Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization
Abstract
:1. Introduction
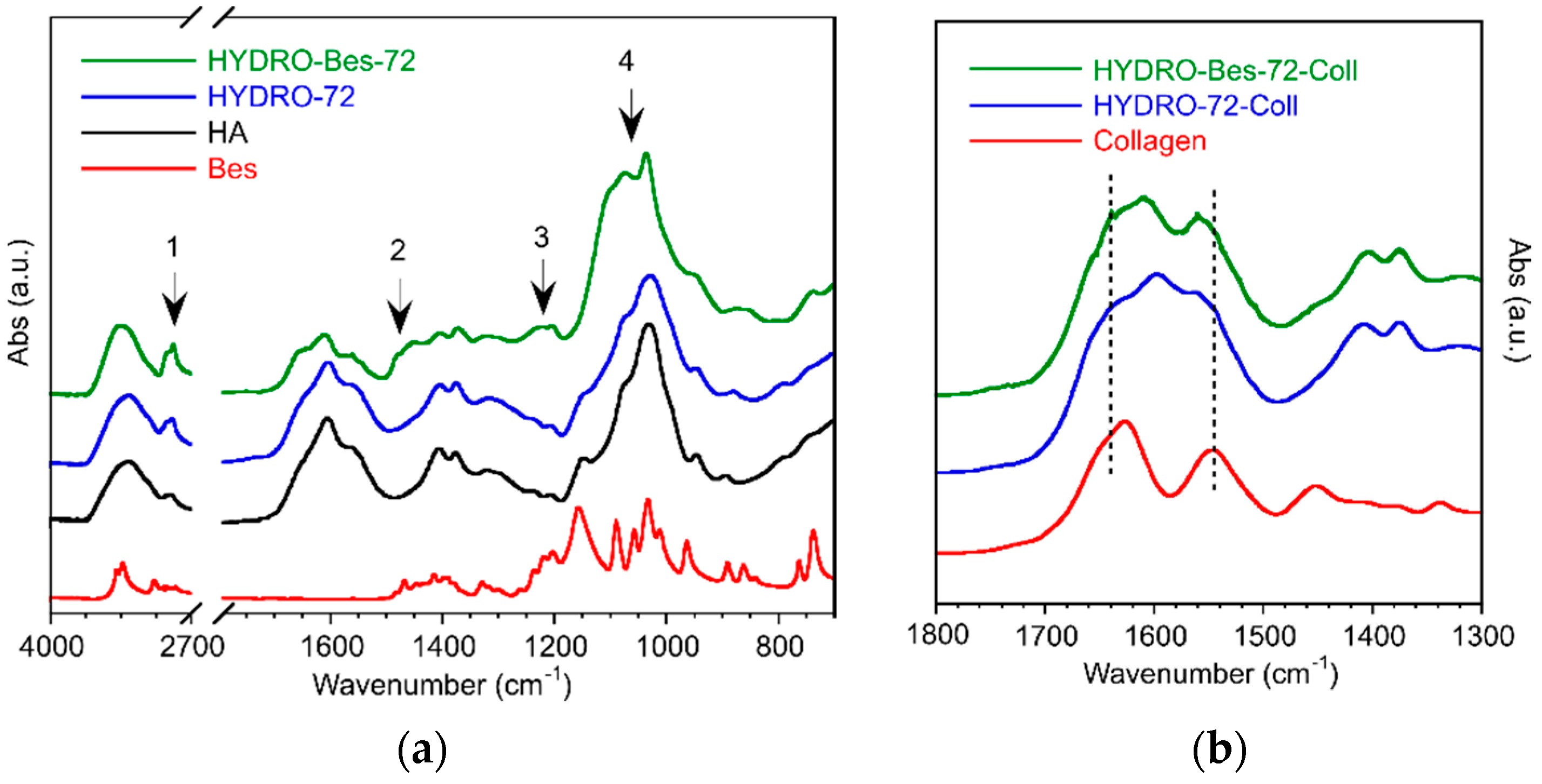
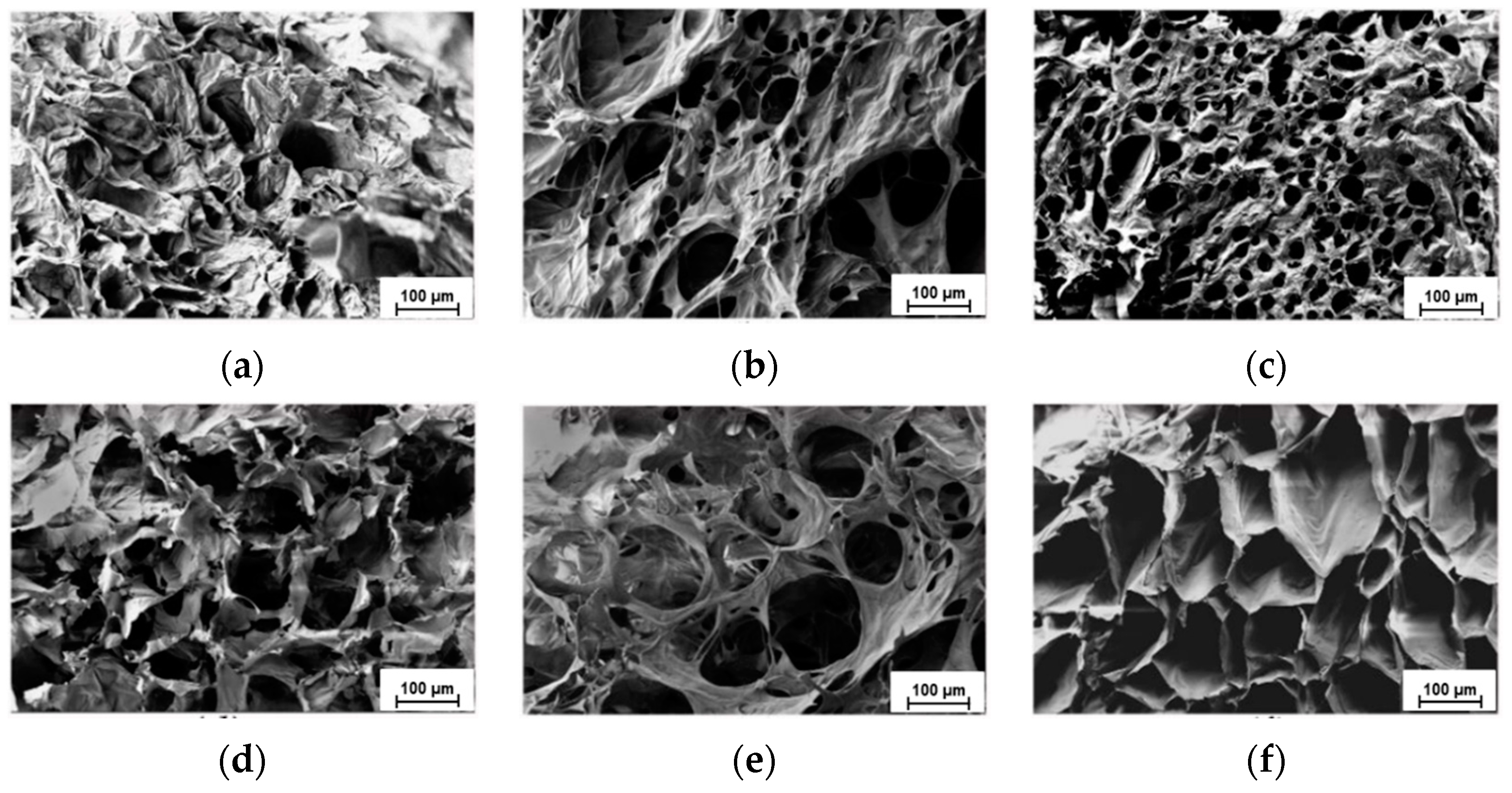
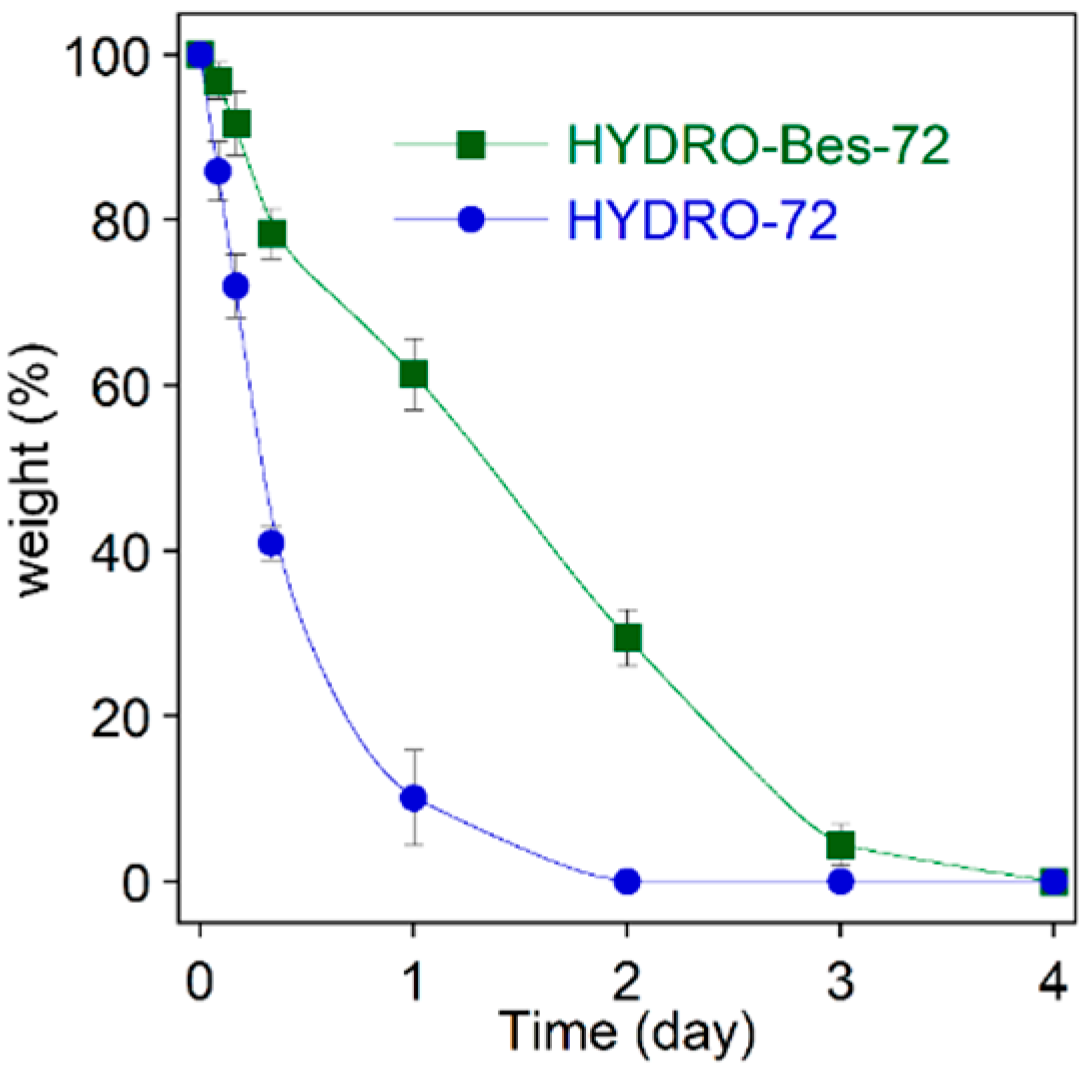
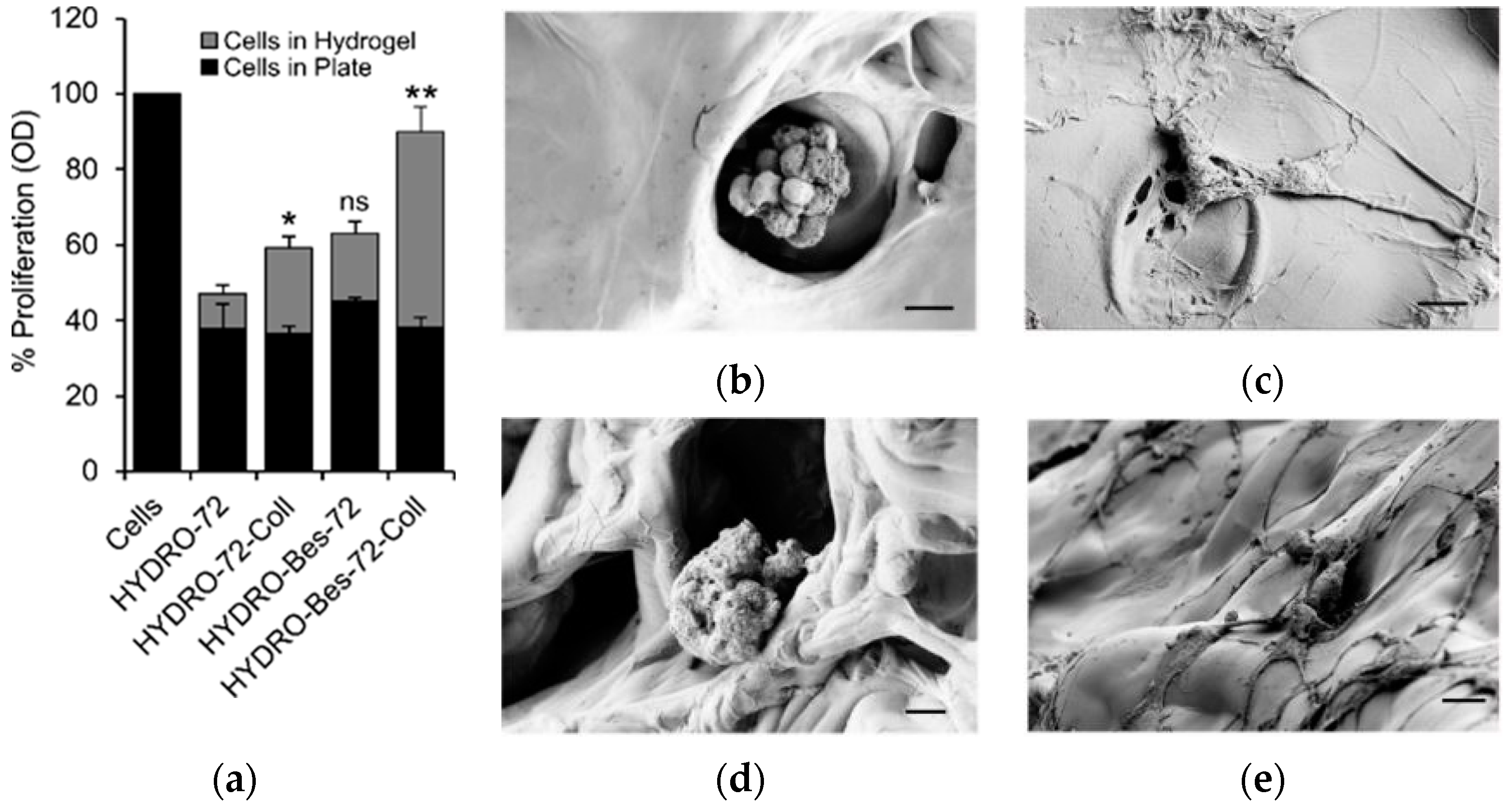
2. Results and Discussion
3. Conclusions
- -
- the formation of a more regular porous structure with interconnected pores;
- -
- a decrease of the equilibrium water uptake and, therefore, an increase of gel mechanical stability;
- -
- a higher hydrogel stability towards enzymatic degradation;
- -
- a greater collagen absorption by electrostatic interaction of the protein with the sulfonic groups, in anionic form even at low pH;
- -
- a resulting increase of viability of fibroblasts cultured for 72 h into the hydrogel with collagen;
- -
- a more stable attachment of fibroblasts on the gel surface, as evidenced by cell morphological changes and formation of filopodia.
