g ± 0.57 | 0.282 b ± 0.02 | 12.98 f ± 0.01 | 2.43 j ± 0.19 | 0.53 a,b ± 0.02 |
| B18 | 96.20 c,d ± 1.17 | 8.38 h,i ± 1.11 | 222.60 c,d,e,f,g ± 3.63 | 0.131 k ± 0.10 | 6.44 m ± 0.01 | 4.54 i ± 0.36 | 0.53 a,b ± 0.01 |
| B19 | 85.20 g,h ± 1.94 | 18.86 d,e ± 1.84 | 176.93 f,g ± 1.17 | 0.135 i.j ± 0.10 | 5.04 o ± 0.02 | 11.44 f ± 0.18 | 0.28 d ± 0.02 |
| Sample | Transmittance, % | Opacity, A × mm−1 | Color | ||
|---|---|---|---|---|---|
| L* | a* | b* | |||
| B1 | 49.40 i ± 0.10 | 3.31 e ± 0.04 | 88.79 b,c ± 0.18 | −0.49 a ± 0.02 | 10.76 e ± 0.31 |
| B2 | 40.66 k ± 0.75 | 3.59 b ± 0.12 | 88.99 b,c ± 0.16 | −0.42 a ± 0.24 | 10.61 e ± 0.29 |
| B3 | 51.00 g ± 0.20 | 3.21 f ± 0.14 | 88.30 b,c,d ± 0.39 | −0.54 a ± 0.02 | 11.33 d,e ± 0.48 |
| B4 | 52.60 e ± 0.20 | 3.49 c ± 0.22 | 88.87 b,c ± 0.96 | −0.61 a ± 0.05 | 10.72 e ± 0.53 |
| B5 | 53.10 e ± 0.10 | 3.47 c ± 0.15 | 88.88 b,c ± 0.73 | −0.51 a ± 0.86 | 9.35 e ± 0.65 |
| B6 | 51.73 f ± 0.15 | 3.50 c ± 0.10 | 89.17 b ± 0.63 | −0.48 a ± 0.15 | 9.85 e ± 0.73 |
| B7 | 50.16 h ± 0.11 | 3.26 e,f ± 0.20 | 89.17 b ± 0.30 | −0.48 a ± 0.16 | 10.03 e ± 0.28 |
| B8 | 50.83 g,h ± 0.11 | 3.02 h ± 0.09 | 88.58 b,c ± 0.92 | −0.49 a ± 0.02 | 11.21 d,e ± 0.83 |
| B9 | 50.43 g,h ± 0.15 | 3.39 d ± 0.08 | 88.50 b,c,d ± 0.93 | −0.47 a ± 0.03 | 11.16 d,e ± 1.02 |
| B10 | 52.73 e ± 0.11 | 3.03 g,h ± 0.16 | 88.31 b,c,d ± 0.68 | −0.52 a ± 0.01 | 10.73 e ± 0.65 |
| B11 | 71.63 c ± 0.11 | 2.53 j ± 0.08 | 92.25 a ± 0.79 | −5.78 c ± 0.07 | 13.70 b,c ± 0.53 |
| B12 | 73.56 b ± 0.12 | 1.88 l ± 0.21 | 92.18 a ± 0.15 | −5.79 c ± 0.02 | 12.84 c,d ± 0.52 |
| B13 | 70.01 a ± 0.03 | 3.99 a ± 0.12 | 91.00 a ± 0.64 | −5.76 c ± 0.01 | 15.26 b ± 0.94 |
| B14 | 18.33 l ± 0.21 | 3.08 g ± 0.12 | 92.07 a ± 0.28 | −5.85 c ± 0.03 | 13.61 b,c ± 0.37 |
| B15 | 41.30 k ± 0.10 | 1.52 m ± 0.17 | 92.19 a ± 0.28 | −5.83 c ± 0.03 | 13.11 c,d ± 0.42 |
| B16 | 46.60 j ± 0.20 | 1.15 n ± 0.12 | 91.83 a ± 0.15 | −5.76 c ± 0.08 | 14.31 b,c ± 1.17 |
| B17 | 73.5 b ± 0.20 | 2.87 i ± 0.23 | 92.09 a ± 0.44 | −5.91 c ± 0.10 | 12.84 c,d ± 0.67 |
| B18 | 40.73 k ± 0.11 | 1.5 m ± 0.13 | 86.97 d ± 1.09 | −5.18 b ± 0.22 | 21.97 a ± 1.09 |
| B19 | 68.83 d ± 0.11 | 1.97 k ± 0.11 | 87.63 c,d ± 0.41 | −0.62 a ± 0.02 | 10.32 e ± 0.56 |
| Sample | TC | EC | ETC | CF | YM | X-SA | LM |
|---|---|---|---|---|---|---|---|
| B1 | 1 | - | - | 1 | - | - | - |
| B2 | - | - | - | - | - | - | - |
| B3 | 15 | - | - | - | - | - | - |
| B4 | 21 | - | - | - | - | 1 | - |
| B5 | - | - | - | - | - | - | - |
| B6 | 7 | - | - | - | - | - | - |
| B7 | 2 | - | - | - | - | - | - |
| B8 | - | - | - | - | - | - | - |
| B9 | 13 | - | - | 2 | - | - | - |
| B10 | 1 | - | - | - | - | - | - |
| B11 | - | - | - | 2 | - | - | - |
| B12 | 8 | - | - | - | - | - | - |
| B13 | 19 | - | - | - | - | 1 | - |
| B14 | 6 | - | - | - | - | - | - |
| B15 | - | - | - | - | - | - | - |
| B16 | - | - | - | - | - | - | - |
| B17 | - | - | - | - | - | - | - |
| B18 | - | - | - | - | - | - | - |
| B19 | 28 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puscaselu, R.G.; Lobiuc, A.; Gutt, G. The Future Packaging of the Food Industry: The Development and Characterization of Innovative Biobased Materials with Essential Oils Added. Gels 2022, 8, 505. https://doi.org/10.3390/gels8080505
Puscaselu RG, Lobiuc A, Gutt G. The Future Packaging of the Food Industry: The Development and Characterization of Innovative Biobased Materials with Essential Oils Added. Gels. 2022; 8(8):505. https://doi.org/10.3390/gels8080505
Chicago/Turabian StylePuscaselu, Roxana Gheorghita, Andrei Lobiuc, and Gheorghe Gutt. 2022. "The Future Packaging of the Food Industry: The Development and Characterization of Innovative Biobased Materials with Essential Oils Added" Gels 8, no. 8: 505. https://doi.org/10.3390/gels8080505
APA StylePuscaselu, R. G., Lobiuc, A., & Gutt, G. (2022). The Future Packaging of the Food Industry: The Development and Characterization of Innovative Biobased Materials with Essential Oils Added. Gels, 8(8), 505. https://doi.org/10.3390/gels8080505






