Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Rheological Behavior
2.1.1. Linear Viscoelastic Properties
2.1.2. Viscous Flow Behavior and Thixotropic Properties
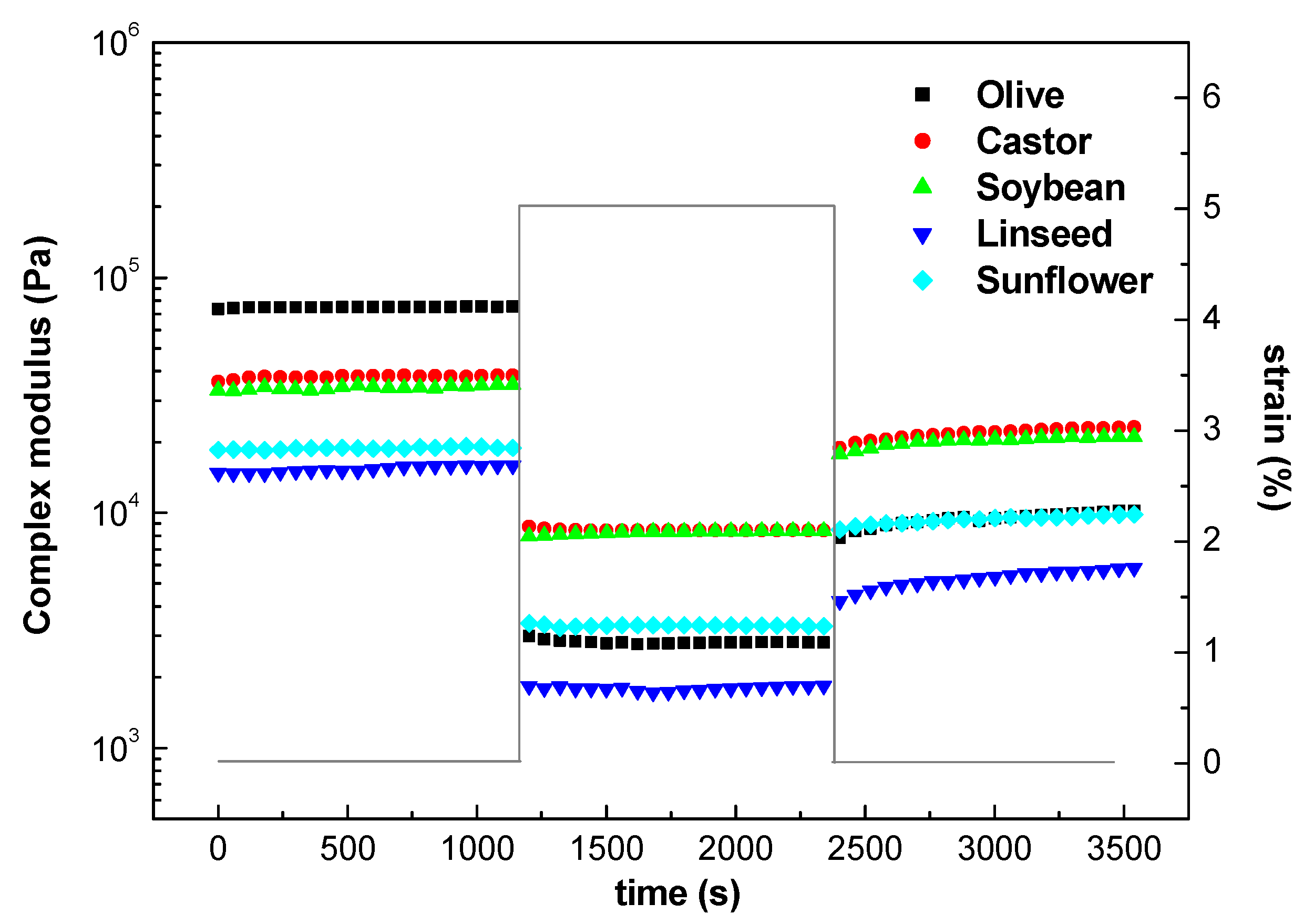
2.1.3. Structural Recovery
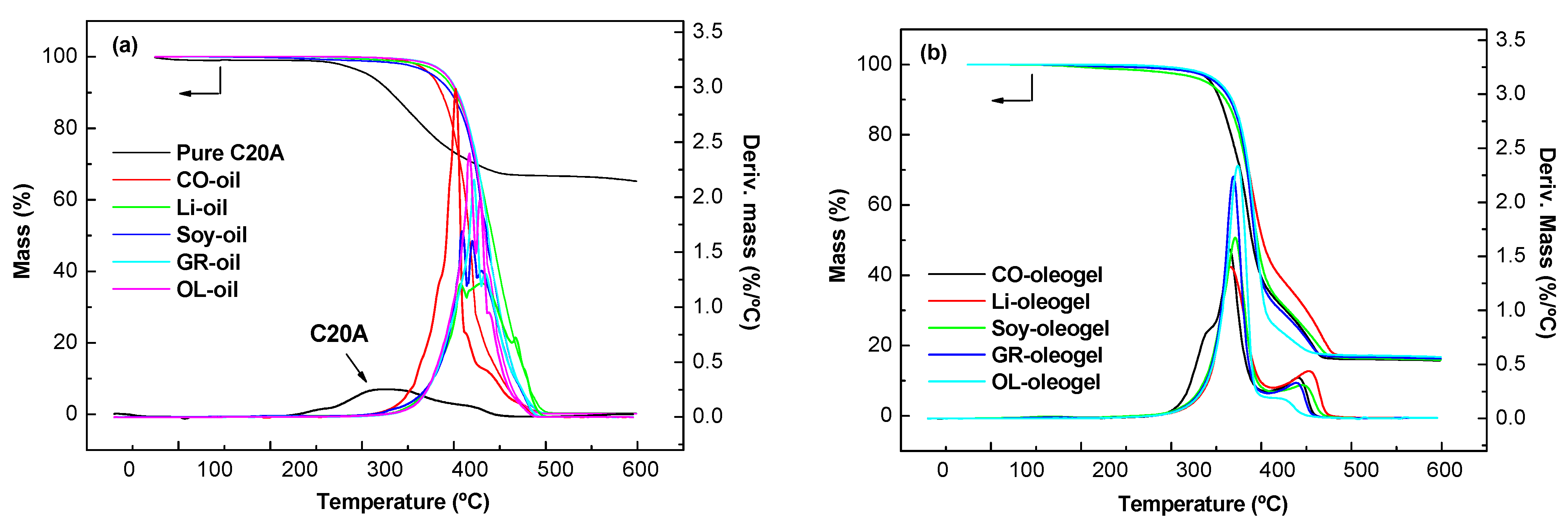
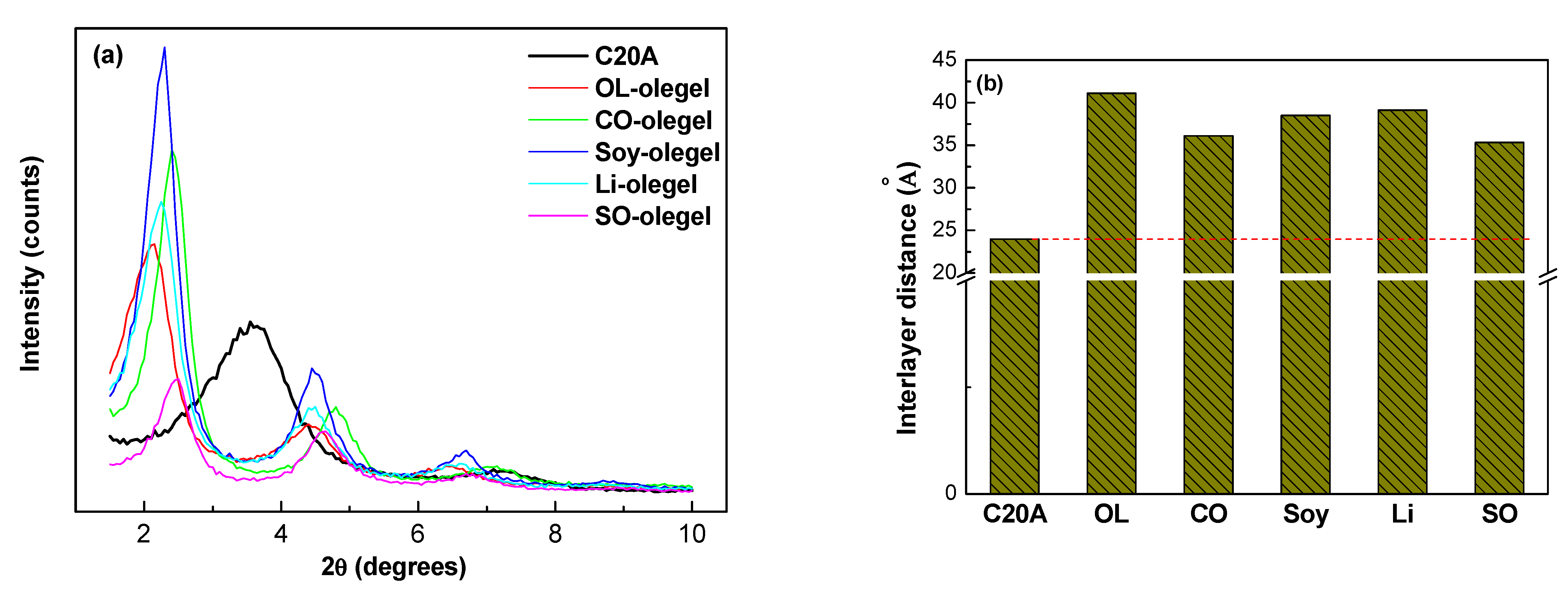
2.2. Thermal and Chemical Properties of Oleogel
2.3. Tribological Properties of Oleogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Oleogel Formulation
4.3. Experimental Methods
4.3.1. Rheological Characterization
4.3.2. Thermogravimetric Analysis (TGA)
4.3.3. X-ray Diffraction Analysis (XRD)
4.3.4. Penetration Test and Tribological Characterization
4.3.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Zhang, J.; Dong, S.; Li, Y.; Slaný, M.; Chen, G. Use of betaine-based gel and its potential application in enhanced oil recovery. Gels 2022, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Zhu, X.; Zhou, F.; Yan, Z.; Evelina, A.; Liu, J.; Wei, Z.; Ma, L. Preparation and Hydrogelling Performances of a New Drilling Fluid Filtrate Reducer from Plant Press Slag. Gels 2022, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Martín-Alfonso, J.E.; López-Beltrán, F.; Valencia, C.; Franco, J.M. Effect of an alkali treatment on the development of cellulose pulp-based gel-like dispersions in vegetable oil for use as lubricants. Tribol. Int. 2018, 123, 329. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In green the 21st century materials word. Prog. Mater. Sci. 2005, 50, 962. [Google Scholar]
- Nourmoradi, H.; Avazpour, M.; Ghasemian, N.; Heidari, M.; Moradnejadi, K.; Khodarahmi, F.; Javaheri, M.; Mohammadi Moghadam, F. Surfactant modified montmorillonite as a low cost adsorbent for 4-chlorophenol: Equilibrium, kinetic and thermodynamic study. J. Taiwan Inst. Chem. Eng. 2016, 59, 244. [Google Scholar] [CrossRef]
- Saurabh, C.K.; Gupta, S.; Bahadur, J.; Mazumder, S.; Variyar, P.S.; Sharma, A. Mechanical and barrier properties of guar gumbased nano-composite films. Carbohydr. Polym. 2015, 124, 77. [Google Scholar] [CrossRef] [PubMed]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 2804. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Prog. Polym. Sci. 2009, 34, 125. [Google Scholar] [CrossRef]