4. Materials and Methods
4.1. Materials
4.2. Hydrogel Preparation
4.3. Degree of Sulfonation Determination
4.4. Infrared Spectroscopy (FTIR-ATR)
4.5. Ninhydrin Test
4.6. Water Swelling Determination
4.7. Mechanical Measurements
4.8. SEM Morphological Analysis
4.9. In Vitro Degradation
4.10. Cell Culture
4.11. MTT Assay
4.12. Cells Morphology (SEM)
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering-A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Pérez González, R.; Sáez-Martínez, V.; Ruiz Pérez, J.; Vilas-Vilela, J.L. Synthesis and characterization of covalently crosslinked pH-responsive hyaluronic acid nanogels: Effect of synthesis parameters. Polymers 2019, 11, 742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Dalir, E.; Vahedi, P.; Hasanzadeh, A.; Samiei, M. The Potential Applications of Hyaluronic Acid Hydrogels in Biomedicine. Drug Res. 2020, 70, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Leonelli, F.; La Bella, A.; Francescangeli, A.; Joudioux, R.; Capodilupo, A.L.; Quagliariello, M.; Migneco, L.M.; Bettolo, R.M.; Crescenzi, V.; De Luca, G.; et al. A new and simply available class of hydrosoluble bioconjugates by coupling paclitaxel to hyaluronic acid through a 4-hydroxybutanoic acid derived linker. Helv. Chim. Acta 2005, 88, 154–159. [Google Scholar] [CrossRef]
- La Gatta, A.; Schiraldi, C.; Papa, A.; D’Agostino, A.; Cammarota, M.; De Rosa, A.; De Rosa, M. Hyaluronan scaffolds via diglycidyl ether crosslinking: Toward improvements in composition and performance. Carbohydr. Polym. 2013, 96, 536–544. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Kafedjiiski, K.; Jetti, R.K.R.; Föger, F.; Hoyer, H.; Werle, M.; Hoffer, M.; Bernkop-Schnürch, A. Synthesis and in vitro evaluation of thiolated hyaluronic acid for mucoadhesive drug delivery. Int. J. Pharm. 2007, 343, 48–58. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, X.; Fan, D.; Zhu, C.; Deng, J.; Hui, J.; Ma, P. Synthesis and characterization of hyaluronic acid/human-like collagen hydrogels. Mater. Sci. Eng. C 2014, 43, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Melnik, T.; Ameur, S.B.; Kanfar, N.; Vinet, L.; Delie, F.; Jordan, O. Bioadhesive Hyaluronic Acid/Dopamine Hydrogels for Vascular Applications Prepared by Initiator-Free Crosslinking. Int. J. Mol. Sci. 2022, 23, 5706. [Google Scholar] [CrossRef]
- Zhang, F.; He, C.; Cao, L.; Feng, W.; Wang, H.; Mo, X.; Wang, J. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int. J. Biol. Macromol. 2011, 48, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.D.; Tirelli, N.; Hubbell, J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 2003, 24, 893–900. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, L.; Duan, Z.; Rohani, S. Hyaluronic acid hydrogels, as a biological macromolecule-based platform for stem cells delivery and their fate control: A review. Int. J. Biol. Macromol. 2021, 189, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, S.; Pei, M.; Yang, H.; Gu, S.; Tao, Y.; Ye, D.; Zhou, Y.; Xu, W.; Xiao, P. Dopamine-Modified Hyaluronic Acid Hydrogel Adhesives with Fast-Forming and High Tissue Adhesion. ACS Appl. Mater. Interfaces 2020, 12, 18225–18234. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; So, Y.K.; Lee, Y.M. Preparation of porous collagen/hyaluronic acid hybrid scaffolds for biomimetic functionalization through biochemical binding affinity. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 506–518. [Google Scholar] [CrossRef]
