Structure and Adsorption Performance of Cationic Entermorpha prolifera Polysaccharide-Based Hydrogel for Typical Pollutants: Methylene Blue, Cefuroxime, and Cr (VI)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of EPS and CAEPS
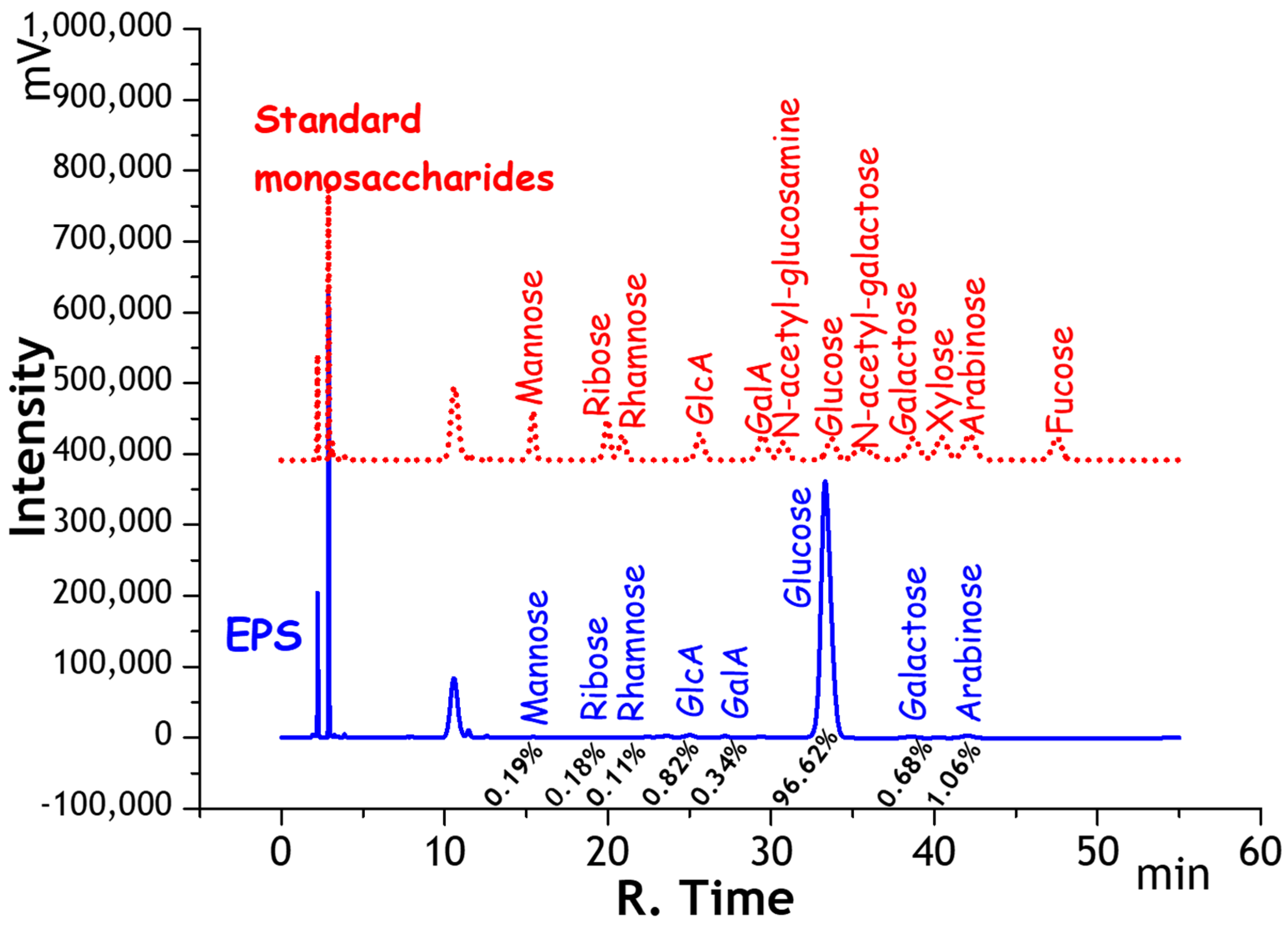
2.1.1. Monosaccharide Composition of EPS
2.1.2. AFM
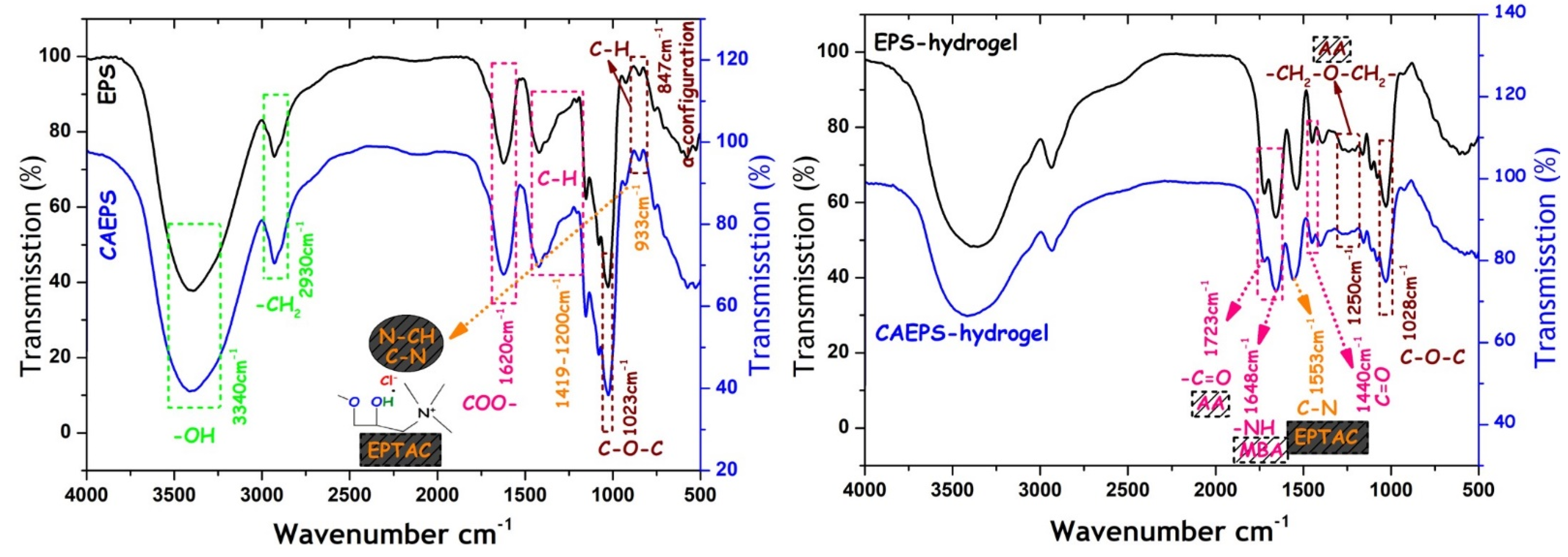
2.1.3. FT-IR
2.1.4. TG
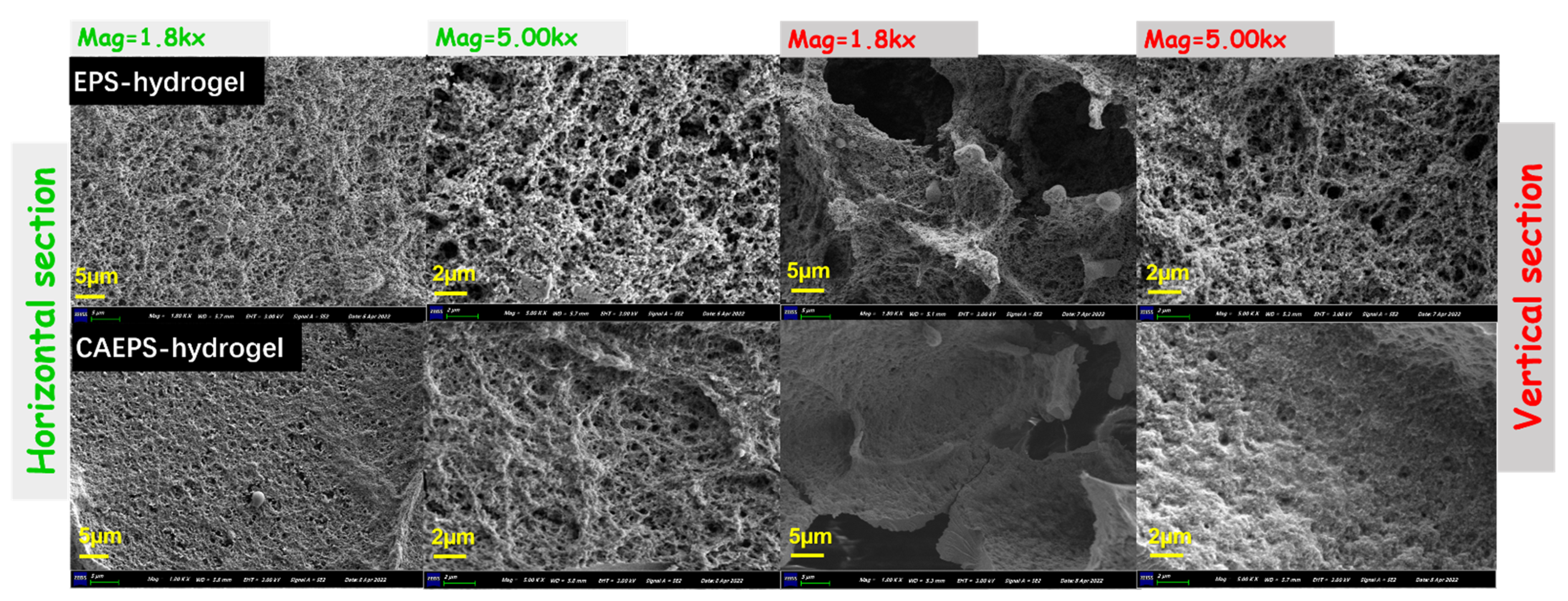
2.1.5. Morphological Observations
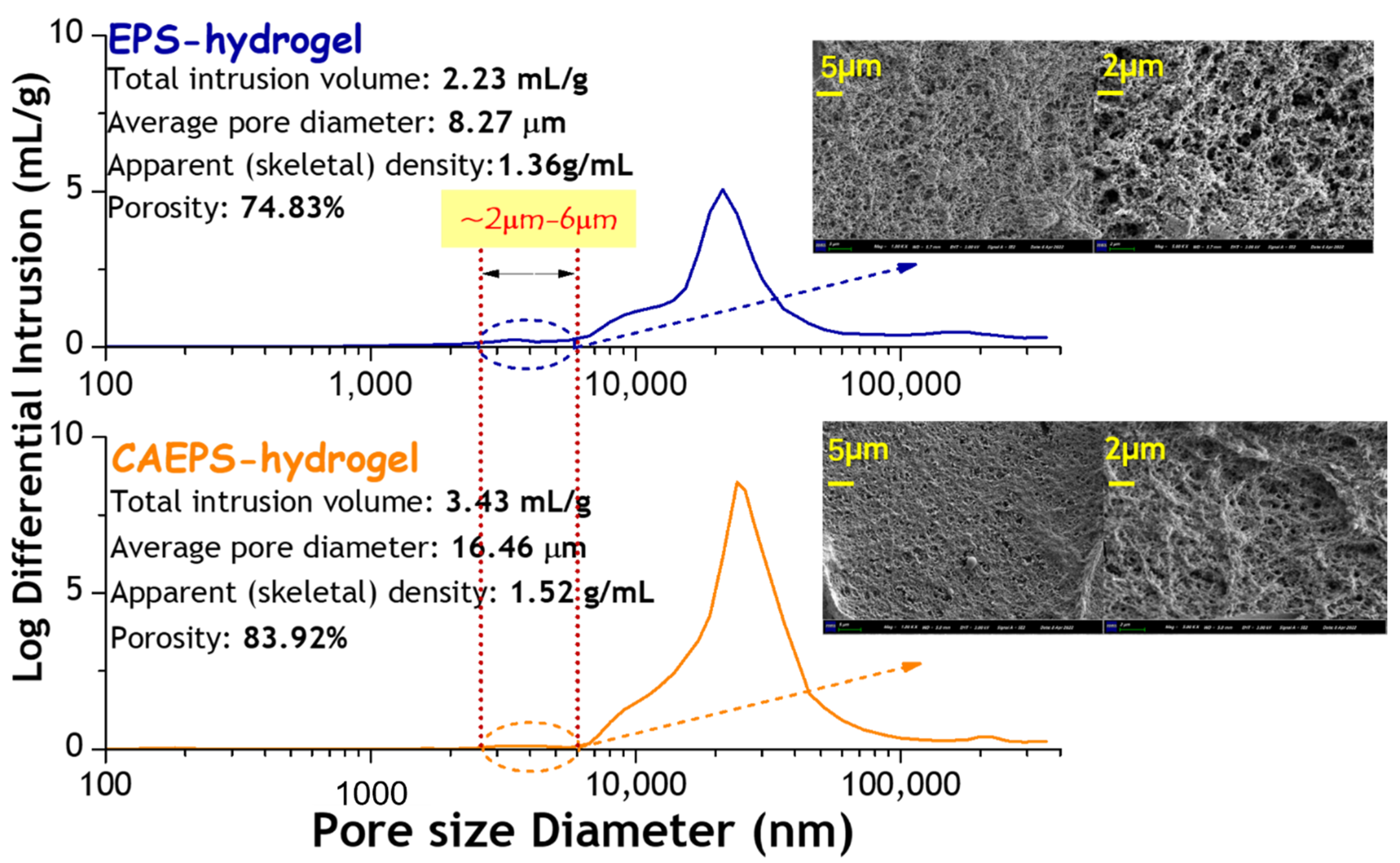
2.1.6. Pore Size Distribution Analysis of EPS- and CAEPS-Hydrogel
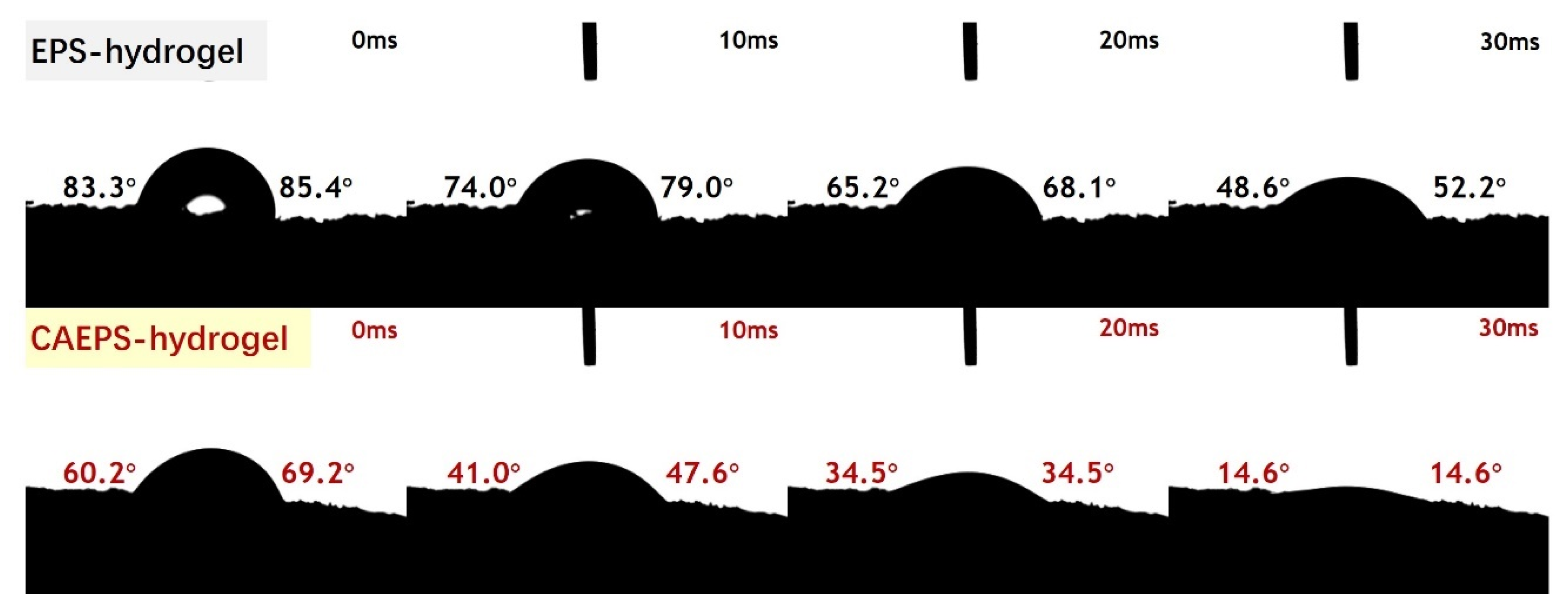
2.1.7. Surface Wettability of EPS-Xerogels and CAEPS-Xerogels
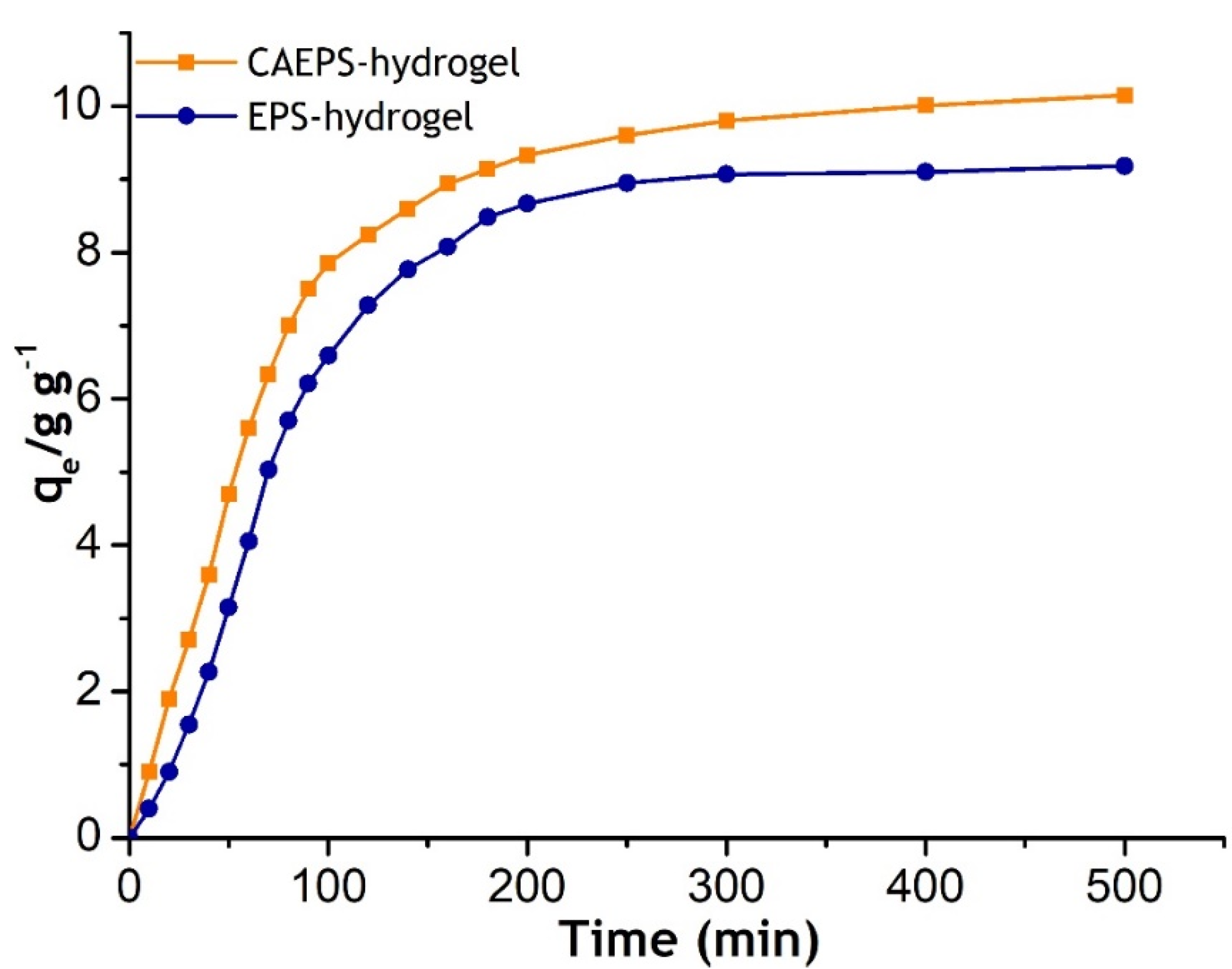
2.2. Swelling Behaviors of the EPS- and CAEPS-Hydrogels
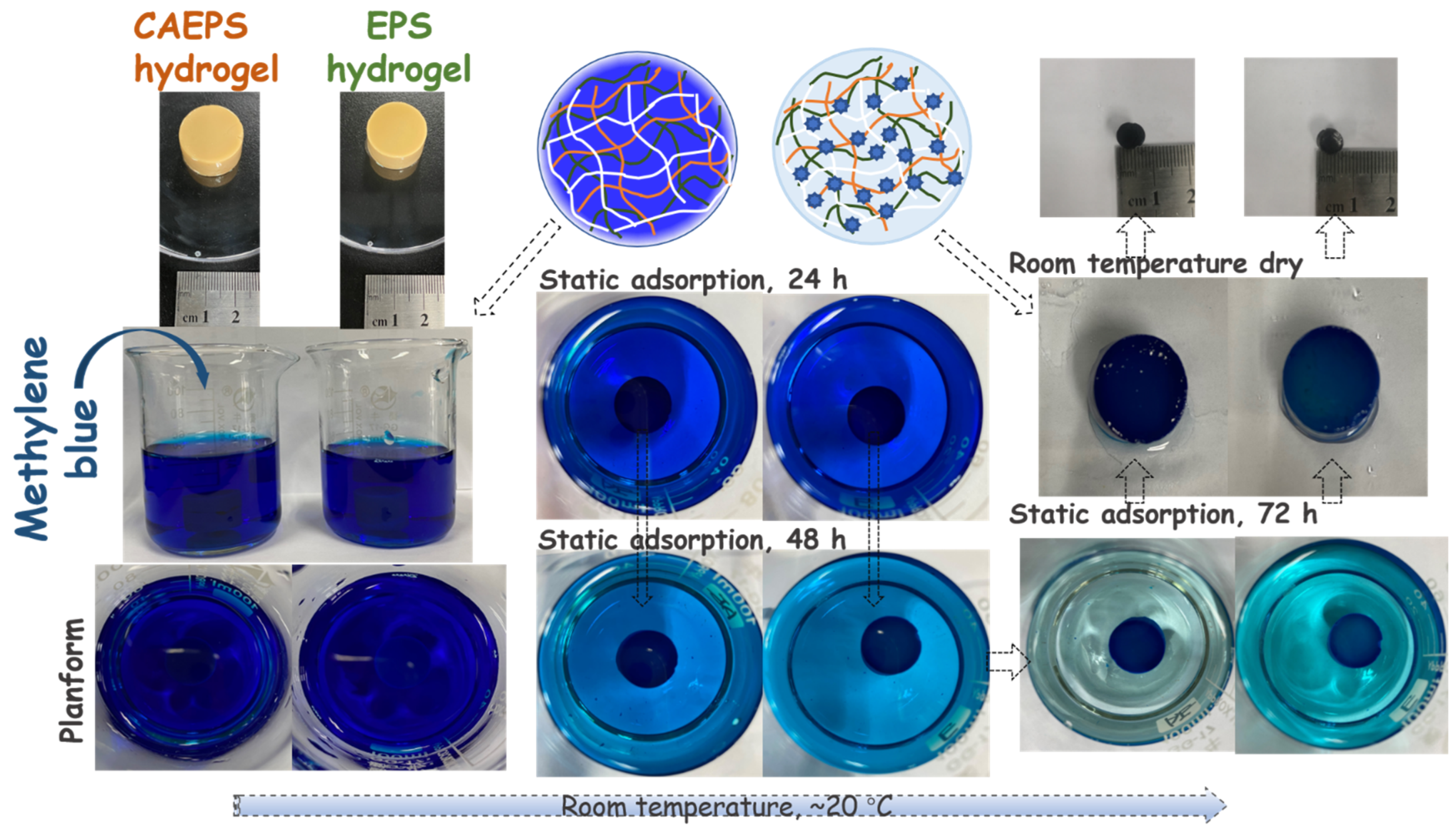
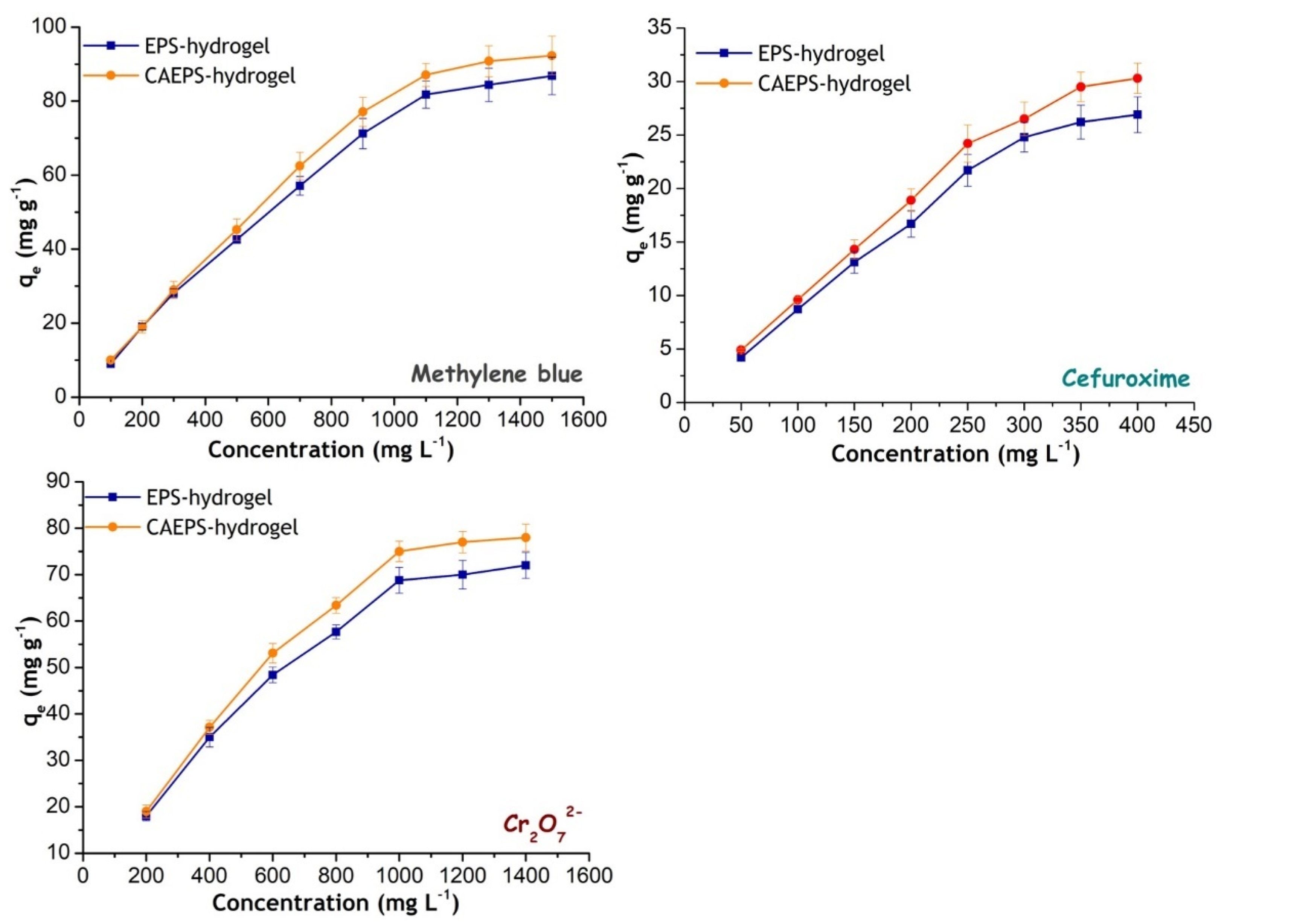
2.3. Adsorption Properties of EPS- and CAEPS-Hydrogels on Methylene Blue, Cefuroxime, and Cr (VI) (Na2Cr2O7)
3. Conclusions
4. Experiments
4.1. Experimental Materials
4.2. Extraction of Enteromorpha Prolifera Polysaccharide (EPS)
4.3. Preparation of Cationic Entermorpha Polysaccharide and Cationic Entermorpha Polysaccharide Hydrogel (EPS- and CAEPS-Hydrogel)
4.4. Experiment and Reaction Mechanism
4.5. Characterization of EPS, CAEPS, EPS-, and CAEPS-Hydrogel
4.5.1. Monosaccharide Composition Analysis of EPS
4.5.2. AFM Analysis
4.5.3. FT-IR Analysis
4.5.4. TG Analysis
4.5.5. SEM-EDS Analysis
4.5.6. Contact Angle Measurement
4.5.7. Mercury Intrusion Analysis of Pore Size Distribution
4.6. Hydrogel Swelling Capacity
4.7. Adsorption Properties of Methylene Blue, Cefuroxim, and Cr (VI) onto EPS- and CAEPS-Hydrogel
