Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants
Abstract
:1. Introduction
2. Results and Discussion
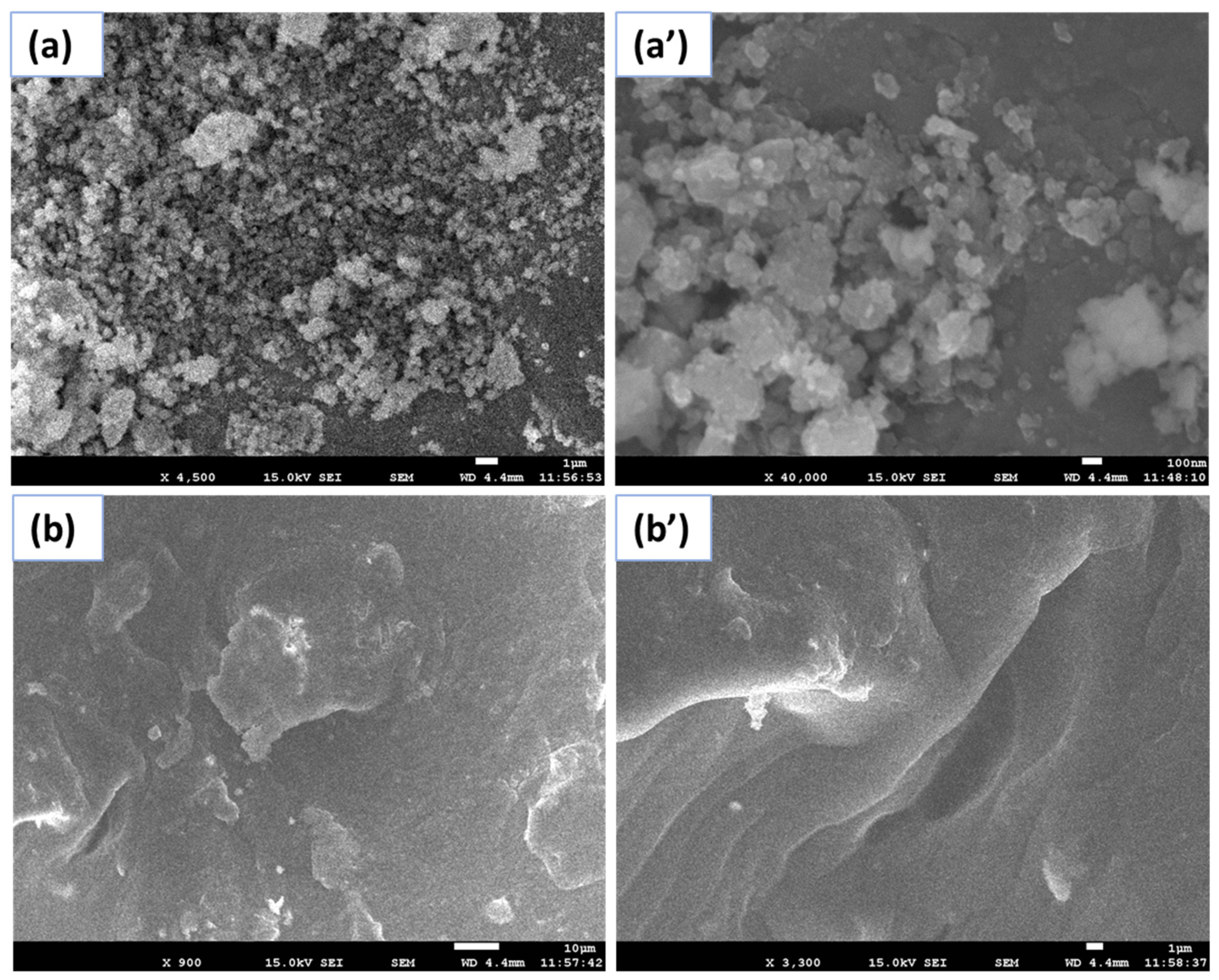
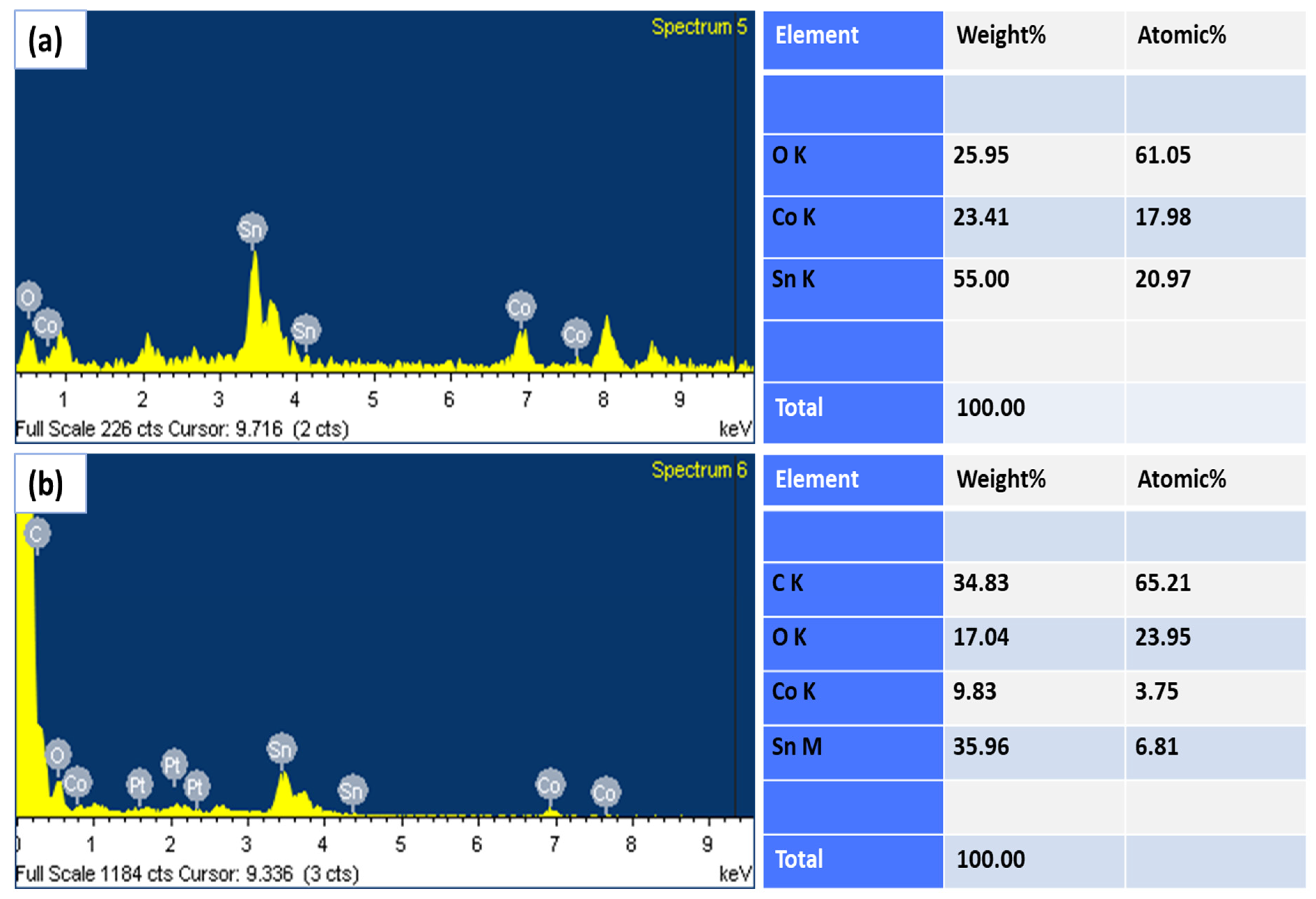
2.1. FESEM and EDX
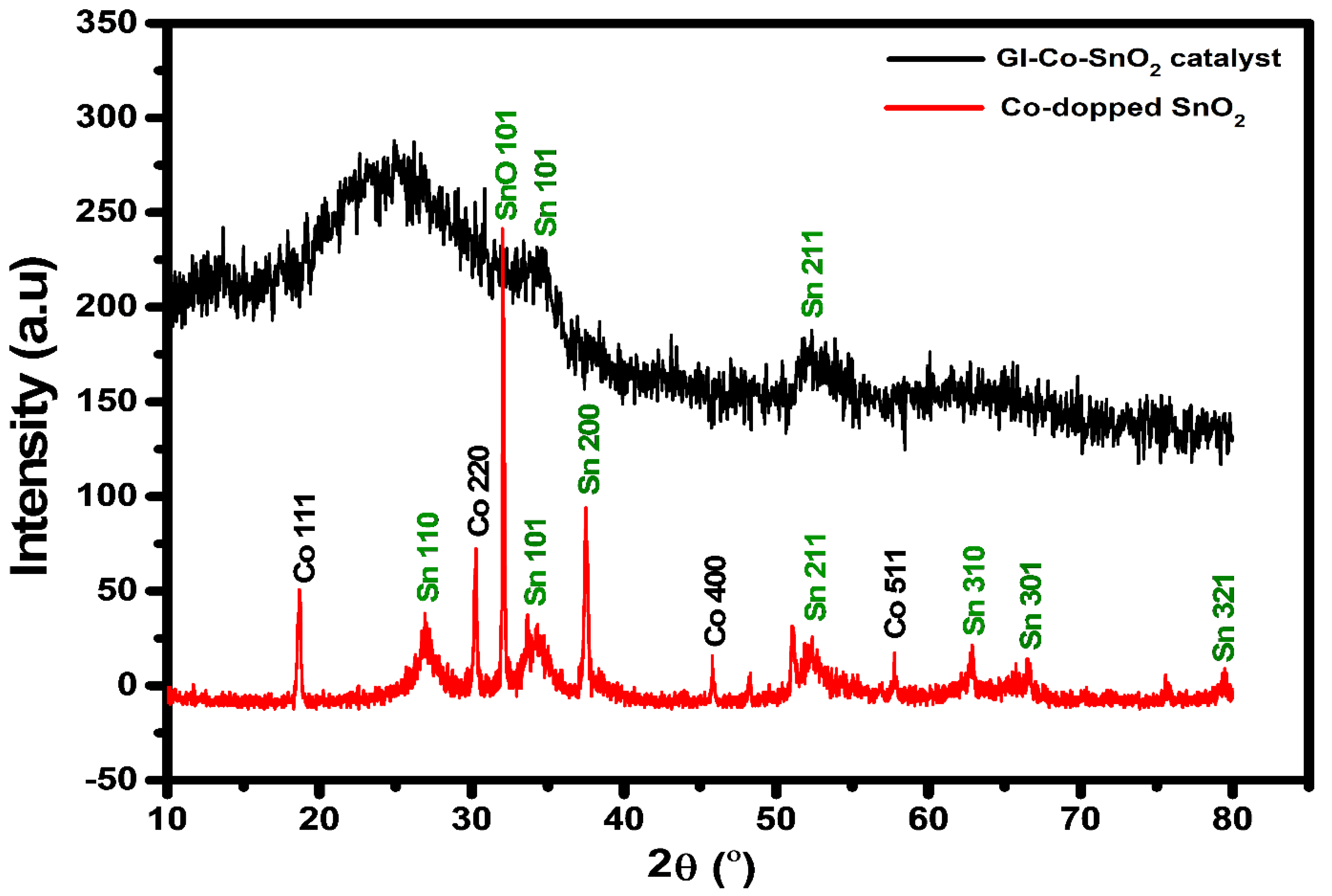
2.2. XRD
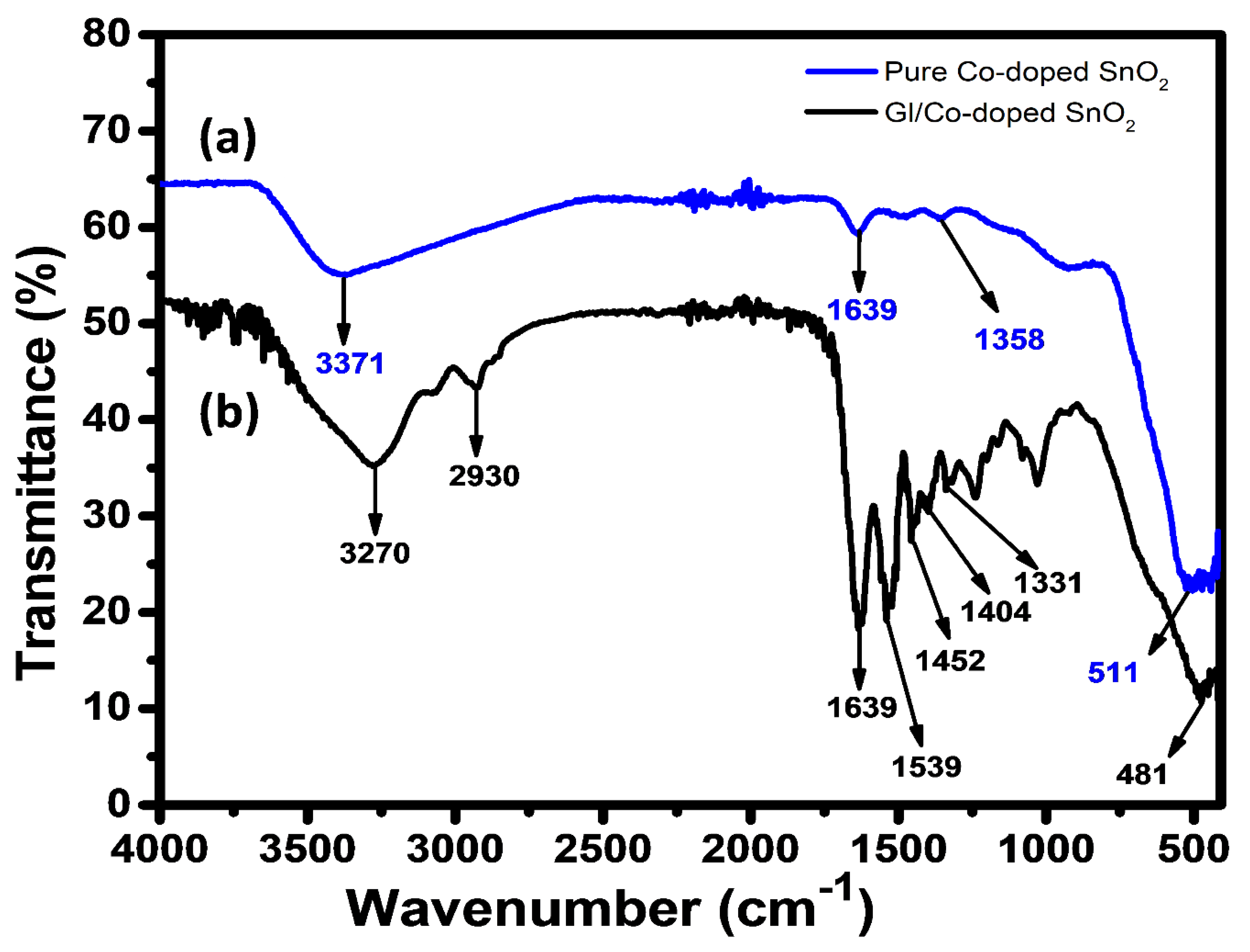
2.3. ATR-IR
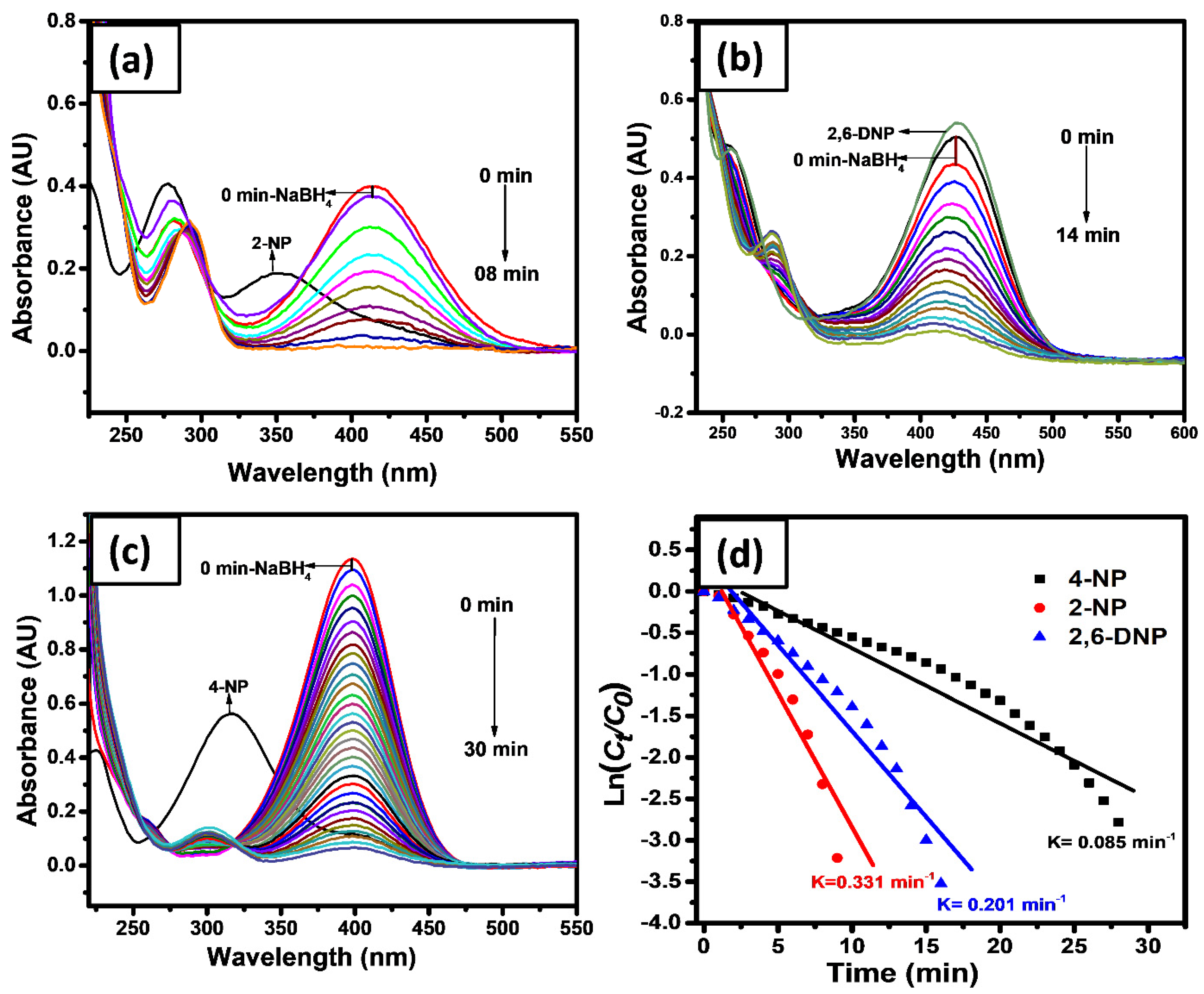
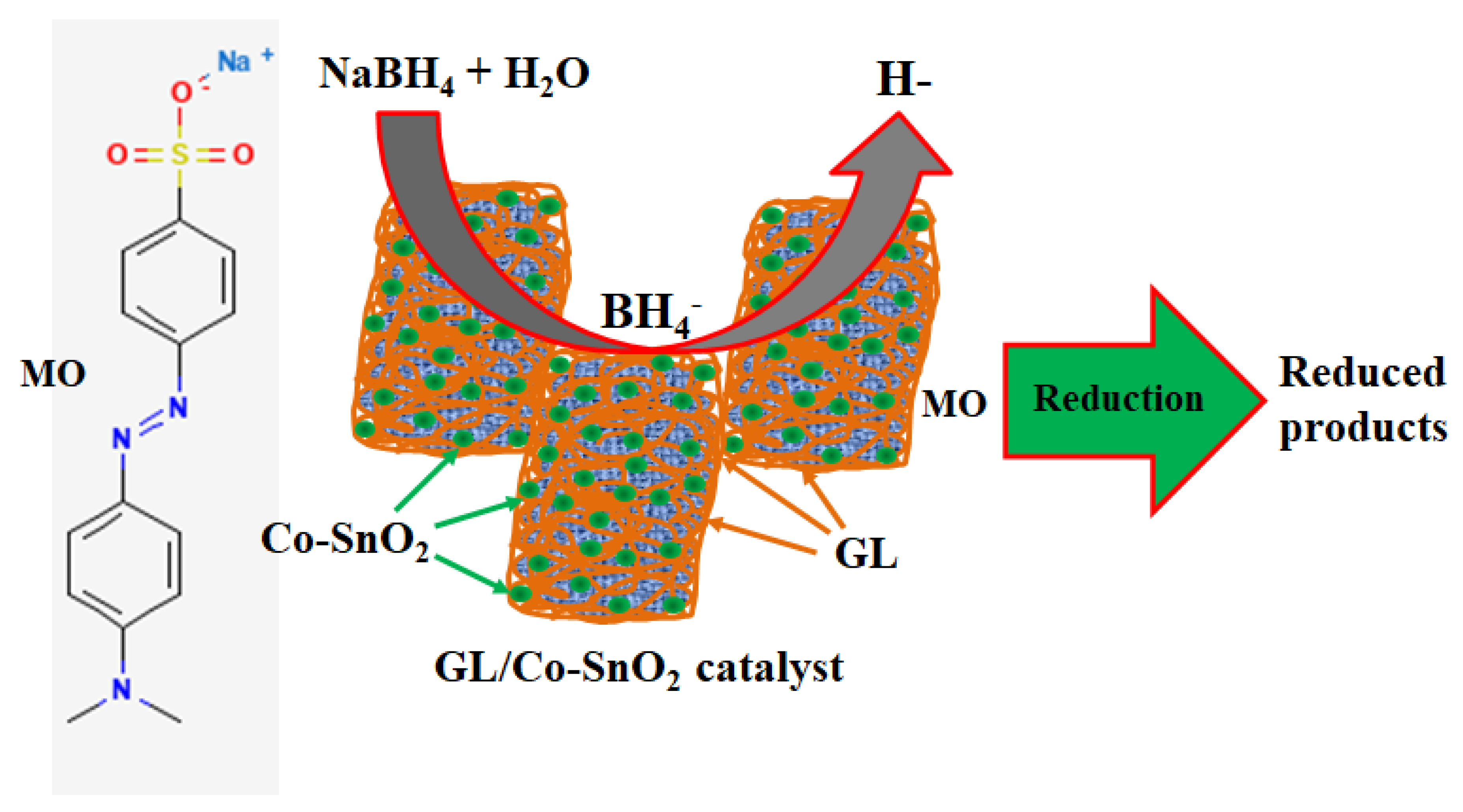
2.4. Catalytic Reduction
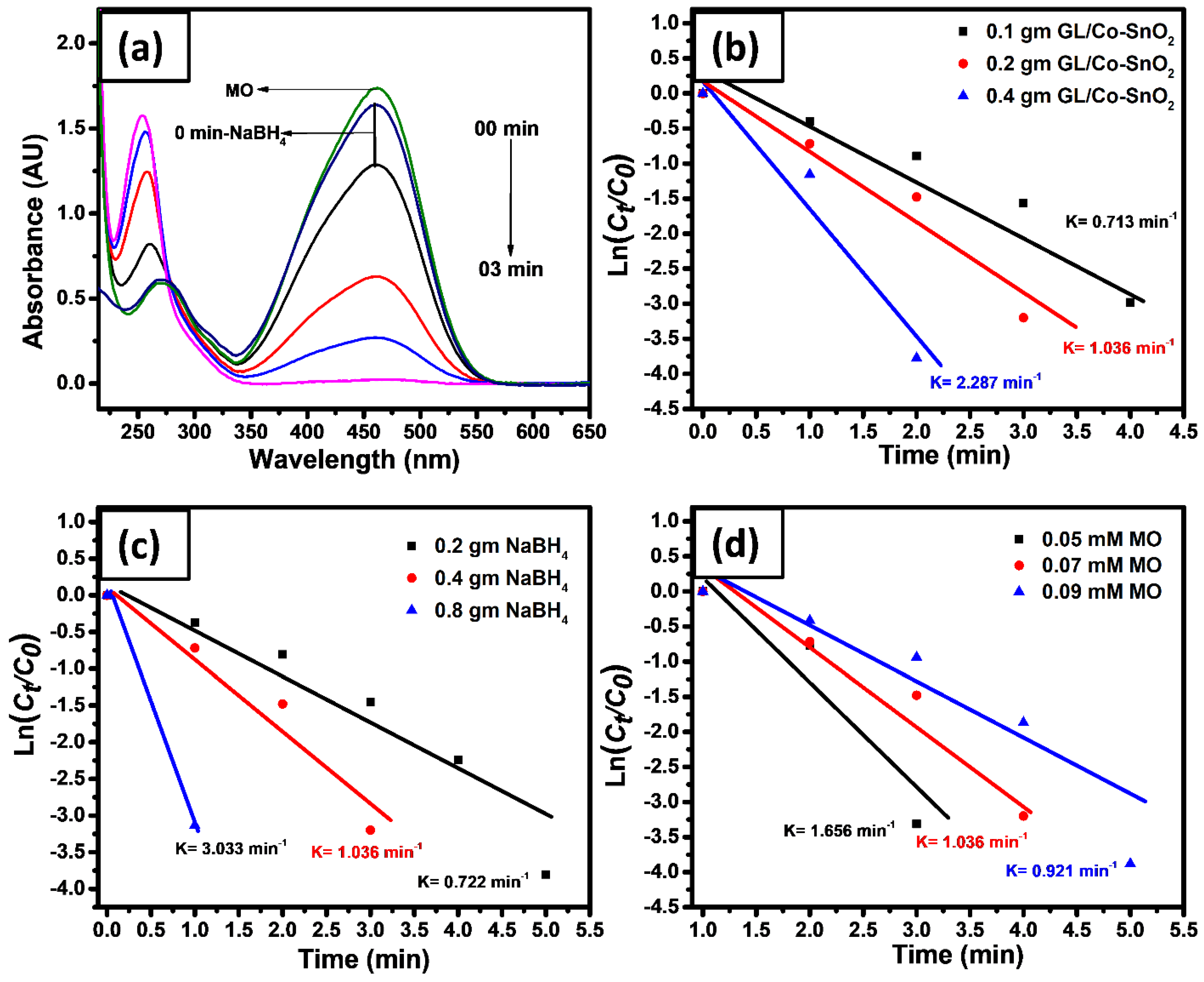
2.5. Catalytic Reduction of GL/Co-SnO2 Nanocomposite Material
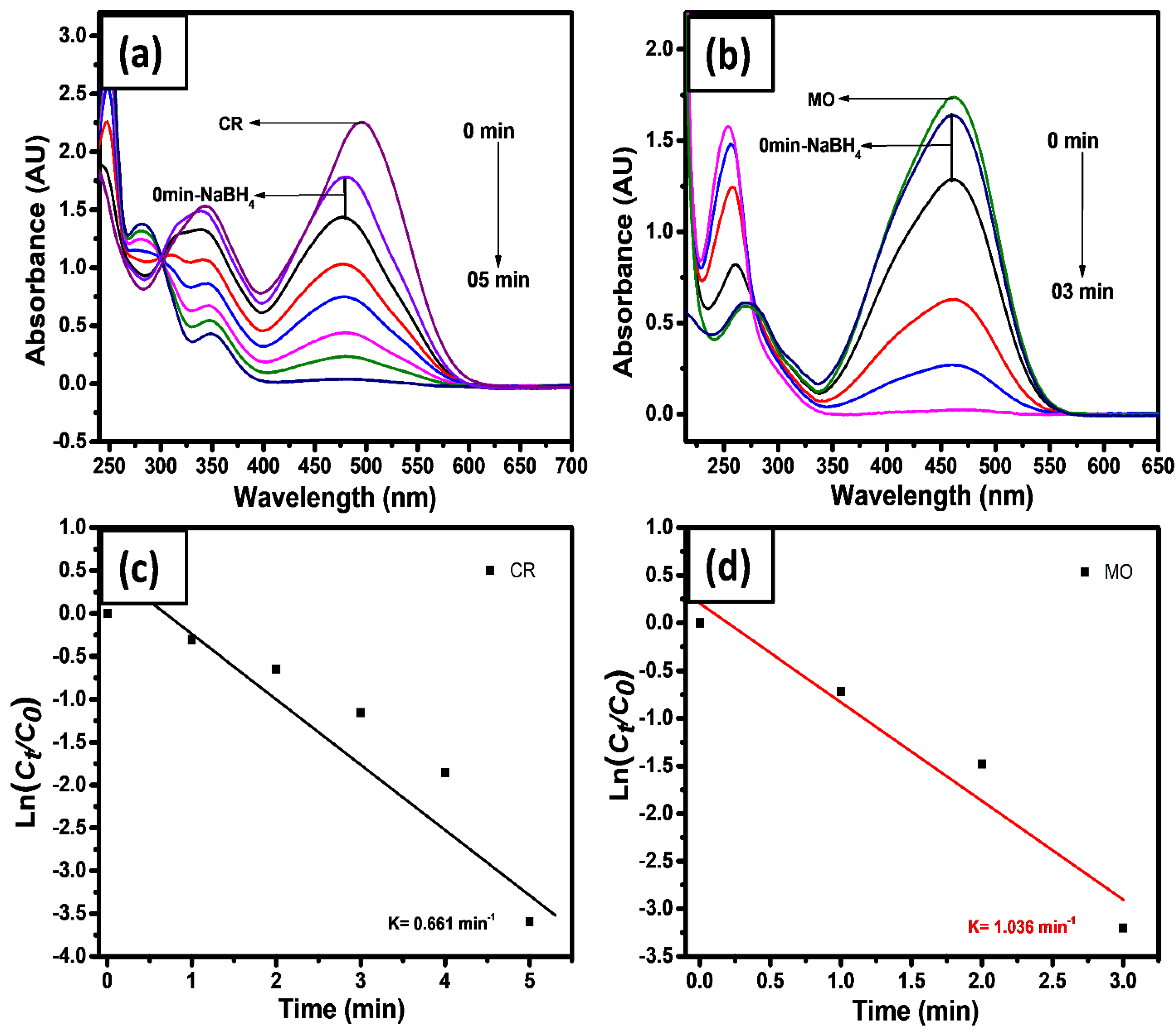
2.6. Catalytic Reduction of Azo Dyes
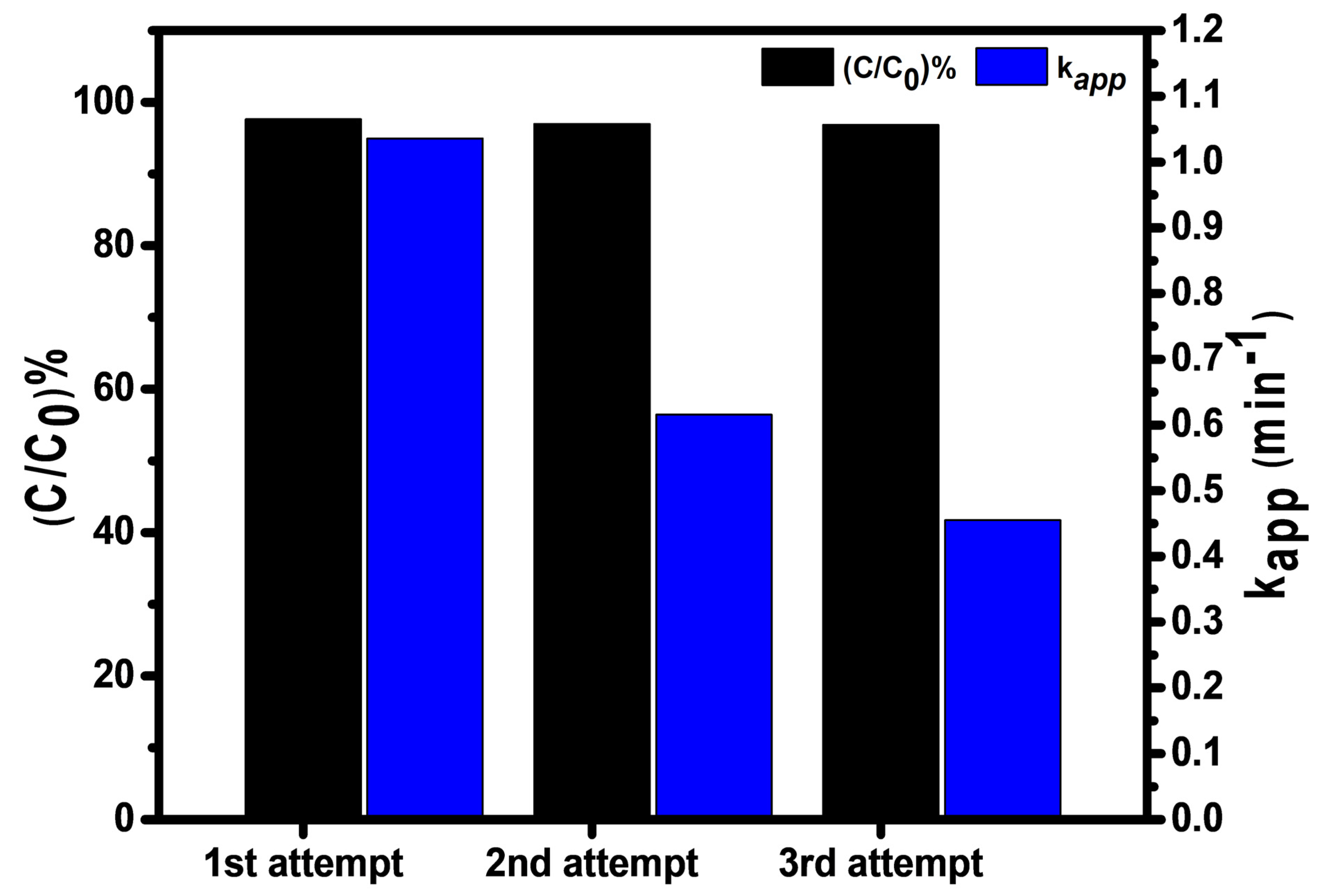
2.7. Recyclability
2.8. Mechanism of Reduction
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
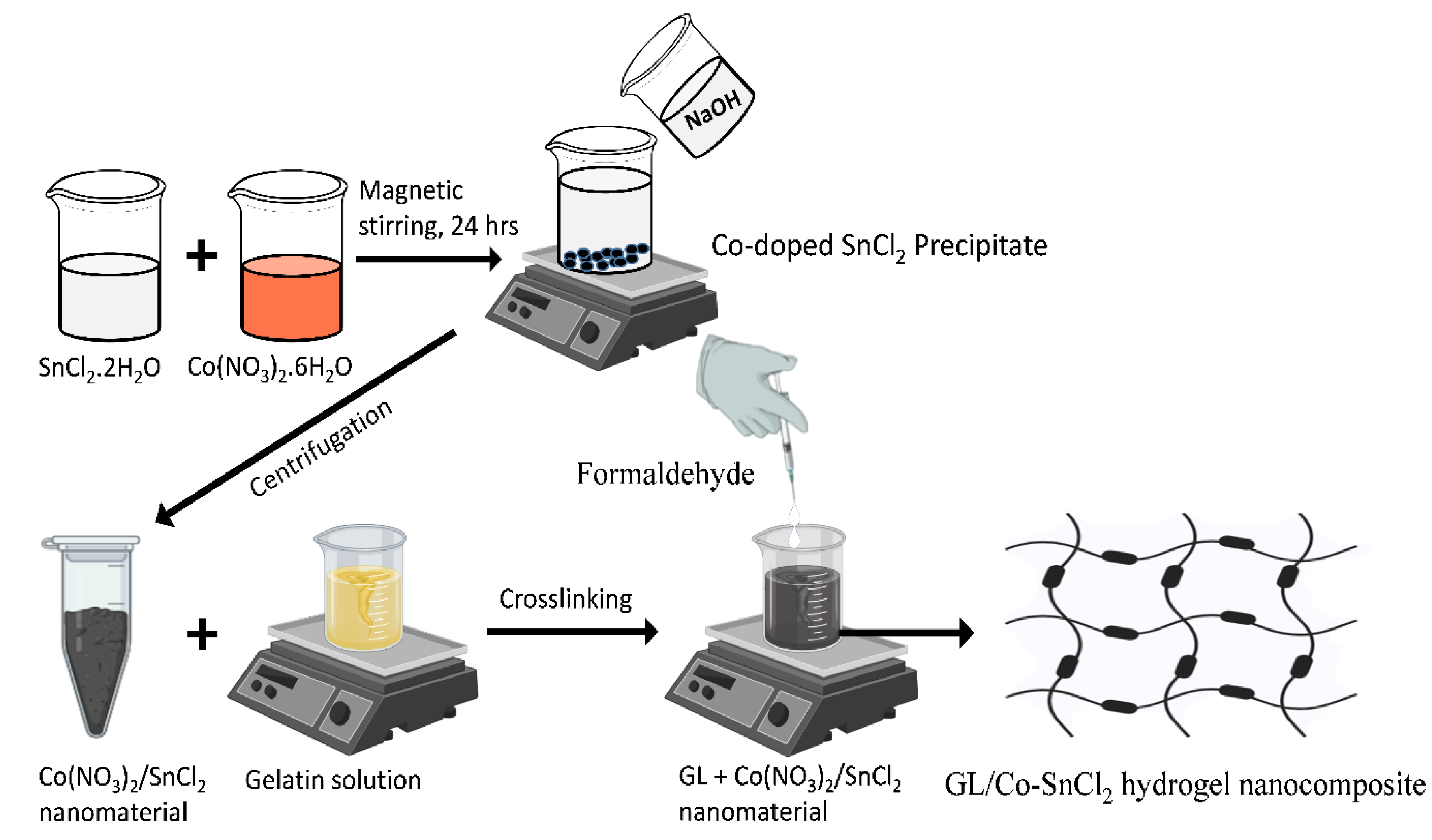
4.2. Preparation of Co-Doped SnO2 Nanomaterial with Hydrogel Nanocomposite
4.3. Catalytic Performance of Co-Doped SnO2 Nanocomposite
4.4. Characterizations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorption of Organic Contaminants by Biopolymers: Role of Polarity, Structure and Domain Spatial Arrangement. Available online: https://www.ncbi.nlm.nih.gov/pubmed/17095043 (accessed on 13 February 2020).
- Khan, S.B.; Ali, F.; Kamal, T.; Anwar, Y.; Asiri, A.M.; Seo, J. CuO embedded chitosan spheres as antibacterial adsorbent for dyes. Int. J. Biol. Macromol. 2016, 88, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Park, H.J.; Hong, S.I.; Byun, Y.J.; Darby, D.O.; Kimmel, R.M.; Whiteside, W.S. Effect of clay content, homogenization RPM, pH, and ultrasonication on mechanical and barrier properties of fish gelatin/montmorillonite nanocomposite films. LWT Food Sci. Technol. 2009, 42, 1179–1186. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2019, 135, 1162–1170. [Google Scholar] [CrossRef]
- Kamal, T.; Ul-Islam, M.; Khan, S.B.; Asiri, A.M. Adsorption and photocatalyst assisted dye removal and bactericidal performance of ZnO/chitosan coating layer. Int. J. Biol. Macromol. 2015, 81, 584–590. [Google Scholar] [CrossRef]