- Kim, T.G.; Chung, H.J.; Park, T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008, 4, 1611–1619. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef] [Green Version]
- Abatangelo, G.; Barbucci, R.; Brun, P.; Lamponi, S. Biocompatibility and enzymatic degradation studies on sulphated hyaluronic acid derivatives. Biomaterials 1997, 18, 1411–1415. [Google Scholar] [CrossRef]
- Jha, A.K.; Altiok, E.I.; Jackson, W.M.; Healy, K.E. Ethylsulfonated hyaluronic acid biopolymers and methods of use thereof. U.S. Patent No. 10,000,582, 19 June 2018. [Google Scholar]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Mater. Today Bio 2022, 13, 100183. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, C.; Bellini, D.; Longinotti, C.; Martinelli, A.; Matricardi, P. Preparation and characterization of a new gellan gum and sulphated hyaluronic acid hydrogel designed for epidural scar prevention. J. Mater. Sci. Mater. Med. 2011, 22, 263–271. [Google Scholar] [CrossRef]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef]
- De Felice, A.C.; Di Lisio, V.; Francolini, I.; Mariano, A.; Piozzi, A.; D’abusco, A.S.; Sturabotti, E.; Martinelli, A. One-Pot Preparation of Hydrophilic Polylactide Porous Scaffolds by Using Safe Solvent and Choline Taurinate Ionic Liquid. Pharmaceutics 2022, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, L.; Cocchiola, R.; Francolini, I.; Lopreiato, M.; Piozzi, A.; Zanoni, R.; Scotto d’Abusco, A.; Martinelli, A. Taurine grafting and collagen adsorption on PLLA films improve human primary chondrocyte adhesion and growth. Colloids Surf. B Biointerfaces 2017, 158, 643–649. [Google Scholar] [CrossRef]
- Barbucci, R.; Rappuoli, R.; Borzacchiello, A.; Ambrosio, L. Synthesis, chemical and rheological characterization of new hyaluronic acid-based hydrogels. J. Biomater. Sci. Polym. Ed. 2000, 11, 383–399. [Google Scholar] [CrossRef] [PubMed]
- De Boulle, K.; Glogau, R.; Kono, T.; Nathan, M.; Tezel, A.; Roca-Martinez, J.X.; Paliwal, S.; Stroumpoulis, D. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol. Surg. 2013, 39, 1758–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels: A strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef]
- Malson, T.; Lindqvist, B.L. Gel of crosslinked hyaluronic acid for use as a vitreous humor substitute. U.S. Patent No. 4,716,154, 29 December 1987. [Google Scholar]
- Tuin, A.; Zandstra, J.; Kluijtmans, S.G.; Bouwstra, J.B.; Harmsen, M.C.; van Luyn, M.J.A. Hyaluronic acid-recombinant gelatin gels as a scaffold for soft tissue regeneration. Eur. Cells Mater. 2012, 24, 320–330. [Google Scholar] [CrossRef]
- Uspenskii, S.A.; Kil’deeva, N.R.; Maslova, M.V.; Demina, T.S.; Vikhoreva, G.A. A study of the viscosity of hyaluronic acid solutions for the preparation of polyelectrolyte complexes with chitosan. Russ. Chem. Bull. 2016, 65, 273–276. [Google Scholar] [CrossRef]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on hyaluronan behavior in aqueous solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.L.; Nyström, B. Effect of pH on the behavior of hyaluronic acid in dilute and semidilute aqueous solutions. Macromol. Symp. 2008, 274, 131–140. [Google Scholar] [CrossRef]