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, W.; Ma, W.; Hu, Y.; Jin, M.; Li, K.; Chang, X.; Yu, X. Production planning for stochastic manufacturing/remanufacturing system with demand substitution using a hybrid ant colony system algorithm. J. Clean. Prod. 2019, 213, 999–1010. [Google Scholar] [CrossRef]
- Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Dual ionic cross-linked alginate/clinoptilolite composite microbeads with improved stability and enhanced sorption properties for methylene blue. React. Funct. Polym. 2017, 116, 31–40. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, C.; Li, J.; Pan, X.; Zhai, X.; Jiao, Y.; Qi, X. Highly efficient removal of antibiotic from biomedical wastewater using Fenton-like catalyst magnetic pullulan hydrogels. Carbohydr. Polym. 2021, 262, 117951. [Google Scholar] [CrossRef] [PubMed]
- Baig, U.; Faizan, M.; Sajid, M. Effective removal of hazardous pollutants from water and deactivation of water-borne pathogens using multifunctional synthetic adsorbent materials: A review–ScienceDirect. J. Clean. Prod. 2021, 302, 126735. [Google Scholar] [CrossRef]
- Singh, R.; Khangarot, R.K.; Singh, A.K.; Kumar, K. Metal Organic Frameworks Based Nanomaterial: Synthesis and Applications; Removal of Heavy Metal Ions from Waste Water. In Metal Nanocomposites for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Raschip, I.E.; Fifere, N.; Varganici, C.D.; Dinu, M.V. Development of antioxidant and antimicrobial xanthan-based cryogels with tuned porous morphology and controlled swelling features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef]
- Ghaedi, M.; Nasab, A.G.; Khodadoust, S.; Rajabi, M.; Azizian, S. Application of activated carbon as adsorbents for efficient removal of methylene blue: Kinetics and equilibrium study. J. Ind. Eng. Chem. 2014, 20, 2317–2324. [Google Scholar] [CrossRef]