- Ali, N.; Kamal, T.; Ul-Islam, M.; Khan, A.; Shah, S.J.; Zada, A. Chitosan-coated cotton cloth supported copper nanoparticles for toxic dye reduction. Int. J. Biol. Macromol. 2018, 111, 832–838. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M. Chitosan-titanium oxide fibers supported zero-valent nanoparticles: Highly efficient and easily retrievable catalyst for the removal of organic pollutants. Sci. Rep. 2018, 8, 6260. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Asiri, A.M.; Sobahi, T.R.A. Chitosan coated cotton cloth supported zero-valent nanoparticles: Simple but economically viable, efficient and easily retrievable catalysts. Sci. Rep. 2017, 7, 16957. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Rodrigues, J.R. Kinetic analysis of metal uptake by dry and gel alginate particles. Biochem. Eng. J. 2009, 46, 320–326. [Google Scholar] [CrossRef]
- Yuan, S.; Xiong, G.; Roguin, A.; Choong, C. Immobilization of gelatin onto poly (glycidyl methacrylate)-grafted polycaprolactone substrates for improved cell–material interactions. Biointerphases 2012, 7, 30. [Google Scholar] [CrossRef]
- Echave, M.C.; Burgo, L.S.; Pedraz, J.L.; Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 2017, 23, 3567–3584. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of highly crosslinked gelatin hydrogel and its influence on chondrocyte proliferation and phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- Al-Mubaddel, F.S.; Haider, S.; Aijaz, M.O.; Haider, A.; Kamal, T.; Almasry, W.A.; Javid, M.; Khan, S.U.-D. Preparation of the chitosan/polyacrylonitrile semi-IPN hydrogel via glutaraldehyde vapors for the removal of Rhodamine B dye. Polym. Bull. 2017, 74, 1535–1551. [Google Scholar] [CrossRef]
- Ali, N.; Ismail, M.; Khan, A.; Khan, H.; Haider, S.; Kamal, T. Spectrophotometric methods for the determination of urea in real samples using silver nanoparticles by standard addition and 2nd order derivative methods. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2018, 189, 110–115. [Google Scholar] [CrossRef]