- Ghosh, S.; Kobal, I.; Zanette, D.; Reed, W.F. Conformational contraction and hydrolysis of hyaluronate in sodium hydroxide solutions. Macromolecules 1993, 26, 4685–4693. [Google Scholar] [CrossRef]
- Ström, A.; Larsson, A.; Okay, O. Preparation and physical properties of hyaluronic acid-based cryogels. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Preparation of cross-linked hyaluronic acid films of low water content. Biomaterials 1997, 18, 189–195. [Google Scholar] [CrossRef]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Hausman, R.; Digman, B.; Escobar, I.C.; Coleman, M.; Chung, T.S. Functionalization of polybenzimidizole membranes to impart negative charge and hydrophilicity. J. Memb. Sci. 2010, 363, 195–203. [Google Scholar] [CrossRef]
- Lee, C.H.; Parkm, H.B.; Chung, Y.S.; Lee, Y.M.; Freeman, B.D. Water sorption, proton conduction, and methanol permeation properties of sulfonated polyimide membranes cross-linked with N,N-Bis(2-hydroxyethyl)-2- ammoethanesulfonic acid (BES). Macromolecules 2006, 39, 755–764. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, K.; Sionkowska, A.; Grabska, S.; Kaczmarek, B.; Michalska, M. The miscibility of collagen/hyaluronic acid/chitosan blends investigated in dilute solutions and solids. J. Mol. Liq. 2016, 220, 726–730. [Google Scholar] [CrossRef]
- Baek, J.; Fan, Y.; Jeong, S.H.; Lee, H.Y.; Jung, H.D.; Kim, H.E.; Kim, S.; Jang, T.S. Facile strategy involving low-temperature chemical cross-linking to enhance the physical and biological properties of hyaluronic acid hydrogel. Carbohydr. Polym. 2018, 202, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Song, S.J.; Lee, K.J.; Park, M.H.; Lee, S.H.; Hahn, S.K.; Kim, S.; Kim, B.S. Mechanical properties and degradation behaviors of hyaluronic acid hydrogels cross-linked at various cross-linking densities. Carbohydr. Polym. 2007, 70, 251–257. [Google Scholar] [CrossRef]
- Casolaro, M.; Casolaro, I. Multiple stimuli-responsive hydrogels for metal-based drug therapy. Polymers 2012, 4, 964–985. [Google Scholar] [CrossRef] [Green Version]
- Barbucci, R.; Magnani, A.; Consumi, M. Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Study of the effect of mixing approach on cross-linking efficiency of hyaluronic acid-based hydrogel cross-linked with 1,4-butanediol diglycidyl ether. Eur. J. Pharm. Sci. 2016, 91, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Al-Sibani, M.; Al-Harrasi, A.; Neubert, R.H.H. Effect of hyaluronic acid initial concentration on cross-linking efficiency of hyaluronic acid-Based hydrogels used in biomedical and cosmetic applications. Pharmazie 2017, 72, 81–86. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.G.; Schilling, A.F. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef]