- Gerasin, V.A.; Kurenkov, V.V.; Pashkov, O.V.; Ilyin, S.O. Structure and rheology of aqueous poly (vinyl acetate) dispersions modified with montmorillonite. Colloid J. 2017, 79, 588. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Brantseva, T.V.; Gorbunova, I.Y.; Antonov, S.V.; Korolev, Y.M.; Kerber, M.L. Epoxy reinforcement with silicate particles: Rheological and adhesive properties—Part I: Characterization of composites with natural and organically modified montmorillonites. Int. J. Adhes. Adhes. 2015, 61, 127. [Google Scholar] [CrossRef]
- Rehbinder, P. Coagulation and thixotropic structures. Discuss. Faraday Soc. 1958, 18, 151. [Google Scholar] [CrossRef]
- Zeichner, G.R.; Schowalter, W.R. Effects of hydrodynamic and colloidal forces on the coagulation of dispersions. J. Colloid Interface Sci. 1979, 71, 237. [Google Scholar] [CrossRef]
- Varga, Z.; Swan, J.W. Large scale anisotropies in sheared colloidal gels. J. Rheol. 2018, 62, 405. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Yarmush, Y.M.; Ilyin, S.O. Rheology and tribology of ester-based greases with microcrystalline cellulose and organomodified montmorillonite. Tribol. Int. 2020, 148, 106318. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C.; Franco, J.M. Composition-property relationship of gel-like dispersions based on organo-bentonite, recycled polypropylene and mineral oil for lubricant purposes. Appl. Clay Sci. 2014, 87, 265. [Google Scholar] [CrossRef]
- Chizhik, P.; Dietzel, D.; Bill, S.; Schirmeisen, A. Tribological properties of a phyllosilicate based microparticle oil additive. Wear 2019, 426, 835. [Google Scholar] [CrossRef]
- Singh, H.; Bhowmick, H. Influence of nanoclay on the thermophysical properties and lubricity characteristics of mineral oil. Mater. Today Proc. 2019, 18 Pt 3, 1058–1066. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, Y.; Xi, X. Nano-montmorillonite-doped lubricating grease exhibiting excellent insulating and tribological properties. Friction 2017, 5, 219. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Hao, Q.; Lei, W. Tribological properties of the functionalized graphene/montmorillonite nanosheets as a lubricant additive. Tribol. Lett. 2021, 69, 117. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Modification of epoxidized soybean oil for lubricant formulations with improved oxidative stability and low pour point. J. Am. Oil Chem. Soc. 2001, 78, 1179. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Chhibber, V.K. Non-edible oil as a source of bio-lubricant for industrial applications: A Review. Int. J. Eng. Sci. Innov. Technol. 2013, 1, 299. [Google Scholar]