- Liu, C.; Omer, A.M.; Ouyang, X.K. Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 2017, 106, 823–833. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Gupta, P.; Sawant, S.Y.; Lee, S.M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315. [Google Scholar] [CrossRef]
- Yimin, D.; Jiaqi, Z.; Danyang, L.; Lanli, N.; Liling, Z.; Yi, Z.; Xiaohong, Z. Preparation of Congo red functionalized Fe3O4@SiO2 nanoparticle and its application for the removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2018, 550, 90–98. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Composites 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2017, 250, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gan, L.; Owens, G.; Chen, Z. New nano-biomaterials for the removal of malachite green from aqueous solution via a response surface methodology. Water Res. 2018, 146, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Andreas, A.; Winata, Z.G.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ju, Y.H. Biocomposite hydrogel beads from glutaraldehyde-crosslinked phytochemicals in alginate for effective removal of methylene blue. J. Mol. Liq. 2021, 329, 115579. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Abu, E.; Sabaa, M.W.; Saad, G.R. Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 2018, 25, 6513–6529. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Su, T.; Lin, L.; Gong, Q. Honeycomb-like hydrogel adsorbents derived from salecan polysaccharide for wastewater treatment. Cellulose 2019, 26, 8759–8773. [Google Scholar] [CrossRef]