- Fukushima, Y.; Inagaki, S. Synthesis of an intercalated compound of montmorillonite and 6-polyamide. In Inclusion Phenomena in Inorganic, Organic, and Organometallic Hosts; Springer: Berlin/Heidelberg, Germany, 1987; pp. 365–374. [Google Scholar]
- Lan, T.; Pinnavaia, T.J. Clay-Reinforced Epoxy Nanocomposites. Chem. Mater. 1994, 6, 2216–2219. [Google Scholar] [CrossRef]
- Tenório-Neto, E.T.; Lima, D.d.; Guilherme, M.R.; Lima-Tenório, M.K.; Scariot, D.B.; Nakamura, C.V.; Kunita, M.H.; Rubira, A.F. Synthesis and drug release profile of a dual-responsive poly (ethylene glycol) hydrogel nanocomposite. RSC Adv. 2017, 7, 27637–27644. [Google Scholar] [CrossRef]
- Khan, M.S.J.; Khan, S.B.; Kamal, T.; Asiri, A.M. Agarose biopolymer coating on polyurethane sponge as host for catalytic silver metal nanoparticles. Polym. Test. 2019, 78, 105983. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Agar hydrogel supported metal nanoparticles catalyst for pollutants degradation in water, Desalin. Water Treat. 2018, 136, 290–298. [Google Scholar] [CrossRef]
- Khan, M.S.J.; Kamal, T.; Ali, F.; Asiri, A.M.; Khan, S.B. Chitosan-coated polyurethane sponge supported metal nanoparticles for catalytic reduction of organic pollutants. Int. J. Biol. Macromol. 2019, 132, 772–783. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Hom, W.L.; Bhatia, S.R. Significant enhancement of elasticity in alginate-clay nanocomposite hydrogels with PEO-PPO-PEO copolymers. Polymer 2017, 109, 170–175. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Memic, A.; Alhadrami, H.A.; Hussain, M.A.; Aldhahri, M.; al Nowaiser, F.; Al-Hazmi, F.; Oklu, R.; Khademhosseini, A. Hydrogels 2.0: Improved properties with nanomaterial composites for biomedical applications. Biomed. Mater. 2015, 11, 014104. [Google Scholar] [CrossRef] [PubMed]
- Phan, V.G.; Thambi, T.; Gil, M.S.; Lee, D.S. Temperature and pH-sensitive injectable hydrogels based on poly (sulfamethazine carbonate urethane) for sustained delivery of cationic proteins. Polymer 2017, 109, 38–48. [Google Scholar] [CrossRef]