- Pindit, K.; Thanapimmetha, A.; Saisriyoot, M.; Srinopakun, P. Biolubricant basestocks synthesis using 5-step reaction from jatropha oil, soybean oil, and palm fatty acid distillate. Ind. Crops Prod. 2021, 166, 113484. [Google Scholar] [CrossRef]
- Nie, J.Y.; Shen, J.H.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Synthesis of trimethylolpropane esters by base-catalyzed transesterification. Eur. J. Lipid Sci. Technol. 2020, 122, 1900207. [Google Scholar] [CrossRef]
- Qiao, S.; Shi, Y.; Wang, X.; Lin, Z.; Jiang, Y. Synthesis of biolubricant trimethylolpropane trioleate and its lubricant base oil properties. Energy Fuels 2017, 31, 7185. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Martín-Alfonso, M.J.; Franco, J.M. Tunable rheological-tribological performance of “green” gel-like dispersions based on sepiolite and castor oil for lubricant applications. Appl. Clay Sci. 2020, 192, 105632. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Martín-Alfonso, M.J.; Valencia, C.; Cuberes, M.T. Rheological and tribological approaches as a tool for the development of sustainable lubricating greases based on nano-montmorillonite and castor oil. Friction 2021, 9, 415. [Google Scholar] [CrossRef]
- Saxena, A.; Kumar, D.; Tandon, N. Development of eco-friendly nano-greases based on vegetable oil: An exploration of the character via structure. Ind. Crops Prod. 2021, 172, 114033. [Google Scholar] [CrossRef]
- Alfonso, J.E.M.; Valencia, C.; Valencia, C. Linear and nonlinear viscoelasticity of oleogels based on vegetable oil and ethylene vinyl acetate copolymer/isotactic polypropylene blends. J. Appl. Polym. Sci. 2015, 132, 42477. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Franco, J.M. Influence of polymer reprocessing cycles on the microstructure and rheological behavior of polypropylene/mineral oil oleogels. Polym. Test. 2015, 45, 12. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Franco, J.M. Ethylene-vinyl acetate copolymer (EVA)/sunflower vegetable oil polymer gels: Influence of vinyl acetate content. Polym. Test. 2014, 37, 78. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Franco, J.M.; Valencia, C.; Gallegos, C.; Urquiola, F.; Urchegui, R. Atomic force microscopy and thermo-rheological characterisation of lubricating greases. Tribol. Lett. 2011, 41, 463. [Google Scholar] [CrossRef]
- Wu, S. Chain structure and entanglement. J. Polym. Sci. B Polym. Phys. 1989, 27, 723. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1980. [Google Scholar]