- Hachet, E.; Van Den Berghe, H.; Bayma, E.; Block, M.R.; Auzély-Velty, R. Design of biomimetic cell-interactive substrates using hyaluronic acid hydrogels with tunable mechanical properties. Biomacromolecules 2012, 13, 1818–1827. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.D.; Cho, H.J.; Balakrishnan, P.; Chung, C.W.; Yoon, I.S.; Oh, Y.K.; Byun, Y.; Kim, D.D. Cross-linked hyaluronic acid-based flexible cell delivery system: Application for chondrogenic differentiation. Colloids Surf. B Biointerfaces 2012, 91, 106–113. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.S.; Chung, C.W.; Sung, J.H.; Cho, H.J.; Kim, J.S.; Shim, W.S.; Shim, C.K.; Chung, S.J.; Kim, D.D. Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. J. Biosci. Bioeng. 2011, 112, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, D.; Zhu, C.; Ma, X. Effects of self-assembled fibers on the synthesis, characteristics and biomedical applications of CCAG hydrogels. J. Mater. Chem. B 2014, 2, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W. Methods for making injectable polymer hydrogels. U.S. Patent Application No. 11/132,604, 19 May 2005. [Google Scholar]
- Kenne, L.; Gohil, S.; Nilsson, E.M.; Karlsson, A.; Ericsson, D.; Kenne, A.H.; Nord, L.I. Modification and cross-linking parameters in hyaluronic acid hydrogels-Definitions and analytical methods. Carbohydr. Polym. 2013, 91, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Martins Shimojo, A.A.; Moraga Galdames, S.E.; da Silva Santos Duarte, A.; Pina, L.M.; Rodrigues, A.A.; Malheiros Luzo, Â.C.; Belangero, W.D.; Andrade, S.; Maria, H. The Structuring of High Molecular Weight Hyaluronic Acid in Microparticles or Sponges Improves its Performance when Associated with Platelet-Rich Plasma. Trends Biomater. Artif. Organs 2015, 29, 160–169. [Google Scholar]




| HA (wt/v%) | HA:BDDE:Bes (Molar Ratio) | tcross (h) | Gel Formation |
|---|---|---|---|
| 5 | 1:1:0.5 | 24, 72, 96 | No |
| 8 | 1:1:0.5 | 24, 72, 96 | Yes (soft gel) |
| 10 | 1:1:0.5 | 24, 72, 96 | Yes |
| 10 | 1:1:0.8 | 24, 72, 96 | No |
| 10 | 1:1:1 | 24, 72, 96 | No |
| Sample | HA:BDDE:Bes * (mol) | tcross (h) | Collagen |
|---|---|---|---|
| HYDRO-24 | 1:1:0 | 24 | - |
| HYDRO-72 | 1:1:0 | 72 | - |
| HYDRO-96 | 1:1:0 | 96 | - |
| HYDRO-72-Coll | 1:1:0 | 72 | Yes |
| HYDRO-Bes-24 | 1:1:0.5 | 24 | - |
| HYDRO-Bes-72 | 1:1:0.5 | 72 | - |
| HYDRO-Bes-96 | 1:1:0.5 | 96 | - |
| HYDRO-Bes-72-Coll | 1:1:0.5 | 72 | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturabotti, E.; Consalvi, S.; Tucciarone, L.; Macrì, E.; Di Lisio, V.; Francolini, I.; Minichiello, C.; Piozzi, A.; Vuotto, C.; Martinelli, A. Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization. Gels 2022, 8, 480. https://doi.org/10.3390/gels8080480
Sturabotti E, Consalvi S, Tucciarone L, Macrì E, Di Lisio V, Francolini I, Minichiello C, Piozzi A, Vuotto C, Martinelli A. Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization. Gels. 2022; 8(8):480. https://doi.org/10.3390/gels8080480
Chicago/Turabian StyleSturabotti, Elisa, Silvia Consalvi, Luca Tucciarone, Elisa Macrì, Valerio Di Lisio, Iolanda Francolini, Carmen Minichiello, Antonella Piozzi, Claudia Vuotto, and Andrea Martinelli. 2022. "Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization" Gels 8, no. 8: 480. https://doi.org/10.3390/gels8080480
APA StyleSturabotti, E., Consalvi, S., Tucciarone, L., Macrì, E., Di Lisio, V., Francolini, I., Minichiello, C., Piozzi, A., Vuotto, C., & Martinelli, A. (2022). Synthesis of Novel Hyaluronic Acid Sulfonated Hydrogels Using Safe Reactants: A Chemical and Biological Characterization. Gels, 8(8), 480. https://doi.org/10.3390/gels8080480