- Barkhordari, S.; Yadollahi, M.; Namazi, H. pH sensitive nanocomposite hydrogel beads based on carboxymethyl cellulose/layered double hydroxide as drug delivery systems. J. Polym. Res. 2014, 21, 454. [Google Scholar] [CrossRef]
- Pagonis, K.; Bokias, G. Temperature- and solvent-sensitive hydrogels based on N-isopropylacrylamide and N,N-dimethylacrylamide. Polym. Bull. 2007, 58, 289–294. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K.; Kumar, A.; Kummara, M.R.; Kamal, T.; Han, S.S. A novel use of cellulose based filter paper containing silver nanoparticles for its potential application as wound dressing agent. Int. J. Biol. Macromol. 2018, 108, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Mousavi, S.B.; Karimi, F.; Marandi, G.B.; Garabaghi, H.; Shahabvand, S. Synthesis of porous poly (acrylamide) hydrogels using calcium carbonate and its application for slow release of potassium nitrate. Express Polym. Lett. 2009, 3, 279–285. [Google Scholar] [CrossRef]
- Kamal, T.; Anwar, Y.; Khan, S.B.; Chani, M.T.S.; Asiri, A.M. Dye adsorption and bactericidal properties of TiO2/chitosan coating layer. Carbohydr. Polym. 2016, 148, 153–160. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, S.B.; Kamal, T.; Asiri, A.M. Visible light activated degradation of organic pollutants using zinc-iron selenide. J. Mol. Liq. 2017, 229, 429–435. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, Q.; Ye, D.; Chang, C. Tunicate cellulose nanocrystals reinforced nanocomposite hydrogels comprised by hybrid cross-linked networks. Carbohydr. Polym. 2017, 169, 139–148. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, T.; Hu, D.; Chang, C. Dual physically cross-linked nanocomposite hydrogels reinforced by tunicate cellulose nanocrystals with high toughness and good self-recoverability. ACS Appl. Mater. Interfaces 2017, 9, 24230–24237. [Google Scholar] [CrossRef] [PubMed]