- Li, D.; Li, Q.; Mao, D.; Bai, N.; Dong, H. A versatile bio-based material for efficiently removing toxic dyes, heavy metal ions and emulsified oil droplets from water simultaneously. Bioresour. Technol. 2017, 245, 649–655. [Google Scholar] [CrossRef]
- Toledo, P.; Limeira, D.; Siqueira, N.C.; Petri, D. Carboxymethyl cellulose/poly(acrylic acid) interpenetrating polymer network hydrogels as multifunctional adsorbents. Cellulose 2019, 26, 597–615. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Development of chitosan-poly(ethyleneimine) based double network cryogels and their application as superadsorbents for phosphate. Carbohydr. Polym. 2019, 210, 17–25. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Hu, E.; Wah, C.; Yuen, M.; Jiang, S.X. Konjac glucomannan/graphene oxide hydrogel with enhanced dyes adsorption capability for methyl blue and methyl orange. Appl. Surf. Sci. 2015, 357, 866–872. [Google Scholar] [CrossRef]
- Shen, X.; Xie, Y.; Wang, Q.; Yi, X.; Shamshina, J.L.; Rogers, R.D. Enhanced heavy metal adsorption ability of lignocellulosic hydrogel adsorbents by the structural support effect of lignin. Cellulose 2019, 26, 4005–4019. [Google Scholar] [CrossRef]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.; Kipper, M.J.; Martins, A.F. New magnetic chitosan/alginate/Fe3O4@SiO2 hydrogel composites applied for removal of Pb(II) ions from aqueous systems. Chem. Eng. J. 2017, 337, 595–608. [Google Scholar] [CrossRef]
- Song, L.; Liu, F.; Zhu, C.; Li, A. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition. Chem. Eng. J. 2019, 369, 641–651. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Zhang, M.; Tong, X.; Jiang, N.; Pan, W.; Xu, L. Efficient decontamination of heavy metals from aqueous solution using pullulan/polydopamine hydrogels–ScienceDirect. Int. J. Biol. Macromol. 2020, 145, 1049–1058. [Google Scholar] [CrossRef]