- Yalcin, H.; Toker, O.S.; Dogan, M. Effect of oil type and fatty acid composition on dynamic and steady shear rheology of vegetable oils. J. Oleo Sci. 2012, 61, 181. [Google Scholar] [CrossRef] [PubMed]
- Yeong, S.K.; Luckham, P.F.; Tadros, T.F. Steady flow and viscoelastic properties of lubricating grease containing various thickener concentrations. J. Colloid Interface Sci. 2004, 274, 285. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Mohanty, S. Dynamic mechanical, rheological, and thermal properties of intercalated polystyrene/organomontmorillonite nanocomposites: Effect of clay modification on the mechanical and morphological behaviors. J. Appl. Polym. Sci. 2009, 112, 778. [Google Scholar] [CrossRef]
- Yoon, J.T.; Jo, W.H.; Lee, M.S.; Ko, M.B. Effects of comonomers and shear on the melt intercalation of styrenics/clay nanocomposites. Polymer 2001, 42, 329. [Google Scholar] [CrossRef]
- Ortega, F.J.; Navarro, F.J.; García-Morales, M.; McNally, T. Thermo-mechanical behaviour and structure of novel bitumen/nanoclay/MDI composites. Compos. Part B Eng. 2015, 76, 192. [Google Scholar] [CrossRef]
- NLGI. Lubricating Greases Guide, 5th ed.; National Lubricating Grease Institute: Kansas City, MO, USA, 2006. [Google Scholar]
- Gow, G. Lubricating Grease in Chemistry and Technology of Lubricants, 3rd ed.; Mortier, R.M., FoxStefan, M.F., Orszulik, T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 411–432. [Google Scholar]
- Brandão, J.A.; Meheux, M.; Ville, F.; Seabra, J.H.O.; Castro, J. Comparative overview of five gear oils in mixed and boundary film lubrication. Tribol. Int. 2012, 47, 50. [Google Scholar] [CrossRef]
- Martín-Alfonso, J.E.; Valencia, C. Tribological, rheological, and microstructural characterization of oleogels based on EVA copolymer and vegetables oils for lubricant applications. Tribol. Int. 2015, 90, 426. [Google Scholar] [CrossRef]
- Zhang, T.; Guan, E.; Yang, Y.; Liu, F.; Zhang, L.; Pang, J.; Bian, K. Fatty acid profiles of vegetable oils from four different plant sources and their effects on dough rheology and chinese steamed bread quality. Int. J. Food Sci. Technol. 2021, 56, 2407. [Google Scholar] [CrossRef]
- ASTM D 217-88; Standard Test Methods for Cone Penetration of Lubricating Grease. Annual Book of ASTM Standards. American Society for Testing and Materials: Philadelphia, PA, USA, 1991.