- Marandi, G.B.; Kermani, Z.P.; Kurdtabar, M. Fast and Efficient Removal of Cationic Dyes From Aqueous Solution by Collagen-Based Hydrogel Nanocomposites. Polym. Plast. Technol. Eng. 2013, 52, 310–318. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.G.; Gostynska, N.; Montesi, M.; Panseri, S.; Sprio, S.; Kon, E.; Marcacci, M.; Tampieri, A.; Sandri, M. Investigation of different cross-linking approaches on 3D gelatin scaffolds for tissue engineering application: A comparative analysis. Int. J. Biol. Macromol. 2017, 95, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly (vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Inoue, M.; Sasaki, M.; Nakasu, A.; Takayanagi, M.; Taguchi, T. An Antithrombogenic Citric Acid-Crosslinked Gelatin with Endothelialization Activity. Adv. Healthc. Mater. 2012, 1, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Khan, M.S.J.; Khan, S.B.; Asiri, A.M.; Chani, M.T.S.; Ullah, M.W. Silver Nanoparticles Embedded in Gelatin Biopolymer Hydrogel as Catalyst for Reductive Degradation of Pollutants. J. Polym. Environ. 2019, 28, 399–410. [Google Scholar] [CrossRef]
- Luque, P.A.; Nava, O.; Soto-Robles, C.A.; Chinchillas-Chinchillas, M.J.; Garrafa-Garrafa, H.E.; Baez-Lopez, Y.A.; Valdez-Nunez, K.P.; Vilchis-Nestor, A.R.; Castro-Beltran, A. Improved photocatalytic efficiency of SnO2 nanoparticles through green synthesis. Optik 2020, 206, 164299. [Google Scholar] [CrossRef]
- Cheng, B.; Russell, J.M.; Shi, W.; Zhang, L.; Samulski, E.T. Large-scale, solution-phase growth of single-crystalline SnO2 nanorods. J. Am. Chem. Soc. 2004, 126, 5972–5973. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Z.; Jiang, W.; Zou, Y.; Wang, Y.; Lu, C. Sandwich-like CNTs@SnO2 /SnO/Sn anodes on three-dimensional Ni foam substrate for lithium ion batteries. J. Electroanal. Chem. 2016, 767, 49–55. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; Yunus, W.M.Z.W.; Kassim, A.; Zainal, Z. Synthesis of Nanocrystalline SnOx (x = 1–2) Thin Film Using a Chemical Bath Deposition Method with Improved Deposition Time, Temperature and pH. Sensors 2011, 11, 9207–9216. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Ryabtsev, S.V.; Domashevskaya, E.P.; Seredin, P.V. Investigation of structural and optical properties of powder tin oxide (SnOx) annealed in air. КОНДЕНСИРОВАННЫЕ 2009, 7, 10–15. [Google Scholar]
- Santhi, K.; Rani, C.; Karuppuchamy, S. Synthesis and characterization of a novel SnO/SnO2 hybrid photocatalyst. J. Alloy. Compd. 2016, 662, 102–107. [Google Scholar] [CrossRef]
- Liang, H.; Raitano, J.M.; Zhang, L.; Chan, S.-W. Controlled synthesis of Co3O4 nanopolyhedrons and nanosheets at low temperature. Chem. Commun. 2009, 48, 7569–7571. [Google Scholar] [CrossRef]
- Sahoo, S.; Satpati, A.K. Electrochemical capacitance properties of cobalt oxide entangled over MWCNT and cobalt oxide AC composites. J. Electroanal. Chem. 2017, 801, 416–424. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamal, T.; Khan, S.B.; Asiri, A.M. An efficient and easily retrievable dip catalyst based on silver nanoparticles/chitosan-coated cellulose filter paper. Cellulose 2016, 23, 3577–3588. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kamal, T.; Khan, S.A.; Anwar, Y.; Saeed, M.T.; Asiri, A.M.; Khan, S.B. Assessment of Anti-bacterial Ni-Al/chitosan Composite Spheres for Adsorption Assisted Photo-Degradation of Organic Pollutants. Curr. Nanosci. 2016, 12, 569–575. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Anwar, Y.; Alamry, K.A.; Asiri, A.M. Bactericidal and catalytic performance of green nanocomposite based on chitosan/carbon black fiber supported monometallic and bimetallic nanoparticles. Chemosphere 2017, 188, 588–598. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Kamal, T.; Ul-Islam, S.; Shah, N.; Park, J.K. Recent Advancement in Cellulose based Nanocomposite for Addressing Environmental Challenges. Recent Pat. Nanotechn. 2016, 10, 169–180. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Kamal, T.; Asiri, A.M.; Akhtar, K. Recent Development of Chitosan Nanocomposites for Environmental Applications. Recent Pat. Nanotechn. 