- Sethi, S.; Kaith, B.S.; Saruchi; Kumar, V. Fabrication and characterization of microwave assisted carboxymethyl cellulose-gelatin silver nanoparticles imbibed hydrogel: Its evaluation as dye degradation. React. Funct. Polym. 2019, 142, 134–146. [Google Scholar] [CrossRef]
- Mittal, H.; Al-Alili, A.; Morajkar, P.P.; Alhassan, S.M. Graphene oxide crosslinked hydrogel nanocomposites of xanthan gum for the adsorption of crystal violet dye. J. Mol. Liq. 2020, 323, 115034. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Sun, Y.; Yu, F.; Ma, J. Ionically Cross-linked Sodium Alginate/-Carrageenan Double-Network Gel Beads with Low-swelling, Enhanced Mechanical Properties, and Excellent Adsorption Performance. Chem. Eng. J. 2019, 372, 1091–1103. [Google Scholar] [CrossRef]
- Sun, J.; Cui, L.; Gao, Y.; He, Y.; Liu, H.; Huang, Z. Environmental application of magnetic cellulose derived from Pennisetum sinese Roxb for effcient tetracycline removal. Carbohydr. Polym. 2021, 251, 117004. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; Guan, Y. Excellent AdsorptionDesorption of Ammonium by a Poly(acrylic acid)-Grafted Chitosan and Biochar Composite for Sustainable Agricultural Development. ACS Sustain. Chem. Eng. 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Su, B.; Ge, B.; Li, M.; Chen, Y.; Chen, Y.; Zhang, J.; Chen, H.; Li, J. Extraction, characterization and deoxy-liquefaction of crude polysaccharide from Enteromorpha prolifera to high-quality liquid oil. Fuel 2019, 237, 763–768. [Google Scholar] [CrossRef]