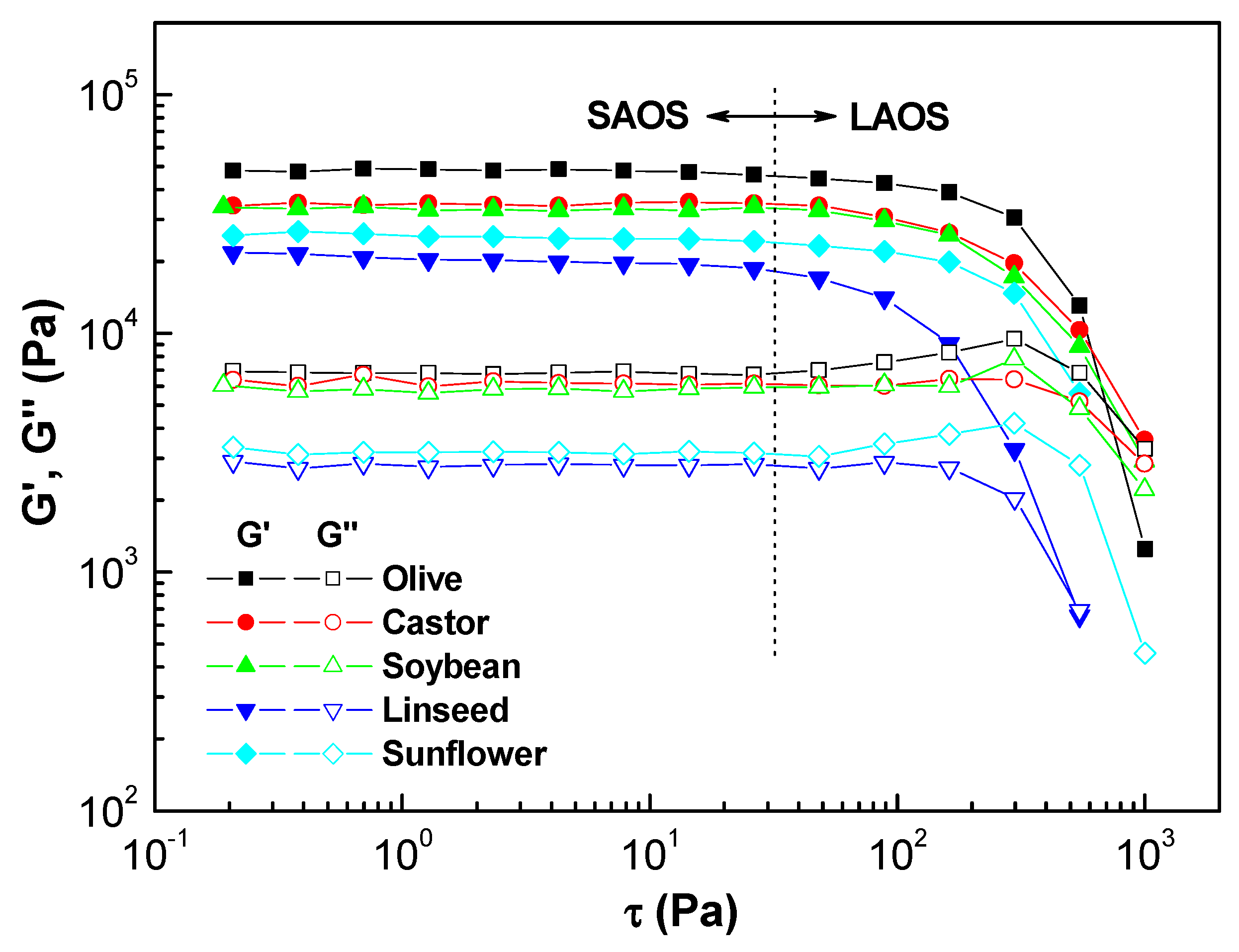
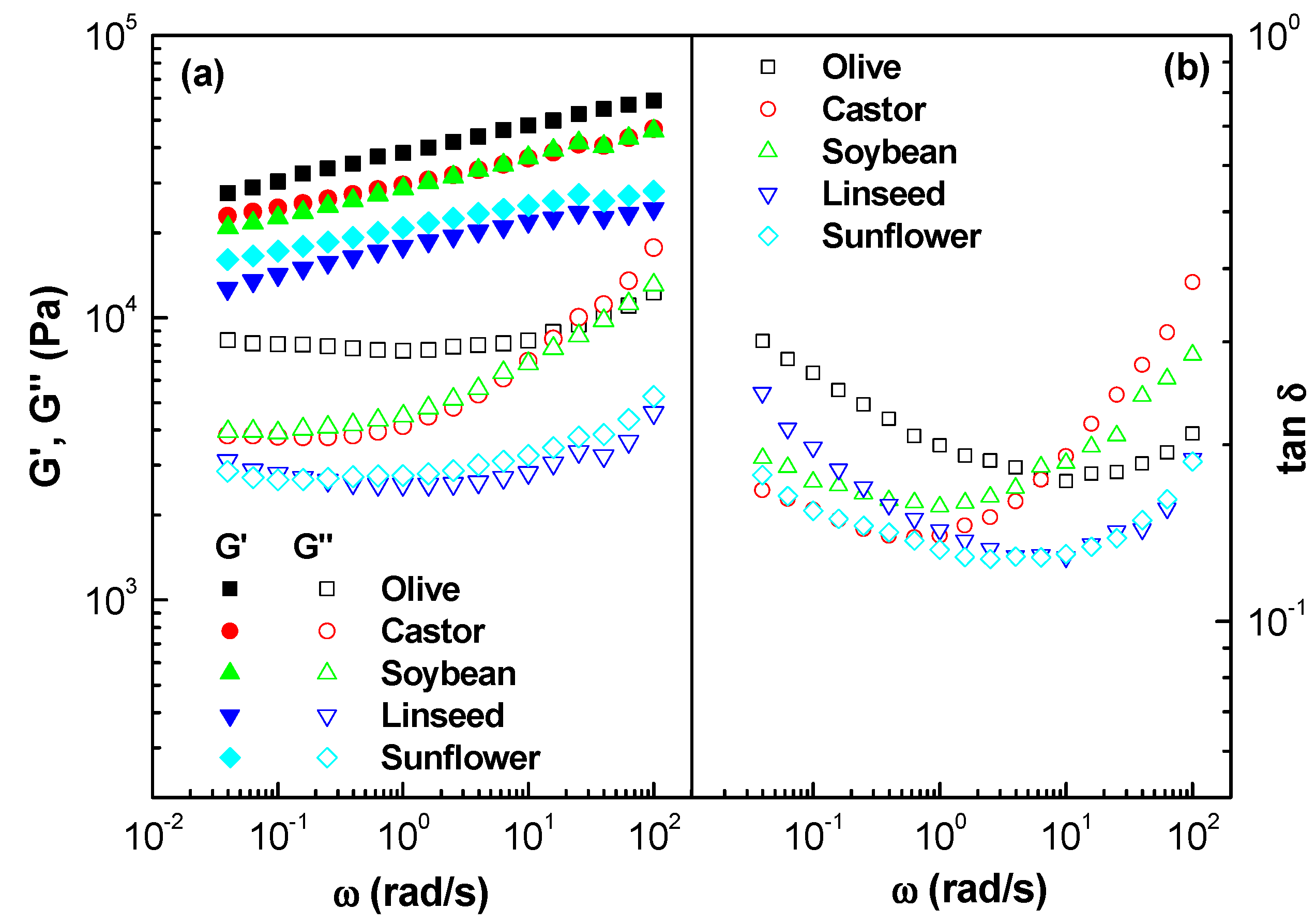
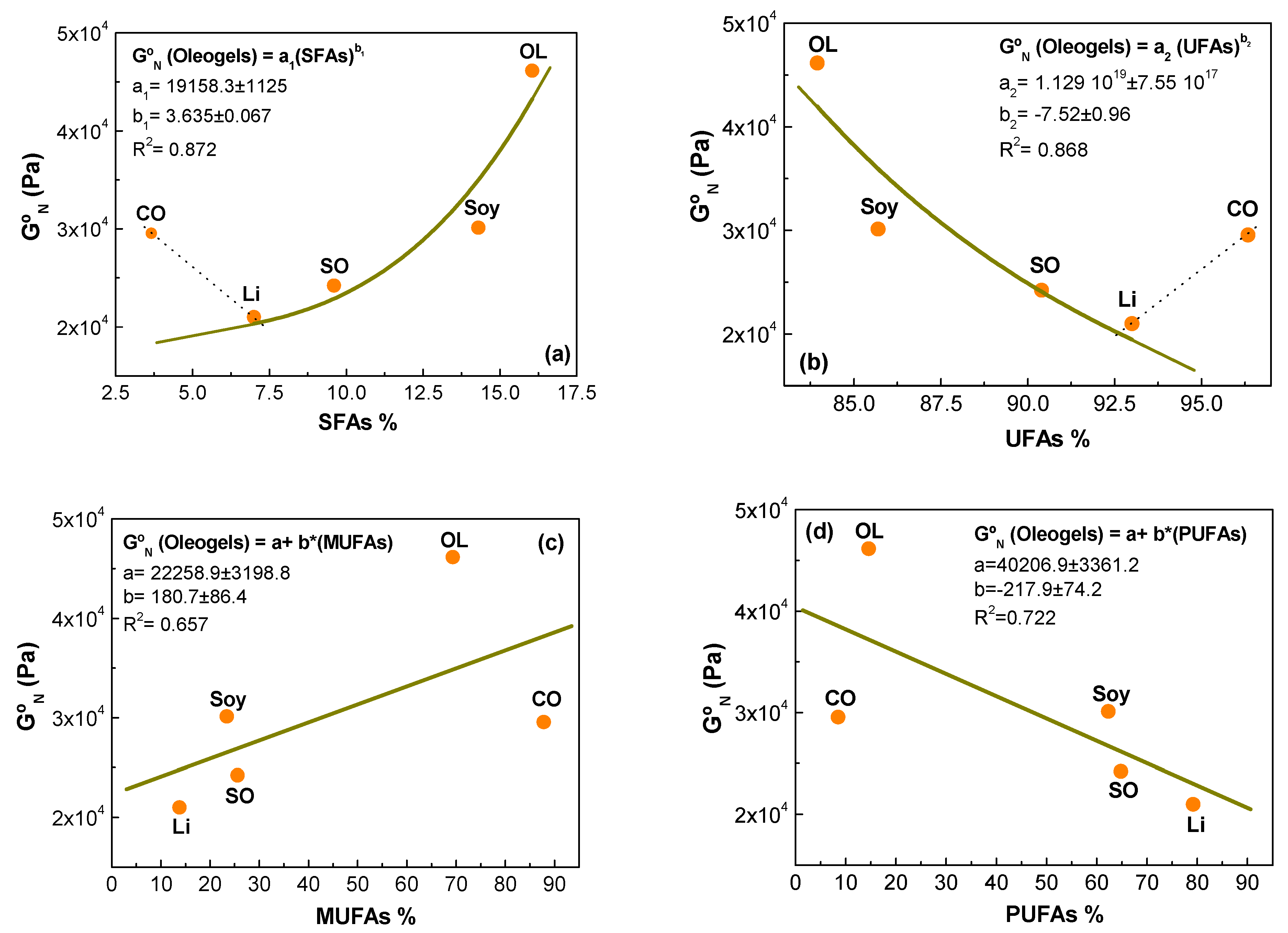
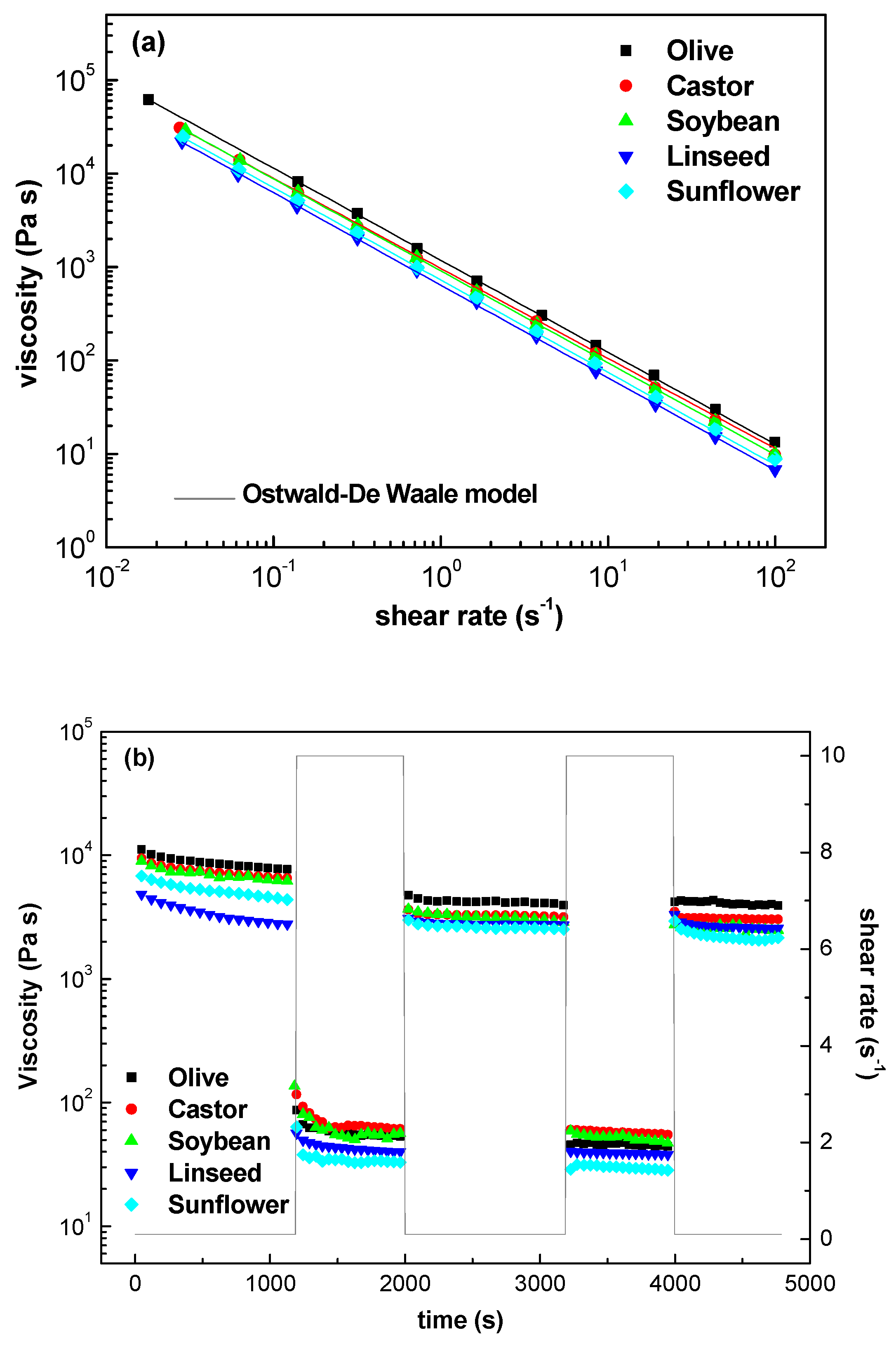


| Samples | τc (Pa) 1 | K (Pa∙sn) 2 | n 3 | Destruction (%) | Recovery (%) |
|---|---|---|---|---|---|
| Olive | 73.8 a | 1179.5 A | 0.015 aa | 96.3 AA | 9.39 α |
| Castor | 80.6 b | 964.7 B | 0.028 bb | 75.7 BB | 44.4 β |
| Soybean | 79.4 c | 907.9 C | 0.018 cc | 77.9 BB | 46.5 β |
| Linseed | 51.9 d | 637.8 D | 0.010 dd | 88.6 CC | 26.9 ᵩ |
| Sunflower | 78.3 c | 726.9 E | 0.011 dd | 82.2 DD | 40.1 γ |
| Samples | T5 (°C) 1 | T10 (°C) 2 | Tmax (°C) 3 | CY 4 |
|---|---|---|---|---|
| C20A | 280 | 307 | 331/450 | 65.8 |
| OL-oil | 372 | 385 | 416/429 | 0.023 |
| CO-oil | 354 | 367 | 382/401 | 0.026 |