2016, 10, 181–188. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, S.B.; Kamal, T.; Yasir, M.; Asiri, A.M. Antibacterial nanocomposites based on chitosan/Co-MCM as a selective and efficient adsorbent for organic dyes. Int. J. Biol. Macromol. 2016, 91, 744–751. [Google Scholar] [CrossRef]
- Khan, S.B.; Khan, S.A.; Marwani, H.M.; Bakhsh, E.M.; Anwar, Y.; Kamal, T.; Asiri, A.M.; Akhtar, K. Anti-bacterial PES-cellulose composite spheres: Dual character toward extraction and catalytic reduction of nitrophenol. RSC Adv. 2016, 6, 110077–110090. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lin, Y.-W. Permeation of Hair Dye Ingredients, p-Phenylenediamine and Aminophenol Isomers, through Protective Gloves. Ann. Occup. Hyg. 2009, 53, 289–296. [Google Scholar] [CrossRef]
- Swathi, T.; Buvaneswari, G. Application of NiCo2O4 as a catalyst in the conversion of p-nitrophenol to p-aminophenol. Mater. Lett. 2008, 62, 3900–3902. [Google Scholar] [CrossRef]
- Lu, H.; Yin, H.; Liu, Y.; Jiang, T.; Yu, L. Influence of support on catalytic activity of Ni catalysts in p-nitrophenol hydrogenation to p-aminophenol. Catal. Commun. 2008, 10, 313–316. [Google Scholar] [CrossRef]
- Contreras-Cáceres, R.; Sánchez-Iglesias, A.; Karg, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Pacifico, J.; Hellweg, T.; Fernández-Barbero, A.; Liz-Marzán, L.M. Encapsulation and Growth of Gold Nanoparticles in Thermoresponsive Microgels. Adv. Mater. 2008, 20, 1666–1670. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Nickel nanoparticles-chitosan composite coated cellulose filter paper: An efficient and easily recoverable dip-catalyst for pollutants degradation. Environ. Pollut. 2016, 218, 625–633. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Haider, S.; Alghamdi, Y.G.; Asiri, A.M. Thin layer chitosan-coated cellulose filter paper as substrate for immobilization of catalytic cobalt nanoparticles. Int. J. Biol. Macromol. 2017, 104, 56–62. [Google Scholar] [CrossRef]