- Sun, J.; Gao, B.; Luo, Y.; Xue, M.; Xing, X.; Yue, Q.; Yan, W. Application and mechanism of polysaccharide extracted from Enteromorpha to remove nano-ZnO and humic acid in coagulation process. Front. Environ. Sci. Eng. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Comparisons of porous, surface chemistry and adsorption properties of carbon derived from Enteromorpha prolifera activated by H4P2O7 and KOH. Chem. Eng. J. Laussane 2013, 232, 582–590. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.S.; Sang, G.Y. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.L.; Zhou, Q.W.; Hao, S.X.; Zhou, T.; Xie, H.J. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2015, 107, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.L.; Xu, J.; Shi, M.J.; Wang, X.L.; Li, Y.T.; Kong, L.M.; Hider, R.C.; Zhou, T. Preparation, antioxidant and antimicrobial evaluation of hydroxamated degraded polysaccharides from Enteromorpha prolifera. Food Chem. 2017, 237, 481. [Google Scholar] [CrossRef]
- Dinari, M.; Shirani, M.A.; Maleki, M.H.; Tabatabaeian, R. Green cross-linked bionanocomposite of magnetic layered double hydroxide/guar gum polymer as an efficient adsorbent of Cr(VI) from aqueous solution. Carbohydr. Polym. 2020, 236, 116070. [Google Scholar] [CrossRef]
- Duman, O.; Polat, T.G.; Diker, C.Z.; Tun, S. Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int. J. Biol. Macromol. 2020, 160, 823–835. [Google Scholar] [CrossRef]
- Sarmah, D.; Karak, N. Double network hydrophobic starch based amphoteric hydrogel as an effective adsorbent for both cationic and anionic dyes. Carbohydr. Polym. 2020, 242, 116320. [Google Scholar] [CrossRef]