| Soy-oil | 356 | 378 | 409/420 | 0.031 |
| Li-oil | 367 | 383 | 408/431 | 0.028 |
| SO-oil | 369 | 384 | 421/434 | 0.038 |
| OL-oleogel | 330 | 346 | 374/412 | 16.8 |
| CO-oleogel | 321 | 332 | 368/402 | 17.7 |
| Soy-oleogel | 316 | 338 | 370/445 | 15.9 |
| Li-oleogel | 329 | 346 | 365/452 | 16.3 |
| SO-oleogel | 327 | 344 | 369/443 | 16.3 |
| Oleogel | Penetration Index (dmm) | NLGI Grade | Friction Coefficient |
|---|---|---|---|
| Olive | 255 a | 2–3 | 0.096 A |
| Castor | 273 b | 2 | 0.119 B |
| Soybean | 281 b | 2 | 0.101 A |
| Linseed | 302 c | 1–2 | 0.113 B |
| Sunflower | 294 d | 2 | 0.108 B |
| Property | Olive | Castor | Soybean | Linseed | Sunflower |
|---|---|---|---|---|---|
| Dynamic viscosity at 40 °C (mPa s) | 38.3 | 230.0 | 31.4 | 30.2 | 31.1 |
| Density at 15 °C (g/cm3) | 0.9164 | 0.9630 | 0.9256 | 0.9416 | 0.9216 |
| Myristic 1 14:0 2 | 0.80 | - | 0.1 | - | - |
| Palmitic 16:0 | 13.15 | 1.70 | 10.8 | 4.09 | 6.19 |
| Stearic 18:0 | 2.10 | 1.96 | 3.40 | 2.91 | 3.41 |
| Oleic 18:1 | 69.35 | 5.34 | 23.4 | 13.8 | 25.6 |
| Ricinoleic (18:1 -OH) | - | 82.48 | - | - | - |
| Linoleic 18:2 | 14.60 | 7.01 | 55.6 | 14.6 | 64.8 |
| Linoleic 18:3 | - | 1.51 | 6.70 | 64.6 | - |
| Saturated (SFAs) | 16.05 | 3.66 | 14.3 | 7.01 | 9.60 |
| Monounsaturated (MUFAs) | 69.35 | 87.82 | 23.4 | 13.8 | 25.6 |
| Polyunsaturated (PUFAs) | 14.60 | 8.52 | 62.3 | 79.2 | 64.8 |
| Unsaturated/saturated ratio | 5.23 | 26.32 | 5.99 | 13.29 | 9.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E. Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels. Gels 2022, 8, 504. https://doi.org/10.3390/gels8080504
Martín-Alfonso MA, Rubio-Valle JF, Hinestroza JP, Martín-Alfonso JE. Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels. Gels. 2022; 8(8):504. https://doi.org/10.3390/gels8080504
Chicago/Turabian StyleMartín-Alfonso, M. A., José F. Rubio-Valle, Juan P. Hinestroza, and José E. Martín-Alfonso. 2022. "Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels" Gels 8, no. 8: 504. https://doi.org/10.3390/gels8080504
APA StyleMartín-Alfonso, M. A., Rubio-Valle, J. F., Hinestroza, J. P., & Martín-Alfonso, J. E. (2022). Impact of Vegetable Oil Type on the Rheological and Tribological Behavior of Montmorillonite-Based Oleogels. Gels, 8(8), 504. https://doi.org/10.3390/gels8080504