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Synthesis and catalytic properties of silver nanoparticles supported on porous cellulose acetate sheets and wet-spun fibers. Carbohydr. Polym. 2017, 157, 294–302. [Google Scholar] [CrossRef]
- Khan, F.U.; Khan, S.B.; Kamal, T.; Asiri, A.M.; Khan, I.U.; Akhtar, K. Novel combination of zero-valent Cu and Ag nanoparticles @ cellulose acetate nanocomposite for the reduction of 4-nitro phenol. Int. J. Biol. Macromol. 2017, 102, 868–877. [Google Scholar] [CrossRef]
- Kamal, T.; Khan, S.B.; Asiri, A.M. Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: Evaluation of azo dyes catalytic reduction. Cellulose 2016, 23, 1911–1923. [Google Scholar] [CrossRef]
- Jana, S.; Ghosh, S.K.; Nath, S.; Pande, S.; Praharaj, S.; Panigrahi, S.; Basu, S.; Endo, T.; Pal, T. Synthesis of silver nanoshell-coated cationic polystyrene beads: A solid phase catalyst for the reduction of 4-nitrophenol. Appl. Catal. A Gen. 2006, 313, 41–48. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Scurrell, M. Silver nanoparticle catalysed redox reaction: An electron relay effect. Mater. Chem. Phys. 2006, 97, 283–287. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marwani, H.M.; Ahmad, S.; Rahman, M.M. Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants. Gels 2022, 8, 479. https://doi.org/10.3390/gels8080479
Marwani HM, Ahmad S, Rahman MM. Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants. Gels. 2022; 8(8):479. https://doi.org/10.3390/gels8080479
Chicago/Turabian StyleMarwani, Hadi M., Shahid Ahmad, and Mohammed M. Rahman. 2022. "Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants" Gels 8, no. 8: 479. https://doi.org/10.3390/gels8080479
APA StyleMarwani, H. M., Ahmad, S., & Rahman, M. M. (2022). Fabrication of 3D Gelatin Hydrogel Nanocomposite Impregnated Co-Doped SnO2 Nanomaterial for the Catalytic Reduction of Environmental Pollutants. Gels, 8(8), 479. https://doi.org/10.3390/gels8080479