- Niu, Y.; Han, X.; Song, J.; Huang, L. Removal of methylene blue and lead(II) via PVA/SA double-cross-linked network gel beads loaded with Fe3O4@KHA nanoparticles. New J. Chem. 2021, 45, 5605–5620. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, F.; Wang, D.; Liu, X.; Jin, J. Polydopamine-mediated surface-functionalization of graphene oxide for heavy metal ions removal. J. Solid State Chem. 2015, 224, 88–93. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, H.; Wang, Y.; Yan, L.; Dong, W. Magnetic chitosan/anaerobic granular sludge composite: Synthesis, characterization and application in heavy metal ions removal. J. Colloid Interface Sci. 2017, 508, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ouyang, X.K.; Yang, L.Y.; Omer, A.M. Fabrication of a magnetic cellulose nanocrystal/metal-organic framework composite for removal of Pb(II) from water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Effective adsorption of methylene blue dye from water solution using renewable natural hydrogel bionanocomposite based on tragacanth gum: Linear-nonlinear calculations. Int. J. Biol. Macromol. 2021, 187, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xie, B.; Jin, D.; Zhang, A.; Hao, J.; Wang, S.; Jing, W.; Cao, J. The Anti-Inflammatory Effect and Structure of EPCP1-2 from Crypthecodinium cohnii via Modulation of TLR4-NF-κB Pathways in LPS-Induced RAW 264.7 Cells. Mar. Drugs 2017, 15, 376. [Google Scholar] [CrossRef] [PubMed]
- Xiaolei, M.; Duomo, D.; Xi, C.; Xuemin, F.; Ma, Y. A polysaccharide-based bioflocculant BP50-2 from banana peel waste: Purifcation, structure and flocculation performance. Int. J. Biol. Macromol. 2022, 205, 11. [Google Scholar]
- Mallakpour, S.; Tabesh, F. Green and plant-based adsorbent from tragacanth gum and carboxyl-functionalized carbon nanotube hydrogel bionanocomposite for the super removal of methylene blue dye-ScienceDirect. Int. J. Biol. Macromol. 2021, 166, 722–729. [Google Scholar] [CrossRef]



| Adsorption | MB | Cefuroxim | Cr2O72− | |||
|---|---|---|---|---|---|---|
| EPS-Hydrogel | CAEPS-Hydrogel | EPS-Hydrogel | CAEPS-Hydrogel | EPS-Hydrogel | CAEPS-Hydrogel | |
| Nonlinear-Langmuir | ||||||
| qm,L (mg g−1) | 95.28 | 96.41 | 33.92 | 35.78 | 80.82 | 83.67 |
| KL (L mg−1) | 0.0146 | 0.008 | 0.0389 | 0.1056 | 0.0139 | 0.0263 |
| R2 | 0.9690 | 0.9798 | 0.9883 | 0.9837 | 0.9867 | 0.9853 |
| Nonlinear-Freundlich | ||||||
| KF ((mg g−1) (L mg−1)1/n) | 11.06 | 6.6152 | 4.14 | 8.29 | 9.21 | 14.25 |
| 1/n | 0.3328 | 0.3954 | 0.4086 | 0.3174 | 0.3349 | 0.2865 |
| R2 | 0.9639 | 0.9188 | 0.9053 | 0.8767 | 0.9378 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Duan, D.; Chen, J.; Xie, B. Structure and Adsorption Performance of Cationic Entermorpha prolifera Polysaccharide-Based Hydrogel for Typical Pollutants: Methylene Blue, Cefuroxime, and Cr (VI). Gels 2022, 8, 546. https://doi.org/10.3390/gels8090546
Ma X, Duan D, Chen J, Xie B. Structure and Adsorption Performance of Cationic Entermorpha prolifera Polysaccharide-Based Hydrogel for Typical Pollutants: Methylene Blue, Cefuroxime, and Cr (VI). Gels. 2022; 8(9):546. https://doi.org/10.3390/gels8090546
Chicago/Turabian StyleMa, Xiaolei, Duomo Duan, Jinbin Chen, and Baolong Xie. 2022. "Structure and Adsorption Performance of Cationic Entermorpha prolifera Polysaccharide-Based Hydrogel for Typical Pollutants: Methylene Blue, Cefuroxime, and Cr (VI)" Gels 8, no. 9: 546. https://doi.org/10.3390/gels8090546
APA StyleMa, X., Duan, D., Chen, J., & Xie, B. (2022). Structure and Adsorption Performance of Cationic Entermorpha prolifera Polysaccharide-Based Hydrogel for Typical Pollutants: Methylene Blue, Cefuroxime, and Cr (VI). Gels, 8(9), 546. https://doi.org/10.3390/gels8090546






